Abstract
Objective: Atherosclerotic plaques and neovascularization play an important role in the course of coronary atherosclerosis. This study evaluated the effect of recombinant endostatin on experimental atherosclerotic plaques and neovascularization in rabbits. Methods: Eighteen healthy male rabbits were divided into three groups: control group, atherosclerotic model group, and recombinant endostatin treated group. The atherosclerotic model was established via a high-cholesterol diet after balloon catheter injury. The subject weights, serum total cholesterol, creatine kinase-myocardial band fraction (CKMB), and matrix metalloproteinase-2 (MMP-2) were measured. Six weeks after treatment, the aortic roots were taken for pathological assay. The thickness ratio of the intima to media was measured by hematoxylin and eosin (HE) staining, and the number of neovessels was measured by immunohistochemistry via monoclonal antibody CD31 staining. Results: The weight, plasma total cholesterol, and CKMB were not significantly different between the atherosclerotic model group and the recombinant endostatin treated group, but much higher than those of the control group (P<0.05). The thickness ratio of the intima to media in the recombinant endostatin treated group was distinctly less than that in the atherosclerotic model group (P<0.05). The number of neovessels decreased dramatically (P<0.05) and the content of MMP-2 decreased slightly without statistical difference (P>0.05) in the recombinant endostatin treated group, compared to the atherosclerotic model group. Conclusions: Recombinant endostatin is able to inhibit the growth of neovascularization in the atherosclerotic plaque and the development of plaque.
Keywords: Recombinant endostatin, Atherosclerosis, Neovessels
1. Introduction
Coronary heart disease is a major cause of morbidity and mortality. In recent years, the incidence rate and death rate of coronary heart disease have been increasing rapidly, despite the progress in prevention and treatment. The main pathologic and physiologic cause of coronary heart disease is atherosclerosis. The mechanism of the atherosclerosis remains unclear. Inflammation, thin fiber caps and large lipid core, exfoliated intima aggregated by platelet, and plaque rupture, hemorrhage or harm are the direct causes of instability of plaques (Naghavi et al., 2003a; 2003b). According to recent studies, with the occurrence and development of atherosclerosis, new vessels grow underneath the intima, a phenomenon even more evident in patients with plaque rupture, vessel wall hemorrhage, or instable angina (Chen et al., 2005). Meanwhile, the development of vessels is also relevant to the reconstruction of the tissues around the plaques and the activation of metal protease, factors irritating the foundation of plaque vessels and leading to plaque rupture. Thus, inhibition of neovascularization in the atherosclerotic plaque is one goal of treatment of coronary heart disease.
The current study deals with the effect of recombinant human endostatin, an angiogenesis inhibitor, on rabbit atherosclerotic plaques and subintimal neovessels, and also specifically examines subject weights, serum total cholesterol, creatine kinase-myocardial band fraction (CKMB), and matrix metalloproteinase-2 (MMP-2).
2. Materials and methods
2.1. Experiment appliances
The chief instruments included an automatic biochemical instrument (Olympus AU640, Japan), microscope (Olympus, Japan), an automatic enzyme-linked immunosorbent assay (ELISA) analyzer (BIO-RAD Model 680, USA), and a coronal artery sacculus tube of 2.5 Fr (1 Fr=0.33 mm).
2.2. Experiment reagents and animals
We used recombinant human endostatin (Shandong Xiansheng Maidejin Biological Pharmaceutical Co., Ltd., China), mouse monoclonal (JC70A) to CD31 (Abcam Hong Kong Ltd., China), and rabbit MMP-2 ELISA kit which consists of standard, standard diluent, horseradish peroxidase (HRP)-conjugate, wash solution, sample diluent, developers A and B, stop buffer, and microelisa stripplate (R&D Systems Company, USA). Eighteen healthy male rabbits (about 2.5 kg in weight), purchased from Zhejiang Chinese Medical University Animal Experiment Center, were used and given a high-fat feed, 2% (w/w) cholesterin+98% (w/w) normal feed (Shanghai CapitalBio Co. Ltd., China).
