Abstract
In this study, we examined the protective effects of Danshen both on endothelial progenitor cells (EPCs) in patients with hypercholesterolemia and on in-vitro EPCs of healthy volunteers. In the clinical study, we randomly divided 24 subjects with hypercholesterolemia into two groups (the control group and the Danshen-treated group). At the end of two weeks of treatment, the EPC cellular functions of both groups were tested. The results indicated that, compared to the control group, EPCs in the Danshen-treated group showed significantly better cellular functions, which was manifested in the cloning number, the proliferation capacity, the number of EPC adhesions, and cell migration. In the subsequent in-vitro experiments, EPCs were treated with vehicle, oxidized low-density lipoprotein (Ox-LDL, 100 μg/ml), or Ox-LDL (100 μg/ml) plus different concentrations of Danshen (Danshensu 2, 10, or 50 μg/ml, respectively) for 24 h. The results showed that Danshen treatments can prevent the detrimental effects of Ox-LDL on EPC cellular functions measured by proliferation capacity (0.24±0.08, 0.37±0.11, 0.30±0.04 vs. 0.13±0.02, P<0.05, P<0.01, and P<0.01, respectively), and adhesion ability (63.00±11.60, 70.00±10.80, 85.50±11.41 vs. 40.50±6.85, all P<0.01). Compared to the group treated with Ox-LDL alone, Danshen treatment significantly decreased the lipid peroxidation end product malondialdehyde (MDA) [(4.34±0.54), (3.98±0.47), (3.46±0.31) vs. (5.57±0.64) nmol/ml, all P<0.01], increased the production of superoxide dismutase (SOD) [(29.74±0.71), (31.09±0.83), (30.41±0.65) vs. (14.76±3.99) U/ml, all P<0.01], and lowered the expression of interleukin-6 (IL-6) [(24.62±7.69), (27.04±3.14), (33.38±18.86) vs. (230.67±33.53) pg/ml, all P<0.01] and tumor necrosis factor-α (TNF-α) [(41.72±6.10), (17.02±6.82), (3.73±2.26) vs. (228.71±41.53) pg/ml, all P<0.01] in Ox-LDL treated EPCs. These results suggest that Danshen may exert a protective effect through its antioxidant and anti-inflammatory features.
Keywords: Danshen, Endothelial progenitor cells, Oxidized low-density lipoprotein, Hypercholesterolemia
1. Introduction
Myocardial ischemia accounts for an increasing mortality and morbidity within the societies living developed and modern lifestyles. Atherosclerotic plaque formation in the coronary artery is the pathological basis of the disease. Among other etiological factors such as hypertension, aging, and smoking, hypercholesterolemia is a major risk factor for atherosclerosis. Oxidized low-density lipoprotein (Ox-LDL) is known to cause endothelial dysfunction via reactive oxygen species (Harrison, 1997; Galle et al., 2000). More and more evidences indicate that replenishment of normal functional endothelial cells by endothelial progenitor cells (EPCs) is very crucial for repairing arterial walls, and therefore for the recovery of coronary artery function (Asahara et al., 1997; Rauscher et al., 2003; Xu et al., 2003). It has been demonstrated that EPCs of peripheral blood in patients with hypercholesterolemia were decreased (Chen et al., 2004), and their functions such as adhesion, migration, and proliferation were impaired (Zhu et al., 2006; Pirro et al., 2008).
Danshen, a traditional medicine, is widely used in cerebrovascular and cardiovascular diseases (Du et al., 2000; Jiang et al., 2005; Zhou et al., 2005; Fish et al., 2006). For the past few years, the pharmacological research of Danshen has made progress. It has been shown that Danshen has pharmacological effects such as anti-atherosclerosis, anti-inflammatory, and anti-oxidative damage (Liu H.B. et al., 2002; Liu C.L. et al., 2007; Baek et al., 2009). A series of studies have shown that Danshen has a protective effect against the damage of endothelial cells caused by a variety of factors (Xu et al., 2001; Wu and Wang, 2002; Zhang et al., 2004). Our preliminary studies found that Danshen at low concentrations was able to evidently increase the number of EPCs and enhance their adhesion and colony-forming abilities (Ji et al., 2006). In this study, we attempt to inspect the therapeutic effects of Danshen and explore its underlying mechanism.
