Abstract
Objective
With improved technology, the values of intraoperative computed tomography (iCT) have been reevaluated. We describe our early clinical experience with a mobile CT (mCT) system for iCT and discuss its clinical applications, advantages and limitations.
Methods
Compared with intraoperative magnetic resonance imaging, this mCT system has no need for major reconstruction of a preexisting operating room for shielding, or for specialized instruments or equipment. Patients are placed on a radiolucent head clamp that fits within the gantry. Because it consists simply of a scanner and a workstation, it can be moved between locations such as an operating room, an intensive care unit (ICU) or an emergency room without difficulty. Furthermore, it can achieve nearly all types of CT scanning procedures such as enhancement, temporal bone imaging, angiography and three-dimensional reconstruction.
Results
For intracranial surgery, mCT can be used for intraoperative real-time neuronavigation by interacting with preoperative images. It can also be used for intraoperative confirmation of the extent of resection of intracranial lesions and for immediate checks for preventing intraoperative unexpected accidents. Therefore, the goals of maximal resection or optimal treatment can be achieved without any guesswork. Furthermore, mCT can achieve improved patient care with safety and faster diagnosis for patients in an ICU who might be subjected to a ventilator and/or various monitoring devices.
Conclusion
Our initial experience demonstrates that mCT with high-quality imaging offers very useful information in various clinical situations.
Keywords: Intraoperative computed tomography, Mobile computed tomography
INTRODUCTION
Over the past couple of decades, intraoperative neuronavigation has been used widely as a standard procedure for precise guidance, orientation and localization in most neurosurgical operations1,2,9). The importance of obtaining real-time intraoperative imaging is being emphasized increasingly instead of being limited to neuronavigation. There could be several reasons for this. Existing neuronavigation systems cannot reflect exactly the "brain shifts" resulting from the loss of cerebrospinal fluid (CSF) or the removal of an intracranial lesion during an operation, so the acquisition of intraoperative images is mandatory for more accurate intraoperative neuronavigation2,13). As a result, intraoperative assessment of the precise orientation or localization of structures and the extent of resection of intracranial lesions such as brain tumors can be improved. These approaches are also useful for intraoperative monitoring of various unexpected intraoperative brain events including acute hemorrhages, diffuse brain swelling and acute hydrocephalus.
Intraoperative imaging systems can be divided into several types, such as magnetic resonance imaging (MRI), computed tomography (CT) and ultrasound13). Among them, intraoperative MRI (iMRI) has been used more extensively than other systems. However, with modern technology, systems based on intraoperative CT (iCT) are now more common. Here, we describe our early clinical experience of iCT with a mobile CT (mCT) system and discuss its clinical applications, advantages and limitations.
MATERIALS AND METHODS
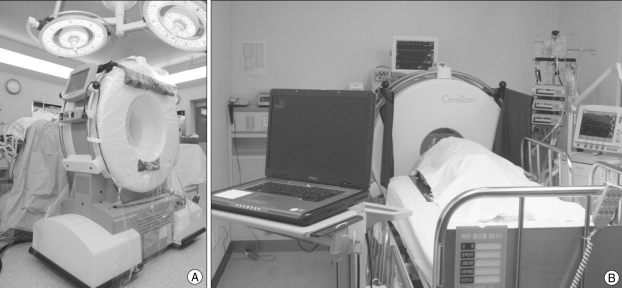
For intraoperative imaging scanning, we used a portable CT scanner, the CereTom (Neurologica, Danvers, MA, USA). This is one of a number of recently developed mobile units and is designed for multi-slice scanning of the head and neck (Fig. 1A). It comprises a mobile system with a portable workstation. It is smaller than other portable CT units, with a weight of 362 kg, a height of 153 cm, a length of 134 cm, a width of 72 cm and a gantry diameter of 32 cm10). Thus, it requires only one person for its transport and can be moved to any places including an operating room (OR), an intensive care unit (ICU) or an emergency room (ER) without difficulty (Fig. 1B). Typical scanning parameters are 120 kV, 7 mA, a scanning time of 2 s and a 10-mm-wide collimated beam with eight 1.25-mm-wide detectors. It can provide a reconstructed section thickness of 1.25 mm, as described10). The average patient effective dose for a routine head CT examination on the CereTom is -1.7 mSV, typical for head examinations performed on any CT scanner during which adults receive between 1 and 2 mSV10).