2.3. Preparation of the models
Eighteen healthy male rabbits were randomly divided into control group (Group A, n=6), atherosclerotic model group (Group B, n=6), and recombinant endostatin treated group (Group C, n=6). All groups initially received normal feed. Groups B and C were anesthetized by 3% (w/v) pentobarbital (1 ml/kg) by mainline from ear edges, and then the femoral artery was separated under aseptic technique, a small cut was made, and a coronal artery sacculus tube of 2.5 Fr was inserted to 15 cm. A force pump was connected to inject physiological saline to create 8–10 atm (1 atm=1.01325×105 Pa) pressure in the sacculus, then the tube was withdrawn to the cut. The process above was repeated three times, then the pressure pump was pulled back to pull out the tube, the femoral artery was ligated, and the cut was sewn up by layers. After the operation, each rabbit was injected with 400 000 U/d of benzylpenicilin sodium by intramuscular injection for three continuous days. From the second day after the operation, Groups B and C were fed with high-fat feed, 150 g/d, while Group A was still fed with normal feed. After eight weeks, in Groups B and C, the intima of artery was seen to be apparently thicker with abundant foam cells under the microscope, the endotheliocytes were scattered disorderly and discontinuously, and a large amount of smooth muscle cells had migrated to intima, which meant that a successful model had been created. Then Group C was injected with recombinant human endostatin 4.872 mg/(kg·d) from veins on the ear edges for six successive weeks.
2.4. Morphologic measurement
The rabbits were killed after six weeks of administration. The aortas were separated carefully, taken out, cut open, and observed, then put into 10% (w/v) neutral formalin for 1 d. A total of 0.5 cm of the root of the aorta was cut. Then samples underwent washing, dehydration, clearing, waxdipping, embedding, slicing, coating, grilling, and HE staining. Finally, the thicknesses of the intima and media were observed with the Image-Pro Plus 5.1 image operation system under 1/10 mm eye lens by 10×10 amplification. The average value of the thicknesses of multiple sections was taken.
2.5. Measurement of immunohistochemistry
The slices were first dewaxed and washed with distilled water. The antigen was repaired by quick cooling 3 min after the highly compressed heating. Mouse monoclonal to CD31 was added. After diaminobenzidine (DAB) coloration, it was processed with hematoxylin light staining for 2 min, followed by bluing, dehydration, clearing, and mounting. Finally, we observed the morphologic changes of the vessel walls: six high power fields (40×) were taken for each slice, and the numbers of positively-stained cells in the intima and media, respectively, were counted. The count was taken six times, then averaged.
2.6. Measurement of total cholesterol, CKMB, and MMP-2
The rabbits were weighed before the experiment and before and after medication, while 5 ml blood was taken from the vein at the ear edge, and total cholesterol and CKMB were measured. MMP-2 was measured with ELISA method according to the directions. We immediately read the optical density (OD) value of each hole at 450 nm in the automatic ELISA analyzer. We took the OD value as the ordinate and the density of the diluted standard substance as the abscissa to draw the standard curves.
2.7. Statistical analysis
The statistical analysis was conducted with SPSS 16.0 software, and all data are expressed as mean±standard deviation (SD). Group mean comparisons were accomplished by analysis of variance (ANOVA), Student-Newman-Keuls (SNK) method, and non-paired t-test, with P<0.05 set for statistical significance.
3. Results
3.1. Changes in the weights and plasma cholesterol levels of the rabbits
The changes in the weights of the rabbits before the operation and before and after medication are shown in Table 1, and the changes of total cholesterol levels in Table 2. As shown in these tables, there was no significant difference in the weights or cholesterol levels between groups before the operation, while before and after medication, the weights and cholesterol levels of the control group were significantly lower than those of the model group and the recombinant endostatin treated group (P<0.05), but there was no statistical difference between the model group and the recombinant endostatin treated group.
Table 1.
Changes in the weights of the rabbits
| Group | Weight (kg) |
||
| Before operation | Before medication | After medication | |
| A | 2.31±0.13 | 2.57±0.19 | 2.73±0.16 |
| B | 2.30±0.07 | 3.01±0.25* | 3.14±0.31* |
| C | 2.35±0.11 | 3.05±0.25* | 3.18±0.28* |
| P | >0.05 | <0.05 | <0.05 |
Significant difference from the control group (P<0.05)
Table 2.
Changes in plasma cholesterol levels
| Group | Plasma cholesterol (mmol/L) |
||
| Before operation | Before medication | After medication | |
| A | 1.035±0.263 | 1.178±0.255 | 1.027±0.312 |
| B | 1.095±0.323 | 22.350±0.681* | 24.300±0.615* |
| C | 0.958±0.311 | 22.343±0.726* | 24.243±0.734* |
| P | >0.05 | <0.05 | <0.05 |
Significant difference from the control group (P<0.05)
3.2. Changes in the CKMB levels of the rabbits
The changes of the myocardial enzymes of the rabbits before the operation and before and after medication are shown in Table 3. There was no significant difference in the CKMB levels between the three groups of rabbits before the operation and before medication. After medication use, the CKMB levels of the control group were significantly lower than those of the model group and the recombinant endostatin treated group (P<0.05), while there was no statistical difference between the model group and the recombinant endostatin treated group.