2. Patients and methods
2.1. Clinical study
We selected 12 pairs (24) of hypercholesteremia inpatients who were matched in age, sex, and disease conditions. Patients with recent surgical trauma, ulcers, retinal disease, tumors, acute myocardial infarction, and angina pectoris were excluded. Each of the pairs was randomly allocated into a control group or therapeutic group. The subjects in the control group received conventional therapy (atorvastatin calcium tablets, 10 mg, qn) and the therapeutic group received Danshen pills (270 mg, tid) and conventional therapy. After two weeks of treatments, we took 20 ml samples of peripheral blood from each patient. The EPCs were cultured and the changes in colony-forming, proliferative, adhesive, and migratory capacities were analyzed.
2.2. In-vitro experiments
To further explore the underlying mechanism of Danshen, we also took 20 ml of peripheral blood from six healthy volunteers to culture EPCs. After culturing for 7 d, cells were digested into cell suspension by 0.25% (w/v) trypsin, then an equal number of cells were plated into 24-well plates and divided into five groups, treated with vehicle, Ox-LDL (100 μg/ml), or Ox-LDL (100 μg/ml) plus different concentrations of Danshen (Danshensu 2, 10, or 50 μg/ml, respectively). After 24 h, the proliferative and adhesive capacities of EPCs were detected, and cell supernate from each group was taken to carry out the superoxide dismutase (SOD), malondialdehyde (MDA), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) content examinations as described below.
2.3. EPC culture
Human peripheral blood mononuclear cells were isolated using a Ficoll gradient (Hill et al., 2003). The cells were resuspensed in M199 (Sigma), which contains vascular endothelial growth factor (VEGF; 10 ng/ml, Chemicon, USA), fetal calf serum (FCS, 10%, w/v), and anti-biotics, and seeded into six-well culture dishes which were pre-coated with fibronectin (Roche, USA) for culture.
2.4. Flow cytometry assays of CD34, VE-cadherin, VEGFR-2, and AC133
After culture for 6 d, mononuclear cells (MNCs) were digested and washed with Hank’s solution two times. Then the cells were resuspensed with phosphate buffer solution (PBS) in 1×106 cells/ml single cell suspension. Fluorescent markers of cluster of differentiation 34 (CD34), vascular endothelial cadherin (VE-cadherin), vascular endothelial growth factor receptor-2 (VEGFR-2), and AC133 (10 μl, 1 mg/ml) were added in the medium. The cells were incubated at room temperature for 30 min. Then the supernatant was discarded, the cells were centrifugated and resuspensed with PBS once again. Eventually, the expressions of CD34, VE-cadherin, VEGFR-2, and AC133 were detected by flow cytometry (Vasa et al., 2001; Wang et al., 2004), using GSC7901 (a gastric cancer cell line) as control.
2.5. Immunocytochemistry
After culture for 6 d, adherent MNCs were incubated with the fluorescent probe 1,1′-dioctadecyl-1-3,3,3′,3′-tetramethyl-indo-carbocyanine perchlorate-acetylated-LDL (DiI-ac-LDL; 2.4 μg/ml, Molecular Probe) at 37 °C for 4 h (Vasa et al., 2001). After staining, samples were viewed with an inverted fluorescent microscope (Leica, Wetzlar, Germany).
2.6. VIII-related antigen immunohistochemistry
After cultured cells were fixed with 95% (v/v) alcohol for 30 min, 0.5% (v/v) H2O2 methanol inactivated endogenous peroxidase, plus the normal goat serum closed, the first antibody (1:1000) was incubated overnight at 4 °C, and the second antibody and strept actividin-biotin complex (SABC) liquid were incubated for 20 min in the oven at 37 °C, plus A, B, and C mixtures according to standard protocols. Positive cells were stained brown. Gastric cancer cells (GSC7901) acted as the negative control.
2.7. Colony-forming ability
After culture for 4 d, a typical cell mass can be observed in an inverted microscope, while a large number of round cells are in the middle, and spindle cells diffuse outwards. A typical cell mass containing more than 50 cells was considered to be a colony (Ji et al., 2006). We took every field of a random vision (×40) in the dish up and down, in five positions, to count colonies, and then averaged them.
2.8. EPC adhesion detection
Adhesive cells were digested with 0.25% (w/v) trypsin, suspended in 500 μl culture medium and counted, then an equal number of EPCs were seeded into fibronectin-coated culture plates, and cultured at 37 °C for 30 min, with unattached cells being washed away. The number of adhesive cells was counted with an inverted microscope in 10 randomly selected vision fields (×200) (Walter et al., 2002).
2.9. EPC migration detection
As mentioned above, the attached cells were digested and counted. Culture medium (100 μl) and VEGF (50 ng/ml) were added into the lower room of modified Boyden chamber (Vasa et al., 2001), while 150 μl culture medium containing 2×104 EPCs was injected into the upper room. After culture for 24 h, the cells that did not move were scraped off. The cells that moved into the lower room were fixed with methanol, stained with Giemsa, and then counted with and inverted microscope in five randomly selected vision fields (×200).
