Abstract
Objective: Although drug-eluting stent (DES) implantation is the primary treatment modality for bare-metal stent (BMS) in-stent restenosis (ISR), little is known about the efficacy and safety profile of DES in the treatment of DES-ISR. The goal of this study was to compare the clinical outcomes following DES treatment for BMS-ISR and DES-ISR. Methods: Rates of major adverse cardiac events (MACE) were compared in 97 consecutive patients who underwent DES implantation for the treatment of ISR (56 BMS-ISR and 41 DES-ISR) from January 2004 to December 2008. Results: Baseline clinical and procedural characteristics were comparable, except that the DES used in the BMS-ISR group was longer and had a larger diameter. The length of follow-up was (28.60±1.96) and (20.34±1.54) months for the BMS-ISR and DES-ISR groups, respectively. One patient (1.8%) experienced non-cardiac mortality and one (1.8%) had target-vessel revascularization (TVR) in the BMS-ISR group. In the DES-ISR group, three patients (7.3%) died of sudden death with a documented acute ST-segment elevation myocardial infarction, and three suffered TVR (7.3%). Kaplan-Meier analysis indicated that cumulative survival probability and MACE-free probability were both significantly lower for the DES-ISR group (log rank test P=0.047 and P=0.005, respectively). In Cox regression analysis, DES-ISR remained an independent predictor for future MACE occurrence after adjustment for other factors (compared with BMS-ISR, risk ratio (RR)=8.743, 95% confidence interval (CI) 1.54–49.54, P=0.014). Switching to a different type of DES to treat DES-ISR did not improve the prognosis. Conclusion: DES-ISR patients had a poorer prognosis than BMS-ISR patients after DES therapy.
Keywords: Atherosclerosis, In-stent restenosis, Bare-metal stent, Drug-eluting stent
1. Introduction
In-stent restenosis (ISR) occurs at a rate of 15% to 50% after bare-metal stent (BMS) implantation (Kastrati et al., 2001) and remains the major drawback of BMS treatment of coronary artery disease. Several randomized clinical trials, such as SISR (sirolimus-eluting stent vs. intravascular brachytherapy in in-stent restenotic coronary artery lesions), ISAR-DESIRE (sirolimus-eluting stent or paclitaxel-eluting stent vs. balloon angioplasty for prevention of recurrence in patient with coronary in-stent restenosis), RIBS-II (restenosis intrastent balloon angioplasty vs. elective sirolimus-eluting stenting), and TAXUS-V-ISR (paclitaxel-eluting stents vs. vascular brachytherapy for in-stent restenosis within bare-metal stents), and some real-world registries, such as TROPICAL (a study of the Cypher SES to treat restenotic native coronary artery lesions), TRUE (Tuscany registry of sirolimus for unselected in-stent restenosis registry), and RESEARCH (rapamycin-eluting stent evaluated at Rotterdam cardiology hospital registry), have compared the efficacy of different catheter-based strategies for the treatment of BMS-ISR (Saia et al., 2004; Kastrati et al., 2005a; Neumann et al., 2005; Alfonso et al., 2006; Holmes et al., 2006; Liistro et al., 2006; Stone et al., 2006). The results were similar: repeat percutaneous coronary intervention (PCI) with a drug-eluting stent (DES) for the treatment of a BMS-ISR lesion is superior to other therapies, including balloon angioplasty, intravascular coronary brachytherapy, or implantation of another BMS. DES therefore became the primary treatment modality for BMS-ISR treatment.
However, there is no consensus on how to treat DES-related ISR (DES-ISR). Emboldened by the positive results achieved in BMS-ISR patients, a few groups have reported using another DES to treat DES-ISR lesions. Moreover, this “DES in DES” technique may be more widely used to handle DES-ISR in clinical practice.
The goal of our study was to compare the clinical outcomes of DES treatment between BMS-ISR and DES-ISR patients. Our results lead one to question the rationale of simply copying the “DES in original stent” strategy for DES-ISR patients.