Table 3.
Changes of CKMB levels in blood plasma
| Group | CKMB (U/L) |
||
| Before operation | Before medication | After medication | |
| A | 651.23±239.91 | 715.60±254.90 | 658.52±293.89 |
| B | 631.18±188.19 | 1784.70±1025.57 | 1990.20±985.40* |
| C | 701.47±204.80 | 1817.00±947.36 | 2401.20±810.96* |
| P | >0.05 | >0.05 | <0.05 |
Significant difference from the control group (P<0.05)
3.3. Macroscopic observation
In the control group, the lumens were unobstructed, and the vessel walls were thin and smooth without isabeling irregular apophysis. In the model group and the recombinant endostatin treated group, the lumen cross-sectional areas were reduced, and the vessel walls were unsmooth with circle or strip isabeling irregular apophysis to the lumens. The apophysis was in infiltrative growth, and it was most apparent at the roots of aorta, iliac artery, and the crotches of the blood vessels. The concentric plaques were mostly seen to be covering the whole surface of the lumen at the root of the aorta, while the plaques at the abdominal aorta were mostly diffusely distributed on one side of the lumen, with several small plaques on the iliac artery.
3.4. Light microscopic analysis of pathological section HE staining
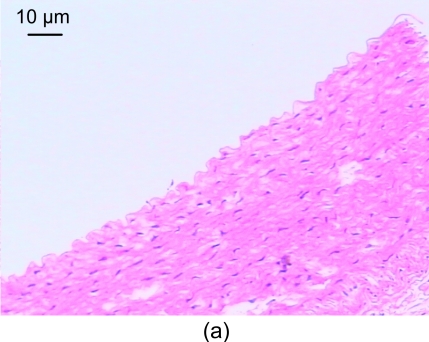
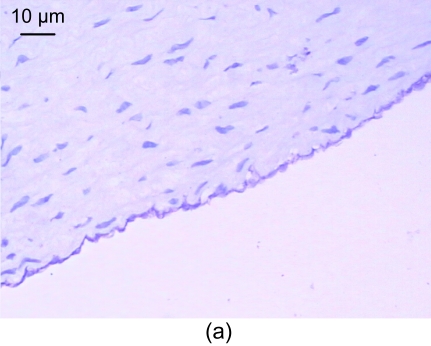
In the control group, the vessel walls were round with even thicknesses. The inner and outer elastic plates were clear and complete, the endotheliocyte core was blue-stained and evenly arranged, and the media smooth muscle cell cores were spindle-shaped. Also, no smooth muscle cells were seen underneath the endoderm (Fig. 1a).
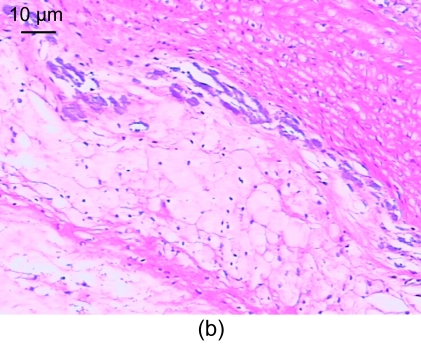
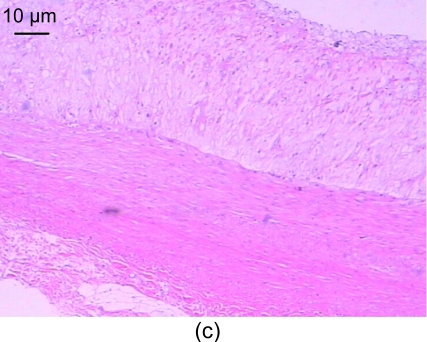
Fig. 1.
Light microscopic analysis of pathological section HE staining in the control group (a), the model group (b), and the recombinant endostatin treated group (c)
(a) In the control group, the vessel walls were thin and smooth with even thicknesses; (b) In the model group, there are many foam cells, atheronecrosis substances, and calcification in the intima. A large amount of smooth muscle cells growing towards the intima are shown; (c) In the recombinant endostatin treated group, the thickness ratio of the intima to media is less than that of the model group
In the model group, the vessel walls were unsmooth, the intima revealed hyperplasia, and the thickness is uneven. There were many foam cells and atheronecrosis substances under the fiber caps, where cholesterol crystals, calcification, and a few inflammatory cells were seen. The inner elastic plates were broken, a large amount of smooth muscle cells with spindle-shape cores were arranged in disorder, the smooth muscles were arranged somewhat lengthwise, and a tendency of growth towards the intima was seen. There was apparent hyperplasia of involucrum capillaries (Fig. 1b).
