Abstract
Intracellular pathogens and endogenous danger signals in the cytosol engage NOD-like receptors (NLRs), which assemble inflammasome complexes to activate caspase-1 and promote the release of proinflammatory cytokines IL-1β and IL-18. However, the NLRs that respond to microbial pathogens in vivo are poorly defined. We show that the NLRs NLRP3 and NLRC4 both activate caspase-1 in response to Salmonella typhimurium. Responding to distinct bacterial triggers, NLRP3 and NLRC4 recruited ASC and caspase-1 into a single cytoplasmic focus, which served as the site of pro–IL-1β processing. Consistent with an important role for both NLRP3 and NLRC4 in innate immune defense against S. typhimurium, mice lacking both NLRs were markedly more susceptible to infection. These results reveal unexpected redundancy among NLRs in host defense against intracellular pathogens in vivo.
IL-1β is a central orchestrator of immunity against various classes of pathogens and a key trigger of inflammatory diseases. It is produced by activated macrophages as a proprotein, which is proteolytically processed to its active form by the cysteine protease caspase-1. Caspase-1 itself is synthesized as a zymogen and must be activated by autocatalytic cleavage. This activation involves recruitment of pro–caspase-1 into multiprotein complexes called inflammasomes, which are comprised of the adapter protein ASC and a NOD-like receptor (NLR), with the latter conferring specificity for microbial and endogenous products (Mariathasan and Monack, 2007).
The NLR family of cytosolic receptors responds to a variety of pathogens and endogenous danger signals (Brodsky and Monack, 2009). For example, NLRC4 (also known as Ipaf) is required by cultured primary macrophages to activate caspase-1 after infection with Salmonella spp., Pseudomonas aeruginosa, Listeria monocytogenes, and Legionella pneumophila. NLRC4 is thought to recognize bacterial flagellin that is injected accidentally into the cytoplasm by bacterial virulence-associated secretion systems (Amer et al., 2006; Franchi et al., 2006; Miao et al., 2006; Ren et al., 2006). Recently, it has been reported that NLRC4 also detects the rod subunit of certain type 3 secretion systems (T3SSs; Miao et al., 2010), which explains how NLRC4 can detect nonflagellated bacteria such as Shigella flexneri and some strains of P. aeruginosa (Sutterwala et al., 2007; Suzuki et al., 2007). In contrast to NLRC4, NLRP3 (also known as Nalp3) is activated by diverse molecules originating from viruses (Sendai, influenza, and adenoviral strains; Kanneganti et al., 2006; Muruve et al., 2008), fungi (Candida albicans and Saccharomyces cerevisiae; Gross et al., 2009; Hise et al., 2009), and bacteria (Staphylococcus aureus, Neisseria gonorrhoeae, and L. monocytogenes; Mariathasan et al., 2006; Warren et al., 2008; Duncan et al., 2009). The precise signal that is detected by NLRP3 remains unclear. Common terminal signals appear to involve damage to membranes and/or changes in potassium levels (Brodsky and Monack, 2009). Other NLRs include NLRP1, which is activated by anthrax lethal toxin, and Aim2, which appears to recognize double-stranded DNA in the cytoplasm (Fernandes-Alnemri et al., 2009; Hornung et al., 2009).
Although several different inflammasomes have been described, the precise molecular structure and composition of inflammasomes remains largely unknown. In vitro studies with purified inflammasome components suggest that NLRs bind their ligands and oligomerize to form a platform for dimerization and autoproteolytic cleavage of pro–caspase-1 (Faustin et al., 2007). Under certain lysis conditions, inflammasome components can form structures between 500 and 700 kD in size (Martinon et al., 2004; Hsu et al., 2008). Overexpressed ASC also forms large aggregates (Masumoto et al., 1999; Richards et al., 2001; Fernandes-Alnemri et al., 2007), although the physiological significance of these aggregates is unclear.
Caspase-1 is important for innate immune defense against many pathogens, but the contributions of specific NLRs to caspase-1 activation during animal infections are less clear. Previous in vivo studies indicated a role for NLRC4 in defense against L. pneumophila and P. aeruginosa but not Salmonella (Amer et al., 2006; Lara-Tejero et al., 2006; Sutterwala et al., 2007). This last finding was surprising, given that NLRC4 was critical for caspase-1 activation by macrophages infected with Salmonella in culture (Mariathasan et al., 2004). Thus far, the only described roles for NLRP3 in vivo during microbial infections are in defense against C. albicans, Plasmodium, and influenza A virus infections (Allen et al., 2009; Dostert et al., 2009; Gross et al., 2009; Hise et al., 2009; Joly et al., 2009; Shio et al., 2009; Thomas et al., 2009), whereas no role has been described in bacterial infections so far.
Salmonella enterica serovar typhimurium (Stm) is an intracellular Gram-negative bacterium that causes infections in human and animal hosts. After initial colonization of the gastrointestinal tract, Stm persists in tissue macrophages. Salmonella virulence requires two T3SSs encoded on Salmonella pathogenicity islands: SPI-1 and SPI-2. SPI-1 is necessary to invade epithelial cells in the gut, whereas SPI-2 is necessary for replication in macrophages (Beuzón et al., 2000). Inflammasomes play an important role in innate immune defense against Stm because mice lacking caspase-1, IL-1β, or IL-18 succumb earlier to infection and have higher bacterial loads (Raupach et al., 2006). Stm expressing the SPI-1 T3SS induces caspase-1– and NLRC4–dependent cell death and IL-1β processing in cultured macrophages. Because NLRC4 is not essential for Stm clearance in mice (Lara-Tejero et al., 2006), we investigated whether multiple NLRs respond to Stm infection in the whole animal.
RESULTS
NLRP3 and NLRC4 recognize intracellular Stm
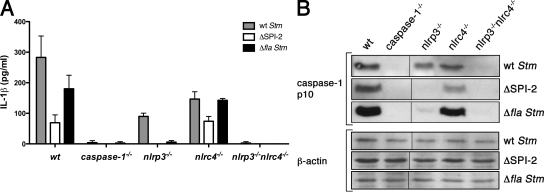
Previous studies with Stm focused on the rapid SPI-1–dependent activation of caspase-1, which is important for host protective responses during the gastrointestinal phase of murine Stm infections (Winter et al., 2009) and results in significant IL-1β production by macrophages infected in culture (Mariathasan et al., 2004; Fig. S1 A). It is known, however, that Stm also induces SPI-1–independent caspase-1 activation and IL-1β release between 17 and 20 h after infection (Monack et al., 2001; Fig. S1 B). Thus, WT Stm grown to stationary phase (which results in decreased SPI-1 gene expression; Lundberg et al., 1999) and an isogenic SPI-1 mutant Stm strain elicited similar amounts of IL-1β from cultured macrophages at 17 h after infection (Fig. S1 C). This SPI-1–independent release of IL-1β required caspase-1 because WT, but not caspase-1−/− macrophages, released mature IL-1β when infected with WT Stm (Fig. 1 A gray bars; and Fig. S1, B and D; unless stated otherwise, all experiments in this study used Stm not expressing SPI-1). The extent of IL-1β secretion correlated with processing of caspase-1 to its active form as determined by Western blotting for the caspase-1 p10 subunit in the culture supernatant (Fig. 1 B, top, first and second lane). Detection of caspase-1 processing is routinely performed on culture supernatants because active caspase-1 is released via a secretion pathway that has yet to be characterized (Keller et al., 2008). WT and caspase-1−/− macrophages contained similar numbers of bacteria at 17 h after infection (unpublished data), indicating that decreased IL-1β secretion was not linked to reduced bacterial replication. To gain insight into why NLRC4-deficient mice can clear an Stm infection as effectively as WT mice, we decided to focus on the identification of the NLRs that are engaged by Stm in a SPI-1–independent manner and on how these NLRs are activated.