2.10. EPC proliferation detection
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was applied to determine the proliferation of EPCs (Wang et al., 2004). As above mentioned, the attached cells were digested and counted. Then equal amounts of EPCs were seeded into fibronectin-coated 96-well culture plates, with 10 μl MTT (5 mg/ml) being added into each well. After culture for 24 h, the supernatant was discarded and then 150 μl dimethyl sulfoxide (DMSO) was supplemented into each well. The 96-well culture plates were fully oscillated for 10 min with trace oscillator, after which optical density (OD) values at 490 nm were detected by enzyme-linked immuno-sorbent assay (ELISA).
2.11. Supernatant cell SOD, MDA, IL-6, and TNF-α content detections
To detect the contents of SOD and MDA, the cell culture supernatant of each group was collected, followed by placing at a 95 °C water bath for 40 min, water-cooling, and 3500 r/min centrifugation. We took out the supernatant after centrifugation, and measured the absorbance value by a visible light spectrophotometer. To detect the contents of the IL-6 and TNF-α, the cell supernatants of all groups were collected, and treated according to standard operating procedures. Then the values of OD were read on the ELISA, and the concentrations of IL-6 and TNF-α were determined by a standard curve.
2.12. Data analysis
SPSS 13.0 statistical software was used for statistical analysis. Measurement data are presented as mean±standard deviation (SD). Differences between group means were evaluated by analysis of variance (ANOVA).
3. Results
3.1. Characterization of EPCs
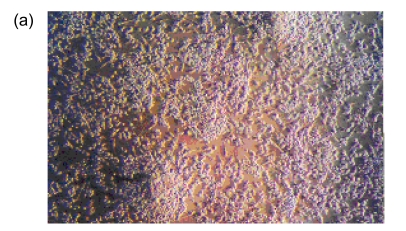
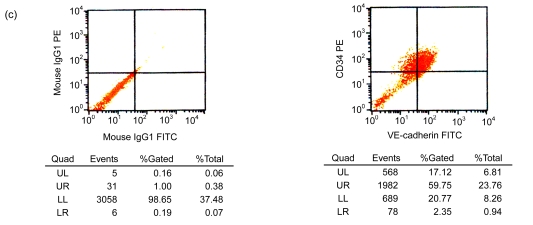
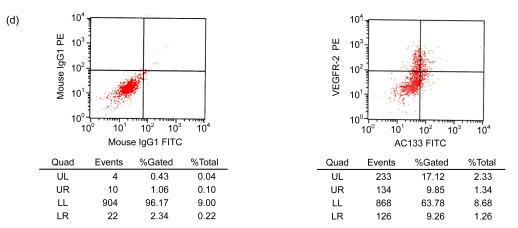
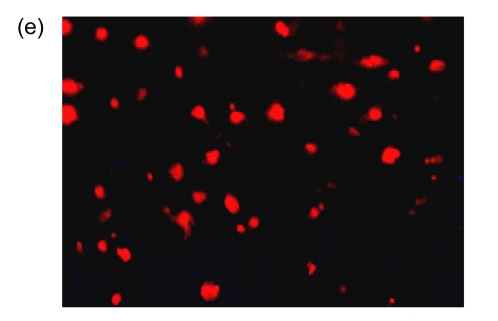
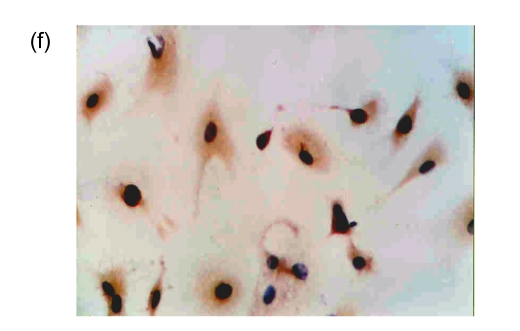
After cultivation for 6 d, culture medium and the red blood cell debris were washed away. A total of 80–100 colonies that were round cells (Fig. 1a) were obtained from each blood sample. The volume and number of the cells were increased after two weeks. The cells connected to sheets and formed the typical cobblestone shape of the endothelial cells (Fig. 1b). In the third week, some of the proliferating cells were then rounded individual cells or small colonies, slightly higher than the training plane. The cells formed an endothelial cell-specific structure of the lumen after long-distance cultivation. After cultivation for 6 d, the flow cytometry analyzed the cells that were expressed by CD34, VE-cadherin, VEGFR-2, and AC133 (Figs. 1c and 1d). Fluorescent microscopy showed that, with cultivating time, more and more cells swallowed DiI-ac-LDL; red was stimulated by fluorescence microscope (Fig. 1e), while the blank and negative control were not. VIII-related antigen Immunohistochemistry showed that positive cells were stained brown (Fig. 1f).
