Abstract
Mesenchymal stem cell (MSC) transplantation has shown a therapeutic potential to repair the ischemic and infracted myocardium, but the effects are limited by the apoptosis and loss of donor cells in host cardiac microenvironment. The aim of this study is to explore the cytoprotection of heat shock protein 90 (Hsp90) against hypoxia and serum deprivation-induced apoptosis and the possible mechanisms in rat MSCs. Cell viability was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Apoptosis was assessed by Hoechst 33258 nuclear staining and flow cytometric analysis with annexin V/PI staining. The gene expression of Toll-like receptor-4 (TLR-4) and V-erb-b2 erythroblastic leukemia viral oncogene homolog 2 (ErbB2) was detected by real-time polymerase chain reaction (PCR). The protein levels of cleaved caspase-3, Bcl-2, Bcl-xL, Bax, total-ERK, phospho-ERK, total-Akt, phospho-Akt, and Hsp90 were detected by Western blot. The production of nitric oxide was measured by spectrophotometric assay. Hsp90 improves MSC viability and protects MSCs against apoptosis induced by serum deprivation and hypoxia. The protective role of Hsp90 not only elevates Bcl-2/Bax and Bcl-xL/Bax expression and attenuates cleaved caspase-3 expression via down-regulating membrane TLR-4 and ErbB2 receptors and then activating their downstream PI3K/Akt and ERK1/2 pathways, but also enhances the paracrine effect of MSCs. These findings demonstrated a novel and effective treatment strategy against MSC apoptosis in cell transplantation.
Keywords: Heat shock protein, Apoptosis, Stem cell, Hypoxia, Phosphoinositide-3-kinase/protein kinase B (PI3K/Akt), Extracellular-signal-regulate kinase (ERK)
1. Introduction
Transplantation of bone marrow-derived mesenchymal stem cells (MSCs) has been proposed as a strategy for cardiac repair following myocardial damage. However, poor cell viability after transplantation limited the reparative capacity of these cells in vivo (van der Bogt et al., 2009). Myocardial necrosis induces complement activation and free radical generation, triggering a cytokine cascade, and then donor cell apoptosis (Frangogiannis et al., 2002). Neovascularization can provide the implanted cells with adequate microenvironment to enhance survival and function, whereas exchange vessel loss and scar formation attenuate the ability to nourish the implanted cells. The donor cell growth appears tenuous and their cardiac reparative benefits are transient (Dai et al., 2005). Preconditioning MSCs with some physical or cytochemical stimuli may improve the therapeutic efficacy of cell therapy, including hypoxia (Hu et al., 2008), growth factors (Hahn et al., 2008), and some cytokines (Gui et al., 2007; Pasha et al., 2008; Liu et al., 2009a), more available for translational application than gene transfection.
Heat shock protein 90 (Hsp90) is deemed as the most active molecular chaperone which plays a critical role in the development and progression of cancer (Tsutsumi et al., 2009). It also acts as a checkpoint, leading to survival or death under stress stimulus. Hsp90 expression can increase manifold in response to many stress stimuli and elicit a protective role, helping the cells to cope with lethal conditions (Calderwood and Ciocca, 2008). Cytoprotection by Hsp90 is largely explained by its anti-apoptotic function through a multitude of intracellular signaling pathways (Bishop et al., 2007). Lee et al. (2001) demonstrated that Hsp90 can protect neuronal cells against 3-hydroxy-kynurenine induced apoptosis in several neurodegenerative disorders. Hsp90 is an endogenous inhibitor of FKBP38 that in turn increases cell survival rates of neuroblastoma cells in post-stimulation apoptosis (Erdmann et al., 2007). Recently, Hsp90 was found to exert a cardioprotective effect via the PI3K/Akt pathway (Wang et al., 2009). We hypothesized that preconditioning with Hsp90 could protect MSCs against the post-infarcted myocardial microenvironment.
Here, we established an in vitro apoptosis model induced by hypoxia and serum deprivation to investigate the role of exogenous Hsp90 in rat bone marrow MSCs on the apoptosis and signaling molecules involved.
2. Materials and methods
2.1. Animals and cell preparation
Male Sprague-Dawley rats (80–100 g) were purchased from the Experimental Animal Center of Zhejiang Medical Sciences Academy (Hangzhou, China). All studies were performed with the approval of the Institutional Animal Care and Use Committee of Zhejiang University. Rat bone marrow-derived MSCs were harvested from femora and tibia by density gradient centrifugation according to previously described methods (Makino et al., 1999). According to adherence growth properties, MSCs were purified and expanded during the passages and incubation in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% (v/v) heat-inactivated fetal calf serum (FCS), 100 U/ml penicillin, and 100 µg/ml streptomycin (Gibco, USA). Cells at passages 3–5 were characterized by fluorescence activating cell sorting (Beckman Coulter, USA) analysis using conjugated antibodies against anti-rat CD44, CD45, and CD90 (Caltag Laboratories Inc., USA).
2.2. Recombinant human Hsp90α (rhHsp90α) pre-conditioning and in vitro apoptosis model establishment
RhHsp90α (Assay Designs, USA) was dissolved in 0.1 mmol/L phosphate buffer saline (PBS, pH 7.4) and was added to each well of culture plates containing 1.5×105 cells to obtain the required final rhHsp90α concentrations (0, 0.01, 0.1, 1, and 10 μmol/L). MSCs were preconditioned with rhHsp90α for 24 h and then exposed to hypoxia (<0.5% O2) and serum deprivation for another 24 h at 37 °C in an airtight chamber (Billups-Rothenberg, USA).
