Abstract
Signaling through the adaptor protein myeloid differentiation factor 88 (MyD88) promotes carcinogenesis in several cancer models. In contrast, MyD88 signaling has a protective role in the development of azoxymethane (AOM)/dextran sodium sulfate (DSS) colitis-associated cancer (CAC). The inability of Myd88−/− mice to heal ulcers generated upon injury creates an altered inflammatory environment that induces early alterations in expression of genes encoding proinflammatory factors, as well as pathways regulating cell proliferation, apoptosis, and DNA repair, resulting in a dramatic increase in adenoma formation and progression to infiltrating adenocarcinomas with frequent clonal mutations in the β-catenin gene. Others have reported that toll-like receptor (Tlr) 4–deficient mice have a similar susceptibility to colitis to Myd88-deficient mice but, unlike the latter, are resistant to CAC. We have observed that mice deficient for Tlr2 or Il1r do not show a differential susceptibility to colitis or CAC. However, upon AOM/DSS treatment Il18−/− and Il18r1−/− mice were more susceptible to colitis and polyp formation than wild-type mice, suggesting that the phenotype of Myd88−/− mice is, in part, a result of their inability to signal through the IL-18 receptor. This study revealed a previously unknown level of complexity surrounding MyD88 activities downstream of different receptors that impact tissue homeostasis and carcinogenesis.
Although inflammatory bowel diseases (IBDs) are associated with a high risk of colon carcinogenesis, the mechanisms underlying this association have yet to be fully elucidated. Intestinal microbiota appear to be a key pathogenic trigger in the development of IBD, a fact which implicates a contribution of innate and adaptive immune responses in which Toll-like receptors (TLRs) may function as primary sensors of microbial colonization. Most of these receptors, as well as all the receptors for the proinflammatory cytokines of the IL-1 family, use a common signal transduction molecule called myeloid differentiation factor 88 (MyD88). Association of MyD88 with the intracellular Toll-like–IL-1 resistance domain of the receptors results in activation of the NF-κB and AP-1 signaling pathways, leading to the initiation of the proinflammatory response. Although Myd88−/− mice are viable and are apparently normal in the absence of infections, early studies (Araki et al., 2005) highlighted an important role of MyD88 in maintenance of intestinal homeostasis, in that γ irradiation or administration of dextran sodium sulfate (DSS) resulted in severe ulceration and inflammation, accompanied by bleeding and high lethality. Several studies demonstrated that recognition of commensal luminal bacteria by TLRs is crucial for colonic epithelial cell regeneration upon DSS injury (Rakoff-Nahoum et al., 2004; Pull et al., 2005; Brown et al., 2007). The ability of MyD88 to signal through NF-κB suggests that its role in controlling mucosal homeostasis may be dependent on the activity of this transcription factor in enterocyte survival. Mice in which the canonical pathway of NF-κB activation is completely blocked in enterocytes (by tissue-specific deletion of Nemo or both Ikka and Ikkb) spontaneously developed colitis that was, however, prevented in mice that were also systemically deficient for MyD88, suggesting that the lack of NF-κB renders the enterocytes susceptible to inflammatory mediators produced by nonepithelial cells triggered by ligands of TLRs or IL-1Rs (Nenci et al., 2007). Mice with enterocyte-specific depletion of Ikkb did not spontaneously develop colitis and were not more susceptible to DSS-induced colitis but developed a lower number of colonic polyps than WT mice when treated with the chemical carcinogen azoxymethane (AOM) followed by DSS administration (Greten et al., 2004). When Ikkb was specifically deleted in myeloid cells, the number of polyps was not affected but their size was decreased, suggesting that NF-κB–dependent myeloid-derived factors promote tumor growth (Greten et al., 2004).
Recently, several studies highlighted an important role of MyD88 in tumor promotion. In the two-stage cutaneous chemical carcinogenesis model, MyD88 has a promoting role in cancer, as indicated by inhibition of tumor induction in MyD88 genetically deficient mice compared with WT mice (Swann et al., 2008). Similarly, in the diethylnitrosamine-induced liver cancer model, MyD88 deficiency diminished the development of hepatic cancer in male mice via inhibition of IL-6 production (Naugler et al., 2007). Additionally, in the ApcMin/+ mouse model of spontaneous intestinal tumorigenesis, MyD88 signaling contributed to adenoma growth and progression. Furthermore, reduced colon tumor growth was observed in Myd88−/− mice subjected to multiple injections of AOM in comparison with WT controls (Rakoff-Nahoum and Medzhitov, 2007). MyD88 signaling was also shown to be required for AOM-enhanced colon carcinogenesis in Il10−/− mice (Uronis et al., 2009). Deficiency in MyD88 was found not to affect the spontaneous development of colonic dysplasia and rectal adenocarcinoma in T-bet−/− and Rag2−/− ulcerative colitis mice (Garrett et al., 2009). Nevertheless, the role of MyD88 in chronic colitis-induced cancer remained to be studied.
Unlike previous studies in other genetic or chemical carcinogenesis models, in this paper we describe a protective role of MyD88 in the development of the colonic tumors that develop after AOM/DSS treatment. The inability of Myd88−/− mice to heal ulcers generated upon injury creates an altered inflammatory environment that exacerbates the mutation rate in mucosal epithelial cells and results in augmented adenoma formation and cancer progression. We also report that mice deficient in Il18 and Il18r1 display upon AOM/DSS treatment an increased susceptibility to colitis and polyp formation, with a similar but not identical molecular profile to that observed in Myd88−/− mice, suggesting that the susceptibility of the latter mice to colitis and colitis-associated cancer (CAC) is in part a result of their inability to signal through the IL-18 receptor.
RESULTS
Myd88−/− mice show increased susceptibility to develop colonic tumors after AOM/DSS administration
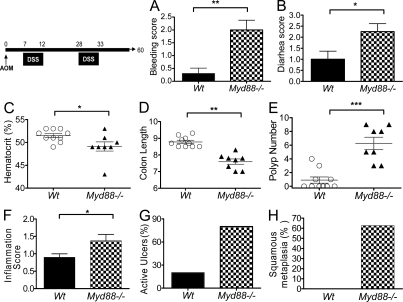
Myd88−/− mice are highly susceptible to the development of colitis induced by irradiation or DSS as a result of reduced ability to repair the mucosal integrity after injury with significant morbidity and mortality (Rakoff-Nahoum et al., 2004; Araki et al., 2005). In our studies, we were able to maintain survival of the majority of the Myd88−/− mice after DSS treatment by administering wet feed and saline injections in case of dehydration. Myd88−/− and WT mice were systemically injected with the carcinogen AOM followed by induction of colitis by DSS administration in the drinking water in 5-d cycles. As expected, a high frequency of intestinal bleeding resulting in anemia, marked diarrhea, and a decrease in the length of the colons was observed in Myd88−/− mice (Fig. 1, A–D). Macroscopic analysis of the colons at day 60 after AOM administration indicated a much higher mean number of colonic polyps in Myd88−/− mice than in WT B6 mice associated with a modest increase in local inflammation score (Fig. 1, E and F). Furthermore, unlike WT mice, the majority of Myd88−/− mice presented extensive active ulcers and squamous metaplasia localized mainly in the last third of the distal colon (Fig. 1, G and H). To limit the variability of the intestinal commensal microbiota in the immunodeficient Myd88−/− mice compared with the WT mice, the experiments were repeated using littermates from Myd88+/− parents. Severe colitis and increased frequency of tumor growth were obtained when Myd88−/− mice were compared with their corresponding WT or Myd88+/− littermates (Fig. S1). These data suggest that MyD88 deficiency has a direct effect on the susceptibility of the mice to colitis and carcinogenesis that is likely not a result of broad changes in the environment or in the type of commensal microbiota.
Figure 1.