Figure 1.
NLRP3 and NLRC4 recognize distinct Stm signals. BM-derived macrophages (BMDMs) of the genotypes indicated were infected with WT Stm for 17 h. Secretion of IL-1β (A) or the processed p10 caspase-1 subunit (B) into the culture supernatant was determined by ELISA and Western blotting, respectively. Equal loading was controlled for by Western blotting for β-actin in the corresponding cell lysates. Gray, white, and black bars in A represent BMDMs infected with WT Stm, a SPI-2 mutant, or a flagellin-deficient strain for 17 h, respectively. Data in A are representative of at least five independent experiments. Data in B are representative of at least two independent experiments. Error bars represent the mean SD of triplicate wells. Black lines indicate that intervening lanes were spliced out.
To identify the NLRs engaged by intracellular Stm upstream of caspase-1 activation, we first infected macrophages from WT, caspase-1–, NLRP3-, and NLRC4-deficient mice with WT Stm. Both nlrc4−/− and nlrp3−/− macrophages processed caspase-1 less efficiently than WT macrophages (Fig. 1 B, top), resulting in decreased IL-1β secretion (Fig. 1 A, gray bars; and Fig. S1 D). These data suggest that WT Stm engages NLRP3 in addition to NLRC4. Consistent with this notion, infected macrophages deficient in both NLRP3 and NLRC4 neither processed caspase-1 nor released IL-1β (Fig. 1, A and B). IL-18 secretion, like IL-1β secretion, was reduced in both nlrp3−/− and nlrc4−/− macrophages and completely abolished in macrophages lacking both NLRP3 and NLRC4 (Fig. S1 E). In addition, the macrophages released comparable levels of TNF in response to Stm infections (Fig. S2 A) and responded normally to infections with Francisella novicida, which has been previously shown to activate the inflammasome independently of NLRP3 and NLRC4, but dependent on ASC (Fig. S2 B). We conclude that NLRP3 and NLRC4 each contribute to caspase-1 activation in response to intracellular Stm and that no additional NLRs are necessary.
NLRP3 and NLRC4 recognize distinct Stm signals
To determine if the SPI-2 T3SS is involved in triggering caspase-1 activation at 17 h after infection, we infected macrophages with isogenic ΔSPI-2 mutant Stm. WT macrophages infected with ΔSPI-2 Stm released IL-1β, albeit significantly less than cells infected with WT Stm (Fig. 1 A, compare gray and white bars), indicating that SPI-1–independent inflammasome activation by intracellular Stm involves both SPI-2–dependent and SPI-2–independent mechanisms.
To determine whether NLRP3 and/or NLRC4 acts downstream of the SPI-2–dependent Stm signal, we compared WT, nlrc4−/−, and nlrp3−/− macrophages infected with ΔSPI-2 Stm (Fig. 1, A and B). Unlike their WT counterparts, nlrp3−/− macrophages did not process caspase-1 (Fig. 1 B) and release IL-1β (Fig. 1 A) when infected with ΔSPI-2 Stm. Because nlrp3−/− macrophages can only activate NLRC4 in response to Stm, these results indicate that NLRC4 is normally engaged by the SPI-2–dependent Stm signal. In contrast to nlrp3−/− macrophages, nlrc4−/− macrophages secreted similar amounts of IL-1β compared with WT macrophages when infected with ΔSPI-2 Stm, indicating that NLRP3 mediates caspase-1 activation in response to the SPI-2–independent Stm signal. In sum, NLRP3 and NLRC4 respond to distinct Stm signals.
To determine whether flagellin, which is one known substrate for NLRC4, is injected into the cytosol by the SPI-2 T3SS during Stm infections, we also compared the response of WT, nlrc4−/−, nlrp3−/−, and caspase-1−/− macrophages to an Stm strain lacking the flagellin genes (Δfla; Fig. 1, A and B). Similarly to nlrp3−/− macrophages infected with ΔSPI-2 Stm, nlrp3−/− macrophages infected with Δfla Stm did not process caspase-1 or release IL-1β, suggesting that flagellin is indeed injected by the SPI-2 T3SS during Stm infections and recognized by NLRC4. Consistent with these results, WT and nlrc4−/− macrophages infected with Δfla Stm processed comparable levels of caspase-1 (Fig. 1 B) and released similar amounts of IL-1β (Fig. 1 A). Surprisingly, WT macrophages infected with Δfla Stm released approximately twofold more IL-1β than WT cells infected with ΔSPI-2 Stm (Fig. 1 A). We attribute this difference in IL-1β secretion to SPI-2 being necessary for bacterial replication in macrophages (Beuzón et al., 2000), whereas WT and Δfla Stm replicate to similar numbers (unpublished data).
ASC is critical for Stm-induced IL-1β secretion
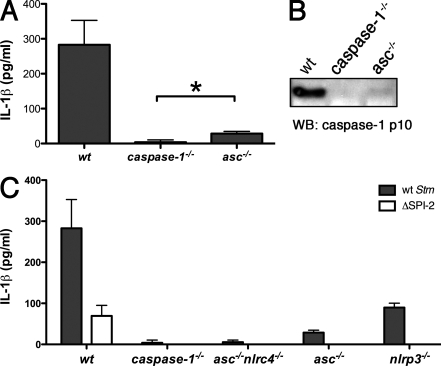
Caspase-1 activation by most NLRs requires ASC, an adapter protein which is believed to bridge NLRs and caspase-1. asc−/− macrophages infected with WT Stm released significantly less IL-1β than WT macrophages but slightly more than caspase-1−/− macrophages (Fig. 2 A). Consistent with these data, we detected extremely weak processing of caspase-1 in infected asc−/− macrophages (Fig. 2 B). These results indicate a critical role for ASC in Stm-induced caspase-1 activation. In addition, our data suggest that a minor ASC-independent signaling pathway might also be triggered. NLRP3 contains a Pyrin domain, which is thought to engage the Pyrin domain of ASC, leaving the caspase activation and recruitment domain (CARD) of ASC to interact with the CARD-containing prodomain of caspase-1. NLRC4, however, has a CARD rather than a Pyrin domain and, interestingly, this can interact directly with the caspase-1 CARD in overexpression studies (Poyet et al., 2001). We found that macrophages lacking both ASC and NLRC4, like caspase-1−/− macrophages, did not release IL-1β after infection with Stm (Fig. 2 C). This finding indicates that NLRP3 cannot activate caspase-1 in the absence of ASC but NLRC4 can. However, ASC likely enhances caspase-1 activation by NLRC4 because nlrp3−/− and asc−/− macrophages, which both express NLRC4, secrete different amounts of IL-1β during infection with Stm (Fig. 2 C). Specifically, asc−/− macrophages secrete approximately fivefold less IL-1β than nlrp3−/− macrophages.