2.3. Cell morphology and viability analysis
Cell morphology in culture plates was assessed with a Zeiss inverted microscope. To evaluate cell viability, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was performed as first described by Mosmann (1983) with the modifications suggested by Denizot and Lang (1986). MSCs were seeded at a density of 1×104 per well in 96-well culture plates (BD Falcon, USA) and incubated under normoxia and serum-contained conditions. Cells were preconditioned with different concentrations of rhHsp90α and then exposed to hypoxia concomitant with serum deprivation before MTT (0.5 mg/ml) incubation for 4 h. Dimethyl sulfoxide (DMSO) was added in each well, and plates were vortexed for 10 min, protected from light. The optical density (OD) value was measured with a spectrophotometer (Bio-Rad, USA) at 490 nm. The cell number of each sample was normalized with the normoxia and serum-contained control to determine the percent viability. The calculated cell viability percentages from the three parallel experiments were averaged for each set of experimental conditions.
2.4. Apoptosis analysis
Annexin V/propidium iodide (PI) apoptosis detection kit (BD Pharmingen, USA) was introduced to detect cell apoptosis at a very early stage according to the manufacturer’s instructions. Cells were resuspended with annexin V binding buffer and incubated with 5 μl of fluorescein isothiocyanate (FITC) annexin V/100 μl of binding buffer plus 5 μl PI for 15 min at room temperature in the dark. Cells were washed again with annexin V binding buffer, and the percentages of annexin V+ and PI+ cells were determined by flow cytometry with a flow cytometry assay (FACSCalibur, Becton Dickinson, USA). Hoechst 33258 staining (Molecular Probes, USA) was used to visualize nuclear changes and apoptotic body formation that are characteristics of apoptosis. Cells were immersed in 0.5 ml of methanol, followed by staining with Hoechst 33258 (1 μg/ml) at room temperature for 15 min in the dark. Cells were viewed under Zeiss fluorescence microscopy at 350 nm of excitation wavelength and 460 nm of emission wavelength, respectively. The apoptotic cells characterized by pyknotic and fragmented nuclei were counted blindly in six random high-power fields.
2.5. Quantitative real-time polymerase chain reaction (RT-PCR)
Cell samples collected from three independent experiments were run in triplicate and amplified for the genes of interest V-erb-b2 erythroblastic leukemia viral oncogene homolog 2 (ErbB2) and Toll-like receptor-4 (TLR-4) and the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Total RNA was extracted from cells using TRIzol reagent (Invitrogen, USA), and the concentration was determined by a spectrophotometer. A total of 1 μg RNA was used for first strand cDNA synthesis with the BioRT cDNA first strand synthesis kit (Bioer Technology, Hangzhou, China). Real-time amplification and detection were performed with the Applied Biosystems (ABI) Step One Plus (ABI, USA) sequence detection system and BioEasy SYBR green I RT-PCR kit (Bioer Technology). All primers are listed 5′→3′. Primers used were ErbB2f (CCCATCTGGAAGTACCCGGA), ErbB2r (GGACGCCCACTACAGTTGCA), TLR-4f (GCAAAATCCCTCATGACATCCC), TLR-4r (GGTTTAAGCCATGCCATGCC), GAPDHf (TGAAGGTCGGAGTCAACGG), GAPDHr (TGGAAGATGGTGATGGGAT). Cycling conditions used were 2 min at 94 °C, 10 s at 94 °C, and then 40 cycles of 10 s denaturation at 94 °C and 30 s annealing at 60 °C. The comparative C
t method for multiplex PCR was performed, and the fold-change between each sample and the reference sample was calculated as 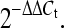
2.6. Western blot analysis
Proteins were extracted from the cells by ice-cold lysis buffer [1% (w/v) Triton X-100, 20 mmol/L 4-(2-hydroxyethyl)-1-piperazineethanesul-fonic acid (HEPES), 5 mmol/L MgCl2, 1 mmol/L ethylene-diaminetetraacetic acid (EDTA), 1 mmol/L ethylene glycol tetraacetic acid (EGTA), 1 mmol/L DL-dithiothreitol (DTT), 1 mmol/L phenylmethanesulfonyl fluoride (PMSF), and 1 mg/ml each of leupeptin, aprotinin, and pepstatin] centrifuging at 13 000×g at 4 °C for 25 min. Samples were subjected to 10% (w/v) sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride (PVDF) membranes. Filters were then blocked in 5% (v/v) non-fat milk/Tris-buffered saline (TBS)-0.05% (w/v) Tween 20 for 1 h and incubated with primary antibodies overnight, including rabbit anti-rat cleaved caspase-3, Bcl-2, Bax, Bcl-xL, ERK, phospho-ERK, Akt, phospho-Akt monoclonal antibodies [Cell Signaling Technology, Danvers, MA, USA (1:1000)], and mouse anti-rat Hsp90 antibody [BD Bioscience, San Jose, CA, USA (1:1000)]. Membranes were washed four times in TBS-Tween 20 and incubated for 2 h with the appropriate horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse IgG [Santa Cruz Biotechnology, CA, USA (1:5000)]. Bands were visualized by enhanced chemoluminescence assay (Amersham, USA), exposed to the Kodak radiography film, and then analyzed by densitometry (ImageJ 1.17 software, NIH). Some inhibitors were used to further explain the role of rhHsp90 on signaling molecules involved in apoptosis, including 17-(allylamino)-17-demethoxygeldanamycin (17-AAG, an Hsp90 inhibitor, Sigma, USA Cat#A8476), wortmannin (a PI3K inhibitor, Alexis Pharmaceuticals, USA Cat#350-020), and U0126 (an MEK1/2 inhibitor, CST, USA Cat#9904).
2.7. Colorimetric analysis
Nitric oxide (NO) assay kit (Merck-Calbiochem®, Darmstadt, Germany) was used for the spectrophotometric measurement of the NO production in the cell culture supernatants as to the manufacturer’s instructions. Cell supernatant samples and standards were added into the wells with reconstituted nitrate reductase followed by nicotinamide adenine dinucleotide (NADH). Sample NO production in each cell culture supernatant was determined by interpolation from the incubated supernatant with color reagents and the absorbance at 540 nm was measured with a spectrophotometer. Then we obtained the standard curve. Each assay was conducted in duplicate and the results are expressed as µmol/L protein in the cells.