Myd88−/− mice develop colonic polyps after AOM/DSS administration. Cohorts of 8–10 WT and Myd88−/− mice per group were injected i.v. with AOM on day 0 followed by two DSS cycles administered in drinking water. At the completion of the first DSS cycle, mice were monitored for bleeding (A) and diarrhea (B). 60 d after AOM administration, hematocrits were measured (C), mice were euthanized and colons were resected and measured (D), and macroscopic polyps were counted (E). Colon sections were fixed in formalin and stained with hematoxylin-eosin. Sections were analyzed for inflammation degree (F), extent of ulceration (G), and squamous metaplasia (H). The data shown correspond to a representative experiment out of four performed. Data represent means ± SE (and in some panels also individual mice results). *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Previous studies have shown that Myd88−/− mice are resistant to different chemical or genetic models of carcinogenesis, including intestinal carcinogenesis in Apcmin/+ mice, Il10−/− mice, or after repeated treatment with the carcinogen AOM (Rakoff-Nahoum and Medzhitov, 2007; Uronis et al., 2009). Thus, to exclude the possibility that our apparently contradictory results in the AOM/DSS system were a result of differences in our animal colony conditions or of genetic drifting in our Myd88−/− mice, we subjected Myd88−/− mice to repeated treatments of AOM administration and, in agreement with the previously reported data, we observed a decreased incidence of colonic tumor (Fig. S2). In contrast, we observed that administration of DSS alone unexpectedly elicited the formation of colonic tumors in a small proportion of Myd88−/− mice but not in WT mice, although the difference did not reach statistical significance (Fig. S2). Thus, it is possible to speculate that the inflammatory effect elicited by exposure of DSS is sufficient to induce a low frequency of colonic tumors in Myd88−/− mice and that this inflammation enhanced their susceptibility to the carcinogenic effects of AOM, whereas the generation of tumors after administration of AOM in the absence of DSS-induced inflammation required intact signaling through Myd88.
Myd88−/− mice exhibit decreased colonic epithelial cell proliferation and enhanced apoptosis after AOM/DSS administration, triggering mucosal ulceration associated with local inflammation
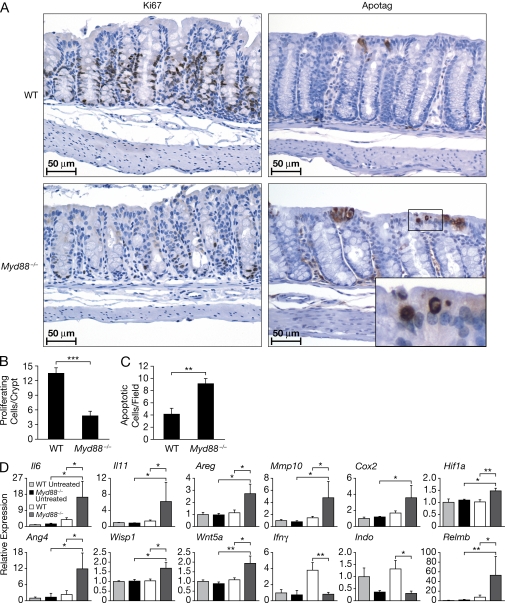
Previous studies indicated that engagement of TLR by commensal microbiota is required to protect the intestinal mucosa from damage induced by DSS or irradiation. Basal colonic mucosal epithelial cell proliferation was shown to be increased in untreated Myd88−/− and Tlr4−/− mice, but the mucosal repair after injury by irradiation in Myd88−/− mice (Rakoff-Nahoum et al., 2004) or by DSS in Tlr4−/− mice (Fukata et al., 2005) was impaired, which is consistent with the reduced ability of these mice to repair their mucosa after injury. We observed that colonic mucosal epithelial cell proliferation, as indicated by Ki67 staining, was decreased approximately threefold at the third day of DSS administration after AOM treatment in Myd88−/− mice compared with WT controls (Fig. 2, A and B), whereas apoptosis was increased (Fig. 2, A and C). Thus, the inability of epithelial cells to proliferate efficiently in Myd88−/− mice during the repair reaction after DSS-induced mucosal injury is likely to contribute to the severe mucosal erosion and bleeding observed in these animals.
Figure 2.
AOM/DSS treatment of Myd88−/− mice results in decreased proliferation and enhanced apoptosis of colonic epithelial cells and increased expression of mitogenic, angiogenic, and pro-tumorigenic genes. Cohorts of 10 WT and Myd88−/− mice were injected i.v. with AOM on day 0 followed by 3 d of DSS administration in drinking water. Thereafter, colons were resected, fixed in formalin, and sections were stained with Ki67 or ApopTag antibodies. Photomicrographs of representative sections from the respective groups are shown at 400× magnification (A). The number of proliferating cells per crypt and the number of apoptotic cells was determined by counting 20 consecutive crypts/section (B and C). Gene expression profile in colon tissue after AOM/DSS treatment was analyzed by real-time RT-PCR. The gene expression was normalized to Gapdh levels, and the expression of each gene relative to untreated WT mice is depicted (D). The data shown in A–D correspond to a representative experiment out of two performed. Data represent means ± SE. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
The decreased reactive epithelial cell proliferation and increased apoptosis was difficult to reconcile with the increased susceptibility of Myd88−/− mice to the development of polyps after AOM/DSS treatment. Thus, gene expression profiling of total colon in AOM/DSS-treated Myd88−/− and WT mice was performed (Fig. S3) and expression of cell proliferation–associated genes was compared. Although the use of RNA from total tissues in microarray gene expression analysis has the limitation of failing to identify the cellular types expressing the different genes, it is the preferred approach in many studies, it avoids the modification of gene expression taking place during cell purification procedure, and it is been shown to be able to provide important information regarding the main molecular pathways affected in different experimental or pathological conditions. The epidermal growth factor receptor (EGFR), Met, and β-catenin pathways and their ligands were observed to be preferentially induced in Myd88−/− mice after AOM/DSS administration (Fig. S4), indicating that compensatory mechanisms are apparently activated in the abortive attempt to maintain the integrity of the colonic mucosa. In addition, mRNA expression of cytokine genes and genes of mitogenic responsiveness involved in wound healing, such as those encoding IL-6, IL-11, EGFR ligands including amphiregulin and heparin-binding EGF, as well as factors involved in tissue remodeling and angiogenesis including matrix metalloproteinase (MMP) 10, angiogenin 4, cyclooxygenase 2 (COX-2), and hypoxia-inducible factor (HIF)-1α, were also up-regulated in treated Myd88−/− mice (Fig. 2 D). Importantly, increased expression of antibacterial peptides, including angiogenin 4 and Relm-β, were also induced in AOM/DSS-treated Myd88−/− mice, possibly in response to bacterial translocation to the colonic mucosa (Fig. 2 D).
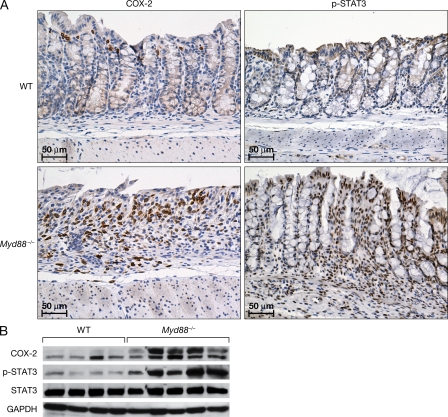
It is of note that expression of genes associated with promotion of colon tumorigenesis, including those encoding amphiregulin, Wnt-5a, Wisp1, HIF-1α, COX-2, MMP-10, and STAT-3, were induced in Myd88−/− mice early in response to colonic injury, which could potentially also drive the formation of colonic polyps (Fig. 2 D). After treatment, elevated expression of COX-2 and pSTAT3 proteins were also detected in Myd88−/− versus WT mice, as shown by immunohistological staining and Western blot analysis (Fig. 3). In Myd88−/− mice, COX-2 expression was observed mainly in the areas where inflammatory cells infiltrated the ulcerated areas of the mucosa, although no colocalization of COX-2 and macrophage staining was observed (Fig. 3 and not depicted). Phospho-STAT3 was strongly and rapidly expressed in the nuclei of most epithelial cells of Myd88−/− mice upon AOM/DSS treatment (as well as in many stromal and infiltrating cells). In WT mice, pSTAT3 was present only in few infiltrating cells and was not expressed on epithelial cells until much later when it was present in the epithelial cells involved in polyp formation but not in the surrounding normal mucosa (Fig. 3 and not depicted). A reduced expression of signaling Smads and enhanced expression of suppressive Smads suggest that TGF-β signaling might be inhibited in the colon of AOM/DSS-treated Myd88−/− mice (unpublished data). Also, Rb expression was reduced, whereas that of E2f was enhanced (Fig. 4).