Fig. 1.
Characterization of human endothelial progenitor cells (EPCs)
(a) After cultivation for 6 d, 80–100 colonies could be obtained from each blood sample (×100); (b) The cells connected to sheets and formed a typical cobblestone shape of the endothelial cells after two weeks (×200); (c, d) After cultivation for 6 d, the cell flow cytometry analyzed the cells that were expressed by CD34 and VE-cadherin (c), and VEGFR-2 and AC133 (d). UL: upper left; UR: upper right; LL: lower left; LR: lower right; PE: phycoerythrin; FITC: fluorescein isothiocyanate; (e) Fluorescent microscopy showed that, with cultivating time, more and more cells took in DiI-ac-LDL. Red was stimulated by the fluorescence microscope (×400); (f) VIII-related antigen immunohistochemistry showed that positive cells were stained brown (×200)
3.2. Danshen pills on the function of peripheral blood EPCs
The analysis of the EPC cultivation of the peripheral blood in 24 hypercholesterolemia subjects showed that, compared with the control group, a two-week treatment with Danshen caused a significant increase in the cloning number of EPCs of peripheral blood in hypercholesterolemia patients (4.47±0.94 vs. 3.38±0.57, P<0.01); the OD value of proliferation was increased (0.27±0.04 vs. 0.20±0.03, P<0.01), and the numbers of EPC adhesion (11.81±2.29 vs. 10.03±1.32, P<0.05) and EPC migration (15.75±2.27 vs. 11.95±1.28, P<0.01) were also increased significantly.
3.3. Effect of Danshen on the adhesion ability of EPCs injured by Ox-LDL
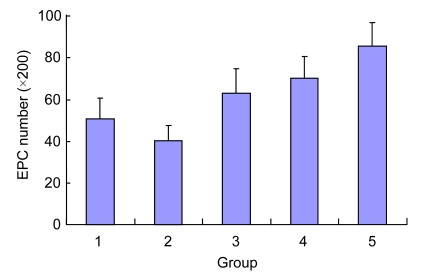
Compared with the control group, Ox-LDL reduced the adhesion force of EPCs significantly (40.50±6.85 vs. 50.50±10.12, P<0.05). After the intervention of different concentrations of Danshen for 24 h, adhesion ability of EPCs was increased significantly, and the greatest effect was achieved in the 50 μg/ml group (compared with the Ox-LDL group, P<0.01). Also, the cell adhesion forces of the Danshen groups were significantly higher than that of the control group (P<0.01) (Fig. 2).
Fig. 2.
Effect of Danshen on the adhesion ability of EPCs injured by Ox-LDL
Group: 1. control; 2. Ox-LDL; 3. 2 µg/ml Danshen; 4. 10 µg/ml Danshen; 5. 50 µg/ml Danshen
3.4. Effect of Danshen on the proliferation ability of EPCs injured by Ox-LDL
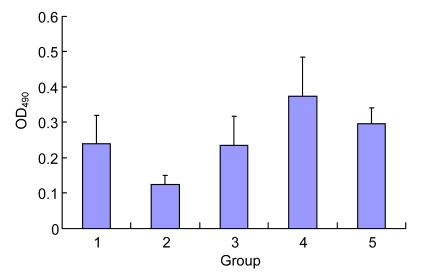
Compared with the control group, Ox-LDL reduced the proliferation ability of EPCs significantly (0.13±0.02 vs. 0.24±0.08, P<0.05). After the intervention of different concentrations of Danshen for 24 h, their proliferation had increased significantly, and the greatest effect was achieved in the 10 μg/ml group (compared with the Ox-LDL group, P<0.01), while the effect in the 50 μg/ml group was dropped, but still higher than that in the control group (Fig. 3).
Fig. 3.