2.8. Statistical analysis
Mean and standard error of mean (SEM) were calculated for each parameter. Data were tested for parametric or non-parametric post hoc analyses and then analyzed by one-way analysis of variance (ANOVA) or Student’s t-test using SPSS 13.0 software (Chicago, IL, USA). P<0.05 was considered to be statistically significant.
3. Results
3.1. MSC morphology and identification
Rat MSC attached on culture plates sparsely and the majority of the cells displayed a spindle-like shape. The recovered cells at passages 3–5 were stably positive for the markers CD90 and CD44 and negative for CD45. Cell morphology and membrane CD marker expression did not alter by hypoxia and serum deprivation.
3.2. Protection of MSCs against hypoxia and serum deprivation-induced apoptosis by preconditioning with rhHsp90α
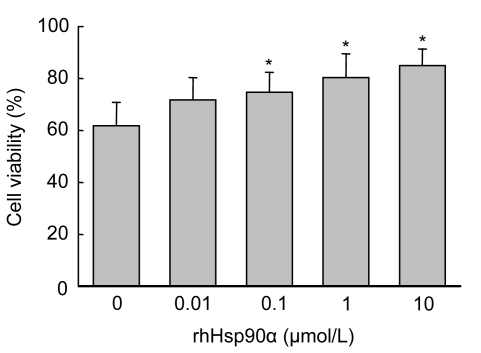
To test whether hypoxia and serum deprivation induced apoptosis, MSCs were plated at 1.5×105 cells per well in six-well plates. Hypoxia concomitant with serum deprivation for 24 h induced (38.3±5.4)% of apoptosis in rat MSCs. MSC preconditioning with rhHsp90α led to an improvement of cell viability in a dose-dependent manner. Preconditioning with rhHsp90α at 1–10 μmol/L significantly preserved MSCs from hypoxia and serum deprivation-induced apoptosis (P<0.05). The concentration of rhHsp90α at 10 μmol/L had the best anti-apoptotic effect on MSCs against hypoxia and serum deprivation (Fig. 1).
Fig. 1.
Protection of MSCs against hypoxia and serum deprivation-induced apoptosis by preconditioning with rhHsp90α
Rat MSC viability was evaluated by MTT assay. Cell viability was calculated as the percentage of viable cell number of each sample divided by that of the normoxia and serum-contained control. Cell viability of MSCs preconditioned with rhHsp90α at the dosage of 0.1–10 μmol/L was significantly increased compared to that of control (* P<0.05)
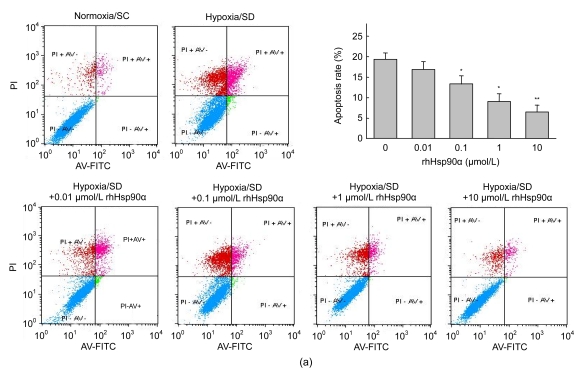
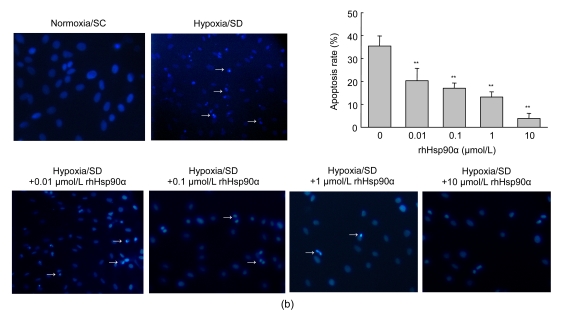
To assess the apoptosis in the early stage, annexin V was evaluated on MSCs by FACS. Hypoxia and serum deprivation showed a 4.6-fold increase of annexin V expression on MSC membranes compared to normoxia and serum-contained control [(19.3±1.6)% vs. (4.2±1.0)%, P<0.05]. Preconditioning with rhHsp90α had a dose-dependent anti-apoptotic effect on MSCs, especially at the concentration of 10 μmol/L (P<0.01, Fig. 2a). It was consistent that rhHsp90α pretreatment prevented MSCs from in vitro apoptosis by Hoechst 33258 fluorescent staining, which was used to visualize nuclear changes and apoptotic body formation as characteristics of apoptosis at the late stage. Preconditioning with rhHsp90α at the concentrations of 0.01–10 μmol/L markedly attenuated apoptotic body formation in rat MSCs (P<0.01, Fig. 2b).
Fig. 2.
