Abstract
Objective: This study aims to determine the mechanisms underlying restenosis and ischemia-reperfusion injury of the myocardium after percutaneous coronary intervention (PCI). Methods: The present study examined serial changes (5 min, 30 min, 2 h, 6 h, and 24 h after PCI) in circulating P-selectin, plasminogen activator inhibitor-1 (PAI-1), magnesium (Mg), and creatine kinase-myocardial band fraction (CK-MB) levels, which may be associated with restenosis and myocardial injury in patients undergoing PCI. The occurrence rates of major adverse cardiovascular events were collected over a six-month follow-up. Results: PCI induced an early elevation of P-selectin, which correlated positively with the inflation pressure used in the PCI procedure. PCI also caused a significant and sustained decrease in serum Mg in PCI patients, without an effect on PAI-1. An increase in CK-MB was observed in PCI patients, although values were within normal reference range. In addition, elevated P-selectin and decreased Mg measured shortly after the coronary angioplasty procedure were associated with recurrent treatment and heart failure, respectively. Conclusions: Our study demonstrates that PCI induces temporal changes of P-selectin, Mg, and CK-MB, which may be involved in restenosis and ischemia-reperfusion injury. These findings highlight the need for using antiplatelet therapy and Mg to reduce the risks associated with PCI.
Keywords: Percutaneous coronary intervention, Restenosis, Ischemia-reperfusion injury, P-selectin, Magnesium
1. Introduction
Percutaneous coronary intervention (PCI) has been a common therapeutic method in the treatment of coronary artery disease since introduced in 1977 (Gruntzig, 1978). However, late restenosis is still quite frequent despite successful angioplasty (Serruys et al., 1988; Pendyala et al., 2008). Another risk is ischemia-reperfusion injury of the myocardium occurring in the immediate stage after successful angioplasty, despite extensive efforts to reduce its incidence (Park and Lucchesi, 1999). Restenosis and ischemia-reperfusion injury of the myocardium may be viewed as multifactorial responses of the vascular tissue to balloon and stent injury and reflow, which include endothelial dysfunction, platelet activation, disorder of electrolytes, and clotting-plasminogen imbalance (Monnink et al., 2003; Bhoday et al., 2006; Ma et al., 2006; Thanyasiri et al., 2007). However, the exact underlying mechanisms are not clear.
It has been reported that the platelet activity and the plasmin activation system play pivotal roles in the development of the restenosis after PCI (Stone and Aronow, 2006; Katsaros et al., 2008). Increased platelet activity, evidenced by membrane P-selectin expression on circulating platelets, is associated not only with a higher risk of stent thrombosis, but also with a higher risk of later restenosis development (Tschoepe et al., 1993; Murasaki et al., 2007; Osmancik et al., 2008). Plasminogen activator inhibitor-1 (PAI-1), an important member of the plasmin activation system, is a predictor of restenosis after stent placement (Katsaros et al., 2008). Both P-selectin and PAI-1 are stored in the granule of static platelets or Weibel-Palade bodies of endothelial cells, and they may act jointly in the development of the restenosis. Nevertheless, little is known on their simultaneous expression pattern after PCI.
Our previous study has shown that the administration of magnesium (Mg) inhibits the increase in P-selectin expression on platelets and endothelium induced by ischemia-reperfusion injury in rats (Ying et al., 2007). Some other studies have demonstrated the cardioprotective effects of Mg after ischemia-reperfusion injury (Headrick et al., 1998; Ravn et al., 1999; Moens et al., 2005) and that intravenous or oral application of Mg has marked antithrombotic effects (Shechter et al., 1999). A significant decrease in serum Mg levels at 3 and 30 min after coronary artery bypass graft (CABG) surgery has been reported (Satur et al., 1994), but limited data exist on changes in serum Mg concentrations in patients after PCI.
In the present study, we investigated serial changes of circulating P-selectin, PAI-1, Mg, and creatine kinase-myocardial band fraction (CK-MB) (a sensitive marker of injured myocardium) in patients undergoing PCI. The occurrence rates of heart failure, re-admission due to cardiovascular events (CVs), recurrent treatment with PCI or CABG, and cardiovascular death were collected over a six-month follow-up. This study will help better understand the dynamic changes of these molecules, which are involved in restenosis and ischemia-reperfusion injury after PCI, and will provide evidence on applying suitable pharmacological interventions to reduce the risks associated with PCI.
2. Patients and methods
2.1. Study population
Subjects were recruited from consecutive in-patients undergoing coronary angiography (CAG) in the Second Affiliated Hospital, School of Medicine, Zhejiang University. Our study population consisted of 34 subjects with positive CAG (more than 50% stenosis in coronary arteries): 24 (22 men and 2 women) undergoing successful PCI, with a mean age of (62.7±10.9) years (range 28–86 years), were included as “the PCI group”; the remaining 10 (9 men and 1 woman) without PCI because of severe lesions of the coronary artery such as left main, multi-vessel, and diffuse lesions, with a mean age of (67.4±1.1) years (range 45–86 years), were included as the “positive CAG group”. The other 16 subjects (14 men and 2 women) with negative CAG (less than 50% stenosis of coronary arteries) were included as the “negative CAG group”, with a mean age of (61.0±10.7) years (range 43–78 years).