Effect of Danshen on the proliferation ability of EPCs injured by Ox-LDL
Group: 1. control; 2. Ox-LDL; 3. 2 µg/ml Danshen; 4. 10 µg/ml Danshen; 5. 50 µg/ml Danshen
3.5. Effect of Danshen on the SOD content of EPCs injured by Ox-LDL
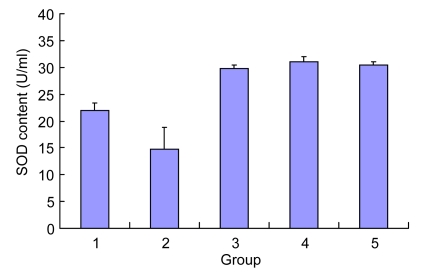
Compared with the normal group, Ox-LDL reduced the SOD content in the cell supernatant significantly. After the intervention of Danshen for 24 h, the level of SOD was significantly increased (compared with the Ox-LDL group, P<0.01), and each group was markedly higher than the control group (Fig. 4).
Fig. 4.
Effect of Danshen on the SOD content of EPCs injured by Ox-LDL
Group: 1. control; 2. Ox-LDL; 3. 2 µg/ml Danshen; 4. 10 µg/ml Danshen; 5. 50 µg/ml Danshen
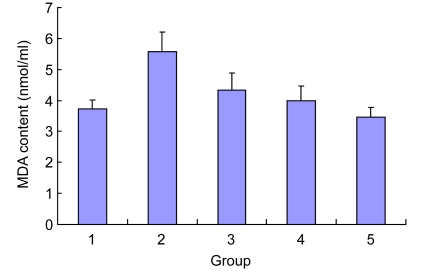
3.6. Effect of Danshen on the MDA content of EPCs injured by Ox-LDL
Compared with the control group, Ox-LDL improved the MDA content in the cell supernatant significantly [(3.72±0.30) vs. (5.57±0.64) nmol/ml, P<0.01). After the intervention with Danshen for 24 h, the level of MDA was reduced significantly (compared with the Ox-LDL group, P<0.01). No statistically significant differences were observed between the Danshen groups and the control group (P>0.05), and the effect between the different Danshen groups also showed no difference (P>0.05) (Fig. 5).
Fig. 5.
Effect of Danshen on the MDA content of EPCs injured by Ox-LDL
Group: 1. control; 2. Ox-LDL; 3. 2 µg/ml Danshen; 4. 10 µg/ml Danshen; 5. 50 µg/ml Danshen
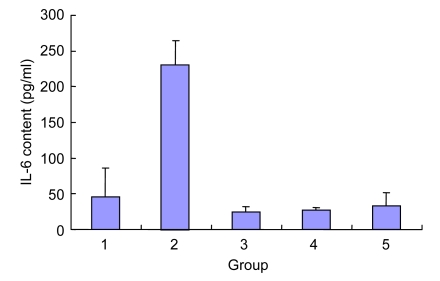
3.7. Effect of Danshen on the IL-6 content of EPCs injured by Ox-LDL
Compared with the control group, Ox-LDL significantly improved the level of IL-6 of the EPC supernatant (P<0.001). After the intervention with Danshen at different concentrations for 24 h, the level of IL-6 of supernatant was significantly reduced (P<0.05), and no statistically significant differences were observed between the groups of Danshen at different concentrations (P>0.05) (Fig. 6).
Fig. 6.
Effect of Danshen on the IL-6 content of EPCs injured by Ox-LDL
Group: 1. control; 2. Ox-LDL; 3. 2 µg/ml Danshen; 4. 10 µg/ml Danshen; 5. 50 µg/ml Danshen
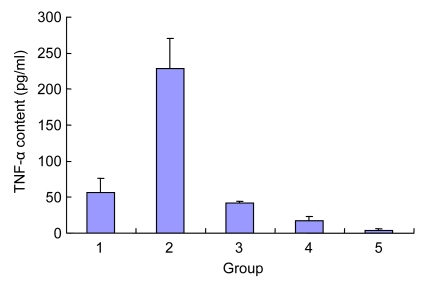
3.8. Effect of Danshen on the TNF-α content of EPCs injured by Ox-LDL
Compared with the control group, Ox-LDL significantly improved the level of TNF-α of EPC supernatant (P<0.001). After Danshen at different concentrations for 24 h, the level of TNF-α of supernatant was significantly reduced, and had a negative correlation with the concentration of Danshen (P<0.05) (Fig. 7).
Fig. 7.
Effect of Danshen on the TNF-α content of EPCs injured by Ox-LDL
Group: 1. control; 2. Ox-LDL; 3. 2 µg/ml Danshen; 4. 10 µg/ml Danshen; 5. 50 µg/ml Danshen
4. Discussion
Ox-LDL causes endothelial cell dysfunction by oxidative damage to endothelial cells and induction of endothelial cell apoptosis. It was known that endothelial cell injury or dysfunction is the initiating agent to atherosclerosis (Ross, 1993). Previous studies (Griese et al., 2003; Werner et al., 2003) showed that accelerated re-endothelialization by EPCs effectively inhibited SMC migration, proliferation, and neointima formation, and therefore prevented the development of the early stages of restenosis after vascular injury. EPCs can also promote angiogenesis of ischemic disease, especially in ischemic cardiovascular and cerebrovascular disease (Kawamoto et al., 2001; Murayama et al., 2002; Kawamoto et al., 2003; Suzuki et al., 2003). They play a momentous role in the prevention of early atherosclerosis and the treatment of restenosis after angioplasty.