Figure 2.
ASC is critical for Stm-induced IL-1β secretion. BMDMs of the genotypes indicated were infected with WT Stm for 17 h. Secretion of IL-1β (A and C) or the processed p10 caspase-1 subunit (B) into the culture supernatant was determined by ELISA and Western blotting, respectively. White bars in C represent BMDMs infected with SPI-2 mutant Stm for 17 h. Equal loading was controlled for by Western blotting for β-actin in the corresponding cell lysates. Data in A and C are representative of five independent experiments. The Western blot in B is representative of three independent experiments. Error bars represent the mean SD of triplicate wells, Statistical significance was determined using the unpaired Mann-Whitney U test. *, P < 0.05.
ASC relocalizes in macrophages infected with Stm
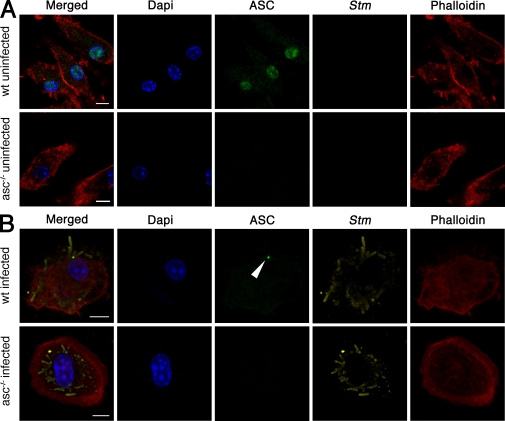
ASC overexpressed in THP-1 cells was shown to form large structures, designated pyroptosomes, when the cells were treated with LPS and ATP or transfected with RNA (Fernandes-Alnemri et al., 2007; Bryan et al., 2009). We investigated whether ASC might form similar structures in primary macrophages infected with Stm. Immunofluorescence microscopy of uninfected WT macrophages revealed endogenous ASC in the nucleus and the cytoplasm, which is in agreement with previously published results (Fig. 3 A; Bryan et al., 2009). This staining was specific for ASC because the antibody did not stain asc−/− macrophages. Macrophages infected with Stm for 17 h exhibited markedly less ASC in the nucleus. Instead, between 8 and 10% of infected cells contained a single cytoplasmic focus of ASC (Fig. 3 B; Fig. 4 A; and Fig. S3 C). These ASC foci also formed within 1 and 2 h after infection during SPI-1–dependent caspase-1 activation (Fig. S3 A). Surprisingly, a large number of cells, up to 40%, formed ASC foci in response to SPI-1 expressing Stm (Fig. S3, B and C). To determine whether ASC focus formation was specific to Salmonella infections, we also stained macrophages infected with F. novicida, an intracellular pathogen which triggers ASC-dependent, but NLRP3- and NLRC4-independent, activation of caspase-1 (Henry et al., 2007). WT macrophages infected with F. novicida formed ASC foci similar to those observed in Stm-infected macrophages (Fig. S4), showing that ASC relocalization is common to infections with at least two different intracellular pathogens.
Figure 3.
ASC relocalizes in macrophages infected with Stm. Fluorescence microscopy of WT and asc−/− BMDMs not infected (A) or infected with WT Stm for 17 h (B). Cells were stained for ASC, Stm, actin (with phalloidin), and DNA (with DAPI). The arrowhead points to an ASC focus. Images in A and B are representative of three independent experiments (original magnification, 63×). Bars, 10 µm.
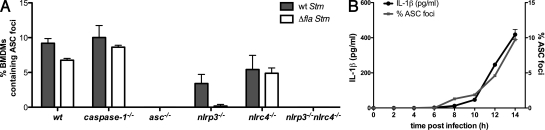
Figure 4.
ASC focus formation requires NLRP3 and NLRC4 and correlates with IL-1β release. (A) Percentage of BMDMs of the genotypes indicated forming ASC foci after infection with WT and Δfla Stm for 17 h. Numbers represent two independent fluorescence microscopy experiments, with at least 500 cells counted in each experiment. (B) Time course of ASC focus formation and IL-1β release in WT BMDMs infected with WT Stm. The percentage of macrophages containing an ASC focus (gray line) was determined by fluorescence microscopy. IL-1β released into the culture supernatant (black line) was determined by ELISA. Data in B are representative of two independent experiments. Error bars represent the mean SD of triplicate wells.
ASC focus formation requires the NLRs NLRP3 and NLRC4
To assess the contribution of NLRs to the formation of ASC foci, we compared ASC focus formation in Stm-infected WT, nlrc4−/−, nlrp3−/−, nlrp3−/−nlrc4−/−, and caspase-1−/− macrophages. Significantly fewer ASC foci formed in nlrp3−/− and nlrc4−/− macrophages when compared with WT macrophages, and no ASC foci were detected in nlrc4−/−nlrp3−/− macrophages (Fig. 4 A). Therefore, NLRC4 and NLRP3 are essential for ASC focus formation during Stm infection. Furthermore, the incidence of ASC foci correlated with the amount of mature IL-1β and IL-18 released, supporting our contention that ASC focus formation is linked to IL-1β and IL-18 processing by caspase-1 (Fig. 1 B and Fig. S1 E). Importantly, even though one NLR was absent in nlrp3−/− or nlrc4−/− macrophages, the ASC foci that formed were a similar size to those in WT macrophages.
We also examined ASC focus formation in macrophages infected with Δfla Stm (Fig. 4 A, white bars). nlrp3−/− macrophages infected with Δfla Stm did not form any ASC foci, which is consistent with their inability to secrete IL-1β (Fig. 1 A) as a result of the absence of flagellin to engage NLRC4. In macrophages infected with SPI-1 expressing Stm (Fig. S5 A), NLRC4 was essential for ASC focus formation but NLRP3 was dispensable, which is consistent with NLRC4 sensing the SPI-1–dependent translocation of flagellin and activating caspase-1 (Franchi et al., 2006; Miao et al., 2006; Fig. S1 A).
Next, we determined the kinetics of ASC focus formation in macrophages infected with Stm not expressing SPI-1. In this context, the first ASC foci were detected between 8 and 10 h after infection (Fig. 4 B), which correlated with IL-1β release. These results suggest that IL-1β processing and release are dependent on ASC focus formation.
Caspase-1 is activated in ASC foci
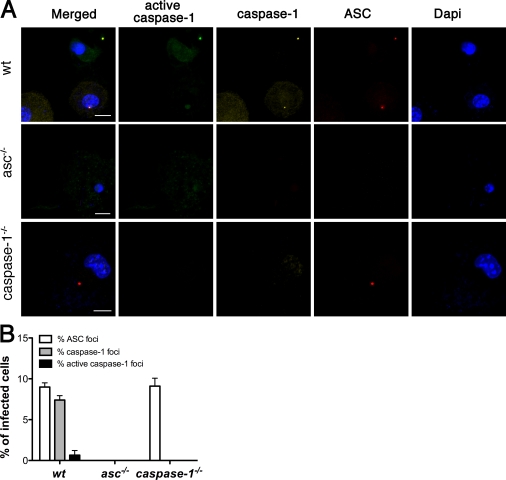
IL-1β processing and release require caspase-1 (Eder, 2009), so we determined whether caspase-1 colocalized with ASC in Stm-infected WT macrophages (Fig. 5 A). We found that ∼80% of ASC foci stained with an antibody recognizing both pro–caspase-1 and cleaved caspase-1, and ∼10% of ASC foci contained active caspase-1 based on labeling with the fluorescent caspase-1 probe FAM-YVAD-FMK, which binds specifically and irreversibly to the active site of processed caspase-1 (Fig. 5 B). Surprisingly, ASC focus formation was independent of caspase-1 because foci were observed at normal frequency in caspase-1−/− macrophages infected with Stm (Fig. 4 A and Fig. 5 B). Comparable results were obtained at earlier time points with SPI-1–expressing Stm, although a much higher percentage of ASC foci costained with the active caspase-1 probe FAM-YVAD-FMK (Fig. S5 A).