Attenuated apoptosis of MSCs exposed to hypoxia and serum deprivation by exogenous rhHsp90α preconditioning
(a) Annexin V/PI binding by flow cytometry was performed to detect the early apoptosis of MSCs under hypoxia and serum deprivation (SD) conditions or normoxia and serum-contained conditions (SC) as control. Annexin V+ and PI+ cells were counted as the apoptotic cells (right top), and the percentage of apoptosis cells was calculated. The apoptosis percentage of MSCs preconditioned with rhHsp90α (0.01–10 μmol/L) decreased compared to those without rhHsp90α preconditioning, as exposed to hypoxia and serum deprivation (* P<0.05 for 0.1–1 μmol/L, ** P<0.01 for 10 μmol/L); (b) Hoechst 33258 staining was introduced to detect nuclear apoptotic bodies as the late characteristics of apoptosis. Representative immunofluorescence staining of MSCs depicted the nuclear apoptotic bodies (arrows) in the left panel. Cells with nuclear apoptotic bodies were counted as apoptosis cells, and the percentage of apoptosis cells was calculated (right panel). Compared to those without rhHsp90α preconditioning, the apoptosis percentage in MSCs preconditioned with rhHsp90α decreased as exposed to hypoxia and SD (0.01–10 μmol/L) (** P<0.01). Cells under normoxia and SC as control group
3.3. Endogenous Hsp90 expression and response to exogenous rhHsp90α preconditioning
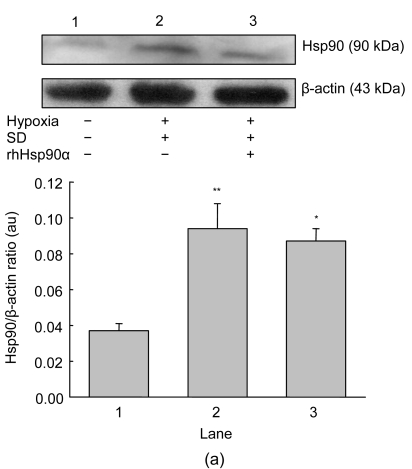
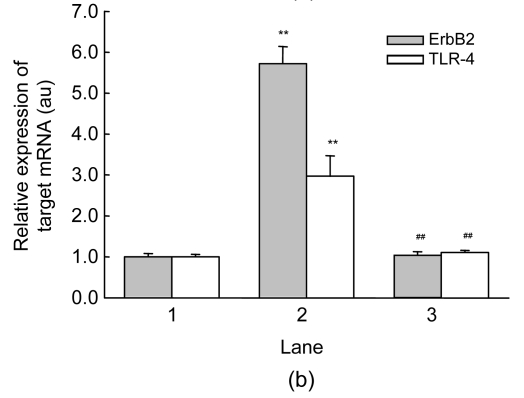
Hypoxia and serum deprivation induced about a three-fold increase in endogenous Hsp90 expression in MSCs (P<0.01, Fig. 3a). Exogenous preconditioning of MSCs with rhHsp90α did not alter endogenous Hsp90 expression in cells. To interpret the anti-apoptotic mechanisms of exogenous rhHsp90α preconditioning, cell signaling molecules responsible for the apoptosis and rhHsp90α preconditioning were investigated. Quantitative RT-PCR was adopted to monitor tyrosine kinase ErbB2 receptor and TLR-4 mRNA induced by apoptosis and rhHsp90α preconditioning. MSCs cultured under normoxia and serum-contained conditions seldom expressed TLR-4 and ErbB2 mRNA, which were 5.7-fold and 3.0-fold increased by hypoxia and serum deprivation, respectively (P<0.01, Fig. 3b). Preconditioning with exogenous rhHsp90α blocked the up-regulation of ErbB2 and TLR-4 mRNA expression on MSCs by hypoxia and serum deprivation. 17-AAG (40 nmol/L), an Hsp90 inhibitor, did not interfere with ErbB2 and TLR-4 mRNA expression on MSCs (data not shown).
Fig. 3.
Expression of endogenous Hsp90 and membrane receptors of ErbB2 and TLR-4
(a) Western blot was run to detect the expression of endogenous Hsp90 on MSCs under normoxia and serum-contained (SC) conditions (Lane 1), hypoxia and serum deprivation (SD) (Lane 2), and preconditioning with exogenous rhHsp90α (10 μmol/L) (Lane 3). Representative bands were shown in the top panel. Hypoxia and SD induced endogenous Hsp90 expression in MSCs compared to normoxia and SC (** P<0.01). Exogenous rhHsp90α preconditioning did not change the Hsp90 expression compared to MSCs without preconditioning (* P>0.05, bottom panel); (b) Cell ErbB2 and TLR-4 receptors were analyzed by quantitative RT-PCR. Hypoxia and SD led to increased expression of ErbB2 and TLR-4 mRNA on MSCs, compared to normoxia and SC (** P<0.01). Expression of ErbB2 and TLR-4 mRNA decreased on MSCs preconditioned with exogenous rhHsp90α, compared to MSCs without preconditioning (## P<0.01)
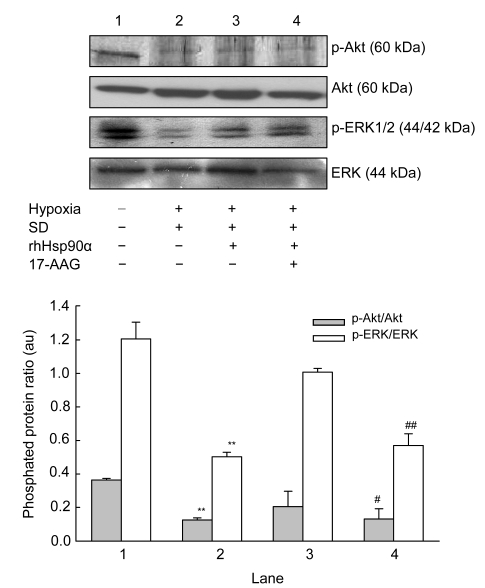
3.4. Signaling of rhHsp90α preconditioning mediated by Akt and ERK kinases
To verify the downstream signaling molecules responsible to the changes of ErbB2 and TLR-4 receptors, PI3K/Akt and ERK1/2 pathways were concerned. In contrast to deactivation of p-Akt/Akt and p-ERK/ERK proteins by hypoxia concomitant with serum deprivation, p-Akt/Akt and p-ERK/ERK proteins were activated in MSCs by rhHsp90α preconditioning (Fig. 4). Incubation with 17-AAG at the concentration of 40 nmol/L prior to rhHsp90α preconditioning attenuated the activation of p-Akt/Akt and p-ERK/ERK proteins compared to that of rhHsp90α preconditioning.
Fig. 4.