Hypercholesterolemia not only causes endothelial cell damage, but also affects the number and function of EPCs. Other research has shown that Ox-LDL is the main reason for the changes of function and number of EPCs in patients with hypercholesterolemia (Wang et al., 2004). The exact mechanism of how Ox-LDL leads to reduced and impaired function of EPCs is unclear. Our data show that, compared with the control group, Ox-LDL markedly reduced the activity of SOD and improved the levels of MDA, IL-6, and TNF-α of the EPC supernatant. At the same time, the proliferation capacity and adhesion ability of EPCs were significantly impaired, which suggests that Ox-LDL may damage EPCs by impairing anti-oxidase activity and promoting the release of the inflammatory factors of EPCs.
In recent years, many studies have shown that Danshen has a protective effect on endothelial cell injuries caused by a variety of factors. However, studies concerning Danshen’s effect on EPCs are few. Our pilot research investigates the effect of Danshen on the function and the number of EPCs (Ji et al., 2006). We found that Danshen at low concentrations (2 μg/ml) can significantly amplify the quantity of cultured EPCs, and enhance adhesion and colony-forming ability. The current study showed that Danshen pills might significantly improve the peripheral blood EPC functions in patients with hypercholesterolemia.
The exact mechanism of Danshen’s protection on the number and function of progenitor cells in patients with hypercholesterolemia is still unclear. This study found that Danshen can not only improve the proliferation and adhesion ability of EPCs injured by Ox-LDL, but also significantly increase the SOD content in the cell supernatant and reduce the level of MDA compared to the control group. SOD, which can remove superoxide radical anions and protect cells from damage, plays a key role in the balance of oxidation and antioxidant of the body. MDA indirectly reflects the severity of cells attacked by free radicals. According to this, it is conjectured that the Danshen can increase the EPC ability to secrete SOD, increase the cells ability to remove oxygen free radicals, and then protect the polyunsaturated fatty acids of cell membranes. Oxygen free radicals also can damage cells through hydrogen peroxide, but Danshen is able to reduce cell supernatant MDA content, suggesting EPCs can partly block the hydrogen peroxide process, and then have a protective effect at the molecular level.
Our data also show that Danshen can also significantly reduce the levels of IL-6 and TNF-α of EPC supernatant. IL-6 is a cytokine of circulation, of which the level appears to reactively rise with acute myocardial infarction (AMI), unstable angina, percutaneous coronary intervention (PCI), and restenosis after PCI. On the one hand, IL-6 stimulates platelet aggregation, and induces tissue factor and macrophage LDL receptors, CRP, and fibrinogen expression. On the other hand, it can also regulate the expression of other inflammatory cytokines such as IL-1 and TNF-α (Ikeda et al., 2001). Also, TNF-α may participate in the occurrence and development processes of atherosclerosis (AS) and coronary heart disease (CHD) through injuring vascular endothelial cells (VEC), promoting clotting, restraining fibrinolysis, and promoting the adhesion of monocyte to endothelial cells (ECs) (Ridker et al., 2000). After Danshen use for 24 h, the levels of IL-6 and TNF-α were significantly reduced, which suggests that Danshen may protect EPCs from the damage caused by Ox-LDL by inhibiting the release of EPCs’ inflammatory factors. Our data also show that compared with the control group, Danshen also significantly improved cell function, and reduced the levels of IL-6 and TNF-α in the EPC supernatant, which suggests that Danshen can not only be used to reduce the damage of EPCs from Ox-LDL, but also significantly enhance the anti-inflammatory ability of EPCs.