Figure 5.
Caspase-1 is activated in ASC foci. (A) Fluorescence microscopy of WT, asc−/−, and caspase-1−/− BMDMs infected with WT Stm for 17 h, stained for active caspase-1 (with FAM-YVAD-FMK), caspase-1, ASC, and DNA (with DAPI). Images are representative of three independent experiments. (B) Percentage of infected cells from A containing foci of ASC, caspase-1, and active caspase-1. Quantification represents mean numbers from three independent experiments, with at least 500 cells counted in each experiment. Images in A were acquired at 63× magnification. Cell counts in B were determined at 40× magnification. Error bars represent the mean SD of triplicate experiments. Bars, 10 µm.
Pro–IL-1β localizes to ASC foci in a caspase-1–dependent manner
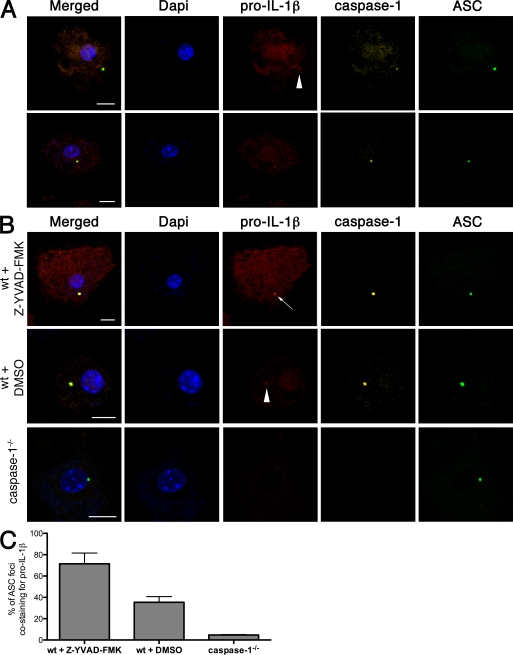
Collectively, our data suggest that the cytoplasmic ASC/caspase-1 focus in an Stm-infected cell may be the site of IL-1β processing. When we stained macrophages infected with WT Stm with an anti–pro–IL-1β antibody, most of the pro–IL-1β was diffuse in the cytoplasm, but about one-third of ASC foci also stained faintly for pro–IL-1β (Fig. 6 A, arrowhead). Faint staining might reflect rapid turnover of IL-1β. Indeed, the percentage of ASC foci containing detectable pro–IL-1β increased to 80% when we treated cells with the caspase-1 inhibitor Z-YVAD-FMK (Fig. 6, B and C). Foci also stained more brightly for pro–IL-1β. We obtained similar results during SPI-1–dependent ASC focus formation (Fig. S5, B and C). Caspase-1 was essential for recruitment of pro–IL-1β to the ASC foci because pro–IL-1β remained diffuse in the cytoplasm of Stm-infected caspase-1−/− macrophages.
Figure 6.
Pro–IL-1β localizes to ASC foci in a caspase-1–dependent manner. (A) Fluorescence microscopy of WT BMDMs infected with WT Stm for 17 h and then stained for DNA (with DAPI), pro–IL-1β, caspase-1, and ASC. The top row shows a cell with weak pro–IL-1β staining of an ASC focus (arrowhead), whereas the bottom row shows a cell with an ASC- and caspase-1–containing focus that is negative for pro–IL-1β. (B) WT and caspase-1−/− BMDMs infected with WT Stm for 17 h were stained for DNA (with DAPI), pro–IL-1β, caspase-1, and ASC. Where indicated, WT BMDMs were infected in the presence of the caspase-1 inhibitor Z-YVAD-FMK or DMSO (vehicle control). An arrow points to an ASC focus staining strongly for pro–IL-1β. An arrowhead marks an ASC focus with a faint pro-IL-1β signal. (C) Percentage of ASC foci in B costaining for pro–IL-1β. Images and numbers are a representative of three independent experiments with a mean of 50 ASC foci counted in each experiment. Error bars represent the mean SD of triplicate experiments. The antibody used for pro–IL-1β immunofluorescence is a polyclonal antibody recognizing both the uncleaved and mature forms of IL-1β. Images and cell counts were acquired at 63× magnification. Bars, 10 µm.
Redundant roles for NLRP3 and NLRC4 in caspase-1 activation during Stm infection in mice
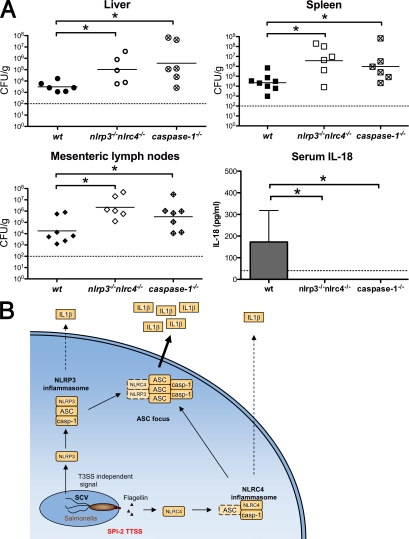
Caspase-1, IL-1β, and IL-18 are critical for innate immune defense against Stm in mice, whereas NLRC4 and also NLRP3 appear to be dispensable in vivo (Lara-Tejero et al., 2006). Given that both NLRP3 and NLRC4 activate caspase-1 in response to intracellular Stm in cultured macrophages (Fig. 1), we determined whether mice lacking both NLRs mount an effective immune response against Stm. WT, caspase-1−/−, and nlrp3−/−nlrc4−/− mice were challenged orally with 2.4 × 107 CFU of WT Stm. At 5 d after infection, caspase-1−/− and nlrp3−/−nlrc4−/− mice both had significantly more bacteria in their mesenteric lymph nodes, spleens, and livers when compared with WT mice (Fig. 7 A). In addition, bacterial clearance in nlrp3−/−nlrc4−/− and caspase-1−/− mice was compromised to a similar extent. In agreement with Lara-Tejero et al. (2006), mice lacking only NLRC4 or NLRP3 had similar bacterial loads to WT mice (Fig. S6, A and B). Unlike WT mice, which contained detectable IL-18 in their sera, IL-18 was not detected in caspase-1−/− and nlrp3−/−nlrc4−/− mice (Fig. 7 A), whereas nlrc4−/− and nlrp3−/− mice had similar IL-18 levels to those of WT animals (Fig. S6, A and B). WT levels of IL-1β were below the limit of detection at this time point (unpublished data). Interestingly, mice lacking ASC had similar bacterial burden compared with WT mice (Fig. S6 C; Lara-Tejero et al., 2006) when infected with WT Stm but showed reduced serum IL-18 levels. These data show that NLRP3 and NLRC4 are redundant for caspase-1 activation in mice infected with Stm and that the loss of both NLRs mimics loss of caspase-1, leading to a severely impaired innate immune response.