Fig. 1.
Photograph of mobile computed tomography (mCT) scanner, gantry and workstation in the operation room and the intensive care unit. A : Intraoperative photograph. For mCT scanning in the operating room, there is a need for additional procedures to confirm safety, such as wrapping the gantry in sterile draping, moving the gantry to the patient's head and performing a simulation without radiation exposure. B : MCT scanning for a patient in an intensive care unit. This demonstrates a portable workstation that can receive images through a wireless system and display three-dimensional reconstructed images.
For acquiring mCT images in the OR, only a few additional procedures are required as follows. The gantry needs to be wrapped in sterile drapes to maintain sterile conditions during the scanning procedure. The gantry of the mCT is then moved to the patient's head to perform a simulation scan without using radiation and to confirm the safety of the position. Contrast medium can be infused intravenously as needed. A required preoperative procedure for intraoperative CT scanning is that the patient must be placed on a conventional operating table fitted with a radiolucent extension or a radiolucent head fixator.
RESULTS
Between January and August 2009, 63 patients underwent scanning using the mCT system in the OR or the ICU. Among these, 20 patients with postoperative routine CT scanning were excluded. Among the remaining 43 patients, we measured the extent of resection of intracranial lesions in 22 (51%). Nine patients (21%) underwent scanning in the ICU, eight (19%) underwent ventricular catheter placement surgery and four (9%) were scanned for monitoring intraoperative brain events. Among the 22 procedures for assessing the extent of resections, there were 10 pituitary adenomas, four vestibular schwannomas, four meningiomas, two intracranial hematomas and one each of a brain metastasis and an abscess.
Assessing the extent of resection
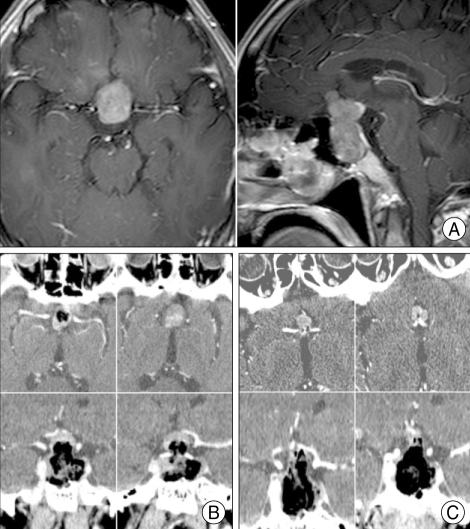
We used the mCT for assessing the extent of resection. For example, a 61-year-old male patient underwent an operation to remove a pituitary macroadenoma that had extended considerably to the suprasellar area (Fig. 2A). After an initial surgical resection using a transsphenoidal approach, an iCT scan was performed using the mCT for assessing the extent of resection of the tumor. After the injection of contrast medium, a residual enhanced lesion was detected over the suprasellar area and surgical resection was resumed for additional resection (Fig. 2B). After a second intraoperative CT scan, the surgeon decided to finish the operation without additional resection because of the close vicinity of the tumor to the anterior cerebral artery, although some residual lesion remained (Fig. 2C).
Fig. 2.
Mobile CT for a pituitary macroadenoma. A : Preoperative axial and sagittal images with contrast enhancement. B : Initial intraoperative mobile computed tomography (mCT) scanning with contrast enhancement showing axial images (upper) and coronal reconstructed images (lower). A small residual enhancing mass was detected at the top of the lesion. C : After the second intraoperative CT scanning, the small residual enhancing mass was still located close to the anterior communicating artery.
The second case demonstrated another application of the mCT for improving the extent of resection. Patient was a 51-year-old female who had presented with decreased visual acuity of the left eye caused by a tuberculum sellae meningioma (Fig. 3A). We achieved a total removal of the tumor based on additional information from an intraoperative mCT scan, which demonstrated an enhanced residual mass beneath the left optic nerve (Fig. 3B).
Fig. 3.