Footnotes
Project (No. 2007A142) supported by the Health Department of Zhejiang Province, China
References
- 1.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 2.Baek EB, Yoo HY, Park SJ, Chung YS, Hong EK, Kim SJ. Inhibition of arterial myogenic responses by a mixed aqueous extract of Salvia miltiorrhiza and Panax notoginseng (PASEL) showing antihypertensive effects. Korean Journal of Physiology and Pharmacology. 2009;13(4):287–293. doi: 10.4196/kjpp.2009.13.4.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen JZ, Zhang FR, Tao QM, Wang XX, Zhu JH, Zhu JH. Number and activity of endothelial progenitor cells from peripheral blood in patients with hypercholesterolaemia. Clinical Science. 2004;107(3):273–280. doi: 10.1042/CS20030389. [DOI] [PubMed] [Google Scholar]
- 4.Du GH, Qiu Y, Zhang JT. Salvianolic acid B protects the memory functions against transient cerebral ischemia in mice. Journal of Asian Natural Products Research. 2000;2(2):145–152. doi: 10.1080/10286020008039903. [DOI] [PubMed] [Google Scholar]
- 5.Fish JM, Welchons DR, Kim YS, Lee SH, Ho WK, Antzelevitch C. Dimethyl Lithospermate B, an extract of Danshen, suppresses arrhythmogenesis associated with the brugada syndrome. Circulation. 2006;113(11):1393–1400. doi: 10.1161/CIRCULATIONAHA.105.601690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galle J, Lehmann-Bodem C, Hubner U, Heinloth A, Wanner C. CyA and OxLDL cause endothelial dysfunction in isolated arteries through endothelinmediated stimulation of O(2)(−) formation. Nephrology Dialysis Transplantation. 2000;15(3):339–346. doi: 10.1093/ndt/15.3.339. [DOI] [PubMed] [Google Scholar]
- 7.Griese DP, Ehsan A, Melo LG, Kong D, Zhang L, Mann MJ, Pratt RE, Mulligan RC, Dzau VJ. Isolation and transplantation of autologous circulating endothelial cells into denuded vessels and prosthetic grafts:implications for cell-based vascular therapy. Circulation. 2003;108(21):2710–2715. doi: 10.1161/01.CIR.0000096490.16596.A6. [DOI] [PubMed] [Google Scholar]
- 8.Harrison DG. Endothelial function and oxidant stress. Clinical Cardiology. 1997;20(11 Suppl. 2):II-11–II-17. [PubMed] [Google Scholar]
- 9.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. New England Journal of Medicine. 2003;348(7):593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 10.Ikeda U, Ito T, Shimada K. Interleukin-6 and acute coronary syndrome. Clinical Cardiology. 2001;24(11):701–704. doi: 10.1002/clc.4960241103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ji KT, Zhang HQ, Yang PL. Effects of compound salvia injection on number and activity of endothelial progenitor cells. China Journal of Chinese Materia Medica. 2006;31(3):246–249. (in Chinese) [PubMed] [Google Scholar]
- 12.Jiang RW, Lau KM, Hon PM, Mak TC, Woo KS, Fung KP. Chemistry and biological activities of caffeic acid derivatives from Salvia miltiorrhiza . Current Medicinal Chemistry. 2005;12(2):237–246. doi: 10.2174/0929867053363397. [DOI] [PubMed] [Google Scholar]
- 13.Kawamoto A, Gwon C, Iwaguro H, Yamaguchi JI, Uchida S, Masuda H, Silver M, Ma H, Kearney M, Isner JM, et al. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation. 2001;103(5):634–637. doi: 10.1161/01.cir.103.5.634. [DOI] [PubMed] [Google Scholar]
- 14.Kawamoto A, Tkebuchava T, Yamaguchi J, Nishimura H, Yoon YS, Milliken C, Uchida S, Masuo O, Iwaguro H, Ma H, et al. Intramyocardial transplantation of autologous endothelial progenitor cells for therapeutic neovascularization of myocardial ischemia. Circulation. 2003;107(3):461–468. doi: 10.1161/01.CIR.0000046450.89986.50. [DOI] [PubMed] [Google Scholar]
- 15.Liu CL, Xie LX, Li M, Durairajan SS, Goto S, Huang JD. Salvianolic acid B inhibits hydrogen peroxide-induced endothelial cell apoptosis through regulating PI3K/Akt signaling. PLoS ONE. 2007;2(12):e1321. doi: 10.1371/journal.pone.0001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu HB, Wen JK, Han M. Effects of Salvia miltiorrhiza on matrix metalloproteinases and osteopontin gene expression and proliferation of cultured vascular smooth muscle cells. Chinese Journal of Integrated Traditional and Western Medicine. 2002;22(10):764–766. (in Chinese) [Google Scholar]