Figure 7.
Redundant roles for NLRP3 and NLRC4 in caspase-1 activation during Stm infection in mice. (A) WT, isogenic caspase-1−/−, and nlrp3−/−nlrc4−/− mice were orally infected with 2.4 × 107 CFU of WT Stm. Liver, spleen, and mesenteric lymph nodes were collected at day 5 after infection. Organ homogenates were diluted and plated to determine cfu per gram of tissue. Bars represent the mean bacterial load. Blood was collected at day 4 after infection and serum IL-18 was determined by ELISA. Data represent the mean ± SD of six to eight mice of each genotype. Results are representative of two independent experiments, each done with groups of six to eight mice of each genotype. Statistical significance was determined using the unpaired Mann-Whitney U test. *, P < 0.05. Dashed lines represent the detection limit of the CFU counts or IL-18 ELISA, respectively. (B) The role of the ASC focus in Stm-induced cytokine processing in vitro. Intracellular Stm activate NLRP3 and NLRC4. NLRC4 responds to flagellin injected by the SPI-2 T3SS, whereas NLRP3 responds to an undefined T3SS-independent signal. Both receptors induce the assembly of inflammasomes that can process pro–IL-1β and pro–IL-18 to their mature forms. NLRP3 inflammasome assembly is completely dependent on ASC, whereas NLRC4 may assemble an inflammasome without ASC. We speculate that ASC then promotes inflammasome aggregation into a single subcellular focus. This ASC focus likely mediates the bulk of cytokine processing and might serve to increase cytokine production in response to continuous stimulation of NLRs. SCV, Salmonella-containing vacuole; casp-1, caspase-1.
DISCUSSION
The mammalian genome encodes many NLRs, each with the potential to respond independently to a multitude of signals generated during exposure to a pathogen. Previous in vitro studies with Stm expressing the SPI-1 T3SS revealed rapid NLRC4-dependent caspase-1 activation (Mariathasan et al., 2004), so it was surprising that NLRC4 was dispensable for caspase-1–dependent Stm clearance in mice (Lara-Tejero et al., 2006; Raupach et al., 2006). Because SPI-1 is down-regulated after the initial phase of an infection, we investigated NLR involvement in SPI-1–independent caspase-1 activation by Stm. By infecting macrophages from different gene-targeted mouse strains, we found that two NLRs, NLRP3 and NLRC4, activate caspase-1 in response to Stm. Infections with mutant strains of Stm revealed that flagellin secreted by the SPI-2 T3SS engages NLRC4, whereas NLRP3 responds to a yet-to-be-defined T3SS-independent signal (Fig. 1). The ability of NLRP3 and NLRC4 to be engaged by distinct signals likely explains why loss of either gene alone does not compromise Stm clearance in mice (Lara-Tejero et al., 2006), but loss of both genes renders mice as susceptible to infection as caspase-1−/− mice (Fig. 7 A).
The bipartite adapter protein ASC is a critical inflammasome component because it can bridge NLRs and caspase-1 via its Pyrin and CARD domains (Martinon et al., 2009). In the context of Stm infections, ASC was crucial for NLRP3 to activate caspase-1. NLRC4, however, appeared able to activate caspase-1 independently of ASC, albeit poorly (Fig. 2). It is worth noting that ASC increased the efficiency of pro–IL-1β processing by the NLRC4-containing inflammasome about fivefold.
To visualize inflammasome assembly in response to Stm infection directly, we used immunofluorescence microscopy analysis of macrophages stained for ASC, caspase-1, and IL-1β. Endogenous ASC was predominantly nuclear in uninfected cells. Infection caused ASC to relocalize into a single cytoplasmic focus (Fig. 3). This dramatic redistribution of ASC within the cell was also observed during infections with F. novicida, so it will be interesting to see if the same phenomenon is observed with all stimuli that activate caspase-1. Specks of active caspase-1 were reported in macrophages stimulated with Yersinia pseudotuberculosis and Anthrax toxin (Fink et al., 2008), but ASC itself was not examined.
ASC focus formation in the cytoplasm of an Stm-infected macrophage was dependent on the presence of either NLRC4 or NLRP3. In WT macrophages, it is unclear whether NLRC4 and NLRP3 are found together in the one focus or if the first NLR that is engaged is sufficient to drive ASC focus formation. Unfortunately, detection of endogenous NLRC4 and NLRP3 has proven difficult with the antibodies that we have generated (unpublished data). A recent study showed that aggregation of YFP-tagged ASC in HeLa cells was a rapid all-or-none reaction, making it highly unlikely that a second focus would form in the same cell (Cheng et al., 2010), but the relevance of these observations to endogenous ASC in immune cells is unclear.
The first hint that these ASC foci might represent sites of IL-1β and IL-18 processing was the correlation between the percentage of macrophages containing an ASC focus and the amount of IL-1β and IL-18 secreted into the culture supernatant. Immunofluorescence microscopy confirmed that pro–caspase-1 is recruited to and activated within ASC foci (Fig. 5). Although caspase-1 was dispensable for ASC focus formation, it was essential for the recruitment of pro–IL-1β. Inhibition of caspase-1 proteolytic activity caused pro–IL-1β to accumulate in the ASC focus, suggesting that the mature cytokine is released rapidly (Fig. 6). How IL-1β and IL-18 then exit the cell is poorly understood. We speculate that the relocation of inflammasome components into one focus could serve to integrate signals originating from different NLRs and/or enhance inflammasome-mediated cytokine processing (Fig. 7 B). Future studies will address the kinetics of focus formation in primary macrophages and its exact molecular architecture.
To our knowledge, our paper is the first to describe the redundancy of inflammasome receptors in vivo during microbial infections. Previous studies have described other pathogens, such as L. monocytogenes and L. pneumophila, to also activate multiple receptors in cultured macrophages (Case et al., 2009; Warren et al., 2008). Interestingly, the response to L. pneumophila in vivo was dependent on NLRC4 only. NLRC4-deficient mice showed increased bacterial loads and lower IL-18 levels in the lung compared with WT animals (Case et al., 2009). They also reported an intriguing phenotype for ASC-deficient mice, which had comparable bacterial loads to WT animals but had lower serum IL-18 levels when infected with L. pneumophila. This is similar to the phenotype we have observed with asc−/− mice during Stm infections (Fig. S6 C). Therefore, we speculate that ASC plays a role in vivo in the maturation of cytokines, whereas it is not essential for restricting bacterial growth. In addition, this implies that additional ASC-independent pathways could be involved in activating caspase-1 in response to NLRC4 and NLRP3 activation during infections in mice.