2.2. Study protocol
The experimental protocol was approved by the Human Subjects Review Committee of the Second Affiliated Hospital, School of Medicine, Zhejiang University, and all subjects provided written informed consent.
2.3. Angioplasty procedures
All patients received nitroglycerin in the coronary artery (100–200 μg) before angioplasty (at baseline and at follow-up angiography) to avoid coronary spasm. After placement of a vascular sheath, 100 U/10 kg heparin was administered intravenously. Once it was determined that patients were suitable for angioplasty, intracoronary nitroglycerin (0.1 mg) was administered during CAG to all subjects in all groups before performing angiography. CAG was performed using the standard available balloon catheters sized to the normal lumen diameter of the artery to be dilated. Inflation duration and pressure were left to the discretion of the angioplasty operator. Drug-eluting stents were used in all cases in this study. Upon completion of the procedure, 10 min was allowed to pass before repeating the angiograms of the dilated vessel. Selective coronary arteriography was performed in multiple views using the Judkins technique.
2.4. Laboratory tests, patient management, and follow-up
A 19-gauge butterfly catheter was inserted atraumatically without a tourniquet into an antecubital vein. Whole blood samples were taken from the three groups before the procedure and at 5 min, 30 min, 2 h, 6 h, and 24 h after the procedure. A total of 3.6 ml blood was mixed with 0.4 ml 3.8% (w/w) sodium citrate (9:1, v/v) in an anticoagulant tube and citrated plasma was obtained by centrifugation at 2500 r/min for 15 min at 4 °C. Aliquots were stored at −70 °C to allow batch analysis. Soluble P-selectin was measured by the enzyme-linked immunosorbent assay (ELISA) using commercial reagents (R&D Systems, Abingdon, UK) (Li-Saw-Hee et al., 2000). PAI-1 was measured by a luminescence substrate method. Another 3 ml blood was placed in a centrifuge tube without anticoagulants and serum was obtained by centrifugation for the evaluation of full blood chemistry (Beckman Synchron CX3, Beckman Instruments Inc., Brea, CA, USA) including Mg levels (atomic absorption spectrophotometry; Perkin-Elmer 3100, Norwalk, CN, USA) (Roth et al., 1994). Subjects were routinely discharged 3–5 d after CAG. They were seen again at the out-patient clinic at 1, 3, and 6 months after discharge. Laboratory tests and 12-lead electrocardiogram were performed, while medication modification and major adverse CVs, including heart failure, re-admission due to CVs, recurrent treatment with PCI or CABG, and cardiovascular death, were recorded.
2.5. Statistical analysis
All continuous variables were expressed as mean±standard error of the mean (SEM). Data were normally distributed. Student t-test, or paired t-test, or one-way parametric analysis of variance (ANOVA) followed by Newman-Keuls as a post hoc test, was employed to detect differences in parametric variables accordingly. Chi-squared analysis was used to detect differences among the categorical variables. Pearson’s correlation was used to measure correlations between variables. A value of P<0.05 was required to reject the null hypothesis.
3. Results
3.1. Baseline characteristics
Baseline values (including age, gender, type of disease, risk factors such as hypertension, diabetes mellitus, current smoking status, and hyperlipidaemia, etc.) of all the three groups before CAG were compared, and no significant differences were found (all P>0.05) (Table 1).
Table 1.