Mobile computed tomography (mCT) of a tuberculum sellae meningioma. A : A meningioma was located at the tuberculum sellae area and compressed the left optic nerve. B : Intraoperative mCT axial images with contrast enhancement. A small residual enhancing mass was detected beneath the left optic nerve.
Among the 22 procedures in which introperative mCT scan was used to assess the extent of resection, residual lesions were actually detected in 7 (32%) patients. Surgery was continued for additional resection in 3 cases.
Ventricular catheter placement
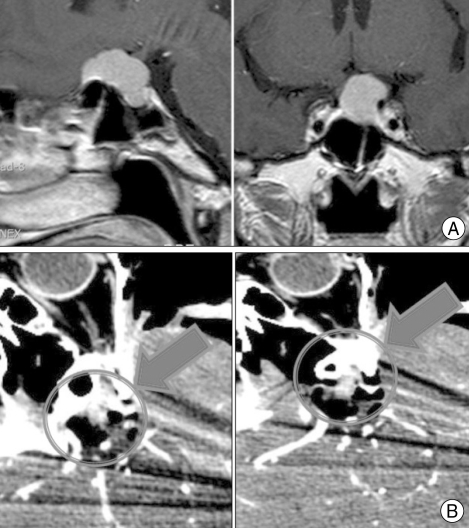
The mCT can be also useful for the intraoperative evaluation of ventricular catheter placement operations such as ventriculo-peritoneal shunting or extraventricular drainage. Fig. 4 shows a patient who underwent a ventriculo-peritoneal shunt operation for the relief of hydrocephalus. Once the ventricular catheter had been placed through the right parieto-occipital point without a problem, the flow of CSF drainage was slightly diminished. An intraoperative mCT scan was performed for immediate review (Fig. 4A). This revealed that the ventricular catheter was located in the temporal horn of the right lateral ventricle. Catheter repositioning of the ventricular catheter into the frontal horn was performed in the OR (Fig. 4B). In another patient undergoing a ventriculo-peritoneal shunt, like aforementioned case, optimal catheter repositioning was achieved through the intraoperative mCT scan. In the 6 remaining cases, intraoperative mCT scan was used due to the difficulty such as the very small size of ventricles to confirm the position of the ventricular catheter.
Fig. 4.
Mobile CT for performing a ventriculo-peritoneal shunt. A : Intraoperative photograph of a ventriculo-peritoneal shunt operation in the operating room. B : The proximal ventricular catheter was located in the right temporal horn (left). Adjustments for correcting malposition of the ventricular catheter were made immediately into the frontal horn in the operation room.
ICU and monitoring intraoperative events
Another application of mCT was for patients in our ICU who underwent insertion of an intracranial cerebral pressure monitor and pentothal coma therapy (Fig. 1B). Nine patients who had taken an ICU management for pentothal coma therapy or monitoring for increased intracranial cerebral pressure received an immediate CT scan without the need for transportation to a remote conventional CT facility.
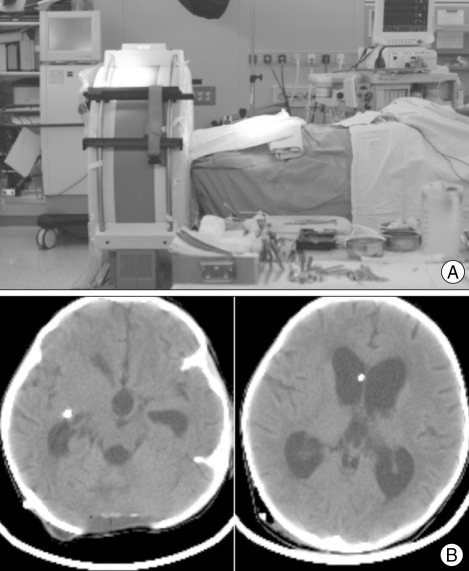
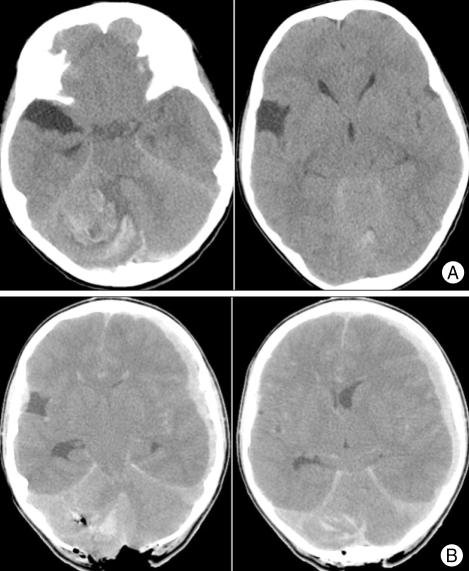
The preoperative CT images of a 7-year-old boy with sudden-onset of projectile vomiting and lethargy had a large hemorrhage in the cerebellum (Fig. 5A). During an emergency evacuation of the hematoma, inexplicable severe cerebellar swelling was observed although a major part of the hematoma had been removed. After intraoperative mCT scanning, a supratentorial subdural hemorrhage was confirmed on the left side (Fig. 5B). After close radiological follow-up without an additional operation, the subdural hemorrhage had disappeared gradually.