Finally, we propose that the redundant roles of NLRC4 and NLRP3 in Stm recognition evolved to allow the host to respond to changes in pathogen-associated molecules. For instance, Stm down-regulates flagellin expression during the systemic phase of an infection (Cummings et al., 2006), perhaps driving the need for NLRP3-mediated pathogen sensing, which is independent of bacterial flagellin. Because we show that NLRP3 is required to respond to Stm lacking a T3SS and these T3SS mutant bacteria do not replicate intracellularly (Beuzón et al., 2000), NLRP3 may detect quiescent persistent pathogens. Stm and other pathogens, such as Mycobacterium tuberculosis, can produce chronic infections, but so far nothing is known about the role of the innate immune system during this process. Interestingly, NLRP3 has been shown to be essential for Mycobacterium marinum–mediated inflammasome activation in cultured macrophages (Koo et al., 2008). Our study shows that mice lacking multiple NLRs will be useful tools for understanding the role of NLRs in defense against acute and persistent microbial infections.
MATERIALS AND METHODS
Bacterial strains.
Bacterial strains include WT Stm SL1344 and the following Stm mutants: ΔSPI-1 (orgA::Tet), ΔSPI-2 (ssaV::Kan), ΔSPI-1ΔSPI-2 (orgA::Tet, ssaV::Kan), Δfla (fljAB::Kan, fliC::Cm), and ΔSPI-1Δfla (orgA::Tet, fljAB::Kan, fliC::Cm). WT Francisella tularensis subspecies novicida U112 was used in this study.
Mice.
Mice lacking NLRP3, NLRC4, caspase-1, or ASC have been previously described (Mariathasan et al., 2004, 2006). nlrp3−/−nlrc4−/− mice were generated by crossing the aforementioned strains. All mouse studies were approved by the institutional animal care and use committees of Genentech Inc. and Stanford University.
Animal infections.
For Stm infection, mice were starved for 12 h and then fed 2.4 × 107 WT Stm SL1344 in PBS. Infected mice were supplied with food and water ad libitum. Mesenteric lymph nodes, spleen, and liver were harvested at day 5 after infection and homogenized in PBS. Dilutions were plated on LB plates containing 100 µg/ml of Streptomycin. Colonies were counted and the CFU/gram of organ was determined. Serum cytokine levels were determined at day 3 after infection.
Cell culture and infections.
BMDMs were differentiated in DME (Invitrogen) with 10% vol/vol FCS (Thermo Fisher Scientific), 10% MCSF (L929 cell supernatant), 10 mM Hepes (Invitrogen), and nonessential aminoacids (Invitrogen). 1 d before infection, macrophages were seeded into 6-, 24-, or 96-well plates at a density of 1.25 × 106, 2.5 × 105, or 5 × 104 per well.
For infections with Stm not expressing the SPI-1 T3SS, Stm was grown overnight in LB at 37°C with aeration. The bacteria were diluted in fresh prewarmed macrophage medium and added to the macrophages at an MOI of 25:1. The plates were centrifuged for 15 min at 500 g, to ensure comparable adhesion of the bacteria to the cells, and placed at 37°C for 30 min. Next, 100 µg/ml gentamycin (Sigma-Aldrich) was added to kill extracellular bacteria in the cultures. After a 90-min incubation, the cells were washed once with DME and given fresh macrophage medium containing 10 µg/ml gentamycin.
Infections with Stm expressing the SPI-1 T3SS were performed as in the previous paragraph, with the following changes: macrophages were cultured for 16 h with 0.1 µg/ml LPS for LPS (Sigma-Aldrich) before infection. To induce SPI-1 expression, Stm were diluted at 1:50 into fresh LB and grown for 3–4 h at 37°C before the infection.
Macrophages infected with Francisella tularensis subspecies novicida were also cultured for 16 h with 0.1 µg/ml LPS (Sigma-Aldrich) before infection. F. novicida were grown overnight at 37°C in tryptic soy broth and macrophages were infected at a MOI of 100:1.
Cytokine measurement.
IL-1β, TNF (R&D systems), and IL-18 (MBL international) were measured by ELISA.
Western blotting.
The caspase-1 p10 subunit and processed IL-1β released into the culture supernatant was determined by Western blotting. Macrophages were washed with plain prewarmed DME lacking serum and phenol red at 8 h after infection. The cells were then cultured in this DME lacking serum and phenol red until 17 h after infection. The supernatant was collected and precipitated with 10% TCA (vol/vol) for 1 h on ice. Precipitated proteins were pelleted at 20,000 g for 30 min at 4°C, washed with ice-cold acetone, air-dried, resuspended in SDS-PAGE sample buffer, and heated to 95°C for 10 min. Protein from 2.5 × 106 macrophages was loaded per well of a 14% acrylamide gel. Western blots were performed with rabbit anti–mouse caspase-1 antibody (sc514; Santa Cruz Biotechnology, Inc.) diluted 1:200 and goat anti–mouse IL-1β antibody (AF-401-NA; R&D Systems) diluted 1:1,000. Cell lysates were probed with anti–β-actin antibodies (Santa Cruz Biotechnology, Inc.).
Statistical analysis.
Statistical data analysis was done using Prism 5.0a (GraphPad Software, Inc.). Statistical significance was determined by the Mann-Whitney U test.
Immunofluorescence.
Macrophages were seeded onto glass coverslips in 24-well plates at a density of 1.25 × 105 per well and infected as described above. Infected cells were washed twice with PBS and fixed for 15 min at 37°C with 4% (vol/vol) paraformaldehyde in PBS. Cells were washed three times with PBS and incubated with primary antibodies for 30 min in blocking buffer (3% BSA and 0.1% Saponin in PBS). Primary antibodies were rat anti–mouse ASC (clone 8E4; Genentech) diluted 1:1,000, rabbit anti–mouse caspase-1 antibody (sc514, Santa Cruz Biotechnology, Inc.) diluted 1:100, and goat anti–mouse IL-1β (AF-401-NA; R&D Systems) diluted 1:100. Cells were washed with PBS three times and incubated with Alexa Fluor–coupled secondary antibodies (1:250; Invitrogen) for 30 min. Cells were washed four times with PBS, stained with DAPI, and imaged with a confocal microscope (LSM700; Carl Zeiss, Inc.). For FAM-YVAD-FMK stainings (FLICA; ImmunoChemistry Technologies), the medium was removed 1 h before the collection time point and replaced with fresh DME containing 5 µM FLICA. For caspase-1 inhibitor treatments, the macrophages were cultured in 20 µM Z-YVAD-FMK in DMSO (EMD) or DMSO throughout the infection.
Online supplemental material.
Fig. S1 shows SPI-1–dependent and –independent inflammasome activation. Fig. S2 shows that macrophages release comparable levels of TNF when infected with Stm and respond normally to F. novicida infections. Fig. S3 shows that ASC foci are formed during Stm SPI-1–mediated inflammasome activation. Fig. S4 shows that ASC foci are also formed during F. novicida–mediated inflammasome activation. Fig. S5 shows characterization of ASC foci formed during SPI-1–mediated inflammasome activation. Fig. S6 shows infections of WT mice and single deficiencies in NLRC4, NLRP3, and ASC with Stm. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20100257/DC1.
Acknowledgments
We thank members of the Monack Laboratory and Daniela Kenzelmann Broz for help with the mouse experiments. We also thank members of the D.M. Monack, G. Barton, R. Vance, A. Sil, J. Cox, and D. Portnoy laboratories for stimulating discussions.
This work was supported by National Institutes of Health National Institute of Allergy and Infectious Diseases (P01 AI063302). D.M. Monack holds an Investigators in the Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund. P. Broz holds a Stanford Institute for Immunity Transplantation and Infection Young Investigator Award and was supported by postdoctoral fellowships from the Swiss National Science Foundation and the Human Frontiers in Science Program.