In the recombinant endostatin treated group, the thickness of the blood vessels was uneven, the thickening of the intima was comparatively smooth, and there were less foam cells under the fiber caps, less atheronecrosis substances, and less inflammatory cells, compared with the model group (Fig. 1c).
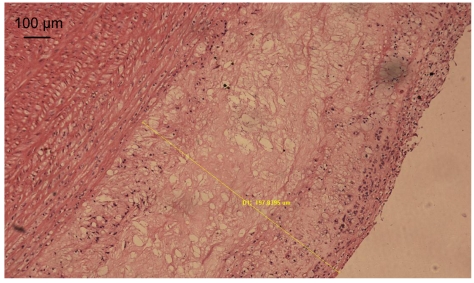
We used Image-Pro Plus 5.1 Image operation system to measure the thicknesses of the intima and media of normal blood vessels and blood vessels with atherosclerosis (Fig. 2). The results are listed in Table 4. The average values of thicknesses of the intima and media of the recombinant endostatin treated group were less than those of the model group, but there was no statistical difference. The thickness ratio of the intima to media of the recombinant endostatin treated group was statistically less than that of the model group.
Fig. 2.
Measurement of the thicknesses of the intima and media
D1 (the thickness of the intima): 197.8395 μm
Table 4.
Changes in the thickness ratio of the intima to media
| Group | IT (μm) | MT (μm) | IT/MT |
| A | − | 365.98±106.73 | 0 |
| B | 868.78±265.07 | 413.10±132.14 | 2.140±0.400* |
| C | 622.18±590.88 | 351.77±162.23 | 1.552±0.615*# |
| P | >0.05 | >0.05 | <0.05 |
IT: intima thickness; MT: media thickness; IT/MT: thickness ratio of the intima to media
Significant difference from the control group (P<0.05)
Significant difference from the model group (P<0.05)
3.5. Immunohistochemistry of CD31
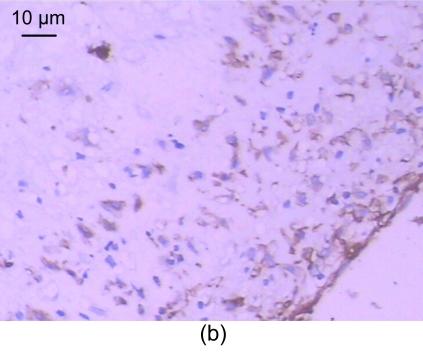
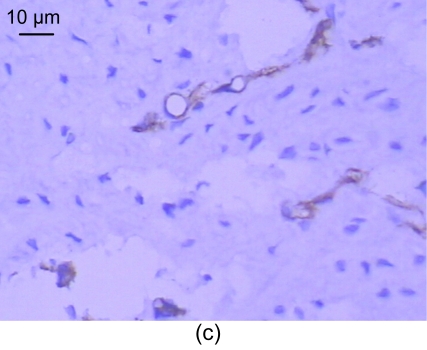
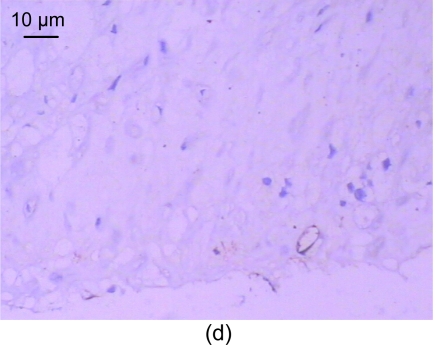
For the standard, the CD31 positive express zone was brown. The changes in the amount of neovessels before and after medication are shown in Table 5. In the control group, no neovessels were seen in the intima and media (Fig. 3a). However, a number of neovessels were seen in the model group (Figs. 3b and 3c) and a few neovessels in the recombinant endostatin treated group (Fig. 3d).
Table 5.
Changes in the amount of neovessels
| Time | Amount of neovessels |
| Before medication | 15.67±8.92 |
| After medication | 5.36±3.18 |
| P | 0.024 |
Fig. 3.