Fig. 5.
Mobile computed tomography (mCT) used for monitoring intraoperative brain events. A : The preoperative CT images showed a large hemorrhage in the cerebellum. B : A diffuse acute subdural hemorrhage was detected in the left fronto-temporal area after intraoperative mCT scanning.
Another monitoring was performed to confirm the site of stereotactic brain biopsy in which the intraoperative frozen biopsy was repeatedly reported as normal brain tissue. Remaining 2 procedures were performed to evaluate a sudden and unexpected brain swelling during the evacuation of epidural hemorrhage and subacute subdural hemorrhage.
DISCUSSION
Since neuronavigation has been introduced to neurosurgery, better clinical outcomes have been achieved through the better guidance, orientation and localization it provides for neurosurgeons5). Such neuronavigation is now a routine method for almost all neurosurgical procedures. However, one of the most important drawbacks for the traditional neuronavigation is undoubtedly the problem of "brain shift". Spatial displacement between the preoperative images and the surgical field can occur after opening of the dura and this will increase gradually because of CSF drainage, changes in arterial pCO2, removal of intracranial lesion and brain edema2,13). When this occurs, there is an inherent risk that any neuronavigation based on preoperative images becomes progressively inaccurate5). The degree of displacement caused by a brain shift is difficult to assess intraoperatively, so the usefulness of neuronavigation may diminish during the surgical procedure2). The best potential approach for solving this problem is the acquisition of intraoperative real-time images showing anatomical structures and intracranial lesions accurately13). These intraoperative real-time images, which facilitate more precise orientation and localization, are increasingly important for contemporary neurosurgical procedures.
Since iMRI was applied to the neurosurgical field in the mid-1990s, it has been more extensively used than other systems, because it offers higher resolution of brain structures and intracranial lesions such as brain tumors than did earlier CT scanners5,12). However, it has some disadvantages such as the need for shielding, which requires major reconstruction of a preexisting OR. It also requires specialized operative instruments and equipment, especially for high-field systems12). Furthermore, it needs considerable expense for installation and maintenance and the installation of iMRI has been limited mainly to a few large hospitals12). On the other hand, although they were introduced in the early 1980s, iCT systems have not been used extensively because they give limited image quality, are time-consuming and give relatively high radiation exposure to patients12). However, with modern technology, iCT systems have become smaller and more compact and can be moved freely in and out of the OR. They do not require specialized equipment as they give a low radiation dose, nor do they need new space for intraoperative imaging9). Thus, the advantage of iCT-based technology compared with iMR imaging is its simplicity combined with adequate image quality. For these reasons, the value of iCT has been reevaluated and the demand for iCT systems is increasing.
Clinical applications
Mobile CT, as one of the iCT systems, was installed at our hospital in late 2008. The clinical applications of mCT can be categorized as follows. First, the acquisition of intraoperative images by mCT can provide more precise information for real-time orientation and localization than does neuronavigation based on preoperative images. Using this information, it is now possible to assess the extent of resection of intracranial lesions such as brain tumors or intracranial hematomas, intraoperatively. Consequently, maximal resection or optimal resection for intracranial lesions can be achieved.