The authors declare no competing financial interests. K. Newton, M. Lamkanfi, S. Mariathasan, and V.M. Dixit are employees of Genentech Inc.
Footnotes
Abbreviations used:
- BMDM
- BM-derived macrophage
- CARD
- caspase activation and recruitment domain
- NLR
- NOD-like receptor
- Stm
- Salmonella enterica serovar typhimurium
- T3SS
- type 3 secretion system
References
- Allen I.C., Scull M.A., Moore C.B., Holl E.K., McElvania-TeKippe E., Taxman D.J., Guthrie E.H., Pickles R.J., Ting J.P. 2009. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 30:556–565 10.1016/j.immuni.2009.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amer A., Franchi L., Kanneganti T.D., Body-Malapel M., Ozören N., Brady G., Meshinchi S., Jagirdar R., Gewirtz A., Akira S., Núñez G. 2006. Regulation of Legionella phagosome maturation and infection through flagellin and host Ipaf. J. Biol. Chem. 281:35217–35223 10.1074/jbc.M604933200 [DOI] [PubMed] [Google Scholar]
- Beuzón C.R., Méresse S., Unsworth K.E., Ruíz-Albert J., Garvis S., Waterman S.R., Ryder T.A., Boucrot E., Holden D.W. 2000. Salmonella maintains the integrity of its intracellular vacuole through the action of SifA. EMBO J. 19:3235–3249 10.1093/emboj/19.13.3235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky I.E., Monack D. 2009. NLR-mediated control of inflammasome assembly in the host response against bacterial pathogens. Semin. Immunol. 21:199–207 10.1016/j.smim.2009.05.007 [DOI] [PubMed] [Google Scholar]
- Bryan N.B., Dorfleutner A., Rojanasakul Y., Stehlik C. 2009. Activation of inflammasomes requires intracellular redistribution of the apoptotic speck-like protein containing a caspase recruitment domain. J. Immunol. 182:3173–3182 10.4049/jimmunol.0802367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case C.L., Shin S., Roy C.R. 2009. Asc and Ipaf Inflammasomes direct distinct pathways for caspase-1 activation in response to Legionella pneumophila. Infect. Immun. 77:1981–1991 10.1128/IAI.01382-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J., Waite A.L., Tkaczyk E.R., Ke K., Richards N., Hunt A.J., Gumucio D.L. 2010. Kinetic properties of ASC protein aggregation in epithelial cells. J. Cell. Physiol. 222:738–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings L.A., Wilkerson W.D., Bergsbaken T., Cookson B.T. 2006. In vivo, fliC expression by Salmonella enterica serovar Typhimurium is heterogeneous, regulated by ClpX, and anatomically restricted. Mol. Microbiol. 61:795–809 10.1111/j.1365-2958.2006.05271.x [DOI] [PubMed] [Google Scholar]
- Dostert C., Guarda G., Romero J.F., Menu P., Gross O., Tardivel A., Suva M.L., Stehle J.C., Kopf M., Stamenkovic I., et al. 2009. Malarial hemozoin is a Nalp3 inflammasome activating danger signal. PLoS One. 4:e6510 10.1371/journal.pone.0006510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J.A., Gao X., Huang M.T., O’Connor B.P., Thomas C.E., Willingham S.B., Bergstralh D.T., Jarvis G.A., Sparling P.F., Ting J.P. 2009. Neisseria gonorrhoeae activates the proteinase cathepsin B to mediate the signaling activities of the NLRP3 and ASC-containing inflammasome. J. Immunol. 182:6460–6469 10.4049/jimmunol.0802696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eder C. 2009. Mechanisms of interleukin-1beta release. Immunobiology. 214:543–553 10.1016/j.imbio.2008.11.007 [DOI] [PubMed] [Google Scholar]
- Faustin B., Lartigue L., Bruey J.M., Luciano F., Sergienko E., Bailly-Maitre B., Volkmann N., Hanein D., Rouiller I., Reed J.C. 2007. Reconstituted NALP1 inflammasome reveals two-step mechanism of caspase-1 activation. Mol. Cell. 25:713–724 10.1016/j.molcel.2007.01.032 [DOI] [PubMed] [Google Scholar]
- Fernandes-Alnemri T., Wu J., Yu J.W., Datta P., Miller B., Jankowski W., Rosenberg S., Zhang J., Alnemri E.S. 2007. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 14:1590–1604 10.1038/sj.cdd.4402194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes-Alnemri T., Yu J.W., Datta P., Wu J., Alnemri E.S. 2009. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 458:509–513 10.1038/nature07710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink S.L., Bergsbaken T., Cookson B.T. 2008. Anthrax lethal toxin and Salmonella elicit the common cell death pathway of caspase-1-dependent pyroptosis via distinct mechanisms. Proc. Natl. Acad. Sci. USA. 105:4312–4317 10.1073/pnas.0707370105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchi L., Amer A., Body-Malapel M., Kanneganti T.D., Ozören N., Jagirdar R., Inohara N., Vandenabeele P., Bertin J., Coyle A., et al. 2006. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in Salmonella-infected macrophages. Nat. Immunol. 7:576–582 10.1038/ni1346 [DOI] [PubMed] [Google Scholar]
- Gross O., Poeck H., Bscheider M., Dostert C., Hannesschläger N., Endres S., Hartmann G., Tardivel A., Schweighoffer E., Tybulewicz V., et al. 2009. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 459:433–436 10.1038/nature07965 [DOI] [PubMed] [Google Scholar]
- Henry T., Brotcke A., Weiss D.S., Thompson L.J., Monack D.M. 2007. Type I interferon signaling is required for activation of the inflammasome during Francisella infection. J. Exp. Med. 204:987–994 10.1084/jem.20062665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hise A.G., Tomalka J., Ganesan S., Patel K., Hall B.A., Brown G.D., Fitzgerald K.A. 2009. An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe. 5:487–497 10.1016/j.chom.2009.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung V., Ablasser A., Charrel-Dennis M., Bauernfeind F., Horvath G., Caffrey D.R., Latz E., Fitzgerald K.A. 2009. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 458:514–518 10.1038/nature07725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu L.C., Ali S.R., McGillivray S., Tseng P.H., Mariathasan S., Humke E.W., Eckmann L., Powell J.J., Nizet V., Dixit V.M., Karin M. 2008. A NOD2-NALP1 complex mediates caspase-1-dependent IL-1beta secretion in response to Bacillus anthracis infection and muramyl dipeptide. Proc. Natl. Acad. Sci. USA. 105:7803–7808 10.1073/pnas.0802726105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly S., Ma N., Sadler J.J., Soll D.R., Cassel S.L., Sutterwala F.S. 2009. Cutting edge: Candida albicans hyphae formation triggers activation of the Nlrp3 inflammasome. J. Immunol. 183:3578–3581 10.4049/jimmunol.0901323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanneganti T.D., Body-Malapel M., Amer A., Park J.H., Whitfield J., Franchi L., Taraporewala Z.F., Miller D., Patton J.T., Inohara N., Núñez G. 2006. Critical role for Cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. J. Biol. Chem. 281:36560–36568 10.1074/jbc.M607594200 [DOI] [PubMed] [Google Scholar]