2. Patients and methods
2.1. Study population and procedures
The present study included consecutive ISR patients treated with DES in two clinical centers (Renji Hospital of Shanghai Jiao Tong University, Puxi and Pudong branches) from the beginning of 2004 to the end of 2008. ISR was defined as greater than 50% luminal narrowing at the segment including the original stent and within 5-mm proximal and distal to the stent edges by visual estimation. The pattern of ISR was classified according to Mehran et al. (1999)’s description. Either a sirolimus-eluting stent (SES) or a paclitaxel-eluting stent (PES) was used according to the operator’s discretion. All procedures and peri-procedural antiplatelet and anticoagulation therapies followed standard guidelines. Therapy at discharge was based on patient comorbidity.
Clinical follow-up for patient status was performed with routine telephone contact. Angiographic follow-up was achieved in available patients either by transcatheter angiography or computed tomography angiography.
The primary outcome of the study was the rate of major adverse cardiac events (MACE), including death, non-fatal myocardial infarction (MI), and target-vessel revascularization (TVR). MI was defined as the presence of prolonged chest pain followed by a total creatinine kinase (CK) level elevation more than three times the upper normal limit, a creatine kinase-myocardial band fraction (CK-MB) level more than two times the upper normal limit, and a positive troponin level (both T and I subtypes), with or without ST-segment elevation in the EKG. TVR was defined as repeat percutaneous or surgical revascularization in any segment of the coronary artery containing the stented lesion.
The authors of this manuscript have certified that they have complied with ethical principles.
2.2. Statistical analysis
Discrete variables are reported as percentages and compared with the Chi-square or the Fisher’s exact tests. Continuous variables are presented as mean±standard deviation (SD) and analyzed using the Student’s t test. Kaplan-Meier survival probability was used to test cumulative rates of death and MACE, respectively. Cox regression was performed for the adjustment of baseline difference. A P value less than 0.05 was considered statistically significant. Data were analyzed with SPSS 13 for Windows (SPSS Inc., Chicago, Illinois, USA).
3. Results
During the study period, 642 patients underwent angiographic follow-up after stent implantation. Among them, a total of 101 patients underwent DES treatment for ISR, with four patients excluded due to loss of contact. Of the remaining 97 patients, 56 developed ISR secondary to BMS implantation and 41 after DES implantation. Complete follow-up data for MACE were obtained.
Baseline clinical characteristics of these two groups of patients are listed in Table 1. Most clinical features in the two groups were well matched, except that a higher proportion of patients in the BMS-ISR group had a history of MI (60.7% vs. 39.0%, P=0.041) while a higher proportion of patients in the DES-ISR group had multivessel coronary disease (63.4% vs. 39.3%, P=0.024). Distributions of clinical presentations of ISR were also similar. Overall, 29.9% presented with stable angina, 33% with unstable angina, 12.4% with acute myocardial infarction (AMI), and 2.1% with heart failure. In total, 22.7% of patients presented with no apparent symptoms, and severe ISR was found only in routine angiographic follow-up; however, the majority of these patients demonstrated ischemic signs with 24-h EKG monitoring or echocardiography examination, thus representing a subset of ISR patients with silent ischemia.