- 17.Murayama T, Tepper OM, Silver M, Ma H, Losordo DW, Isner JM, Asahara T, Kalka C. Determination of bone marrow-derived endothelial progenitor cell significance in angiogenic growth factor-induced neovascularization in vivo. Experimental Hematology. 2002;30(8):967–972. doi: 10.1016/S0301-472X(02)00867-6. [DOI] [PubMed] [Google Scholar]
- 18.Pirro M, Bagaglia F, Paoletti L, Razzi R, Mannarino MR. Hypercholesterolemia-associated endothelial progenitor cell dysfunction. Therapeutic Advances in Cardiovascular Disease. 2008;2(5):329–339. doi: 10.1177/1753944708094769. [DOI] [PubMed] [Google Scholar]
- 19.Rauscher FM, Goldschmidt-Clermont PJ, Davis BH, Wang T, Gregg D, Ramaswami P, Pippen AM, Annex BH, Dong C, Taylor DA. Aging, progenitor cell exhaustion, and atherosclerosis. Circulation. 2003;108(4):457–463. doi: 10.1161/01.CIR.0000082924.75945.48. [DOI] [PubMed] [Google Scholar]
- 20.Ridker PM, Rifai N, Pfeffer M, Sacks F, Lepage S, Braunwald E. Elevation of tumor necrosis factoralph and increased risk of recurrent coronary events after myocardial infarction. Circulation. 2000;101(18):2149–2153. doi: 10.1161/01.cir.101.18.2149. [DOI] [PubMed] [Google Scholar]
- 21.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki T, Nishida M, Futami S, Fukino K, Amaki T, Aizawa K, Chiba S, Hirai H, Maekawa K, Nagai R. Neoendothelialization after peripheral blood stemcell transplantation in humans: a case report of a Tokaimura nuclear accident victim. Cardiovascular Research. 2003;58(2):487–492. doi: 10.1016/S0008-6363(02)00780-0. [DOI] [PubMed] [Google Scholar]
- 23.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circulation Research. 2001;89(1):e1–e7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 24.Walter DH, Rittig K, Bahlmann FH, Kirchmair R, Silver M, Murayama T, Nishimura H, Losordo DW, Asahara T, Isner JM. Statin therapy accelerate reendothelialization: a novel effect involving mobilization and incorporation of bone marrow-derived endothelial progenitor cells. Circulation. 2002;105(25):3017–3024. doi: 10.1161/01.CIR.0000018166.84319.55. [DOI] [PubMed] [Google Scholar]
- 25.Wang XX, Chen JZ, Tao QM, Zhu JH, Shang YP. Effects of Ox-LDL on number and activity of circulating endothelial progenitor cells. Drug and Chemical Toxicology. 2004;27(3):243–255. doi: 10.1081/DCT-120037505. [DOI] [PubMed] [Google Scholar]
- 26.Werner N, Junk S, Laufs U, Link A, Walenta K, Bohm M, Nickenig G. Intravenous transfusion of endothelial progenitor cells reduces neointima formation after vascular injury. Circulation Research. 2003;93(2):e17–e24. doi: 10.1161/01.RES.0000083812.30141.74. [DOI] [PubMed] [Google Scholar]
- 27.Wu XJ, Wang YP. Salvia magnesium acetate of oxidized low density lipoprotein-induced apoptosis of endothelial cells. Chinese Journal of Pathophysiology. 2002;18(13):18–35. (in Chinese) [Google Scholar]
- 28.Xu DB, Zhang JP, Zhang WZ. Experimental study on protective effect of Danshen for the cultured epithelial cells from injury by oxidized low density lipoprotein. Journal of Tianjin Medical University. 2001;7(1):21–23. (in Chinese) [Google Scholar]
- 29.Xu Q, Zhang Z, Davison F, Hu Y. Circulating progenitor cells regenerate endothelium of vein graft atherosclerosis, which is diminished in ApoE-deficient mice. Circulation Research. 2003;93(8):e76–e86. doi: 10.1161/01.RES.0000097864.24725.60. [DOI] [PubMed] [Google Scholar]
- 30.Zhang ZX, Gao FY, Li KM. Effect of magnesium lithospermate B on endothelial cells in human aorta after free radical injury. Chinese Journal of Integrated Traditional and Western Medicine. 2004;24(6):521–524. (in Chinese) [PubMed] [Google Scholar]
- 31.Zhou L, Zuo Z, Chow MS. Danshen: an overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. The Journal of Clinical Pharmacology. 2005;45(12):1345–1359. doi: 10.1177/0091270005282630. [DOI] [PubMed] [Google Scholar]
- 32.Zhu J, Wang X, Chen J, Sun J, Zhang F. Reduced number and activity of circulating endothelial progenitor cells from patients with hyperhomocysteinemia. Archives of Medical Research. 2006;37(4):484–489. doi: 10.1016/j.arcmed.2005.09.017. [DOI] [PubMed] [Google Scholar]