Effect of exogenous rhHsp90α preconditioning on PI3K/Akt and ERK1/2 pathways
Representative bands of the interested proteins are shown on the top panel. Compared to normoxia and serum-contained (SC) conditions (Lane 1), hypoxia and serum-deprivation (SD) (Lane 2) resulted in a 3.0-fold decrease of p-Akt/Akt and p-ERK/ERK expression on MSCs, which was reversed by exogenous rhHsp90α preconditioning (** P<0.01, respectively). MSCs incubated with 17-AAG (40 nmol/L) (Lane 4), an Hsp90 inhibitor, reduced p-Akt/Akt and p-ERK/ERK expression of MSCs preconditioning with exogenous rhHsp90α (10 μmol/L) (Lane 3) (# P<0.05; ## P<0.01)
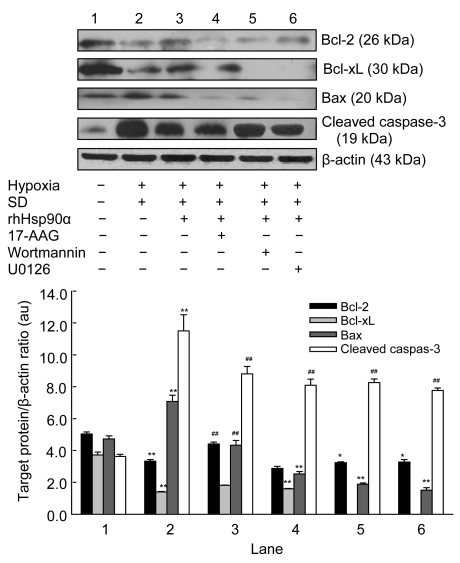
3.5. Critical role of caspase-3 in the anti-apoptosis of rhHsp90α preconditioning
The cytoprotection of rhHsp90α preconditioning led us to hypothesize that caspase-3-dependent pathways may be involved in the anti-apoptosis of rhHsp90α preconditioning, with critical pro-apoptotic proteases that execute cell death signals. We then set out to investigate pro-apoptotic protein Bax, anti-apoptotic proteins Bcl-2 and Bcl-xL, and cleaved caspase-3 to finally execute apoptosis. Bcl-2 and Bcl-xL expression decreased while Bax expression significantly increased in MSCs exposed to hypoxia and serum deprivation, which could be reversed by rhHsp90α preconditioning. Cleaved caspase-3 could not be detected in MSCs under normoxia and serum-contained conditions, but was dramatically expressed in cells exposed to hypoxia and serum deprivation conditions. It was consistent that rhHsp90α preconditioning could alleviate cleaved caspase-3 expression in MSCs. Incubation of MSCs with 17-AAG (40 nmol/L), wortmannin (0.2 μmol/L), or U0126 (10 μmol/L) prior to rhHsp90α preconditioning led to deactivation of Bcl-2 and Bcl-xL and activation of Bax, thus elevating cleaved caspase-3 (Fig. 5).
Fig. 5.
Effect of exogenous rhHsp90α preconditioning on the apoptotic proteins
Representative bands of the interested proteins are shown on the top pannel. Compared to normoxia and serum-contained (SC) (Lane 1), hypoxia and serum deprivation (SD) (Lane 2) decreased the expression of Bcl-2 and Bcl-xL proteins, and increased the expression of Bax and cleaved caspase-3 proteins in MSCs (** P<0.01, respectively). Preconditioning MSCs with exogenous rhHsp90α (10 μmol/L) (Lane 3) elevated the expression of Bcl-2 and Bcl-xL proteins, and alleviated the expression of Bax and cleaved caspase-3 proteins (## P<0.01, respectively), which were reversed by 17-AAG (an Hsp90 inhibitor, 40 nmol/L) (Lane 4), wortmannin (a PI3K inhibitor, 0.2 μmol/L) (Lane 5) and U0126 (an ERK1/2 inhibitor, 10 μmol/L) (Lane 6); ** P<0.01 respectively. β-actin as the internal standard
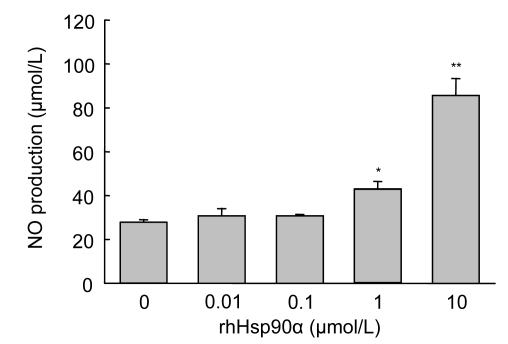
3.6. NO synthesis in MSCs promoted by exogenous rhHsp90α preconditioning
To further explain the anti-apoptotic mechanisms, cell supernatant was collected and the paracrine effect was analyzed. NO is regarded as the “endothelium-derived relaxing factor”, which alleviates the production of superoxide and oxygen free radicals under stress stimuli, and exhibits an anti-apoptotic effect against myocardial injury (Stolzing and Scutt, 2006). The NO concentration was significantly higher in the culture supernatants of MSCs preconditioned with rhHsp90α at 1 (P<0.05) and 10 μmol/L (P<0.01), which indicates that exogenous rhHsp90α preconditioning could dose-dependently trigger NO synthesis and secretion in rat MSCs (Fig. 6).
Fig. 6.