Baseline characteristics of the three groups
| Baseline characteristic | Value* |
||
| PCI (n=24) | Positive CAG (n=10) | Negative CAG (n=16) | |
| Age (year) | 62.7±10.1 | 67.4±10.1 | 61.0±10.7 |
| Gender (M/F) | 22/2 | 9/1 | 14/2 |
| Type of disease | |||
| Acute MI | 6 | 1 | 0 |
| Subacute MI | 6 | 2 | 0 |
| Old MI | 4 | 1 | 0 |
| Unstable angina | 5 | 5 | 0 |
| Stable angian pectoris | 3 | 1 | 0 |
| Risk factors | |||
| Hypertension | 7 | 4 | 4 |
| Diabetes mellitus | 3 | 2 | 2 |
| Smoking | 9 | 5 | 4 |
| Hyperlipidaemia | 4 | 2 | 3 |
| Medication | |||
| Aspirin | 18 | 7 | 11 |
| ADP-receptor antagonist | 20 | 8 | 13 |
| ACEI | 10 | 4 | 6 |
| ARB | 3 | 1 | 2 |
| β-blocker | 6 | 3 | 4 |
| Calcium chanel blocker | 3 | 2 | 3 |
| Statin | 16 | 6 | 9 |
| Nitrate | 7 | 3 | 5 |
Values are expressed as mean±SEM or number
M: male; F: female; PCI: percutaneous coronary intervention; CAG: coronary angiography; MI: myocardial infarction; ADP: adenosine diphosphate; ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker. All P>0.05
3.2. Temporal changes of the plasma P-selectin and PAI-1 levels after PCI
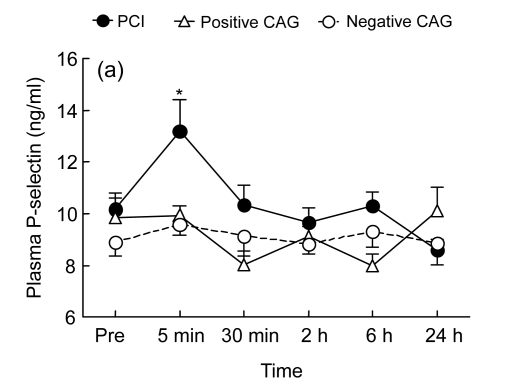
Before the procedure, the plasma concentration of P-selectin was (10.17±0.63), (9.83±0.77), and (8.89±0.54) ng/ml in the PCI, the positive CAG, and the negative CAG groups, respectively (P>0.05, Fig. 1a). P-selectin did not change significantly in the negative CAG and the positive CAG groups after a CAG procedure alone (Fig. 1a). However, in the PCI group, plasma P-selectin increased significantly at 5 min after PCI [(13.20±1.21) ng/ml] compared to the baseline value, indicating an early increase in circulating P-selectin.
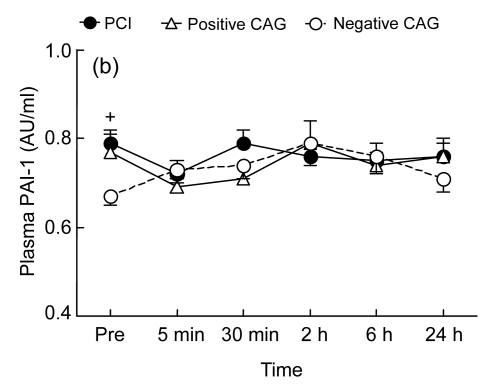
Fig. 1.
Temporal changes in plasma P-selectin (a) and plasminogen activator inhibitor-1 (PAI-1) (b) before the procedure [coronary angiography (CAG) or percutaneous coronary intervention (PCI)] and at 5 min, 30 min, 2 h, 6 h, and 24 h following the procedure in the PCI group (who underwent PCI, n=24), the positive CAG group (who had positive CAG findings, but did not have PCI, n=10), and the negative CAG group (who had negative CAG findings, n=16)
* P<0.05 vs. baseline; + P<0.05 vs. the other non-PCI groups
Basal levels of plasma PAI-1 in two CAD groups [PCI group: (0.79±0.03) AU/ml; positive CAG group: (0.77±0.04) AU/ml] were significantly higher than that in non-CAD group [negative CAG group: (0.67±0.02) AU/ml, P<0.05; Fig. 1b]. PCI did not induce any changes in plasma PAI-1 level in the PCI group, neither did CAG procedure alone in the two non-PCI groups (Fig. 1b).
3.3. Temporal changes of the serum CK-MB and Mg levels after PCI
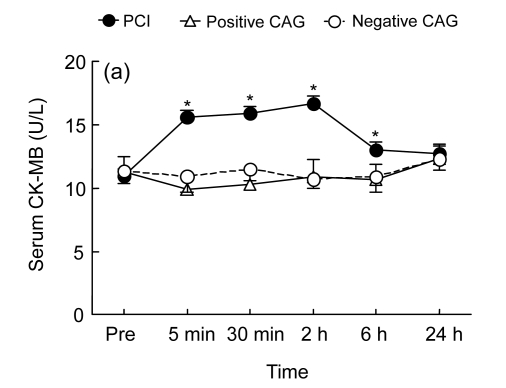
The basal serum CK-MB levels were similar among the three groups (Fig. 2a). In the PCI group, the serum CK-MB levels were significantly increased after the procedure relative to baseline values. The level peaked during the period of 5 min to 2 h after PCI, then declined but remained higher at 6 h after PCI (P<0.01, Fig. 2a). In the two control groups, CAG alone did not induce any changes in serum CK-MB concentrations (Fig. 2a).
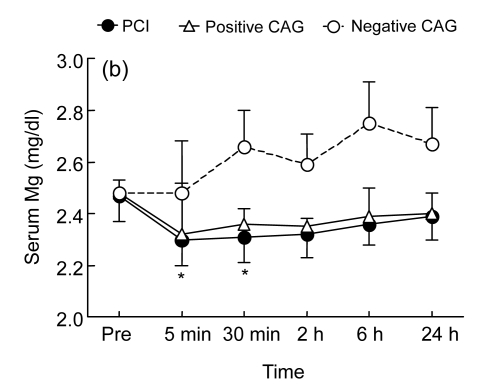
Fig. 2.