Figure 3.
AOM/DSS treatment enhances mucosal expression of COX-2 and phospho-STAT3 in Myd88−/− mice. Cohorts of 5–10 mice were subjected to treatment as indicated in Fig. 2. At day 3 of DSS administration, colons were resected and fixed in formalin or snap frozen for protein extraction. Tissue sections were stained for COX-2 and pSTAT3. Photomicrographs of representative sections from the respective groups are shown at 200× (A). Protein extracts were analyzed by Western blotting for COX-2, STAT3, and pSTAT3 expression. Gapdh was used as a control (B). The data shown in A and B correspond to a representative experiment out of two performed.
Figure 4.
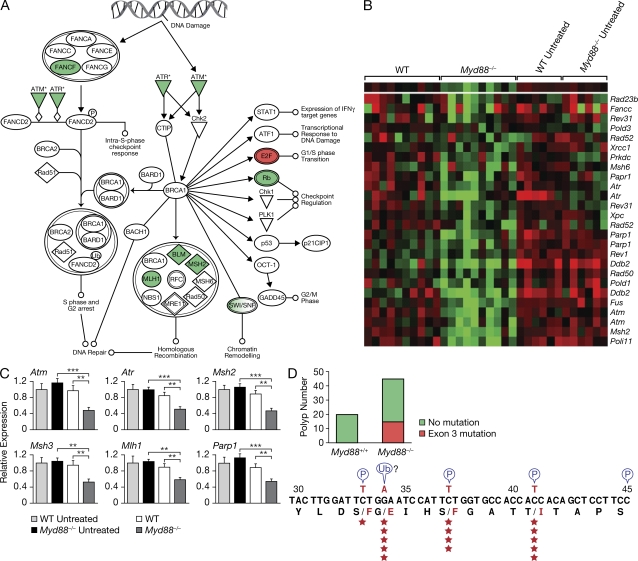
Increased DNA damage is elicited by AOM/DSS treatment in Myd88−/− mice compared with WT mice. RNA was extracted from cohorts of 10 WT or Myd88−/− mice per group treated with AOM/DSS and from cohorts of six nontreated WT or Myd88−/− mice/group. Samples were processed using the Affymetrix microarray platform, and analyzed using the Ingenuity Pathway and Cluster and TreeView Programs. A significant decrease in DNA repair gene expression profile was observed in Myd88−/− mice after AOM/DSS treatment compared with treated WT mice (A and B). The data shown in A and B were obtained from the microarray experiment that was performed once. The pattern of DNA repair gene expression was subsequently confirmed using real-time RT-PCR. The gene expression was normalized to Gapdh levels, and the expression of each gene relative to untreated WT mice is depicted. One out of two representative experiments is shown (C). Data represent means ± SE. **, P < 0.01; ***, P < 0.001. For analysis of β-catenin mutations, cohorts of 15–20 mice/group were subjected to AOM/DSS treatment (two DSS/cycles). 60 d after AOM administration, individual polyps were resected, and DNA was extracted and analyzed for mutation of β-catenin using specific primers for exon 3. The numbers of polyps exhibiting point mutations at positions 33, 34, 37, and 41 in exon 3 are indicated in the scheme by the stars (D). The polyps analyzed for β-catenin mutations were obtained from multiple experiments and each polyp was sequenced for mutations once.
After AOM/DSS treatment, Ifng was up-regulated in WT but not in Myd88−/− colon (Fig. 2 D). Compared with the WT mice, both the basal level and that after AOM/DSS of IFN-responsive genes, including indoleamine-pyrrole 2,3-dioxygenase (Indo), Irf8 (IFN regulatory factor 8), and Igtp (IFN-γ–induced GTPase), were decreased in Myd88−/− mice. In addition, the induction upon AOM/DSS treatment of other IFN-responsive genes, including Irf4 (IFN regulatory factor 4), Ifi30 (IFN-γ inducible protein 30), and Irf2bp2 (IFN regulatory factor 2 binding protein 2), was reduced in Myd88−/− mice (Fig. 2 D and not depicted).
Overall, these data indicate that in Myd88−/− mice the response to the mucosal damaged induced by AOM/DSS, which is characterized by diminished IFN responses, decreased cell proliferation, and enhanced apoptosis, was also associated with the induction of compensatory pathways of tissue self-renewal and wound repair. However, this was not fully effective in controlling the mucosal damage and resulted in a chronic inflammatory state promoting tumor formation.
AOM/DSS treatment results in decreased expression of mismatch DNA repair genes, which correlates with the high frequency of β-catenin mutations and colon adenocarcinoma formation in Myd88−/− mice
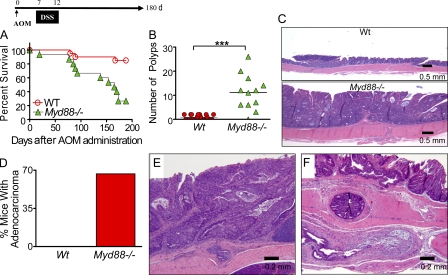
AOM/DSS-induced colitis in Myd88−/− mice was observed to be associated with a coordinated down-regulation of genes involved in mismatch DNA repair, including poly ADP-ribose polymerase (Parp) family members Parp1 and Parp2, Fancb (Fanconi anemia complementation group B), Fancf, Dclre1a (DNA cross-link repair 1A), msh2, msh3, mlh1, and bml, as well as genes involved in double-strand DNA repair including ATM (ataxia telangiectasia mutated) and ATR (ataxia telangiectasia and Rad3 related; Fig. 4). Low levels of DNA mismatch repair gene expression might imply higher genetic instability in the epithelial cells after DNA damage induced by AOM or inflammatory mediators with increased frequency of oncogenic mutations in Myd88−/− mice. Indeed, a screen for β-catenin mutations in exon 3 revealed a much higher frequency of mutations in the polyps resected from Myd88−/− mice (15 out of 45 sequenced) than in those from WT mice (no mutation detected in 20 resected polyps; Fig. 4 D). Importantly, the detected β-catenin mutations were at codons 33, 34, 37, and 41, all of which encode functional residues for phosphorylation by GSK3β or for ubiquitination and, therefore, control β-catenin degradation. Previous studies indicated that functional mutations in the β-catenin gene play a critical role in the progression of colon carcinogenesis (Yamada et al., 2003). We therefore performed long-term experiments to evaluate if the adenomas that developed in MyD88 knockout mice progressed into adenocarcinomas. For this purpose, mice were injected with AOM at day 0, followed by a single DSS cycle from days 7 to 12, and the animal were followed for 6 mo. Significant decreases in survival were observed in Myd88−/− mice in contrast to the WT group (P = 0.0005; Fig. 5 A). Myd88−/− mice exhibited a marked increase in the number of polyps at the time of euthanasia in contrast to the WT control group (mean polyps: 11.16 ± 5.6 in Myd88−/− vs 0.89 ± 0.84 in WT; P < 0.0001; Fig. 5 B). Myd88−/− mice exhibited extensive, large, and confluent adenomas in contrast to WT (Fig. 5 C). 67% of the Myd88−/− mice developed adenocarcinomas characterized by formation of acinar structures within the mucosa and invasion into the submucosa, and even into the serosa, in some cases, with occasional formation of mucin deposits (Fig. 5, D–G).
Figure 5.
AOM/DSS treatment induces increased frequency of colonic adenocarcinomas in Myd88−/− mice. Cohorts of 15 Myd88−/− mice and 20 WT mice were injected i.v. with AOM on day 0 followed by a single DSS cycle. Animals were monitored during 6 mo after treatment for survival (P = 0.0005, WT vs. Myd88−/−; A). All the colons were analyzed for polyps when mice reached any of the end points for euthanasia or at the completion of the experiment (***, P < 0.001; B). Colon sections were fixed and stained with hematoxylin-eosin. 17 polyps from the WT group and 52 polyps from Myd88−/− knockout mice were analyzed histologically. Photomicrographs of representative sections from the respective groups are shown at 20× (C). The percentage of mice developing adenocarcinomas is indicated (D). Photomicrographs of representative adenocarcinomas from multiple of those that developed in Myd88−/− mice are shown at 40× magnification (E–G). The data shown in this figure were obtained from a single experiment performed treating the mice with one DSS cycle but closely mimic the data obtained from two other independent experiments with mice treated with two DSS cycles that are shown in Fig. S1.