Gumprecht and Lumenta3) reported the clinical application of iCT scanning for removing brain tumors. According to their report, the tumors remaining in 32/76 patients (42%) could be detected by intraoperative scanning. Among these, surgery was continued for additional tumor resection in 16 cases. In our study, mCT scanning for the same purpose was performed on 22 patients (51%). Although we cannot confirm that this additional resection results in significantly improved clinical outcomes such as long-term tumor control rate or survival benefits, we believe that at least it provides a realistic possibility for surgeons to avoid unexpected or unwanted residual lesions. The combination of real-time neuronavigation and intraoperative assessing of the extent of resection could be more useful for various operations at the skull base.
Another advantage of mCT is that it can reduce uncertainty in various clinical situations during neurosurgical procedures. Whenever intraoperative images are needed, an immediate imaging check can be performed to check on unexpected complications such as diffuse brain edema, localor more rarely, distant-brain hemorrhages, acute hydrocephalus or ischemia2). The discovery of such an unexpected brain event provides an opportunity for immediate action and helps to avoid serious consequences2). In our study, we could achieve a fast diagnosis and appropriate decision-making without any guesswork through the confirmation of distant intracranial hemorrhages based on intraoperative CT images in the OR.
Although only a few studies for ventricular catheter placement surgery used by iCT have been reported, the usefulness of iCT for these operations is obvious. Ventricular catheter placement surgery is one of the most frequent neurosurgical procedures. In this condition, iCT can help in diagnosing any suboptimal placement of ventricular catheter and complications, allowing for correction before the patient leaves the OR. In particular, it can improve the confidence of a neurosurgeon when placing a ventricular catheter in complex cases or in various operations into nontraditional sites including cysts, small ventricles and Ommaya reservoir insertion. The frequency in use for ventricular catheter placement surgery in our study was around 20%. If there were no mCT available, we would have to use guesswork and decide on a treatment plan only after the confirmation of postoperative CT images.
One of the advantages of our iCT system is its mobility. This mCT technology could provide a better way for evaluating patients in the ICU and emergency department as well as the OR, because it takes a relatively short time to obtain images and it improves the quality of treatment for the patients. Transporting ventilated, sedated patients who are critically ill and physiologically unstable from the ICU to a conventional CT room is often slow, and is a potential risk that can result in aggravated secondary injuries to the brain4,8). Further, mCT could also prove useful in the emergency department for evaluating patients suspected of stroke or head trauma as it decreases the evaluation time10). Rumboldt et al.10) reported that the inherent risks associated with such intrahospital transport could reach more than 50% and adverse events could occur in a further 15% of cases, even with a well-trained transport team. Gunnarsson et al.4) reported that the staff workload for one scanning procedure used by a portable CT system was reduced by 60% to 75%.
The mCT can perform almost all CT scanning procedures including image enhancement, angiography, fluoroscopy, temporal bone scans and various three-dimensional reconstructions10). Through these varied protocols, it can be used extensively not just for patients undergoing neurosurgery, but also for patients in other specialties such as orthopedics, pediatrics and otorhinolaryngology. Hoelzle et al.6) reported that the iCT monitoring of orbital floor fractures is considered a very useful surgical aid for immediate monitoring of a surgical reduction. Stieve et al.11) demonstrated that iCT could assist in exposure of the skull base and lamina papyracea during endonasal surgery or cochlear implantation in otorhinolaryngology. Actually, economic efficacy as well as clinical benefits must be considered. Previous studies have concluded that the introduction of an iCT with or without a portable system in the OR or ICU is feasible and cost-effective4,7,10,12).
Limitations
Because this system is CT-based, it offers limited evaluation for several lesions such as low grade gliomas or poorly delineated lesions and it has an inherent risk of radiation exposure. In addition, because it has a relatively smaller gantry than other iCT equipment in order to permit effective transport, it cannot evaluate other body parts below the head and neck. Because of the initial experience with mCT, it still needs an expert technician in managing of the mCT system for various CT procedures. A long-term follow-up study is mandatory for determining the clinical efficacy, costs and benefits of mCT.