Temporal changes in serum creatine kinase-myocardial band fraction (CK-MB) (a) and magnesium (Mg) (b) before the procedure [coronary angiography (CAG) or percutaneous coronary intervention (PCI)] and at 5 min, 30 min, 2 h, 6 h, and 24 h following the procedure in the PCI group (who underwent PCI, n=24), the positive CAG group (who had positive CAG findings, but did not have PCI, n=10), and the negative CAG group (who had negative CAG findings, n=16)
* P<0.05 vs. baseline
The three groups had similar serum Mg concentrations before the operation (Fig. 2b). Serum Mg levels were decreased after the procedure in the PCI group, the differences being significant when comparing values at 5 and 30 min after PCI vs. baseline value (P<0.05, Fig. 2b) and comparing values at 5 min, 30 min, 2 h, and 6 h after PCI in the PCI and negative control groups (P<0.05, Fig. 2b). There was a trend for a decrease in Mg levels in the positive CAG group from 5 min to 2 h after the procedure, but the differences did not reach significance in comparison with either baseline values or corresponding time points in the negative control group (P>0.05).
In addition, serum Mg had negative, but weak, correlations with plasma P-selectin (R=−0.204, P<0.05) and serum CK-MB (R=−0.341, P<0.05) in those who underwent PCI after CAG.
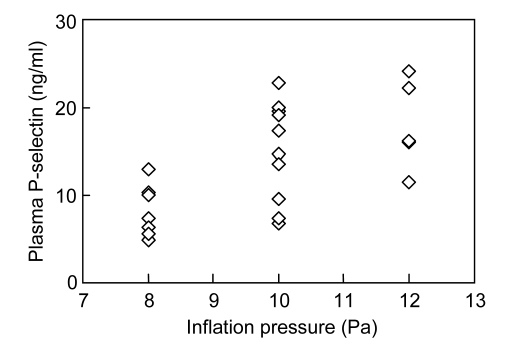
3.4. Correlation between P-selectin and inflation pressure
We analyzed the correlation between the above parameters and inflation pressure and duration used in the PCI procedure. Interestingly, the P-selectin levels at 5 min following PCI were positively correlated with the inflation pressure (R=0.647, P<0.001, Fig. 3). There were no correlations between CK-MB/Mg and the inflation parameters.
Fig. 3.
Correlation between the P-selectin levels and the inflation pressure used in percutaneous coronary intervention (PCI)
The P-selectin levels at 5 min following PCI were positively correlated with inflation pressure (R=0.647, P<0.001, n=24)
3.5. Associations between P-selectin/Mg and adverse cardiovascular events
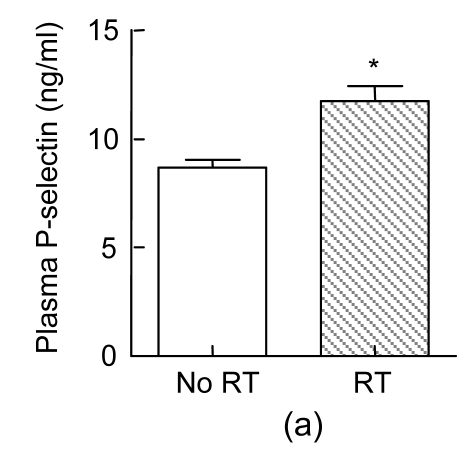
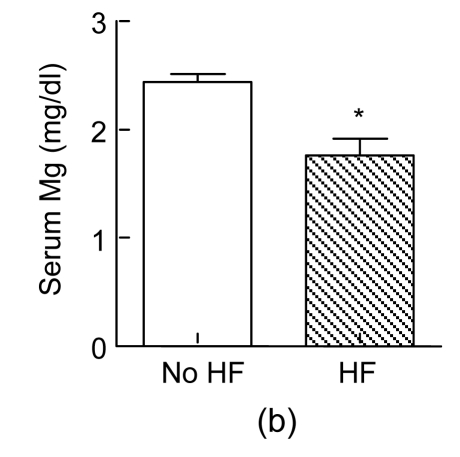
During a six-month follow-up, the prevalences of heart failure and re-admission due to CVs in the positive CAG group were significantly higher than those in the PCI and the negative CAG groups (P<0.05 and P<0.005, respectively), while the occurrence rates of recurrent treatment and death due to CV were not significantly different among the three groups (P>0.05, Table 2). We further tested whether the circulating levels of P-selectin, PAI-1, Mg, and CK-MB had any associations with adverse CVs. Interestingly, subjects who had recurrent treatment at six-month follow-up had elevated P-selectin levels at 24 h after the procedure (P<0.01, Fig. 4a) and those who developed heart failure had lower Mg levels at 5 min after the procedure (P<0.01, Fig. 4b), suggesting that an increase in P-selectin and a decrease in Mg after the procedure are correlated with outcomes.