Il18−/− and Il18r1−/− mice partially mimic the susceptibility of Myd88−/− to AOM/DSS induction of colitis and colon cancer
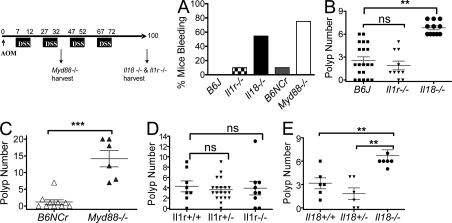
MyD88 mediates signaling for the IL-1 family receptors and TLRs. To identify the upstream MyD88-coupled receptors involved in mediating susceptibility to colitis and colon carcinogenesis, we examined the responses elicited by AOM/DSS treatment in either Il1r1−/− or Il18−/− mice. Cohorts of Myd88−/−, Il18−/−, or Il1r1−/− mice and their respective controls were treated with AOM and subjected to two or four DSS cycles. Compared with WT C57BL6J controls and Il1r−/− animals, Il18−/− mice displayed significantly more intestinal bleeding after DSS administration, mimicking, although to a lesser degree, the phenotype observed in Myd88−/− mice (Fig. 6 A). As result of increased intestinal bleeding, lower hematocrits were displayed by Il18−/− and Myd88−/− mice (unpublished data). In addition, in acute colitis induced by a 5-d treatment with 5% DSS the Il18−/− mice showed higher bleeding, diarrhea, and inflammation histology score as well as shorter colon than WT controls (Fig. S5, A–D). The histology was characterized by an increase in ulceration, reminiscent of the pathology observed in DSS-treated Myd88−/− mice (Fig. S5, E and F). AOM/DSS-treated Il18r1−/− mice also showed more severe colitis than WT mice as manifested by weight loss, bleeding, and lethality (Fig. S6 and not depicted). Similarly to the Myd88−/− mice, a significant decrease of the expression of several DNA damage repair genes (Atm, Atr, Msh2, and Parp1) was observed in AOM/DSS-treated Il18−/− mice (Fig. 7). Most importantly, all the Il18−/− and Il18r1−/− mice exhibited increased numbers of intestinal polyps after four DSS cycles (Fig. 6 B and Fig. S6 C), mimicking the effects observed in Myd88−/− mice after exposure to two DSS cycles. As in Myd88−/− mice, β-catenin mutation was observed in polyps from Il18−/− mice but not from WT mice (unpublished data). Il1r1−/− mice developed similar numbers of polyps to the WT controls. It is of note that Myd88−/− mice developed a higher number of polyps after the second DSS cycle than both Il18−/− and Il18r1−/− mice after four DSS cycles (Fig. 6, B and C; and Fig. S6 C). Additionally, increased frequency of tumor growth was observed when Il18−/− mice were compared with their corresponding WT or Il18+/− littermates (Fig. 6 E). In contrast, there was no difference in the number of polyps among Il1r−/− and their corresponding littermates (Fig. 6 D).
Figure 6.
Il18 deficiency mimics MyD88 deficiency susceptibility to polyp formation. Cohorts of 6–20 mice/group were subjected to AOM/DSS treatment as depicted by the scheme. At the end of the first DSS cycle, mice were analyzed for bleeding (A). 100 d after AOM administration, the Il1r1−/−, Il18−/−, and their corresponding controls were euthanized and colonic polyps were counted (B). For comparison, Myd88−/− mice were tested simultaneously. Because of their susceptibility to the treatment, the number of polyps was scored at the completion of the second DSS cycle (C). The data shown in A–C correspond to a representative experiment out of three performed. Il18−/− and Il1r−/− mice and their corresponding heterozygote and WT littermates were subjected to AOM/DSS treatment as indicated by the scheme. At the completion of the experiment, the number of colonic polyps was counted (D and E). The data shown in D and E correspond to a representative experiment out of two performed using littermate controls. Data represent means ± SE. **, P < 0.01; ***, P < 0.001.
Figure 7.
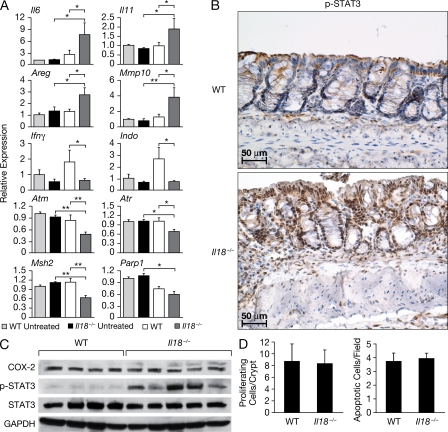
Il18-deficient mice exhibit enhanced expression of mitogenic/inflammatory cytokines and STAT-3 phosphorylation after AOM/DSS treatment. Cohorts of 8–10 WT and Il18−/− mice per group were injected i.v. with AOM on day 0, followed by 3 d of DSS administration in drinking water. Thereafter, colons were resected, RNA was extracted, and RT-PCR was performed using specific primers for the indicated genes. The gene expression was normalized to Gapdh levels, and the expression of each gene relative to untreated WT mice is depicted (A). Tissue sections were stained for pSTAT3. Photomicrographs of representative sections from the respective groups are shown at 200× magnification. Protein extracts were analyzed by Western blotting for COX-2, STAT3, and pSTAT3 expression. Gapdh was used as a control (C). Quantification of colonic epithelial cell proliferation and apoptosis was performed in tissue sections stained with Ki67 or ApopTag antibodies. The number of proliferating cells per crypt and the number of apoptotic cells/field are shown. The data shown in A–D correspond to a representative experiment out of two performed. Data represent means ± SE. *, P < 0.05; **, P < 0.01.
To evaluate whether common mediators are associated with the susceptibility of Il18- and MyD88-deficient mice to colitis, we compared the levels of expression of genes involved in inflammation and wound healing that we had observed to be differentially regulated in Myd88−/− mice. As observed in Myd88−/− mice, increased Il6, Il11, Mmp10, and Areg mRNA expression, as well as enhanced levels of pSTAT-3, was observed in the colonic mucosa of Il18−/− mice. After AOM/DSS treatment, Ifng was up-regulated in WT but not in Il18−/− colon (Fig. 7 A). Also, a decrease in the IFN-dependent gene Indo was found in Il18−/− mice (Fig. 7, A–C). However, unlike that seen in Myd88−/− mice, no decreased proliferation rate or apoptosis in enterocytes was observed in Il18−/− mice after AOM/DSS treatment (Fig. 7 D). In addition, Cox2 expression was up-regulated in Myd88−/− mice after AOM/DSS treatment but not in Il18−/− mice (Fig. 7 C).
Importantly, in contrast to the high susceptibility that Il18−/− mice exhibited in response to DSS, we confirmed the data of others (Dinarello, 2007) that demonstrated that the mice were resistant to trinitrobenzene sulfonic acid (TNBS)–induced colitis (unpublished data), indicating that IL-18 has a pathogenic role in immune-mediated colitis, but it has a protective role in the repair of mucosal damage in DSS-induced ulcerative colitis.
DISCUSSION
Several studies support a causative link between chronic inflammation and cancer, and the identification of inflammatory pathways that contribute to carcinogenesis is of critical importance for cancer prevention and treatment. Pathways that use MyD88 have been implicated in the promotion of cancer in several mouse carcinogenesis models. In this paper, we show that under chronic colitis conditions induced by DSS-induced mucosal damage, MyD88 has a protective role in colon cancer.
The analysis of gene expression profile after AOM/DSS treatment in the colon of Myd88−/− mice compared with WT mice generated informative results that, even if reflecting global gene expression in the colon tissue and not exclusively in epithelial cells, allowed us to propose possible mechanisms explaining the increased susceptibility of Myd88−/− mice to CAC. In response to DSS-induced mucosal damage, the repair mechanism involves an increased proliferation of epithelial cells that was shown to be defective in Tlr4−/− mice and that we now find to also be reduced by almost threefold in AOM/DSS-treated Myd88−/− mice. However, this reduced epithelial cell proliferation was, at 3 d after DSS treatment initiation, already associated with an up-regulation of many of the molecular pathways that are involved in induction and progression into the cell cycle. This seems to indicate an aborted attempt to respond to the damage with increased proliferation. It is not yet obvious what the mechanism is that limits the ability of the cells to proliferate, but it is apparent that they are in a proliferation prone status and that any functional and genetic alteration that would remove or overcome such a block may result in increased and deregulated proliferation.