CD31 expression of the control group (a), the model group (b, c), and the recombinant endostatin treated group (d)
(a) In the control group, no neovessels are seen in the intima and media; (b, c) In the model group, a number of neovessels are seen in the intima and media (b), and neovessels grew towards the intima with smooth muscle cells (c); (d) In the recombinant endostatin treated group, a few neovessels are seen in the intima and media
3.6. MMP-2 measurement
The changes in MMP-2 of the three groups of rabbits before the operation and before and after medication are shown in Table 6. There was no apparent difference in the MMP-2 OD values between the three groups of rabbits before the operation. Before and after medication, however, the MMP-2 OD values of the control group were lower than those of the model group and the recombinant endostatin treated group, while there were no statistical differences between the latter two groups.
Table 6.
Changes in the MMP-2 OD values
| Group | MMP-2 OD value |
||
| Before operation | Before medication | After medication | |
| A | 1.938±0.054 | 1.945±0.083 | 1.939±0.043 |
| B | 1.962±0.074 | 2.762±0.067* | 2.778±0.073* |
| C | 1.919±0.068 | 2.754±0.078* | 2.758±0.019* |
| P | >0.05 | <0.05 | <0.05 |
Significant difference from the control group (P<0.05)
4. Discussion
Endostatin is an endogenic potent vascular endothelial growth inhibitor (O′Reilly et al., 1997) with the gene on the chromosome 21. Individuals with Down syndrome have a significantly higher level of circulating endostatin, about 1/3 higher than normal, attributed to the presence of three copies of collagen XVIII on chromosome 21. An interesting phenomenon is that atherosclerosis is relatively rare in Down syndrome (Chadefaux et al., 1988). Thus, there may be some relationships between endostatin and atherosclerosis. The effect of endostatin on the pathologic mechanism of atherosclerosis from the aspect of angiogenesis is considered below.
4.1. Thickness of the plaques
Atherosclerosis mainly affects the elastic arteries (e.g., aorta) and elastic muscle arteries (e.g., coronary arteries). As the lipid in the blood sediments in the intima of artery, the intima develops diffuse fibrous thickening, the vessel walls harden, and the lumens become narrow. The oxygen and nutrition transported by arteries are indispensible to the normal work of the organs and tissues of human bodies, and the nutrition of the artery walls is supplied by direct expansion of the lumens or vasa vasorum. In most arteries, including coronary arteries, the vasa vasorum occurs in the tunica externa only. Anoxia and ischemia lead to the discharge of cytokines and growth factors, including vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), epidermal growth factor, transforming growth factor, platelet derived growth factor, and so on. These can stimulate endotheliocytes directly, or stimulate the activation, migration, multiplication, capillary morphogenesis, and small vessel branching via accessory cells, including macrophagocytes and matrix cells. Capillaries then grow in the plaques, and form dense, small vessel networks, or vasa vasorum. If the thickness of the artery wall exceeds 0.5 mm, the vasa vasorum has to stretch into the media, or even the intima. According to autopsy reports, there is a strong relationship between the degree of the scleratheroma of the artery walls and the growth of the vasa vasora (Pels et al., 1997).
CD31 is a marker for endotheliocytes. It is mainly distributed on vas cells, and closely related to angiopoiesis. As shown in Fig. 3a, CD31 is positive at sites of endotheliocytes. On normal blood vessel surfaces, CD31 is expressed continuously. In atherosclerotic vessels, at zones without plaques, the endotheliocytes attach to the surfaces of the intact blood vessels, indicating that endostatin does not affect the expression of the normal intima. We infer that endostatin, with a limited density, may not lead to the apoptosis of normal endotheliocytes, but can inhibit its activation, which shows that the inhibition effect of endostatin is only effective on neovessels.
In the plaque zones of atherosclerosis, more CD31 expression can be seen under the intima. By comparing the model group with the recombinant endostatin treated group, it can be concluded that endostatin is able to inhibit the new growth of the blood vessels in the plaques. Without the nourishment from the neovessels, the thickening of the atheromatous plaques was inhibited, the growth rates of both the vascular intima and media were reduced, and the narrowing of the lumens was also less.
The increase of the thickness ratio of intima to media is one of the characteristics of pathological changes of atherosclerosis. By comparing the thicknesses of the intima and media, this experiment shows that the thickness ratio of intima to media of the recombinant endostatin treated group was statistically lower than that of the model group, but the average value of the intima and media thicknesses of the recombinant endostatin treated group was not statistically different from that of the model group. Thus, we infer that endostatin can affect the plaque thickness, which needs to be confirmed by further study.