Table 2.
Major adverse cardiovascular event follow-up of the three groups six months after discharge from the hospital
| Cardiovascular event | Number |
P | ||
| PCI (n=24) | Positive CAG (n=10) | Negative CAG (n=16) | ||
| Re-admission due to CV | 2 | 5 | 2 | <0.05 |
| Heart failure | 1 | 4 | 1 | <0.005 |
| Recurrent treatment | 1 | 3 | 1 | >0.05 |
| Death due to CV | 2 | 1 | 0 | >0.05 |
CV: cardiovascular event
Fig. 4.
Associations between P-selectin (a)/Mg (b) and adverse cardiovascular events
Subjects who had recurrent treatment (RT) at six-month follow-up had elevated P-selectin levels at 24 h after percutaneous coronary intervention (PCI) (P<0.01, n=24), and those who developed heart failure (HF) had lower Mg levels at 5 min after PCI (P<0.01, n=24)
4. Discussion
The present study investigated temporal changes of circulating P-selectin, PAI-1, Mg, and CK-MB in subjects undergoing PCI, and their correlations with future adverse CVs. There were several findings. PCI induced an early increase in P-selectin, a sustained increase in CK-MB, and a significant decrease in Mg in patients with coronary heart diseases, but had no effect on PAI-1. The P-selectin levels at 5 min following PCI were positively correlated with the inflation pressure. Elevated P-selectin and decreased Mg measured shortly after the coronary procedure were associated with recurrent treatment and heart failure, respectively. This study suggests that the changes of P-selectin and Mg induced by PCI may be associated with restenosis and ischemia-reperfusion injury, and provides additional evidence supporting the use of platelet inhibitors and Mg before and/or during the procedure to reduce the risks associated with PCI.
Basal levels of PAI-1 in both CAD groups (PCI and positive CAG groups) were significantly higher than that in the disease-free group, in keeping with a contribution from the imbalance of the coagulation-fibrinolysis system in the development and progression of CAD. Interestingly, PCI did not induce elevations in PAI-1, which suggests that PCI, as a kind of mechanical vascular recurrent treatment, has no direct effect on the coagulation-fibrinolysis system. Since thrombolysis causes an increase in plasma PAI-1 levels (Paganelli et al., 1999), PCI might be superior to drug thrombolysis in this regard.
We detected an early elevation of the plasma P-selectin after PCI, which may be related to immediate platelet activation and injury of endothelial cells. It is known that coronary angioplasty induces platelet activation (Dehmer et al., 1997), and increased platelet activity is associated not only with ischemia-reperfusion injury at the immediate stage, but also with restenosis at the late stage after coronary angioplasty (Tschoepe et al., 1993; Murasaki et al., 2007; Osmancik et al., 2008). In this study, we also found that the plasma P-selectin level at 5 min after PCI was positively correlated to inflation pressure, indicating that the increase in circulating P-selectin levels is partly due to vascular injury caused by coronary angioplasty. These results highlight the importance of using antiplatelet therapy as early as possibly to inhibit the activation of platelets, as well as performing the operation as gently as compatible with a successful dilatation, in order to prevent unnecessary arterial injury.
CK-MB is a sensitive marker of myonecrosis and has been detected in 5%–50% of patients after PCI (Abdelmeguid and Topol, 1996; Califf et al., 1998; Kanaparti and Brown, 2000). The levels of serum CK-MB were significantly elevated after PCI, but values were still within the normal range, indicating that PCI induced a low-grade injury but not necrosis of the myocardium. The elevation of CK-MB after PCI may be due to a burst of oxygen free radicals, calcium overload, and activation of leukocytes and platelets (Piper et al., 1998; Ambrosio and Tritto, 1999; Park and Lucchesi, 1999). In addition, a limitation of blood flow to heart tissue caused by coronary angioplasty may lead to partial leakage of enzyme from the myocardium.
Our study showed lower serum Mg levels in the PCI group from 5 min to 24 h following the procedure (being significant at 5 min and 30 min). Interestingly, Satur et al. (1994) found that the levels of serum Mg were significantly reduced at 3 and 30 min after CABG compared to the preoperative value and lasted for more than 24 h. They proposed that the decrease in serum Mg levels was related to the following factors: (1) blood diluting-induced hypomagnesaemia; (2) paroxysmal ischemia and blood dilution; (3) acute catecholamine secretion, stress, and associated high free fatty acid concentrations (Ryzen et al., 1986). However, the exact mechanism of Mg decline associated with PCI is unclear. Whether those factors underlying CABG-induced Mg deficiency are also involved in the decrease in serum Mg of PCI patients remains unknown and warrants further investigation.