We also observed an unexpected up-regulation of Il6 and Il11 in AOM/DSS-treated Myd88−/− mice that is associated with pSTAT3 expression in almost all nuclei of mucosal epithelial cells. Although it is likely that the induction of these cytokines is dependent on the altered interaction with commensal microbiota, in the absence of signaling through TLRs and IL-1Rs the availability of innate receptors available for signaling is reduced, but the engagement of cytoplasmic receptors, such as the NODs which are known to be associated with IBD and colon carcinoma in humans, remains a likely possibility. The phosphorylation of STAT3 is most likely downstream of IL-6 and IL-11, which are known mediators of gastrointestinal inflammation and tumorigenesis, both of which are induced by and signal through STAT3, likely establishing a self-activating loop (Atreya and Neurath, 2008; Ernst et al., 2008). We observed enhanced expression of several known STAT3-dependent genes (Yu et al., 2009) in addition to Il6 and Il11 in AOM/DSS-treated Myd88−/− mice, including Cox2, Hif1a, and several metalloproteases. STAT3 has also been reported to prevent TGF-β signaling by inducing the suppressor Smad-7 (Jenkins et al., 2005), which is similar to what we have observed in our system. STAT3 activation in both epithelial cells and in stromal and infiltrating cells has been shown to regulate the tumor inflammatory environment as well as to have direct effects on the tumor itself by inducing antiapoptotic, proangiogenic, and proproliferative gene expression programs (Grivennikov et al., 2009; Yu et al., 2009). In contrast with the enhanced expression of the IL-6 family cytokines and the STAT3-dependendent genes, IFN-γ and many IFN-dependent genes were less induced in Myd88−/− mice compared with WT mice. Our findings in the AOM/DSS system are in striking contrast with the data observed in Apcmin/+ mice in which decreased IL-6, STAT3 phosphorylation, and STAT3-dependent gene expression were reported in the absence of MyD88 (Rakoff-Nahoum and Medzhitov, 2007). This observation points to the fact that the mucosal damage induced by DSS in Myd88−/− mice, with the consequent exposure to commensal microbiota resulting in enhanced migration of bacteria to the mesenteric lymph nodes (Vaishnava et al., 2008), is likely responsible for a dramatically different state of inflammation compared with carcinogenesis models not associated with alteration of mucosal integrity.
Unlike in most WT mice in which polyps are not observed until after the third or fourth cycle of DSS and the majority of those are benign adenomas, in Myd88−/− mice polyps are induced after a single DSS cycle and most of the tumors progress to aggressive and invasive adenocarcinomas that cause death starting at 2 mo after treatment. This suggested that in Myd88−/− mice, the polyps may have a higher frequency of oncogenic mutations. Indeed, when we analyzed by PCR and sequencing of total DNA from excised polyps we were able to detect degradation-blocking mutations in exon 3 of β-catenin in one-third of the polyps excised from Myd88−/− mice, but none in those from WT mice. Because the initiating mutations in this model are induced by the mutagen AOM, we analyzed whether defects in DNA repair mechanisms could be present in the Myd88−/− mice. Indeed, we found that a large number of genes encoding factors involved in DNA repair were expressed at significantly lower levels in the colon of Myd88−/− mice than in WT mice after AOM/DSS treatment. It is of interest that all the DNA damage response genes that we have observed to be decreased in the mice corresponded to genes that have been reported to be frequently mutated in human colon cancer or in familiar hereditary nonpolyposis colon cancer syndrome (Bartek et al., 2007; Lynch et al., 2009). It is therefore plausible that one of the mechanisms of the increased susceptibility to AOM/DSS-induced carcinogenesis in Myd88−/− mice is an inefficient response to DNA damage induced either by AOM or by the inflammatory mediators.
IL-18 (IL1F4) is a member of the IL-1 family that, similarly to IL-1β, is translated as a pro-cytokine that requires processing by caspase-1 activated by the inflammasome. IL-18 was originally described as an inducer of IFN-γ acting mostly by amplifying the effect of other IFN-γ inducers such as IL-12. Thus, IL-18 was originally considered a Th-1–inducing cytokine but it was later found that it can also enhance the production of other cytokines, including Th-2 cytokines, and thus it has a much broader effect than originally thought. IL-18 binds to a two-chain receptor that requires the adapter MyD88 for signal transduction, similarly to all the other members of the IL-1 family. The functions of IL-18 are complex and their possible contribution to the maintenance of chronic inflammation in the intestine is unclear. Polymorphism in the gene encoding IL-18Rb has been associated with IBD (Zhernakova et al., 2008), whereas genetics data on polymorphism of the Il18 gene promoter have been controversial (Rodriguez-Bores et al., 2007). Several studies have associated circulating or local IL-18 with IBD severity, suggesting that IL-18 could be an effector cytokine in IBD (Dinarello, 2007). Early studies using Il18- or Caspase-1–deficient mice or blocking IL-18 with antibodies or IL-18 binding protein have indicated that IL-18 is required for TNBS- or DSS-induced colitis (Dinarello, 2007). However, studies in skin wound healing have demonstrated an important role of IL-18 in the early phase of skin wound repair (Kämpfer et al., 2000). Similarly, other studies, unlike the results of some of the early studies, reported that Il18- or Il18R-deficient mice are more susceptible rather than resistant to DSS-induced colitis (Takagi et al., 2003; Reuter and Pizarro, 2004). Recently, it has been shown that mice deficient for the Nalp3 inflammasome or the inflammasome components Asc and caspase 1 (Dupaul-Chicoine et al, 2010; Zaki et al., 2010) are very susceptible to DSS-induced acute colitis and this susceptibility has been attributed, at least in part, to decreased production of IL-18 on the basis of the ability of exogenously administered recombinant IL-18 to partially protect the mice. We now report that not only are Il18−/− and Il18r1−/− mice more susceptible to AOM/DSS colitis with a phenotype that mimics, although it is milder, that of the Myd88−/− mice but also that similar to the Myd88−/−, Il18−/−, and Il18r1−/− mice are much more susceptible to CAC than WT mice. Il1r1−/− mice were not significantly different from WT mice. As a control, we also tested the susceptibility of Il18−/− mice to TNBS-induced colitis, confirming the previously reported data (Dinarello, 2007) that in an immune T cell–mediated type of experimental colitis, IL-18 is an effector molecule probably required for optimal T cell response.
The phenotype observed in Il18−/− and Il18r1−/− mice mimics that of the Myd88−/− mice, although the colitis was less severe and the development of colon polyps was slower and not as aggressive. In both deficient strains, the DSS colitis was characterized by severe bleeding and extensive erosion, likely reflecting that both genes are required for homeostatic control of the colon epithelium and for efficient repair upon injury. However, unlike in Myd88−/− mice, in Il18−/− mice the enterocyte proliferation or apoptosis after AOM/DSS treatment was not significantly altered and COX-2 expression was not enhanced.
In both Myd88−/− and Il18−/− mice, the inflammatory response to AOM/DSS treatment is characterized by an enhanced production of IL-6 family cytokines and STAT3 phosphorylation associated with a decreased expression of IFN-γ and of IFN-dependent genes. Thus, the enhanced susceptibility to carcinogenesis in these mice is surprising because in other models of inflammation-induced colon carcinogenesis, for example, in Socs1-, Sigirr-, and Caspase-12–deficient mice, in which there is an enhanced inflammatory response with inflammasome hyperactivation and/or increased signaling through the TOLL/IL-1 and IFN-γ receptors, the increased susceptibility to colon carcinogenesis is associated with an increased production of proinflammatory cytokines including IFN-γ (Hanada et al., 2006; Xiao et al., 2007; Dupaul-Chicoine et al., 2010). In particular, in the Socs-1–deficient mice, IFN-γ was shown to be required for spontaneous colon tumor formation even when the IL-6/STAT3 axis was still active (Hanada et al., 2006). These data make it unlikely that the increased susceptibility of Myd88−/− and Il18−/− mice to colon carcinogenesis might exclusively be the result of the lack of an immunosurveillance effect of adaptive immunity and IFN-γ (Dunn et al., 2006), particularly when it is considered that Rag−/− immunodeficient mice have reduced rather than increased susceptibility to colon carcinogenesis (unpublished data).