Table 1.
Baseline clinical characteristics of patients
| Variable | Value* |
P | ||
| All (n=97) | BMS-ISR (n=56) | DES-ISR (n=41) | ||
| Age (year) | 64.28±10.85 | 63.73±11.89 | 65.73±9.26 | 0.373 |
| Male (%) | 76.3 | 80.4 | 70.7 | 0.336 |
| Diabetes mellitus (%) | 34.0 | 26.8 | 43.9 | 0.088 |
| Hypertension (%) | 70.1 | 64.3 | 78.0 | 0.180 |
| Renal insufficiency (%) | 3.1 | 1.8 | 4.9 | 0.572 |
| TC (mmol/L) | 4.39±1.04 | 4.44±0.92 | 4.32±1.19 | 0.584 |
| LDL-C (mmol/L) | 2.63±0.83 | 2.68±0.75 | 2.56±0.93 | 0.484 |
| Prior MI (%) | 51.5 | 60.7 | 39.0 | 0.041 |
| Prior CABG (%) | 2.1 | 0 | 4.9 | 0.176 |
| Multivessel disease (%) | 49.5 | 39.3 | 63.4 | 0.024 |
| Presentation of ISR | ||||
| Stable angina (%) | 29.9 | 30.4 | 29.3 | 1.000 |
| Unstable angina (%) | 33.0 | 32.1 | 34.1 | 1.000 |
| AMI (%) | 12.4 | 16.1 | 7.3 | 0.229 |
| Heart failure (%) | 2.1 | 1.8 | 2.4 | 1.000 |
| Severe restenosis without symptom (%) | 22.7 | 19.6 | 26.8 | 0.466 |
Values are expressed as mean±SD or percent
TC: total cholesterol; LDL-C: low density lipoprotein cholesterol; MI: myocardial infarction; CABG: coronary artery bypass graft; AMI: acute myocardial infarction; BMS-ISR: bare-metal stent in-stent restenosis; DES-ISR: drug-eluting stent in-stent restenosis. Renal insufficiency is based on a history of baseline creatinine >1.5 mg/dl at admission. Multivessel disease is based on a history of >70% luminal narrowing in more than two coronary arteries
Table 2 presents baseline angiographic characteristics and procedural parameters. Distributions of lesions and ISR morphologies were comparable in the two groups: ISR occurred more often in the left artery descending (LAD) coronary artery lesions; and the distributions of angiographic patterns of ISR were relatively balanced in each type. One hundred and fourteen DESs were implanted to treat 98 ISR lesions: 28 PESs, 84 SESs, and 2 tacrolimus-eluting stents (TESs). Implanted stents in the BMS-ISR group had a significantly larger diameter [(3.03±0.36) vs. (2.86±0.36) mm, P=0.014] and greater length [(24.6±7.13) vs. (21.11±7.14) mm, P=0.012] than in the DES-ISR group. There were no combinations of different types of DES for a given patient. The procedural success rate was 100% in both groups. The main medical therapy at discharge was similar in the two groups.
Table 2.
Baseline angiographic features and procedural parameters
| Variable | Value* |
P | ||
| All | BMS-ISR | DES-ISR | ||
| ISR lesions as unit | n=98 | n=57 | n=41 | |
| ISR location | ||||
| LM (%) | 1.0 | 1.8 | 0 | 1.000 |
| LAD (%) | 55.1 | 50.9 | 61.0 | 0.411 |
| LCX (%) | 12.1 | 10.5 | 14.6 | 1.000 |
| RCA (%) | 31.6 | 36.8 | 24.4 | 0.271 |
| Angiographic morphology of ISR | ||||
| Focal (%) | 30.6 | 26.3 | 36.6 | 0.374 |
| Diffuse intra-stent (%) | 21.4 | 17.5 | 26.8 | 0.322 |
| Diffuse proliferative (%) | 27.6 | 33.3 | 19.5 | 0.171 |
| Occlusive (%) | 20.4 | 22.8 | 17.1 | 0.614 |
| Stent as unit | n=114 | n=68 | n=46 | |
| Stent size (mm) | 2.96±0.37 | 3.03±0.36 | 2.86±0.36 | 0.014 |
| Stent length (mm) | 23.19±7.31 | 24.6±7.13 | 21.11±7.14 | 0.012 |
| Treatment DES type | ||||
| SES (%) | 73.7 | 73.5 | 73.9 | 1.000 |
| PES (%) | 24.6 | 25.0 | 23.9 | 1.000 |
| TES (%) | 1.8 | 1.5 | 2.2 | 1.000 |
| Patient as unit | n=97 | n=56 | n=41 | |
| Multiple stenting at target vessel (%) | 13.4 | 16.1 | 9.8 | 0.386 |
| Post dilation (%) | 33.0 | 33.9 | 31.7 | 1.000 |
| Therapy at discharge | ||||