Exogenous rhHsp90α preconditioning induced NO synthesis in MSCs exposed to hypoxia and serum deprivation (SD)
NO production in the supernatant of each MSC culture was quantitated by colorimetric analysis. NO levels were significantly elevated in MSCs preconditioned with exogenous rhHsp90α at the dosage of 1–10 μmol/L (* P<0.05, ** P<0.01), compared to 0 μmol/L rhHsp90α group
4. Discussion
MSCs have shown great therapeutic potential because of their ability to regenerate and repopulate the injured myocardium, importantly playing a paracrine role after transplantation into an ischemic or infracted heart (Orlic et al., 2001; Dai et al., 2005; Cheng and Yau, 2008). However, although MSC transplantation has attracted considerable interest, the therapeutic exploitation has been limited by the fact that most of the implanted MSCs do not fully survive and are readily lost, presumably due to cell death induced by the ischemic cardiac microenvironment (Hale et al., 2008). It has been reported that only a small proportion of the cells (<10%) are retained in the donor heart 24 h after transplantation (Hou et al., 2005). Recently, a number of methods have been developed to prevent stem cells from apoptosis and improved their therapeutic potential in the ischemic heart, such as genetic modification, suitable transplantation routes, and retaining viability by in vitro preconditioning. Genetic modification may have values for mediating substantial functional recovery after acute myocardial infarction (Tang et al., 2005; Noiseux et al., 2006; Li et al., 2007), but it cannot be translated into a clinical strategy. One available way to enhance the implanted cell survival is preconditioning MSCs with physiochemical factors or cytokines, including hypoxia (Hou et al., 2005), low-level laser irradiation (Hou et al., 2008), hydrogen peroxide (H2O2) (Li et al., 2009), melatonin (Mias et al., 2008), and trimetazidine (1-[2,3,4-trimethoxybenzyl] piperazine; TMZ) (Wisel et al., 2009). It has also been demonstrated that preconditioning MSCs with stromal-derived factor 1α (SDF-1α) alone or in combination with growth factors such as fibroblast growth factor-2, insulin-like growth factor-1, and bone morphogenetic protein-2 can alleviate the host cell apoptosis and enhance their therapeutic effects (Mias et al., 2008). Our previous studies have shown that some candidates for preconditioning, such as heregulin (Mias et al., 2008), cyclosporin A (Chen et al., 2008), angiopoietin-1 (Liu et al., 2008), and dimethyloxalylglycine (DMOG) (Liu et al., 2009b), can protect MSCs against hypoxia and serum deprivation-induced apoptosis through classic cytoplasmic and/or mitochondrial apoptotic pathways.
The molecular chaperone Hsp90 is deemed as a promising target in cancer therapy. It has demonstrated that young MSCs in vitro culturing at reduced temperature result in the up-regulation of endogenous Hsp90, which has a generally beneficial effect as an anti-apoptotic heat shock protein (Stolzing et al., 2006). In this study, we also showed that endogenous Hsp90α was up-regulated in young MSCs exposed to hypoxia concomitant with serum deprivation, which cannot be affected by exogenous rhHsp90α preconditioning.
It is indicated in our study that exogenous Hsp90 preconditioning can protect MSCs against hypoxia and serum deprivation induced apoptosis via PI3K/Akt and ERK1/2 pathways. The dimeric structure of the growth factor receptor ErbB2 consists of three conservative segments: the extracellular domain, the transmembrane region, and the tyrosine-kinase region (Hu et al., 2005). The molecular chaperone Hsp90 can lead to the phosphorylation of the intracellular tyrosine-kinase by binding to the extracellular domain of ErbB2 receptor, and then activating downstream PI3K/Akt and ERK1/2 pathways. TLR-4 is comprised of an ectodomain with leucine-rich repeats, a transmembrane region, and a cytoplasmic Toll/IL-1R (TIR) domain so named for its homology to the IL-1R (Lasker and Nair, 2006). It has been reported that TLR-4 agonists can strongly induce nuclear factor (NF)-κB activation and NO production in microglia (Jung et al., 2005). Our data implicate that Hsp90 can activate TLR-4 receptor on MSCs and its downstream NF-κB signaling, thus promoting NO synthesis and secretion. According to a previous study showing that TLR-4 can protect cardiomyocytes from stress-induced injury through MyD88-dependent mechanisms (Zhu et al., 2006), our data suggest that Hsp90 can activate PI3K/Akt signaling through TLR-4 and the adaptor protein MyD88, thus exerting an anti-apoptotic effect on MSCs. Hsp90 possesses a conserved adenosine triphosphate (ATP)-binding site at its N-terminal. 17-AAG functionally inhibits Hsp90 by binding at the ATP binding site of the chaperone (Nilapwar et al., 2009). It is consistent with our study that MSCs treated with 17-AAG prior to exogenous Hsp90 preconditioning can abolish the anti-apoptotic effect. The kinase inhibitors, wortmannin or U0126, also show significant inhibition of the cytoprotection by exogenous Hsp90 preconditioning on MSCs exposed to hypoxia and serum deprivation.
Taken together, preconditioning with Hsp90 exerts an anti-apoptotic and paracrine effect on MSCs exposed to hypoxia and serum deprivation through PI3K and ERK1/2 pathways. Since hypoxia and serum deprivation condition can mimic the ischemic myocardium microenvironment, our findings indicate that Hsp90 is a novel, potent survival factor for MSCs. This may prove to be of considerable therapeutic significance in terms of exploiting MSC-based therapy in the infarcted myocardium.
5. Conclusions
We demonstrate for the first time that exogenous rhHsp90α protects MSCs from apoptosis induced by hypoxia and serum deprivation, elevates Bcl-2/Bax and Bcl-xL/Bax expression, and attenuates cleaved caspase-3 expression of MSCs. Also, exogenous rhHsp90α promotes NO synthesis of MSC, via down-regulating membrane ErbB2 and TLR-4 receptors and then activating their downstream PI3K/Akt and ERK1/2 pathways. These findings suggest that the exogenous rhHsp90α preconditioning exerts both protective actions on MSC survival and enhances MSC paracrine activity, representing a novel strategy in cell therapy for ischemic heart disease.
Acknowledgments
We thank Drs. Jing-jia YE and Xing ZHANG (Clinical Research Center, the Second Affiliated Hospital, School of Medicine, Zhejiang University, China) for their technical assistance. We also thank Prof. Alan DAUGHERTY from University of Kentucky for critical review of the manuscript.