4.2. Stability of the plaques
4.2.1. Relationship between blood vessels under intima and the stability of plaques
In normal conditions, the endotheliocytes are generally regarded as being quiescent, and divide once only every seven years (Scott and Harris, 1994). However, the endotheliocytes can be activated with some exterior stimulus. The activated endotheliocytes are characterized by active multiplication and migration to certain places, and they are also involved in a series of biological activities, such as the formation of physiological or pathological neovessels. This process is called “activation”. Then the development of basilar membrane, and the integration of the peri-endothelial cells, pericapillary cells and smooth muscle cells for large blood vessels into the vessel walls are basic steps of the vascular remodeling leading to vascular maturation (McDonald and Choyke, 2003; Kalluri, 2003). The end of this process of maturation is the formation of small and large blood vessels, and the formation of the blood vessel system that meets the functional requirements including the hydrostatic pressure, blood vessel permeability, and tissue perfusion rate. At this time, pro-angiogenesis and anti-angiogenesis reach a balance.
According to some studies, subjects with plaque rupture, vessel wall hemorrhage, or unstable angina have a relatively high detection rate of neovessels in the plaques (Chen et al., 2005). The neovessels are mainly located at the vulnerable regions of the plaques: the “shoulder zones” (Ignatescu et al., 1999), which is in agreement with what the pathology shows. The neovessels are not completely developed, and their growth rate and integrity of their structure are different form normal. They often have only an irregular basilar membrane, which leaks easily, and have no smooth muscle walls, so they easily rupture and bleed. Thus, the neovessels are one of the main causes of instability of the plaques, and they can promote plaque rupture, leading to acute coronary artery syndrome. Meanwhile, the neovessels can also aggravate the inflammatory reaction, and lead to instability of the plaques. According to some studies, the proximity of the neovessels to the atherosclerosis intima leads to both the leakage of the inflammatory cells and the expression of adhesion molecule E-selection (Pang et al., 2002). That is, the inflammation stimulates the formation of neovessels. The neovessels may be the path for the inflammatory cells to infiltrate to the focus, which stimulates positive feedback mechanism (Fleiner et al., 2004), and promotes the development of plaques.
Thus, the fewer blood vessels there are, the more stable the plaques. According to the comparison between the recombinant endostatin treated group and the model group, endostatin has a strong inhibiting effect on blood vessels under the intima. It can inhibit these immature and instable neovessels, and make the plaques more stable. Meanwhile, endostatin can also stabilize the vessel walls and lessen the leakage. According to some studies, there is an increased vascular permeability or vascular leakage in knock-out mice lacking collagen VIII, the maternal substance of endostatin (Moulton et al., 2004). Endostatin prevents the abnormal blood vessel leakage mediated by VEGF and prothrombin activators or induced by tumors, protects the connection among endotheliocytes and the direct contact between endotheliocytes, peri-endothelial cells, and basilar membrane, stabilizes blood vessel walls, and promotes the blood vessels to become mature.
4.2.2. Relationship between MMP-2 and the stability of plaques
The MMPs are a type of zinc-binding endopeptidases that degrade the extracellular matrix, and are able to degrade almost any extracellular constituent. MMPs are proangiogenic. In normal conditions, the MMPs are little expressed by the vascular smooth muscle cells and are in balance with tissue inhibitor of metalloproteinase (TIMP). Among the MMPs, MMP-2 is also called gelatinase A, and it mainly degrades collagen fiber IV.
In atherosclerosis, a large amount of smooth muscle cells can be seen to have lost contractile function. They not only phagocytize the fat and become foam cells, but also secrete a huge amount of MMPs in the process of foam cell formation. The secretion of MMPs has a positive feedback mechanism, and MMPs can activate each other and form a cascade, inhibiting the activity of TIMP, which weakens the capability of synthesizing ground substances. The expression and activity of MMP-2 in atherosclerotic plaques are both higher than those in normal artery walls. The MMP-2 secreted by smooth muscle cells has the following effects: (1) Degrade the basilar membrane effectively, which will promote the migration of large amounts of smooth muscle cells (Mason et al., 1999), make the vascular intima thicken, and lead to the losses of lumens; (2) Degrade the collagen substances in the fibrous membrane, and weaken the mechanical strength of the fiber caps (Rekhter, 1999). As the strenth of the fiber caps weakens, the plaques become vulnerable (Waltenberger, 2001; Galis and Khatri, 2002). In general, a thickness <65 μm of the fiber caps or the cell layer quantity ≤3 can be considered as instable plaques (Moreno and Lodder, 2002); (3) Degrade the extracellular matrix, ensure the migration of endotheliocytes, and promote the angiogenesis. Thus, the more MMPs there are, the thinner the fiber caps are, and the more easily neovessels can develop. At present, studies frequently involve the measurement of the indexes which reflect the pathophysiology of the vulnerable plaques as a surrogate end point to predict the vulnerability of the plaques. However, this approach still requires large scale clinical verification (Schwartz et al., 2003). Our experiment has also found that MMP-2 density may be a discriminator of plaque vulnerability.