Thus, it appears that the increased susceptibility of Il18−/− mice to colitis and CAC in the AOM/DSS model may be dependent on partially overlapping mechanisms in both MyD88- and Il18-deficient mice. The deficient expression of either gene appears to be responsible for deviation of the inflammatory response, as well as alteration of homeostatic and mucosal repair mechanisms and the host interaction with the commensal microbiota (Vaishnava et al., 2008; Dupaul-Chicoine et al., 2010), resulting in increased susceptibility to carcinogenesis. Inability to signal through the IL-18R may represent the central mechanism responsible for the phenotype of Myd88−/− mice, although the milder phenotype of Il18−/− compared with Myd88−/− mice suggests that other MyD88-coupled receptors may act in concert with IL-18 to minimize carcinogenesis. Alternatively, signaling through the IL-18R might be the major factor responsible for carcinogenesis protection, but the lack of signaling through other MyD88-coupled receptors, on epithelial or other cells, may contribute to altering the colon microenvironment and minimizing the effect of the IL-18 signaling deficiency. Our data with Tlr2-deficient mice seems to exclude a major role for this receptor in our model, in contrast to others who have reported that Tlr4−/− mice in part reproduce the phenotype of Myd88−/− mice in terms of inability to efficiently repair the colon mucosa upon DSS-induced injury. However, Tlr4−/−-deficient mice were resistant to CAC (Fukata et al., 2007), thus indicating that the susceptibility to CAC in Myd88−/− mice is not a result of their inability to signal through TLR4-MyD88. The role of TLR5 and TLR9 on CAC remains to be elucidated, although it is possible that TLR5 plays a protective role because Tlr5 deletion resulted in the development of spontaneous colitis (Vijay-Kumar et al., 2007). Tlr9-deficient mice are more resistant to DSS-induced colitis (Obermeier et al., 2005), but TLR9 ligands protect mice from colitis by inducing type I IFN production (Katakura et al., 2005). Obviously, a large number of other TLRs are upstream of MyD88 (up to 15 in mammals). Several other members of the IL-1 family, in addition to IL-1α, IL-1β, and IL-18, could possibly be involved and, by acting on the same or different cell types in the colon, might act synergistically or antagonistically in determining the susceptibility to colitis and CAC. Finally, it remains to be identified in which cell types MyD88 signaling is important for colitis and CAC and, in the case of IL-18, which cells are producing and responding to it.
MATERIALS AND METHODS
Mice.
Myd88−/−, Il1r1−/−, Il18−/−, and Il18r1−/− mice were maintained in a specific pathogen-free (including helicobacter and parvovirus) environment, and generally used between 6 and 10 wk of age. All strains were backcrossed to obtain at least 98% congenicity to either B6J or B6NCr background. As control groups, either littermates or WT mice of identical background were used. Animal studies were approved by the Institutional Animal Care and Use Committee (IACUC) of National Cancer Institute (Frederick, Maryland) and were conducted in accordance with the IACUC guidelines and the National Institutes of Health Guide for the Care and Use of Laboratory. (National Institutes of Health Publication No. 86–23, 1985).
AOM/DSS model of chronic colitis.
Mice 6–8 wk of age were injected i.p. with 10 mg/kg AOM in 0.2 ml saline. 1 wk after AOM administration, DSS at 2% was administered in the drinking water for five consecutive days. Thereafter, mice received reverse osmosis water. Up to four DSS cycles were administered with intervals of 16 d on water between cycles. In some experiments, 10 mg/kg AOM was administered i.p. once a week for six consecutive weeks. During the course of the experiment, mice were monitored for body weight, rectal prolapse, diarrhea, and macroscopic bleeding, as well as occult blood by hemoccult (Beckman Coulter).
At the time of harvest, colons were resected, flushed with PBS, opened longitudinally, and measured. Polyps were counted using a stereomicroscope. Colon sections were fixed in formalin or snap frozen. In some experiments, polyps were harvested individually and frozen in liquid nitrogen.
Gene expression analysis by microarrays and real-time quantitative RT-PCR.
Total RNA was extracted from colon sections and gene expression was analyzed by whole genome mouse microarray (Affymetrix) or by quantitative RT-PCR as detailed in the supplemental material.
Mutation analysis.
PCR products were purified on the Mag-Bind EZ Pure kit (Omega Bio-Tek). The product was then sequenced bidirectionally using BigDye Terminator (version 1.1; Applied Biosystems). The PCR amplification oligonucleotides were used as sequencing primers. Unincorporated terminators were removed from the sequencing product using the Mag Bind SE DTR kit (Omega Bio-Tek) before electrophoresis on a sequencer (ABI3730; Applied Biosystems). Sequences were analyzed using Mutation Surveyor software (SoftGenetics, LLC). Somatic mutations were identified in samples where complementary mosaic peaks were present at the mutation site in both directions. The default settings of Mutation Surveyor (mutation score > 5, peak height > 500, overlap > 0.2) were used to determine mosaic peak presence.
Immunohistology.
Immunohistochemical staining was performed on formalin-fixed paraffin-embedded tissues using rabbit mAb to phospho-STAT3 (Tyr705; clone D3A7; 1:1000; Cell Signaling Technology), rabbit anti–mouse COX-2 (1:1,000; Cayman Chemical), and rabbit anti-Ki67 (1:10,000; Vector Laboratories). The corresponding secondary antibodies were obtained from Vector Laboratories. Apoptosis was detected using the ApopTag kit (Millipore). For detection, the Vectastain ABC kit (Vector Laboratories) and diaminobenzidine (Sigma-Aldrich) were used. The distal and middle sections of colon were assessed for inflammation using a semiquantitative score (0–15) system as follows: distribution of inflammation, 0 = none, 1 = focal, 2 = multifocal, 3 = diffuse; severity of inflammation, 1 = mild, 2 = moderate, 3 = severe; extent of inflammation, 1 = mucosal only, 2 = extends to submucosa, 3 = extends to muscle; ulceration, 0 = none, 1 = focal/locally extensive, 2 = multifocal, 3 = diffuse/extensive; and degree of necrosis, 0 = none, 1 = mild, 2 = moderate, and 3 = severe. Total disease score per mouse was calculated by adding each parameter.
SDS-PAGE and immunoblotting.
Colon sections were lysed in buffer containing 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 150 mM NaCl, 10 mM Tris HCl, pH 7.4, 1 mM EDTA, 1 mM sodium orthovanadate, 0.5 mM PMSF, and complete protection protease inhibitors (Boehringer Mannheim). Protein extracts were centrifugated at 14,000 rpm for 15 min at 4°C. Total protein was determined, samples were subjected to SDS-PAGE, and the proteins were transferred into PDF membranes. After blocking (1% BSA or 5% milk, 2 h, 4°C), the membranes were stained using rabbit mAb to phospho-STAT3 (1:1,000) and rabbit anti–mouse STAT-3 (1:1,000; Cell Signaling Technology), rabbit anti–mouse COX-2 (1:200), and rabbit mAb to GAPDH HRP conjugated (1:1,000; Cell Signaling Technology). Membranes were exposed to the corresponding secondary antibodies and then washed and developed by ECL (GE Healthcare).
Microarray performance and statistical analysis.
Total RNA was extracted using reagent (QIAGEN) according to the manufacturer’s instructions. RNA was digested using the RNase-Free Dnase set and amplified into antisense RNA (aRNA) according to the Affymetrix gene expression protocol. The quality of both total RNA and secondarily amplified RNA was tested using a Bioanalyzer 2000 (Agilent Technologies). Test aRNA was loaded into whole genome mouse arrays 430 2.0 (Affymetrix) and hybridized overnight in a hybridization oven. Posthybridization arrays were labeled using a fluidics wash (Affymetrix) and stain station and were immediately scanned. 430 2.0 whole genome mouse arrays contain 39,000 transcripts on a single array. The sequences from which these probe sets were derived were selected from GenBank, dbEST, and RefSeq. The sequence clusters were created from the UniGene database (Build 107, June 2002) and then refined by analysis and comparison with the publicly available draft assembly of the mouse genome from the Whitehead Institute for Genome Research (April 2002; www.affymetrix.com).