Footnotes
Project supported by the National Natural Science Foundation of China (Nos. 30670868, 30770887, and 30770887/H0220), the Key Lab of Traditional Chinese Medicine of Zhejiang Province (No. ZK23812), and the Qianjiang Talent Scheme Foundation of Zhejiang Province (No. 2009R10069), China
References
- 1.Bishop SC, Burlison JA, Blagg BS. Hsp90: a novel target for the disruption of multiple signaling cascades. Current Cancer Drug Targets. 2007;7(4):369–388. doi: 10.2174/156800907780809778. [DOI] [PubMed] [Google Scholar]
- 2.Calderwood SK, Ciocca DR. Heat shock proteins: stress proteins with Janus-like properties in cancer. International Journal of Hyperthermia. 2008;24(1):31–39. doi: 10.1080/02656730701858305. [DOI] [PubMed] [Google Scholar]
- 3.Chen TL, Wang JA, Shi H, Gui C, Luo RH, Xie XJ, Xiang MX, Zhang X, Cao J. Cyclosporin A pre-incubation attenuates hypoxia/reoxygenation-induced apoptosis in mesenchymal stem cells. Scandinavian Journal of Clinical & Laboratory Investigation. 2008;68(7):585–593. doi: 10.1080/00365510801918761. [DOI] [PubMed] [Google Scholar]
- 4.Cheng AS, Yau TM. Paracrine effects of cell transplantation: strategies to augment the efficacy of cell therapies. Seminars in Thoracic and Cardiovascular Surgery. 2008;20(2):94–101. doi: 10.1053/j.semtcvs.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Dai W, Hale SL, Martin BJ, Kuang JQ, Dow JS, Wold LE, Kloner RA. Allogeneic mesenchymal stem cell transplantation in postinfarcted rat myocardium: short- and long-term effects. Circulation. 2005;112(2):214–223. doi: 10.1161/CIRCULATIONAHA.104.527937. [DOI] [PubMed] [Google Scholar]
- 6.Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. Journal of Immunological Methods. 1986;89(2):271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- 7.Erdmann F, Jarczowski F, Weiwad M, Fischer G, Edlich F. Hsp90-mediated inhibition of FKBP38 regulates apoptosis in neuroblastoma cells. FEBS Letters. 2007;581(29):5709–5714. doi: 10.1016/j.febslet.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 8.Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovascular Research. 2002;53(1):31–47. doi: 10.1016/S0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- 9.Gui C, Wang JA, He AN, Chen TL, Luo RH, Jiang J, Hu XY, Xie XJ. Heregulin protects mesenchymal stem cells from serum deprivation and hypoxia-induced apoptosis. Molecular and Cellular Biochemistry. 2007;305(1-2):171–178. doi: 10.1007/s11010-007-9541-3. [DOI] [PubMed] [Google Scholar]
- 10.Hahn JY, Cho HJ, Kang HJ, Kim TS, Kim MH, Chung JH, Bae JW, Oh BH, Park YB, Kim HS. Pre-treatment of mesenchymal stem cells with a combination of growth factors enhances gap junction formation, cytoprotective effect on cardiomyocytes, and therapeutic efficacy for myocardial infarction. Journal of the American College of Cardiology. 2008;51(9):933–943. doi: 10.1016/j.jacc.2007.11.040. [DOI] [PubMed] [Google Scholar]
- 11.Hale SL, Dai W, Dow JS, Kloner RA. Mesenchymal stem cell administration at coronary artery reperfusion in the rat by two delivery routes: a quantitative assessment. Life Sciences. 2008;83(13-14):511–515. doi: 10.1016/j.lfs.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hou D, Youssef EA, Brinton TJ, Zhang P, Rogers P, Price ET, Yeung AC, Johnstone BH, Yock PG, March KL. Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: implications for current clinical trials. Circulation. 2005;112(9 Suppl. I):150–156. doi: 10.1161/CIRCULATIONAHA.104.526749. [DOI] [PubMed] [Google Scholar]
- 13.Hou JF, Zhang H, Yuan X, Li J, Wei YJ, Hu SS. In vitro effects of low-level laser irradiation for bone marrow mesenchymal stem cells: proliferation, growth factors secretion and myogenic differentiation. Lasers in Surgery and Medicine. 2008;40(10):726–733. doi: 10.1002/lsm.20709. [DOI] [PubMed] [Google Scholar]
- 14.Hu P, Feng J, Zhou T, Wang J, Jing B, Yu M, Hu M, Zhang X, Shen B, Guo N. In vivo identification of the interaction site of ErbB2 extracellular domain with its autoinhibitor. Journal of Cellular Physiology. 2005;205(3):335–343. doi: 10.1002/jcp.20409. [DOI] [PubMed] [Google Scholar]
- 15.Hu X, Yu SP, Fraser JL, Lu Z, Ogle ME, Wang JA, Wei L. Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. The Journal of Thoracic and Cardiovascular Surgery. 2008;135(4):799–808. doi: 10.1016/j.jtcvs.2007.07.071. [DOI] [PubMed] [Google Scholar]
- 16.Jung DY, Lee H, Jung BY, Ock J, Lee MS, Lee WH, Suk K. TLR4, but not TLR2, signals autoregulatory apoptosis of cultured microglia: a critical role of IFN-β as a decision maker. The Journal of Immunology. 2005;174(10):6467–6476. doi: 10.4049/jimmunol.174.10.6467. [DOI] [PubMed] [Google Scholar]
- 17.Lasker MV, Nair SK. Intracellular TLR signaling: a structural perspective on human disease. The Journal of Immunology. 2006;177(1):11–16. doi: 10.4049/jimmunol.177.1.11. [DOI] [PubMed] [Google Scholar]
- 18.Lee MW, Park SC, Chae HS, Bach JH, Lee HJ, Lee SH, Kang YK, Kim KY, Lee WB, Kim SS. The protective role of HSP90 against 3-hydroxy-kynurenine-induced neuronal apoptosis. Biochemical and Biophysical Research Communications. 2001;284(2):261–267. doi: 10.1006/bbrc.2001.4938. [DOI] [PubMed] [Google Scholar]