Some studies have verified that intravenous infusion of Mg inhibits the expression of P-selectin (Gawaz et al., 1996; Rukshin et al., 2003) and alleviates atherosclerosis, myocardial infarction, and ischemia-reperfusion injury of the myocardium (Leor and Kloner, 1995; Murthi et al., 2003). Mg, as a natural antagonist of calcium, can inhibit the overload of calcium in the endothelial cells, attenuate the injury of endothelial and other cells initiated by P-selectin, decrease platelet activation (Shechter et al., 2000), improve myocardial metabolism, and finally prevent ischemia-reperfusion injury. Murasaki et al. (2007) have previously demonstrated that Mg pretreatment plays a cardioprotective role by inhibiting ischemia-reperfusion induced up-regulation of P-selectin. Another study has shown that Mg adminstration before PCI results in beneficial effects on LV function and microvascular function in patients with acute myocardial infarction (Nakashima et al., 2004). The present study shows that decreased Mg levels were accompanied by elevated P-selectin and CK-MB in the PCI group. Taken together, Mg deficiency and elevated P-selectin related to coronary angioplasty could be associated with CK-MB leakage through inducing myocardial injury. Therefore, it is suggested that supplying patients with Mg before the procedure and for a period thereafter could attenuate myocardial injury.
Elevated P-selectin and decreased Mg measured shortly after the CAG procedure are associated with recurrent treatment and heart failure, respectively, in all subjects, which further supports the concept that the administration of platelet inhibitors and Mg is necessary for patients receiving CAG with or without PCI.
The present study has some limitations. It is challenging to seek the association between plasma biomarkers and clinical end points in a small study. Although an association has been shown, it is not clear yet whether these markers are mechanistically causative of the restenosis and ischemic injury following PCI.
In conclusion, this study investigates temporal changes in circulating P-selectin, CK-MB, and Mg levels in patients with coronary heart diseases after PCI. The results provide additional evidence for the application of antiplatelet therapy and Mg to reduce the risks associated with PCI.
Acknowledgments
The authors thank Guang-ming QIN (Department of Cardiology, the Second Affiliated Hospital, School of Medicine, Zhejiang University, China) for his excellent technical assistance.
Footnotes
Project (Nos. 2008CA056 and 391020-W50819) supported by the Medical Science Research Grant of the Health Bureau of Zhejiang Province, China
References
- 1.Abdelmeguid AE, Topol EJ. The myth of the myocardial ‘infarctlet’ during percutaneous coronary revascularization procedures. Circulation. 1996;94(12):3369–3375. doi: 10.1161/01.cir.94.12.3369. [DOI] [PubMed] [Google Scholar]
- 2.Ambrosio G, Tritto I. Reperfusion injury: experimental evidence and clinical implications. American Heart Journal. 1999;138(2):S69–S75. doi: 10.1016/S0002-8703(99)70323-6. [DOI] [PubMed] [Google Scholar]
- 3.Bhoday J, de Silva S, Xu Q. The molecular mechanisms of vascular restenosis: which genes are crucial? Current Vascular Pharmacology. 2006;4(3):269–275. doi: 10.2174/157016106777698397. [DOI] [PubMed] [Google Scholar]
- 4.Califf RM, Abdelmeguid AE, Kuntz RE, Popma JJ, Davidson CJ, Cohen EA, Kleiman NS, Mahaffey KW, Topol EJ, Pepine CJ, et al. Myonecrosis after revascularization procedures. Journal of the American College of Cardiology. 1998;31(2):241–251. doi: 10.1016/S0735-1097(97)00506-8. [DOI] [PubMed] [Google Scholar]
- 5.Dehmer GJ, Nichols TC, Bode AP, Liles D, Sigman J, Li S, Koch G, Tate DA, Griggs TR. Assessment of platelet activation by coronary sinus blood sampling during balloon angioplasty and directional coronary atherectomy. The American Journal of Cardiology. 1997;80(7):871–877. doi: 10.1016/S0002-9149(97)00538-9. [DOI] [PubMed] [Google Scholar]
- 6.Gawaz M, Reininger A, Neumann FJ. Platelet function and platelet-leukocyte adhesion in symptomatic coronary heart disease. Effects of intravenous magnesium. Thrombosis Research. 1996;83(5):341–349. doi: 10.1016/0049-3848(96)00144-2. [DOI] [PubMed] [Google Scholar]