Resulting data files were uploaded to the mAdb databank (http://nciarray.nci.nih.gov) and further analyzed using BRBArrayTools (developed by the Biometric Research Branch, National Cancer Institute; Rubinfeld et al., 1997; http://linus.nci.nih.gov/BRB-ArrayTools.html), Cluster, and TreeView softwares (Eisen et al., 1998). Data were normalized using the median over the entire array as the reference because single color labeling technology was used. Gene ratios were mean corrected across experimental samples and displayed according to an uncentered correlation algorithm (Ross et al., 2000).
Gene expression profiles were compared statistically by the unpaired Student’s t test using the BRB-ArrayTools and Stanford Cluster programs to identify differentially expressed genes among treated and untreated MyD88 knockout and WT mice. The significance cutoff level of P < 0.01 was chosen as demanded by the statistical power of the comparison and sample number. Statistical significance and adjustments for multiple test comparisons were based on univariate and multivariate permutation tests as previously described (Wang et al., 2002; Basil et al., 2006). The microarray data were deposited in the GEO database and the accession no. is GSE19793.
Real-time quantitative RT-PCR.
The cDNA was prepared from 500 ng of total RNA according to the protocol for SuperScript II Reverse transcription (Invitrogen). For each sample, real-time quantitative RT-PCR was then performed using 12.5 ng cDNA, the SYBR Green PCR Master Mix (Applied Biosystems), and the forward and reverse primers, at a final concentration of 0.3 µM, in a sample volume of 25 µl. The primers, which are listed in Table S1, were designed using Primer 3.0 software from mRNA sequences submitted to GenBank and checked in BLAST to confirm the total gene specificity. PCR was performed using a StepOnePlus machine (Applied Biosystems) under the cycling conditions required in the protocol for SYBR Green PCR Master Mix. The expression of candidate genes in each group of mice was normalized to Gapdh to obtain a ΔCt value and to calculate a 2^−(mean ΔCt). Data represent 6 and 10 different mice per group and are relative to the levels of expression of Gapdh in the untreated WT mice. For statistical analysis, a two-tailed Mann Whitney test was performed comparing the ΔCt values.
Online supplemental material.
In Fig. S1, the susceptibility of MyD88-deficient mice to AOM/DSS-induced colitis and CAC is maintained when compared with corresponding littermate WT mice. Fig. S2 shows that Myd88−/− mice are resistance to the development of colonic polyps in response to AOM or DSS treatment alone. Fig. S3 shows an unsupervised heat map of genes differentially expressed among treated WT versus treated Myd88−/− mice. Fig. S4 shows that AOM/DSS treatment enhances the expression of genes involved in the EGFR and B-catenin pathways on Myd88−/− mice compared with WT mice. Fig. S5 shows that Il18−/− mice are highly susceptible to DSS colitis. Fig. S6 shows that Il18r1−/− mice are susceptible to AOM/DSS administration, similar to Il18−/− mice. Table S1 shows real-time PCR primer sequences and experimental conditions. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20100199/DC1.
Acknowledgments
We thank Donna Butcher, Loretta Smith, Megan Karwan, Tammy Beachley, Rodney Wiles, and Sara Bass for technical assistance.
This Research was supported by the Intramural Research Program National Cancer Institute and by funds from the National Cancer Institute, National Institutes of Health, under contract HHSN261200800001E. Mention of trade names, commercial products, services, or organizations does not imply endorsement by the U.S. Government.
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- AOM
- azoxymethane
- CAC
- colitis-associated cancer
- COX-2
- cyclooxygenase 2
- DSS
- dextran sodium sulfate
- EGFR
- epidermal growth factor receptor
- HIF
- hypoxia-inducible factor
- IBD
- inflammatory bowel disease
- Indo
- indoleamine-pyrrole 2,3-dioxygenase
- MMP
- matrix metalloproteinase
- MyD88
- myeloid differentiation factor 88
- Parp
- poly ADP-ribose polymerase
- TLR
- toll-like receptor
- TNBS
- trinitrobenzene sulfonic acid
References
- Araki A., Kanai T., Ishikura T., Makita S., Uraushihara K., Iiyama R., Totsuka T., Takeda K., Akira S., Watanabe M. 2005. MyD88-deficient mice develop severe intestinal inflammation in dextran sodium sulfate colitis. J. Gastroenterol. 40:16–23 10.1007/s00535-004-1492-9 [DOI] [PubMed] [Google Scholar]
- Atreya R., Neurath M.F. 2008. Signaling molecules: the pathogenic role of the IL-6/STAT-3 trans signaling pathway in intestinal inflammation and in colonic cancer. Curr. Drug Targets. 9:369–374 10.2174/138945008784221116 [DOI] [PubMed] [Google Scholar]
- Bartek J., Lukas J., Bartkova J. 2007. DNA damage response as an anti-cancer barrier: damage threshold and the concept of ‘conditional haploinsufficiency’. Cell Cycle. 6:2344–2347 [DOI] [PubMed] [Google Scholar]
- Basil C.F., Zhao Y., Zavaglia K., Jin P., Panelli M.C., Voiculescu S., Mandruzzato S., Lee H.M., Seliger B., Freedman R.S., et al. 2006. Common cancer biomarkers. Cancer Res. 66:2953–2961 10.1158/0008-5472.CAN-05-3433 [DOI] [PubMed] [Google Scholar]
- Brown S.L., Riehl T.E., Walker M.R., Geske M.J., Doherty J.M., Stenson W.F., Stappenbeck T.S. 2007. Myd88-dependent positioning of Ptgs2-expressing stromal cells maintains colonic epithelial proliferation during injury. J. Clin. Invest. 117:258–269 10.1172/JCI29159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C.A. 2007. Interleukin-18 and the pathogenesis of inflammatory diseases. Semin. Nephrol. 27:98–114 10.1016/j.semnephrol.2006.09.013 [DOI] [PubMed] [Google Scholar]
- Dunn G.P., Koebel C.M., Schreiber R.D. 2006. Interferons, immunity and cancer immunoediting. Nat. Rev. Immunol. 6:836–848 10.1038/nri1961 [DOI] [PubMed] [Google Scholar]
- Dupaul-Chicoine J., Yeretssian G., Doiron K., Bergstrom K.S., McIntire C.R., LeBlanc P.M., Meunier C., Turbide C., Gros P., Beauchemin N., et al. 2010. Control of intestinal homeostasis, colitis, and colitis-associated colorectal cancer by the inflammatory caspases. Immunity. 32:367–378 10.1016/j.immuni.2010.02.012 [DOI] [PubMed] [Google Scholar]
- Eisen M.B., Spellman P.T., Brown P.O., Botstein D. 1998. Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. USA. 95:14863–14868 10.1073/pnas.95.25.14863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M., Najdovska M., Grail D., Lundgren-May T., Buchert M., Tye H., Matthews V.B., Armes J., Bhathal P.S., Hughes N.R., et al. 2008. STAT3 and STAT1 mediate IL-11-dependent and inflammation-associated gastric tumorigenesis in gp130 receptor mutant mice. J. Clin. Invest. 118:1727–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata M., Michelsen K.S., Eri R., Thomas L.S., Hu B., Lukasek K., Nast C.C., Lechago J., Xu R., Naiki Y., et al. 2005. Toll-like receptor-4 is required for intestinal response to epithelial injury and limiting bacterial translocation in a murine model of acute colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 288:G1055–G1065 10.1152/ajpgi.00328.2004 [DOI] [PubMed] [Google Scholar]
- Fukata M., Chen A., Vamadevan A.S., Cohen J., Breglio K., Krishnareddy S., Hsu D., Xu R., Harpaz N., Dannenberg A.J., et al. 2007. Toll-like receptor-4 promotes the development of colitis-associated colorectal tumors. Gastroenterology. 133:1869–1881 10.1053/j.gastro.2007.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett W.S., Punit S., Gallini C.A., Michaud M., Zhang D., Sigrist K.S., Lord G.M., Glickman J.N., Glimcher L.H. 2009. Colitis-associated colorectal cancer driven by T-bet deficiency in dendritic cells. Cancer Cell. 16:208–219 10.1016/j.ccr.2009.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greten F.R., Eckmann L., Greten T.F., Park J.M., Li Z.W., Egan L.J., Kagnoff M.F., Karin M. 2004. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 118:285–296 10.1016/j.cell.2004.07.013 [DOI] [PubMed] [Google Scholar]