Although the mechanism of action of endostatin is not very clear, the MPPs could be one of the target points of endostatin. It can inhibit angiogenesis by interrupting the activity of MMP-2 (Nyberg et al., 2003). In this experiment, after the intervention with recombinant human endostatin, the average density value and the SD were both lower than those of the model group, but there was no apparent statistical difference. The possible causes for this include: (1) In the models used here, the content of the blood fat was apparently higher than the normal level, which could affect the activity of the enzymes, so that the effect of the drugs on serum MMPs could be not as readily observed. (2) There is a difference in the expression level of MMP-2 in different tissues. The MMPs mainly come from the secretion of foam cells of arteriosclerotic plaques, and the level of rise or fall may not be so apparent in the serum. The sensitivity could be higher if the in-plaque MMP-2 semiquantitative immunohistochemistry staining method was adopted. (3) The specimens were kept in 4 °C fridge for a long time, and were measured together after the experiment intervention, which made the time of preservation perhaps too long, and may have weakened the immunocompetence of the enzymes. (4) This experiment measured the level of serum MMP-2 by ELISA method, the fundamental principle of which is antigen-antibody reaction, while the MMPs are a type of enzymatic proteins, so the specificity and sensitivity of detecting the activity of enzymes by ELISA method are not as good as those by other methods such as gelatin zymography. This may have led to lower detection of MMP-2. (5) There were not many specimens in the experiment (n=6), and thus the study may have lacked power.
4.3. Cardiac zymogram
The cardiac zymogram is one of the biochemical indexes to measure myocardial damage. The cardiac zymogram of the rabbits was increased after making creating the model, although penicillin intramuscular injection after operation was also one of the reasons why the cardiac zymograms of the model and medical administration groups were higher than that of the control group.
The cardiac zymogram of the recombinant endostatin treated group was slightly higher than that of the model group, but there was no statistical difference. Thus, we believe that six weeks of medication at 4.872 mg/(kg·d) will not likely lead to further damage to cardiac muscles in atherosclerotic rabbits. However, as there were not many specimens (n=6), more studies are needed to confirm this.
5. Conclusions
Endostatin is able to inhibit the development of neovessels in the atherosclerotic plaques and the development of the plaques. By inhibiting angiogenesis, promoting the maturity of the blood vessels, and inhibiting the MMP-2, endostatin can improve the stability of the plaques. Short-term treatment with endostatin does not lead to a rise of cardiac zymogram of rabbits with atherosclerosis and has no effect on total cholesterol of the blood plasma. Therefore, anti-angiogenesis treatment by endostatin may become an effective approach to atherosclerosis.
Footnotes
Project (No. 201026031) supported by the Medicine and Technology Program of Zhejiang Province, China
References
- 1.Chadefaux B, Ceballos I, Hamet M, Coude M, Poissonnier M, Kamoun P, Allard D. Is absence of atheroma in down syndrome due to decreased homocysteine levels? Lancet. 1988;332(8613):741. doi: 10.1016/S0140-6736(88)90211-5. [DOI] [PubMed] [Google Scholar]
- 2.Chen F, Eriksson P, Kimura T, Herzfeld I, Valen G. Apoptosis and angiogenesis are induced in the unstable coronary atherosclerotic plaque. Coronary Artery Disease. 2005;16(3):191–197. doi: 10.1097/00019501-200505000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Fleiner M, Kummer M, Mirlacher M, Sauter G, Cathomas G, Krapf R, Biedermann BC. Arterial neovascularization and inflammation in vulnerable patients: early and late signs of symptomatic atherosclerosis. Circulation. 2004;110(18):2843–2850. doi: 10.1161/01.CIR.0000146787.16297.E8. [DOI] [PubMed] [Google Scholar]
- 4.Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circulation Research. 2002;90(3):251–262. [PubMed] [Google Scholar]