- 7.Gruntzig A. Transluminal dilatation of coronary-artery stenosis. Lancet. 1978;311(8058):263. doi: 10.1016/S0140-6736(78)90500-7. [DOI] [PubMed] [Google Scholar]
- 8.Headrick JP, McKirdy JC, Willis RJ. Functional and metabolic effects of extracellular magnesium in normoxic and ischemic myocardium. AJP-Heart and Circulatory Physiology. 1998;275(3 Pt 2):H917–H929. doi: 10.1152/ajpheart.1998.275.3.H917. [DOI] [PubMed] [Google Scholar]
- 9.Kanaparti PK, Brown DL. Relation between coronary atherosclerotic plaque burden and cardiac enzyme elevation following percutaneous coronary intervention. The American Journal of Cardiology. 2000;86(6):619–622. doi: 10.1016/S0002-9149(00)01039-0. [DOI] [PubMed] [Google Scholar]
- 10.Katsaros KM, Speidl WS, Kastl SP, Zorn G, Huber K, Maurer G, Glogar D, Wojta J, Christ G. Plasminogen activator inhibitor-1 predicts coronary in-stent restenosis of drug-eluting stents. Journal of Thrombosis and Haemostasis. 2008;6(3):508–513. doi: 10.1111/j.1538-7836.2007.02884.x. [DOI] [PubMed] [Google Scholar]
- 11.Leor J, Kloner RA. An experimental model examining the role of magnesium in the therapy of acute myocardial infarction. The American Journal of Cardiology. 1995;75(17):1294–1295. doi: 10.1016/S0002-9149(99)80787-5. [DOI] [PubMed] [Google Scholar]
- 12.Li-Saw-Hee FL, Blann AD, Lip GY. A cross-sectional and diurnal study of thrombogenesis among patients with chronic atrial fibrillation. Journal of the American College of Cardiology. 2000;35(7):1926–1931. doi: 10.1016/S0735-1097(00)00627-6. [DOI] [PubMed] [Google Scholar]
- 13.Ma X, Zhang X, Li C, Luo M. Effect of postconditioning on coronary blood flow velocity and endothelial function and LV recovery after myocardial infarction. Journal of Interventional Cardiology. 2006;19(5):367–375. doi: 10.1111/j.1540-8183.2006.00191.x. [DOI] [PubMed] [Google Scholar]
- 14.Moens AL, Claeys MJ, Timmermans JP, Vrints CJ. Myocardial ischemia/reperfusion-injury, a clinical view on a complex pathophysiological process. International Journal of Cardiology. 2005;100(2):179–190. doi: 10.1016/j.ijcard.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 15.Monnink SH, Tio RA, Veeger NJ, Amoroso G, van Boven AJ, van Gilst WH. Exercise-induced ischemia after successful percutaneous coronary intervention is related to distal coronary endothelial dysfunction. Journal of Investigative Medicine. 2003;51(4):221–226. doi: 10.2310/6650.2003.39196. [DOI] [PubMed] [Google Scholar]
- 16.Murasaki K, Kawana M, Murasaki S, Tsurumi Y, Tanoue K, Hagiwara N, Kasanuki H. High P-selectin expression and low CD36 occupancy on circulating platelets are strong predictors of restenosis after coronary stenting in patients with coronary artery disease. Heart and Vessels. 2007;22(4):229–236. doi: 10.1007/s00380-006-0966-5. [DOI] [PubMed] [Google Scholar]
- 17.Murthi SB, Wise RM, Weglicki WB, Komarov AM, Kramer JH. Mg-gluconate provides superior protection against postischemic dysfunction and oxidative injury compared to Mg-sulfate. Molecular and Cellular Biochemistry. 2003;245(1-2):141–148. doi: 10.1023/A:1022840704157. [DOI] [PubMed] [Google Scholar]
- 18.Nakashima H, Katayama T, Honda Y, Suzuki S, Yano K. Cardioprotective effects of magnesium sulfate in patients undergoing primary coronary angioplasty for acute myocardial infarction. Circulation Journal. 2004;68(1):23–28. doi: 10.1253/circj.68.23. [DOI] [PubMed] [Google Scholar]
- 19.Osmancik PP, Bednar F, Pavkova L, Tousek P, Stros P, Jirasek K. Higher platelet activity is present in patients with restenosis after percutaneous coronary intervention compared with patients with an occlusion of coronary artery bypass graft. Blood Coagulation & Fibrinolysis. 2008;19(8):807–812. doi: 10.1097/MBC.0b013e3283169223. [DOI] [PubMed] [Google Scholar]
- 20.Paganelli F, Alessi MC, Morange P, Maixent JM, Levy S, Vague IJ. Relationship of plasminogen activator inhibitor-1 levels following thrombolytic therapy with rt-PA as compared to streptokinase and patency of infarct related coronary artery. Thrombosis and Haemostasis. 1999;82(1):104–108. [PubMed] [Google Scholar]
- 21.Park JL, Lucchesi BR. Mechanisms of myocardial reperfusion injury. The Annals of Thoracic Surgery. 1999;68(5):1905–1912. doi: 10.1016/S0003-4975(99)01073-5. [DOI] [PubMed] [Google Scholar]