- Keller M., Rüegg A., Werner S., Beer H.D. 2008. Active caspase-1 is a regulator of unconventional protein secretion. Cell. 132:818–831 10.1016/j.cell.2007.12.040 [DOI] [PubMed] [Google Scholar]
- Koo I.C., Wang C., Raghavan S., Morisaki J.H., Cox J.S., Brown E.J. 2008. ESX-1-dependent cytolysis in lysosome secretion and inflammasome activation during mycobacterial infection. Cell. Microbiol. 10:1866–1878 10.1111/j.1462-5822.2008.01177.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara-Tejero M., Sutterwala F.S., Ogura Y., Grant E.P., Bertin J., Coyle A.J., Flavell R.A., Galán J.E. 2006. Role of the caspase-1 inflammasome in Salmonella typhimurium pathogenesis. J. Exp. Med. 203:1407–1412 10.1084/jem.20060206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg U., Vinatzer U., Berdnik D., von Gabain A., Baccarini M. 1999. Growth phase-regulated induction of Salmonella-induced macrophage apoptosis correlates with transient expression of SPI-1 genes. J. Bacteriol. 181:3433–3437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariathasan S., Monack D.M. 2007. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat. Rev. Immunol. 7:31–40 10.1038/nri1997 [DOI] [PubMed] [Google Scholar]
- Mariathasan S., Newton K., Monack D.M., Vucic D., French D.M., Lee W.P., Roose-Girma M., Erickson S., Dixit V.M. 2004. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 430:213–218 10.1038/nature02664 [DOI] [PubMed] [Google Scholar]
- Mariathasan S., Weiss D.S., Newton K., McBride J., O’Rourke K., Roose-Girma M., Lee W.P., Weinrauch Y., Monack D.M., Dixit V.M. 2006. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 440:228–232 10.1038/nature04515 [DOI] [PubMed] [Google Scholar]
- Martinon F., Agostini L., Meylan E., Tschopp J. 2004. Identification of bacterial muramyl dipeptide as activator of the NALP3/cryopyrin inflammasome. Curr. Biol. 14:1929–1934 10.1016/j.cub.2004.10.027 [DOI] [PubMed] [Google Scholar]
- Martinon F., Mayor A., Tschopp J. 2009. The inflammasomes: guardians of the body. Annu. Rev. Immunol. 27:229–265 10.1146/annurev.immunol.021908.132715 [DOI] [PubMed] [Google Scholar]
- Masumoto J., Taniguchi S., Ayukawa K., Sarvotham H., Kishino T., Niikawa N., Hidaka E., Katsuyama T., Higuchi T., Sagara J. 1999. ASC, a novel 22-kDa protein, aggregates during apoptosis of human promyelocytic leukemia HL-60 cells. J. Biol. Chem. 274:33835–33838 10.1074/jbc.274.48.33835 [DOI] [PubMed] [Google Scholar]
- Miao E.A., Alpuche-Aranda C.M., Dors M., Clark A.E., Bader M.W., Miller S.I., Aderem A. 2006. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat. Immunol. 7:569–575 10.1038/ni1344 [DOI] [PubMed] [Google Scholar]
- Miao E.A., Mao D.P., Yudkovsky N., Bonneau R., Lorang C.G., Warren S.E., Leaf I.A., Aderem A. 2010. Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proc. Natl. Acad. Sci. USA. 107:3076–3080 10.1073/pnas.0913087107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monack D.M., Detweiler C.S., Falkow S. 2001. Salmonella pathogenicity island 2-dependent macrophage death is mediated in part by the host cysteine protease caspase-1. Cell. Microbiol. 3:825–837 10.1046/j.1462-5822.2001.00162.x [DOI] [PubMed] [Google Scholar]
- Muruve D.A., Pétrilli V., Zaiss A.K., White L.R., Clark S.A., Ross P.J., Parks R.J., Tschopp J. 2008. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature. 452:103–107 10.1038/nature06664 [DOI] [PubMed] [Google Scholar]
- Poyet J.L., Srinivasula S.M., Tnani M., Razmara M., Fernandes-Alnemri T., Alnemri E.S. 2001. Identification of Ipaf, a human caspase-1-activating protein related to Apaf-1. J. Biol. Chem. 276:28309–28313 10.1074/jbc.C100250200 [DOI] [PubMed] [Google Scholar]
- Raupach B., Peuschel S.K., Monack D.M., Zychlinsky A. 2006. Caspase-1-mediated activation of interleukin-1beta (IL-1beta) and IL-18 contributes to innate immune defenses against Salmonella enterica serovar Typhimurium infection. Infect. Immun. 74:4922–4926 10.1128/IAI.00417-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren T., Zamboni D.S., Roy C.R., Dietrich W.F., Vance R.E. 2006. Flagellin-deficient Legionella mutants evade caspase-1- and Naip5-mediated macrophage immunity. PLoS Pathog. 2:e18 10.1371/journal.ppat.0020018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards N., Schaner P., Diaz A., Stuckey J., Shelden E., Wadhwa A., Gumucio D.L. 2001. Interaction between pyrin and the apoptotic speck protein (ASC) modulates ASC-induced apoptosis. J. Biol. Chem. 276:39320–39329 10.1074/jbc.M104730200 [DOI] [PubMed] [Google Scholar]
- Shio M.T., Tiemi Shio M., Eisenbarth S.C., Savaria M., Vinet A.F., Bellemare M.J., Harder K.W., Sutterwala F.S., Bohle D.S., Descoteaux A., et al. 2009. Malarial hemozoin activates the NLRP3 inflammasome through Lyn and Syk kinases. PLoS Pathog. 5:e1000559 10.1371/journal.ppat.1000559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutterwala F.S., Mijares L.A., Li L., Ogura Y., Kazmierczak B.I., Flavell R.A. 2007. Immune recognition of Pseudomonas aeruginosa mediated by the IPAF/NLRC4 inflammasome. J. Exp. Med. 204:3235–3245 10.1084/jem.20071239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Franchi L., Toma C., Ashida H., Ogawa M., Yoshikawa Y., Mimuro H., Inohara N., Sasakawa C., Nuñez G. 2007. Differential regulation of caspase-1 activation, pyroptosis, and autophagy via Ipaf and ASC in Shigella-infected macrophages. PLoS Pathog. 3:e111 10.1371/journal.ppat.0030111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P.G., Dash P., Aldridge J.R., Jr., Ellebedy A.H., Reynolds C., Funk A.J., Martin W.J., Lamkanfi M., Webby R.J., Boyd K.L., et al. 2009. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity. 30:566–575 10.1016/j.immuni.2009.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren S.E., Mao D.P., Rodriguez A.E., Miao E.A., Aderem A. 2008. Multiple Nod-like receptors activate caspase 1 during Listeria monocytogenes infection. J. Immunol. 180:7558–7564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter S.E., Thiennimitr P., Nuccio S.P., Haneda T., Winter M.G., Wilson R.P., Russell J.M., Henry T., Tran Q.T., Lawhon S.D., et al. 2009. Contribution of flagellin pattern recognition to intestinal inflammation during Salmonella enterica serotype typhimurium infection. Infect. Immun. 77:1904–1916 10.1128/IAI.01341-08 [DOI] [PMC free article] [PubMed] [Google Scholar]