| Aspirin (%) | 97.9 | 98.2 | 97.6 | 1.000 |
| Clopidogrel (%) | 100 | 100 | 100 | N/A |
| ARBS/ACE-I (%) | 57.7 | 60.7 | 53.7 | 0.536 |
| Statin (%) | 84.5 | 78.6 | 92.7 | 1.000 |
Values are expressed as mean±SD or percent
LM: left main; LAD: left artery descending artery; LCX: left circumflex artery; RCA: right coronary artery; SES: sirolimus-eluting stent; PES: paclitaxel-eluting stent; TES: tacrolimus-eluting stent; ARBs: angiotensin II receptor blockers; ACE-I: angiotensin-converting enzyme inhibitor I; BMS-ISR: bare-metal stent in-stent restenosis; DES-ISR: drug-eluting stent in-stent restenosis; N/A: not available
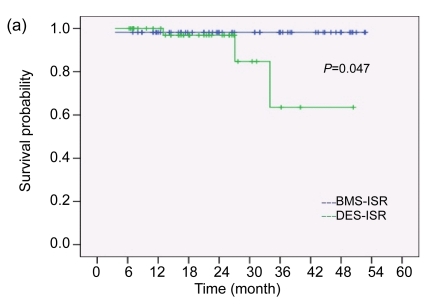
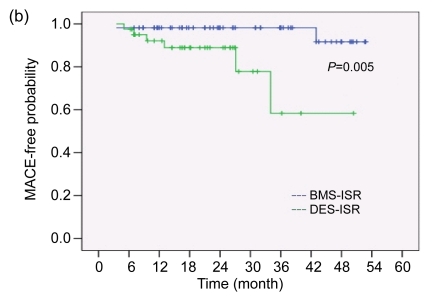
No patients presented with MACE during hospitalization. The mean duration of follow-up was 28.63 months in the BMS-ISR group and 20.34 months in the DES-ISR group. Table 3 compares the cumulative outcomes during follow-up. Patients in the DES-ISR group experienced more MACE than those in the BMS-ISR group. A total of four patients died after treatment: one patient in the BMS-ISR group died of multi-organ failure from severe infection four months after the procedure (1.8%). In the DES-ISR group, three patients died of sudden death in the emergency room secondary to ST-segment elevation MI (7.3%). Clinically-driven TVR also occurred more often in the DES-ISR group than in the BMS-ISR group (7.3% vs. 1.8 %). There were no non-fatal MIs in both groups. The Kaplan-Meier estimates of survival and event-free time were both significantly higher for the BMS-ISR group than for the DES-ISR group (log rank test, P=0.047 for survival and P=0.005 for MACE-free time, Fig. 1). For adjustment of baseline differences, we performed Cox regression analysis to determine predictors of future MACE after therapy of ISR. After the adjustment of variables including age, gender, comorbidity, history of coronary disease, original stent type, presentation, location and morphology of ISR, treatment DES type, procedural parameters, and medications, the original stent type remained an independent predictor of MACE. After DES implantation for ISR, patients with DES-ISR had a higher probability of experiencing MACE than patients with BMS-ISR (risk ratio (RR)=8.743, 95% confidence interval (CI) 1.54–49.54, P=0.014). To further investigate if the use of a different type of DES than the original stent could more influence clinical outcomes in the DES-ISR patients, we compared MACE rates between those cases in which a switch was done and those cases in which it was not. As shown in Table 4, there was a trend toward a lower rate of MACE in patients who received a different type of DES; however, it did not reach statistical significance. Cox regression was not performed in this part of the analysis due to insufficient power.
Table 3.
Clinical outcomes during follow-up
| Variable | Value* |
||
| All patients (n=97) | BMS-ISR (n=56) | DES-ISR (n=41) | |
| Follow-up period (month) | 25.13±1.36 | 28.63±1.96 | 20.34±1.54 |
| Death | 4 (4.1%) | 1 (1.8%) | 3 (7.3%) |
| Non-fatal MI | 0 (0%) | 0 (0%) | 0 (0%) |
| TVR | 4 (4.1%) | 1 (1.8%) | 3 (7.3%) |
| Overall MACE | 8 (8.2%) | 2 (3.6%) | 6 (14.6%) |
Values are expressed as mean±SD or number (percent)
MI: myocardial infarction; TVR: target vessel revascularization; MACE: major adverse cardiac event; BMS-ISR: bare-metal stent in-stent restenosis; DES-ISR: drug-eluting stent in-stent restenosis
Fig. 1.
Kaplan-Meier estimates of survival probability (a) and MACE-free probability (b)
Table 4.