- Grivennikov S., Karin E., Terzic J., Mucida D., Yu G.Y., Vallabhapurapu S., Scheller J., Rose-John S., Cheroutre H., Eckmann L., Karin M. 2009. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 15:103–113 10.1016/j.ccr.2009.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada T., Kobayashi T., Chinen T., Saeki K., Takaki H., Koga K., Minoda Y., Sanada T., Yoshioka T., Mimata H., et al. 2006. IFN-γ–dependent, spontaneous development of colorectal carcinomas in SOCS1-deficient mice. J. Exp. Med. 203:1391–1397 10.1084/jem.20060436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins B.J., Grail D., Nheu T., Najdovska M., Wang B., Waring P., Inglese M., McLoughlin R.M., Jones S.A., Topley N., et al. 2005. Hyperactivation of Stat3 in gp130 mutant mice promotes gastric hyperproliferation and desensitizes TGF-beta signaling. Nat. Med. 11:845–852 10.1038/nm1282 [DOI] [PubMed] [Google Scholar]
- Kämpfer H., Paulukat J., Mühl H., Wetzler C., Pfeilschifter J., Frank S. 2000. Lack of interferon-gamma production despite the presence of interleukin-18 during cutaneous wound healing. Mol. Med. 6:1016–1027 [PMC free article] [PubMed] [Google Scholar]
- Katakura K., Lee J., Rachmilewitz D., Li G., Eckmann L., Raz E. 2005. Toll-like receptor 9-induced type I IFN protects mice from experimental colitis. J. Clin. Invest. 115:695–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch H.T., Lynch P.M., Lanspa S.J., Snyder C.L., Lynch J.F., Boland C.R. 2009. Review of the Lynch syndrome: history, molecular genetics, screening, differential diagnosis, and medicolegal ramifications. Clin. Genet. 76:1–18 10.1111/j.1399-0004.2009.01230.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naugler W.E., Sakurai T., Kim S., Maeda S., Kim K., Elsharkawy A.M., Karin M. 2007. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 317:121–124 10.1126/science.1140485 [DOI] [PubMed] [Google Scholar]
- Nenci A., Becker C., Wullaert A., Gareus R., van Loo G., Danese S., Huth M., Nikolaev A., Neufert C., Madison B., et al. 2007. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 446:557–561 10.1038/nature05698 [DOI] [PubMed] [Google Scholar]
- Obermeier F., Dunger N., Strauch U.G., Hofmann C., Bleich A., Grunwald N., Hedrich H.J., Aschenbrenner E., Schlegelberger B., Rogler G., et al. 2005. CpG motifs of bacterial DNA essentially contribute to the perpetuation of chronic intestinal inflammation. Gastroenterology. 129:913–927 10.1053/j.gastro.2005.06.061 [DOI] [PubMed] [Google Scholar]
- Pull S.L., Doherty J.M., Mills J.C., Gordon J.I., Stappenbeck T.S. 2005. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc. Natl. Acad. Sci. USA. 102:99–104 10.1073/pnas.0405979102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakoff-Nahoum S., Medzhitov R. 2007. Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science. 317:124–127 10.1126/science.1140488 [DOI] [PubMed] [Google Scholar]
- Rakoff-Nahoum S., Paglino J., Eslami-Varzaneh F., Edberg S., Medzhitov R. 2004. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 118:229–241 10.1016/j.cell.2004.07.002 [DOI] [PubMed] [Google Scholar]
- Reuter B.K., Pizarro T.T. 2004. Commentary: the role of the IL-18 system and other members of the IL-1R/TLR superfamily in innate mucosal immunity and the pathogenesis of inflammatory bowel disease: friend or foe? Eur. J. Immunol. 34:2347–2355 10.1002/eji.200425351 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Bores L., Fonseca G.C., Villeda M.A., Yamamoto-Furusho J.K. 2007. Novel genetic markers in inflammatory bowel disease. World J. Gastroenterol. 13:5560–5570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross D.T., Scherf U., Eisen M.B., Perou C.M., Rees C., Spellman P., Iyer V., Jeffrey S.S., Van de Rijn M., Waltham M., et al. 2000. Systematic variation in gene expression patterns in human cancer cell lines. Nat. Genet. 24:227–235 10.1038/73432 [DOI] [PubMed] [Google Scholar]
- Rubinfeld B., Robbins P., El-Gamil M., Albert I., Porfiri E., Polakis P. 1997. Stabilization of beta-catenin by genetic defects in melanoma cell lines. Science. 275:1790–1792 10.1126/science.275.5307.1790 [DOI] [PubMed] [Google Scholar]
- Swann J.B., Vesely M.D., Silva A., Sharkey J., Akira S., Schreiber R.D., Smyth M.J. 2008. Demonstration of inflammation-induced cancer and cancer immunoediting during primary tumorigenesis. Proc. Natl. Acad. Sci. USA. 105:652–656 10.1073/pnas.0708594105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi H., Kanai T., Okazawa A., Kishi Y., Sato T., Takaishi H., Inoue N., Ogata H., Iwao Y., Hoshino K., et al. 2003. Contrasting action of IL-12 and IL-18 in the development of dextran sodium sulphate colitis in mice. Scand. J. Gastroenterol. 38:837–844 10.1080/00365520310004047 [DOI] [PubMed] [Google Scholar]
- Uronis J.M., Mühlbauer M., Herfarth H.H., Rubinas T.C., Jones G.S., Jobin C. 2009. Modulation of the intestinal microbiota alters colitis-associated colorectal cancer susceptibility. PLoS One. 4:e6026 10.1371/journal.pone.0006026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaishnava S., Behrendt C.L., Ismail A.S., Eckmann L., Hooper L.V. 2008. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc. Natl. Acad. Sci. USA. 105:20858–20863 10.1073/pnas.0808723105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijay-Kumar M., Sanders C.J., Taylor R.T., Kumar A., Aitken J.D., Sitaraman S.V., Neish A.S., Uematsu S., Akira S., Williams I.R., Gewirtz A.T. 2007. Deletion of TLR5 results in spontaneous colitis in mice. J. Clin. Invest. 117:3909–3921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E., Miller L.D., Ohnmacht G.A., Mocellin S., Perez-Diez A., Petersen D., Zhao Y., Simon R., Powell J.I., Asaki E., et al. 2002. Prospective molecular profiling of melanoma metastases suggests classifiers of immune responsiveness. Cancer Res. 62:3581–3586 [PMC free article] [PubMed] [Google Scholar]
- Xiao H., Gulen M.F., Qin J., Yao J., Bulek K., Kish D., Altuntas C.Z., Wald D., Ma C., Zhou H., et al. 2007. The Toll-interleukin-1 receptor member SIGIRR regulates colonic epithelial homeostasis, inflammation, and tumorigenesis. Immunity. 26:461–475 10.1016/j.immuni.2007.02.012 [DOI] [PubMed] [Google Scholar]
- Yamada Y., Oyama T., Hirose Y., Hara A., Sugie S., Yoshida K., Yoshimi N., Mori H. 2003. beta-Catenin mutation is selected during malignant transformation in colon carcinogenesis. Carcinogenesis. 24:91–97 10.1093/carcin/24.1.91 [DOI] [PubMed] [Google Scholar]
- Yu H., Pardoll D., Jove R. 2009. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat. Rev. Cancer. 9:798–809 10.1038/nrc2734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki M.H., Boyd K.L., Vogel P., Kastan M.B., Lamkanfi M., Kanneganti T.D. 2010. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity. 32:379–391 10.1016/j.immuni.2010.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhernakova A., Festen E.M., Franke L., Trynka G., van Diemen C.C., Monsuur A.J., Bevova M., Nijmeijer R.M., van ‘t Slot R., Heijmans R., et al. 2008. Genetic analysis of innate immunity in Crohn’s disease and ulcerative colitis identifies two susceptibility loci harboring CARD9 and IL18RAP. Am. J. Hum. Genet. 82:1202–1210 10.1016/j.ajhg.2008.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]