- 22.Pendyala L, Jabara R, Shinke T, Chronos N, Robinson K, Li J, Hou D. Drug-eluting stents: present and future. Cardiovascular & Hematological Agents in Medicinal Chemistry. 2008;6(2):105–115. doi: 10.2174/187152508783955051. [DOI] [PubMed] [Google Scholar]
- 23.Piper HM, Garcia-Dorado D, Ovize M. A fresh look at reperfusion injury. Cardiovascular Research. 1998;38(2):291–300. doi: 10.1016/S0008-6363(98)00033-9. [DOI] [PubMed] [Google Scholar]
- 24.Ravn HB, Moeldrup U, Brookes CI, Ilkjaer LB, White P, Chew M, Jensen L, Johnsen S, Birk-Soerensen L, Hjortdal VE. Intravenous magnesium reduces infarct size after ischemia/reperfusion injury combined with a thrombogenic lesion in the left anterior descending artery. Arteriosclerosis, Thrombosis, and Vascular Biology. 1999;19(3):569–574. doi: 10.1161/01.atv.19.3.569. [DOI] [PubMed] [Google Scholar]
- 25.Roth A, Eshchar Y, Keren G, Kerbel S, Harsat A, Villa Y, Laniado S, Miller HI. Effect of magnesium on restenosis after percutaneous transluminal coronary angioplasty: a clinical and angiographic evaluation in a randomized patient population. A pilot study. European Heart Journal. 1994;15(9):1164–1173. doi: 10.1093/oxfordjournals.eurheartj.a060649. [DOI] [PubMed] [Google Scholar]
- 26.Rukshin V, Santos R, Gheorghiu M, Shah PK, Kar S, Padmanabhan S, Azarbal B, Tsang VT, Makkar R, Samuels B, et al. A prospective, nonrandomized, open-labeled pilot study investigating the use of magnesium in patients undergoing nonacute percutaneous coronary intervention with stent implantation. Journal of Cardiovascular Pharmacology and Therapeutics. 2003;8(3):193–200. doi: 10.1177/107424840300800304. [DOI] [PubMed] [Google Scholar]
- 27.Ryzen E, Elkayam U, Rude RK. Low blood mononuclear cell magnesium in intensive cardiac care unit patients. American Heart Journal. 1986;111(3):475–480. doi: 10.1016/0002-8703(86)90051-7. [DOI] [PubMed] [Google Scholar]
- 28.Satur CM, Anderson JR, Jennings A, Newton K, Martin PG, Nair U, Walker DR. Magnesium flux caused by coronary artery bypass operation: three patterns of deficiency. The Annals of Thoracic Surgery. 1994;58(6):1674–1678. doi: 10.1016/0003-4975(94)91657-8. [DOI] [PubMed] [Google Scholar]
- 29.Serruys PW, Luijten HE, Beatt KJ, Geuskens R, de Feyter PJ, van den Brand M, Reiber JH, ten Katen HJ, van Es GA, Hugenholtz PG. Incidence of restenosis after successful coronary angioplasty: a time-related phenomenon. A quantitative angiographic study in 342 consecutive patients at 1, 2, 3, and 4 months. Circulation. 1988;77(2):361–371. doi: 10.1161/01.cir.77.2.361. [DOI] [PubMed] [Google Scholar]
- 30.Shechter M, Merz CN, Paul Labrador MJ, Meisel SR, Rude RK, Molloy MD, Dwyer JH, Shah PK, Kaul S. Oral magnesium supplementation inhibits platelet-dependent thrombosis in patients with coronary artery disease. The American Journal of Cardiology. 1999;84(2):152–156. doi: 10.1016/S0002-9149(99)00225-8. [DOI] [PubMed] [Google Scholar]
- 31.Shechter M, Merz CN, Rude RK, Paul Labrador MJ, Meisel SR, Shah PK, Kaul S. Low intracellular magnesium levels promote platelet-dependent thrombosis in patients with coronary artery disease. American Heart Journal. 2000;140(2):212–218. doi: 10.1067/mhj.2000.107553. [DOI] [PubMed] [Google Scholar]
- 32.Stone GW, Aronow HD. Long-term care after percutaneous coronary intervention: focus on the role of antiplatelet therapy. Mayo Clinic Proceedings. 2006;81(5):641–652. doi: 10.4065/81.5.641. [DOI] [PubMed] [Google Scholar]
- 33.Thanyasiri P, Kathir K, Celermajer DS, Adams MR. Endothelial dysfunction and restenosis following percutaneous coronary intervention. International Journal of Cardiology. 2007;119(3):362–367. doi: 10.1016/j.ijcard.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 34.Tschoepe D, Schultheiss HP, Kolarov P, Schwippert B, Dannehl K, Nieuwenhuis HK, Kehrel B, Strauer B, Gries FA. Platelet membrane activation markers are predictive for increased risk of acute ischemic events after PTCA. Circulation. 1993;88(1):37–42. doi: 10.1161/01.cir.88.1.37. [DOI] [PubMed] [Google Scholar]
- 35.Ying SQ, Fang L, Xiang MX, Xu G, Shan J, Wang JA. Protective effects of magnesium against ischaemia-reperfusion injury through inhibition of P-selectin in rats. Clinical and Experimental Pharmacology and Physiology. 2007;34(12):1234–1239. doi: 10.1111/j.1440-1681.2007.04697.x. [DOI] [PubMed] [Google Scholar]