- 19.Li S, Deng Y, Feng J, Ye W. Oxidative preconditioning promotes bone marrow mesenchymal stem cells migration and prevents apoptosis. Cell Biology International. 2009;33(3):411–418. doi: 10.1016/j.cellbi.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 20.Li W, Ma N, Ong LL, Nesselmann C, Klopsch C, Ladilov Y, Furlani D, Piechaczek C, Moebius JM, Lutzow K, et al. Bcl-2 engineered MSCs inhibited apoptosis and improved heart function. Stem Cells. 2007;25(8):2118–2127. doi: 10.1634/stemcells.2006-0771. [DOI] [PubMed] [Google Scholar]
- 21.Liu XB, Jiang J, Gui C, Hu XY, Xiang MX, Wang JA. Angiopoietin-1 protects mesenchymal stem cells against serum deprivation and hypoxia-induced apoptosis through the PI3K/Akt pathway. Acta Pharmacologica Sinica. 2008;29(7):815–822. doi: 10.1111/j.1745-7254.2008.00811.x. [DOI] [PubMed] [Google Scholar]
- 22.Liu XB, Hou JF, Shi LH, Chen JH, Sang JL, Hu SS, Cong XF, Chen X. Lysophosphatidic acid protects mesenchymal stem cells against ischemia-induced apoptosis in vivo. Stem Cells and Development. 2009;18(7):947–954. doi: 10.1089/scd.2008.0352. [DOI] [PubMed] [Google Scholar]
- 23.Liu XB, Wang JA, Ogle ME, Wei L. Prolyl hydroxylase inhibitor dimethyloxalylglycine enhances mesenchymal stem cell survival. Journal of Cellular Biochemistry. 2009;106(5):903–911. doi: 10.1002/jcb.22064. [DOI] [PubMed] [Google Scholar]
- 24.Makino S, Fukuda K, Miyoshi S, Konishi F, Kodama H, Pan J, Sano M, Takahashi T, Hori S, Abe H, et al. Cardiomyocytes can be generated from marrow stromal cells in vitro. Journal of Clinical Investigation. 1999;103(5):697–705. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mias C, Trouche E, Seguelas MH, Calcagno F, Dignat-George F, Sabatier F, Piercecchi-Marti MD, Daniel L, Bianchi P, Calise D, et al. Ex vivo pretreatment with melatonin improves survival, proangiogenic/mitogenic activity, and efficiency of mesenchymal stem cells injected into ischemic kidney. Stem Cells. 2008;26(7):1749–1757. doi: 10.1634/stemcells.2007-1000. [DOI] [PubMed] [Google Scholar]
- 26.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of Immunological Methods. 1983;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 27.Nilapwar S, Williams E, Fu C, Prodromou C, Pearl LH, Williams MA, Ladbury JE. Structural-thermodynamic relationships of interactions in the N-terminal ATP-binding domain of Hsp90. Journal of Molecular Biology. 2009;392(4):923–936. doi: 10.1016/j.jmb.2009.07.041. [DOI] [PubMed] [Google Scholar]
- 28.Noiseux N, Gnecchi M, Lopez-Ilasaca M, Zhang L, Solomon SD, Deb AD, Zau VJ, Pratt RE. Mesenchymal stem cells overexpressing Akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Molecular Therapy. 2006;14(6):840–850. doi: 10.1016/j.ymthe.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 29.Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proceedings of the National Academy of Sciences. 2001;98(18):10344–10349. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pasha Z, Wang Y, Sheikh R, Zhang D, Zhao T, Ashraf M. Preconditioning enhances cell survival and differentiation of stem cells during transplantation in infarcted myocardium. Cardiovascular Research. 2008;77(1):134–142. doi: 10.1093/cvr/cvm025. [DOI] [PubMed] [Google Scholar]
- 31.Stolzing A, Scutt A. Effect of reduced culture temperature on antioxidant defences of mesenchymal stem cells. Free Radical Biology and Medicine. 2006;41(2):326–338. doi: 10.1016/j.freeradbiomed.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 32.Stolzing A, Sethe S, Scutt AM. Stressed stem cells: temperature response in aged mesenchymal stem cells. Stem Cells and Development. 2006;15(4):478–487. doi: 10.1089/scd.2006.15.478. [DOI] [PubMed] [Google Scholar]
- 33.Tang YL, Tang Y, Zhang YC, Qian K, Shen L, Phillips MI. Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. Journal of the American College of Cardiology. 2005;46(7):1339–1350. doi: 10.1016/j.jacc.2005.05.079. [DOI] [PubMed] [Google Scholar]
- 34.Tsutsumi S, Beebe K, Neckers L. Impact of heat-shock protein 90 on cancer metastasis. Future Oncology. 2009;5(5):679–688. doi: 10.2217/fon.09.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van der Bogt KE, Schrepfer S, Yu J, Sheikh AY, Hoyt G, Govaert JA, Velotta JB, Contag CH, Robbins RC, Wu JC. Comparison of transplantation of adipose tissue- and bone marrow-derived mesenchymal stem cells in the infarcted heart. Transplantation. 2009;87(5):642–652. doi: 10.1097/TP.0b013e31819609d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang W, Peng Y, Wang Y, Zhao X, Yuan Z. Anti-apoptotic effect of heat shock protein 90 on hypoxia-mediated cardiomyocyte damage is mediated via the phosphatidylinositol 3-kinase/Akt pathway. Clinical and Experimental Pharmacology and Physiology. 2009;36(9):899–903. doi: 10.1111/j.1440-1681.2009.05167. [DOI] [PubMed] [Google Scholar]
- 37.Wisel S, Khan M, Kuppusamy ML, Mohan IK, Chacko SM, Rivera BK, Sun BC, Hideg K, Kuppusamy P. Pharmacological preconditioning of mesenchymal stem cells with trimetazidine (1-[2,3,4-trimethoxybenzyl] piperazine) protects hypoxic cells against oxidative stress and enhances recovery of myocardial function in infarcted heart through Bcl-2 expression. Journal of Pharmacology and Experimental Therapeutics. 2009;329(2):543–550. doi: 10.1124/jpet.109.150839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu X, Zhao H, Graveline AR, Buys ES, Schmidt U, Bloch KD, Rosenzweig A, Chao W. MyD88 and NOS2 are essential for toll-like receptor 4-mediated survival effect in cardiomyocytes. AJP Heart and Circulatory Physiology. 2006;291(4):H1900–H1909. doi: 10.1152/ajpheart.00112.2006. [DOI] [PubMed] [Google Scholar]