- 5.Ignatescu MC, Gharehbaghi-Schnell E, Hassan A, Rezaie-Majd S, Korschineck I, Schleef RR, Glogar HD, Lang IM. Expression of the angiogenic protein, platelet-derived endothelial cell growth factor, in coronary atherosclerotic plaques: in vivo correlation of lesional microvessel density and constrictive vascular remodeling. Arteriosclerosis, Thrombosis, and Vascular Biology. 1999;19(10):2340–2347. doi: 10.1161/01.atv.19.10.2340. [DOI] [PubMed] [Google Scholar]
- 6.Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nature Reviews Cancer. 2003;3(6):422–433. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]
- 7.Mason DP, Kenagy RD, Hasenstab D, Bowen-Pope DF, Seifert RA, Coats S, Hawkins SM, Clowes AW. Matrix metalloproteinase-9 overexpression enhances vascular smooth muscle cell migration and alters remodeling in the injured rat carotid artery. Circulation Research. 1999;85(12):1179–1185. doi: 10.1161/01.res.85.12.1179. [DOI] [PubMed] [Google Scholar]
- 8.McDonald DM, Choyke PL. Imaging of angiogenesis: from microscope to clinic. Nature Medicine. 2003;9(6):713–725. doi: 10.1038/nm0603-713. [DOI] [PubMed] [Google Scholar]
- 9.Moreno PR, Lodder RA. Detection of lipid pool, thin fibrous cap, and inflammatory cells in human aortic atherosclerotic plaques by near-infarcted spectroscopy. Circulation. 2002;105(8):923–927. doi: 10.1161/hc0802.104291. [DOI] [PubMed] [Google Scholar]
- 10.Moulton KS, Olsen BR, Sonn S, Fukai N, Zurakowski D, Zeng X. Loss of collagen XVIII enhances neovascularization and vascular permeability in atherosclerosis. Circulation. 2004;110(10):1330–1336. doi: 10.1161/01.CIR.0000140720.79015.3C. [DOI] [PubMed] [Google Scholar]
- 11.Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, Badimon JJ, Stefanadis C, Moreno P, Pasterkamp G, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I. Circulation. 2003;108(14):1664–1672. doi: 10.1161/01.CIR.0000087480.94275.97. [DOI] [PubMed] [Google Scholar]
- 12.Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, Badimon JJ, Stefanadis C, Moreno P, Pasterkamp G, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part II. Circulation. 2003;108(15):1772–1778. doi: 10.1161/01.CIR.0000087481.55887.C9. [DOI] [PubMed] [Google Scholar]
- 13.Nyberg P, Heikkila P, Sorsa T, Luostarinen J, Heljasvaara R, Stenman UH, Pihlajaniemi T, Salo T. Endostatin inhibits human tongue carcinoma cell invasion and intravasation and blocks the activation of matrix metalloprotease-2, -9, and -13. Journal of Biological Chemistry. 2003;278(25):22404–22411. doi: 10.1074/jbc.M210325200. [DOI] [PubMed] [Google Scholar]
- 14.O′Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88(2):277–285. doi: 10.1016/S0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 15.Pang RQ, Wang HX, Pan XH. Inhibitory effect of panax notoginseng saponins on proliferation of cultured rat vascular smooth muscle cells stimulated by hypercholesterolemic serum. China Journal of Modern Medicine. 2002;12(2):4–6. (in Chinese) [Google Scholar]
- 16.Pels K, Labinaz M, O′Brien ER. Arterial wall neovascularization-potential role in atherosclerosis and restenosis. Japanese Circulation Journal. 1997;61(11):893–904. doi: 10.1253/jcj.61.893. [DOI] [PubMed] [Google Scholar]
- 17.Rekhter MD. Collagen synthesis in atheroselerosis: too much and not enough. Cardiovascular Research. 1999;41(2):378–384. doi: 10.1016/s0008-6363(98)00321-6. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz RS, Bayes-Genis A, Lesser JR, Sangiorgi M, Henry TD, Conover CA. Detecting vulnerable plaque using peripheral blood: inflammatory and cellular markers. Journal of Interventional Cardiology. 2003;16(3):231–242. doi: 10.1034/j.1600-0854.2003.8025.x. [DOI] [PubMed] [Google Scholar]
- 19.Scott PA, Harris AL. Current approaches to targeting cancer using antiangiogenesis therapies. Cancer Treatment Reviews. 1994;20(4):393–412. doi: 10.1016/0305-7372(94)90020-5. [DOI] [PubMed] [Google Scholar]
- 20.Waltenberger J. Pathophysiological bases of unstable coronary syndrome. Herz. 2001;26(Suppl. 1):2–8. doi: 10.1007/PL00014027. (in German) [DOI] [PubMed] [Google Scholar]