Type of DES used in DES-ISR group
| Variable | Value* |
P | |
| Hetero-DES (n=16) | Homo-DES (n=25) | ||
| MACE | 1 (6.25%) | 5 (20%) | 0.376 |
| Event-free | 15 (93.75%) | 20 (80%) | |
Values are expressed as number (percent)
Homo-DES: using the same type of DES as the original DES to treat DES-ISR; Hetero-DES: using another type of DES to treat DES-ISR; MACE: major adverse cardiac events
4. Discussion
The use of DES has led to a remarkable reduction in ISR. However, it is estimated that at a long-term follow-up (>3 years) after DES implantation, more than 10% of lesions still require repeat intervention (Kastrati et al., 2005b; Cosgrave et al., 2007a). Due to the exponential increase of DES implantation in the past decade, ISR secondary to DES is seen much more frequently in clinical practice than before. The main purpose of the present study was to ascertain whether the “another DES” strategy is as safe and effective in DES-ISR patients as it is in BMS-ISR patients. Our data yielded a negative answer to this question. The MACE rate was up to 14.6%, including a 7.3% death rate, in the DES-ISR group over just 20 months of follow-up, while MACE only occurred in 3.6% of the BMS-ISR patients (1.8% of death) after a mean follow-up of more than 28 months.
This may seem counterintuitive, but a careful examination of the issue suggests that different factors that influence secondary ISR formation may be the underlying mechanisms. Because the tubular slotted stent does not recoil, which in turn prevents geometric arterial constriction, ISR is mainly due to simple neointimal hyperplasia after BMS therapy (Dussaillant et al., 1995; Hoffmann et al., 1996), which is the very target of DES. The average late loss of lumen diameter after primary BMS placement was about 0.8 mm (Bestehorn et al., 2004). The use of DES in a native coronary stenosis decreased the lumen diameter loss to about 0.08 mm (Sousa et al., 2001; Moses et al., 2003). This capability of DES to reduce neointimal formation remains powerful when treating BMS-ISR. The lowest late luminal loss after DES implantation in BMS-ISR lesions has been reported as (0.08±0.49) mm, which is on the same order of magnitude as that seen in native lesions (Neumann et al., 2005). Other results yielded ranges from 0.16 to 0.45 mm (Degertekin et al., 2003; Sousa et al., 2003; Kastrati et al., 2005a). This may account for the low rate of TVR in BMS-ISR patients after DES treatment.
However, when ISR occurs within DES, the situation is much more complicated. First, the initial DES failure would be a strong signal that the patient may have an inherent intensive intimal response to stent implantation, which is resistant to the eluting drug. Second, nearly all polymers of DES are associated with prolonged inflammatory reactions in vessels, which have long proved to be a driving stimulus for neointimal thickening (van der Giessen et al., 1996; Kornowski et al., 1998). Third, the development of DES-ISR is likely to be linked to a greater propensity of micro-thrombosis, which is attributed to delayed re-endothelialization over the stent surface. In addition, chemoattractants and growth factors released by activated platelets within the thrombi may contribute to the recruitment of smooth muscle cells and their proliferation within the stent. Based on these points, the rationale to use another DES for the treatment of DES-ISR is theoretically flawed.
Some have presumed that using a type of DES other than that of the original stent would be effective because of the different mechanisms by which to inhibit neointimal hyperplasia. However, this hypothesis has not been proved in clinical observation. Garg et al. (2007), Cosgrave et al. (2007b), and Sardella et al. (2009) reported, respectively, a similar prognosis between homo-DES and hetero-DES strategies in the treatment of DES-ISR. In our study, there was a trend toward lower MACE rates in the DES-ISR patients using a different type of DES, but it did not reach statistical significance. Moreover, the real inflammation-inducing influence of a double layer of polymers is unknown, but the polymer-associated inflammation is very likely to be responsible for the high incidence of repeat TVR in the DES-ISR group. Finally, it should be specially noted that three DES-ISR patients in our study suffered sudden death after a documented acute MI. Therefore, the risk of catastrophic thrombosis due to potentially further postponed re-endothelialization should be carefully evaluated when considering the DES-in-DES modality.
In comparison to previous reports of DES therapy for ISR, the present study found a relatively low TVR rate for both groups of patients despite a similar death rate. The primary reason may be a lower rate of angiographic follow-up after the treatment in this study, which may miss asymptomatic patients with recurrent restenosis. The higher frequency and duration of medical therapy (aspirin, clopidogrel, and statin) compared to previous studies may also contribute to the decreased TVR rate. Despite the differences in the follow-up length and the patient race, results of the present study are consistent with two recent reports, which also documented worse outcomes in DES-ISR patients than in BMS-ISR patients after undergoing DES-sandwich treatment (Whan Lee et al., 2008; Steinberg et al., 2009).