CONCLUSION
Our initial experience shows promising results that mCT with various high-quality images offers a very reasonable technology in various clinical situations such as intraoperative imaging and ICU care.
References
- 1.Enchev Y. Neuronavigation : geneology, reality, and prospects. Neurosurg Focus. 2009;27:E11. doi: 10.3171/2009.6.FOCUS09109. [DOI] [PubMed] [Google Scholar]
- 2.Foroglou N, Zamani A, Black P. Intra-operative MRI (iop-MR) for brain tumour surgery. Br J Neurosurg. 2009;23:14–22. doi: 10.1080/02688690802610587. [DOI] [PubMed] [Google Scholar]
- 3.Gumprecht H, Lumenta CB. Intraoperative imaging using a mobile computed tomography scanner. Minim Invasive Neurosurg. 2003;46:317–322. doi: 10.1055/s-2003-812496. [DOI] [PubMed] [Google Scholar]
- 4.Gunnarsson T, Theodorsson A, Karlsson P, Fridriksson S, Boström S, Persliden J, et al. Mobile computerized tomography scanning in the neurosurgery intensive care unit : increase in patient safety and reduction of staff workload. J Neurosurg. 2000;93:432–436. doi: 10.3171/jns.2000.93.3.0432. [DOI] [PubMed] [Google Scholar]
- 5.Haberland N, Ebmeier K, Hliscs R, Grnewald JP, Silbermann J, Steenbeck J, et al. Neuronavigation in surgery of intracranial and spinal tumors. J Cancer Res Clin Oncol. 2000;126:529–541. doi: 10.1007/s004320000122. [DOI] [PubMed] [Google Scholar]
- 6.Hoelzle F, Klein M, Schwerdtner O, Lueth T, Albrecht J, Hosten N, et al. Intraoperative computed tomography with the mobile CT Tomoscan M during surgical treatment of orbital fractures. Int J Oral Maxillofac Surg. 2001;30:26–31. doi: 10.1054/ijom.2000.0014. [DOI] [PubMed] [Google Scholar]
- 7.Masaryk T, Kolonick R, Painter T, Weinreb DB. The economic and clinical benefits of portable head/neck CT imaging in the intensive care unit. Radiol Manage. 2008;30:50–54. [PubMed] [Google Scholar]
- 8.Matson MB, Jarosz JM, Gallacher D, Malcolm PN, Holemans JA, Leong C, et al. Evaluation of head examinations produced with a mobile CT unit. Br J Radiol. 1999;72:631–636. doi: 10.1259/bjr.72.859.10624318. [DOI] [PubMed] [Google Scholar]
- 9.Nakao N, Nakai K, Itakura T. Updating of neuronavigation based on images intraoperatively acquired with a mobile computerized tomographic scanner : technical note. Minim Invasive Neurosurg. 2003;46:117–120. doi: 10.1055/s-2003-39344. [DOI] [PubMed] [Google Scholar]
- 10.Rumboldt Z, Huda W, All JW. Review of portable CT with assessment of a dedicated head CT scanner. AJNR Am J Neuroradiol. 2009;30:1630–1636. doi: 10.3174/ajnr.A1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stieve M, Schwab B, Haupt C, Bisdas S, Heermann R, Lenarz T. Intraoperative computed tomography in otorhinolaryngology. Acta Otolaryngol. 2006;126:82–87. doi: 10.1080/00016480510040119. [DOI] [PubMed] [Google Scholar]
- 12.Uhl E, Zausinger S, Morhard D, Heigl T, Scheder B, Rachinger W, et al. Intraoperative computed tomography with integrated navigation system in a multidisciplinary operating suite. Neurosurgery. 2009;64:231–239. doi: 10.1227/01.NEU.0000340785.51492.B5. discussion 239-240. [DOI] [PubMed] [Google Scholar]
- 13.Willems PW, van der Sprenkel JW, Tulleken CA, Viergever MA, Taphoorn MJ. Neuronavigation and surgery of intracerebral tumours. J Neurol. 2006;253:1123–1136. doi: 10.1007/s00415-006-0158-3. [DOI] [PubMed] [Google Scholar]