Based on the current evidence, the effect of another DES on DES-ISR treatment is at least suboptimal. Interventional cardiologists should be more cautious about using this technique, considering its high rate of recurrent restenosis and cardiac death. The efficacy of other catheter-based modalities should be re-assessed in the context of DES-ISR treatment. New devices such as drug-coated balloon, polymer-free DES, and bioabsorbable stent as well as pharmacologic approaches may be promising in the treatment of DES-ISR.
However, our present study still has some limitations. Our analysis and conclusion are subject to the inherent limitations of a retrospective study. The relatively small sample size in each group is another limitation.
Acknowledgments
We expressed profound thanks to Mr. Zhi-qiang YIN for his kind help with patient follow-up, and to Dr. Bin-shi GUO (GlaxoSmithKline, China) for his careful language revision.
Footnotes
Project (No. 08XD14026) supported by the Program of Shanghai Subject Chief Scientist, China
References
- 1.Alfonso F, Pérez-Vizcayno MJ, Hernandez R, Bethencourt A, Martí V, López-Mínguez JR, Angel J, Mantilla R, Morís C, Cequier A, et al. A randomized comparison of sirolimus-eluting stent with balloon angioplasty in patients with in-stent restenosis: results of the restenosis intrastent balloon angioplasty versus elective sirolimus-eluting stenting (RIBS-II) trial. Journal of the American College of Cardiology. 2006;47(11):2152–2160. doi: 10.1016/j.jacc.2005.10.078. [DOI] [PubMed] [Google Scholar]
- 2.Bestehorn HP, Neumann FJ, Büttner HJ, Betz P, Stürzenhofecker P, von Hodenberg E, Verdun A, Levai L, Monassier JP, Roskamm H. Evaluation of the effect of oral verapamil on clinical outcome and angiographic restenosis after percutaneous coronary intervention: the randomized, double-blind, placebo-controlled, multicenter verapamil slow-release for prevention of cardiovascular events after angioplasty (VESPA) trial. Journal of the American College of Cardiology. 2004;43(12):2160–2165. doi: 10.1016/j.jacc.2004.02.047. [DOI] [PubMed] [Google Scholar]
- 3.Cosgrave J, Melzi G, Corbett S, Biondi-Zoccai GG, Agostoni P, Babic R, Airoldi F, Chieffo A, Sangiorgi GM, Montorfano M, et al. Comparable clinical outcomes with paclitaxel- and sirolimus-eluting stents in unrestricted contemporary practice. Journal of the American College of Cardiology. 2007;49(24):2320–2328. doi: 10.1016/j.jacc.2007.02.057. [DOI] [PubMed] [Google Scholar]
- 4.Cosgrave J, Melzi G, Corbett S, Biondi-Zoccai GG, Babic R, Airoldi F, Chieffo A, Sangiorgi GM, Montorfano M, Michev I, et al. Repeated drug-eluting stent implantation for drug-eluting stent restenosis: the same or a different stent. American Heart Journal. 2007;153(3):354–359. doi: 10.1016/j.ahj.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 5.Degertekin M, Regar E, Tanabe K, Smits PC, van der Giessen WJ, Carlier SG, de Feyter P, Vos J, Foley DP, Ligthart JM, et al. Sirolimus-eluting stent for treatment of complex in-stent restenosis: the first clinical experience. Journal of the American College of Cardiology. 2003;41(2):184–189. doi: 10.1016/S0735-1097(02)02704-3. [DOI] [PubMed] [Google Scholar]
- 6.Dussaillant GR, Mintz GS, Pichard AD, Kent KM, Satler LF, Popma JJ, Wong SC, Leon MB. Small stent size and intimal hyperplasia contribute to restenosis: a volumetric intravascular ultrasound analysis. Journal of the American College of Cardiology. 1995;26(3):720–724. doi: 10.1016/0735-1097(95)00249-4. [DOI] [PubMed] [Google Scholar]
- 7.Garg S, Smith K, Torguson R, Okabe T, Slottow TL, Steinberg DH, Roy P, Xue Z, Gevorkian N, Satler LF. Treatment of drug-eluting stent restenosis with the same versus different drug-eluting stent. Catheterization and Cardiovascular Interventions. 2007;70(1):9–14. doi: 10.1002/ccd.21106. [DOI] [PubMed] [Google Scholar]
- 8.Hoffmann R, Mintz GS, Dussaillant GR, Popma JJ, Pichard AD, Satler LF, Kent KM, Griffin J, Leon MB. Patterns and mechanisms of in-stent restenosis: a serial intravascular ultrasound study. Circulation. 1996;94(6):1247–1254. doi: 10.1161/01.cir.94.6.1247. [DOI] [PubMed] [Google Scholar]
- 9.Holmes DRJr, Teirstein P, Satler L, Sketch M, O'Malley J, Popma JJ, Kuntz RE, Fitzgerald PJ, Wang H, Caramanica E, et al. Sirolimus-eluting stents vs. vascular brachytherapy for in-stent restenosis within bare-metal stents: the SISR randomized trial. The Journal of the American Medical Association. 2006;295(11):1264–1273. doi: 10.1001/jama.295.11.1264. [DOI] [PubMed] [Google Scholar]
- 10.Kastrati A, Mehilli J, Dirschinger J, Pache J, Ulm K, Schühlen H, Seyfarth M, Schmitt C, Blasini R, Neumann FJ, et al. Restenosis after coronary placement of various stent types. The American Journal of Cardiology. 2001;87(1):34–39. doi: 10.1016/S0002-9149(00)01268-6. [DOI] [PubMed] [Google Scholar]
- 11.Kastrati A, Mehilli J, von Beckerath N, Dibra A, Hausleiter J, Pache J, Schühlen H, Schmitt C, Dirschinger J, Schömig A, et al. Sirolimus-eluting stent or paclitaxel-eluting stent vs. balloon angioplasty for prevention of recurrence in patient with coronary in-stent restenosis: a randomized controlled trial (ISAR-DESIRE) The Journal of the American Medical Association. 2005;293(2):165–171. doi: 10.1001/jama.293.2.165. [DOI] [PubMed] [Google Scholar]
- 12.Kastrati A, Dibra A, Eberle S, Mehilli J, Suárez de Lezo J, Goy JJ, Ulm K, Schömig A. Sirolimus-eluting stents vs. paclitaxel-eluting stents in patients with coronary artery disease: meta-analysis of randomized trials. The Journal of the American Medical Association. 2005;294(7):819–825. doi: 10.1001/jama.294.7.819. [DOI] [PubMed] [Google Scholar]
- 13.Kornowski R, Hong MK, Tio FO, Bramwell O, Wu H, Leon MB. In-stent restenosis: contributions of inflammatory responses and arterial injury to neointimal hyperplasia. Journal of the American College of Cardiology. 1998;31(1):224–230. doi: 10.1016/S0735-1097(97)00450-6. [DOI] [PubMed] [Google Scholar]
- 14.Liistro F, Fineschi M, Angioli P, Sinicropi G, Falsini G, Gori T, Ducci K, Bravi A, Bolognese L. Effectiveness and safety of sirolimus stent implantation for coronary in-stent restenosis: the TRUE (Tuscany registry of sirolimus for unselected in-stent restenosis) Registry. Journal of the American College of Cardiology. 2006;48(2):270–275. doi: 10.1016/j.jacc.2006.03.039. [DOI] [PubMed] [Google Scholar]
- 15.Mehran R, Dangas G, Abizaid AS, Mintz GS, Lansky AJ, Satler LF, Pichard AD, Kent KM, Stone GW, Leon MB. Angiographic patterns of in-stent restenosis: classification and implications for long-term outcome. Circulation. 1999;100(18):1872–1878. doi: 10.1161/01.cir.100.18.1872. [DOI] [PubMed] [Google Scholar]
- 16.Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O'Shaughnessy C, Caputo RP, Kereiakes DJ, Williams DO, Teirstein PS, et al. Sirolimus-luting stents versus standard stents in patients with stenosis in a native coronary artery. The New England Journal of Medicine. 2003;349(14):1315–1323. doi: 10.1056/NEJMoa035071. [DOI] [PubMed] [Google Scholar]
- 17.Neumann FJ, Desmet W, Grube E, Brachmann J, Presbitero P, Rubartelli P, Mügge A, Di Pede F, Füllgraf D, Aengevaeren W, et al. Effectiveness and safety of sirolimus-eluting stents in the treatment of restenosis after coronary stent placement. Circulation. 2005;111(16):2107–2111. doi: 10.1161/01.CIR.0000162467.53001.6B. [DOI] [PubMed] [Google Scholar]
- 18.Saia F, Lemos PA, Arampatzis CA, Hoye A, Degertekin M, Tanabe K, Sianos G, Smits PC, van der Giessen WJ, de Feyter PJ, et al. Routine sirolimus eluting stent implantation for unselected in-stent restenosis: insights from the rapamycin eluting stent evaluated at Rotterdam cardiology hospital (RESEARCH) registry. Heart. 2004;90(10):1183–1188. doi: 10.1136/hrt.2003.025536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sardella G, Colantonio R, de Luca L, Conti G, di Roma A, Mancone M, Canali E, Benedetti G, Fedele F. Comparison between balloon angioplasty and additional coronary stent implantation for the treatment of drug-eluting stent restenosis: 18-month clinical outcomes. Journal of Cardiovascular Medicine (Hagerstown, Md) 2009;10(6):469–473. doi: 10.2459/JCM.0b013e3283297c23. [DOI] [PubMed] [Google Scholar]
- 20.Sousa JE, Costa MA, Abizaid AC, Rensing BJ, Abizaid AS, Tanajura LF, Kozuma K, van Langenhove G, Sousa AG, Falotico R, et al. Sustained suppression of neointimal proliferation by sirolimus-eluting stents: one-year angiographic and intravascular ultrasound follow-up. Circulation. 2001;104(17):2007–2011. doi: 10.1161/hc4201.098056. [DOI] [PubMed] [Google Scholar]
- 21.Sousa JE, Costa MA, Abizaid A, Sousa AG, Feres F, Mattos LA, Centemero M, Maldonado G, Abizaid AS, Pinto I, et al. Sirolimus-eluting stent for the treatment of in-stent restenosis: a quantitative coronary angiography and three-dimensional intravascular ultrasound study. Circulation. 2003;107(1):24–27. doi: 10.1161/01.CIR.0000047063.22006.41. [DOI] [PubMed] [Google Scholar]
- 22.Steinberg DH, Gaglia MAJr, Pinto Slottow TL, Roy P, Bonello L, de Labriolle A, Lemesle G, Torguson R, Kineshige K, Xue Z, et al. Outcome differences with the use of drug-eluting stents for the treatment of in-stent restenosis of bare-metal stents versus drug-eluting stents. The American Journal of Cardiology. 2009;103(4):491–495. doi: 10.1016/j.amjcard.2008.09.107. [DOI] [PubMed] [Google Scholar]
- 23.Stone GW, Ellis SG, O'Shaughnessy CD, Martin SL, Satler L, McGarry T, Turco MA, Kereiakes DJ, Kelley L, Popma JJ, et al. Paclitaxel-eluting stents vs. vascular brachytherapy for in-stent restenosis within bare-metal stents: the TAXUS V ISR randomized trial. The Journal of the American Medical Association. 2006;295(11):1253–1263. doi: 10.1001/jama.295.11.1253. [DOI] [PubMed] [Google Scholar]
- 24.van der Giessen WJ, Lincoff AM, Schwartz RS, van Beusekom HM, Serruys PW, Holmes DRJr, Ellis SG, Topol EJ. Marked inflammatory sequelae to implantation of biodegradable and nonbiodegradable polymers in porcine coronary arteries. Circulation. 1996;94(7):1690–1697. doi: 10.1161/01.cir.94.7.1690. [DOI] [PubMed] [Google Scholar]
- 25.Whan Lee C, Kim SH, Suh J, Park DW, Lee SH, Kim YH, Hong MK, Kim JJ, Park SW, Park SJ. Long-term clinical outcomes after sirolimus-eluting stent implantation for treatment of restenosis within bare-metal versus drug-eluting stents. Catheterization and Cardio-vascular Interventions. 2008;71(5):594–598. doi: 10.1002/ccd.21399. [DOI] [PubMed] [Google Scholar]