Abstract
Since the World Health Organization (WHO) classification for central nervous system neoplasms was declared in 2000, chordoid glioma of the third ventricle has been noted as a newly recognized tumor for central nervous system neoplasms. Although there is not enough universal experience to know the nature of this tumor due to its rarity, the origin of chordoid glioma was guardedly proposed to be the ependymal cells of the third ventricle. Such an idea has been primarily based on the specific location of the tumor, that is, third ventricle, suprasellae, and hypothalamus. However, we report a rare case of histologically confirmed chordoid glioma located in the left thalamus, not attached to any of the midline structures having unusual neuroradiological characteristics.
Keywords: Chordoid glioma, Third ventricle, Radiological feature
INTRODUCTION
In 1998, Brat et al.2) introduced a tumor of the third ventricle presenting with both glial and chordoid features, and a new clinicopathological entity was named as "chordoid glioma of the third ventricle". From then on, approximately 50 cases of chordoid glioma, to our best knowledge, have been reported1-5,7,8,11-18,20-23,25,28). Chordoid glioma is a rare low-grade tumor of the brain, most of which are known to be located in the third ventricle, frequently attached to the hypothalamic or suprasellar area.
Though this neoplasm is currently incorporated into the WHO classification of gliomas according to the context mentioned above, the histogenesis of the tumor is still unclear. Some previous reports have suggested that this tumor might arise from ependymal cells of the subcommissural organ6,17,19,25). However, we present a case of a chordoid glioma of the left thalamus without involvement of the structures of third ventricle and suprasellae.
CASE REPORT
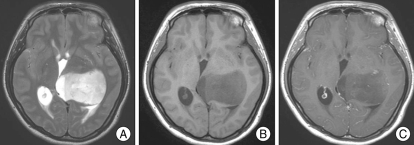
A 27-year-old woman with a two-month history of worsening headache and visual disturbance underwent computed tomography (CT) as recommended by a primary physician. A low-density lesion in left thalamus associated with mild hydrocephalus was found. The patient was then referred to our institution. She had no significant past medical history. Neurological examination revealed a visual field defect on the left temporal side on confrontation test. However, ophthalmologic examination was normal. Magnetic resonance (MR) images demonstrated a well-defined large mass in the left thalamus. The tumor showed high signal intensity on T2-weighted images, and low signal on T1-weighted images (Fig. 1). It was entirely located in the left thalamus, compressing the third ventricle, without any involvement of the third ventricle, hypothalamus and suprasellar region. After gadolinium injection, scattered dot-like subtle enhancement was found (Fig. 1). Since the tumor was presumed to be a diffuse glioma, MR spectroscopy and 18FDG-positron emission tomography (PET) were performed.
Fig. 1.
Magnetic resonance images of the tumor. A : T2-weighted image shows a well-defined high signal mass in the left thalamic pulvinar area without any involvement of the third ventricle. B : T1-weighted image shows low signal intensity of the mass. C : Gadolinium-enhanced T1-weighted image shows scattered dot-like subtle enhancement of the tumor, suggestive of the possibility of intermediate grade glioma.
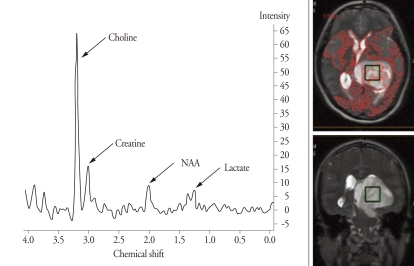
With single-voxel MR spectroscopy with a point-resolved spectroscopy (PRESS) sequence (echo time 288 ms), high choline, low N-acetyl aspartate (NAA) and equivocal lactate were detected (Fig. 2). A choline peak was highest in the central portion of the tumor and so the central portion was thought to be composed of the tumor cells with higher histological grade. Brain 18FDG-PET demonstrated diffuse hypometabolism of the tumor with scattered foci of intermediate metabolism within the tumor in the left posterior thalamus.
Fig. 2.
Magnetic resonance spectroscopy. Single-voxel MR spectroscopy performed in the central portion of the tumor shows high choline, low N-acetyl aspartate (NAA), and equivocal lactate. The box on the tumor indicates the voxel.
The patient underwent a left frontotemporal craniotomy using a transcortical approach via the inferior temporal gyrus. Near total resection of the tumor was performed in spite of the difficult tumor location. Post-operative precontrast CT demonstrated no unusual findings. In addition, her neurological status was not changed after the surgery. Unfortunately, on the third day after the surgery, the patient had respiratory failure of unknown cause. Despite efforts to resuscitate her, she died on the 10th post-operative day.
Pathological findings
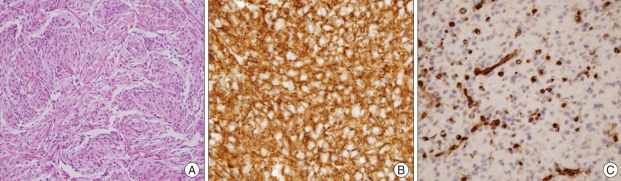
Surgical specimens were fixed in formalin and embedded in paraffin. Hematoxylin-eosin (HE), periodic acid-Schiff (PAS), D-PAS, Alcian blue, and Masson's trichrome stains were performed. In addition, a broad range of immunohistochemical staining was performed. Histologically, the tumor showed clusters and cords of epithelioid cells, abundant myxoid (mucinous) stroma, well developed capillary network, mild nuclear pleomorphism, no mitosis, no vascular endothelial hyperplasia, and no necrosis (Fig. 3A). However, prominent lymphoplasmacytic infiltration with Russell bodies was exceptionally absent in this case.
Fig. 3.
Histological findings. A : Hematoxylin and eosin staining revealed clusters and cords of epithelioid cells, abundant myxoid and mucinous stroma, well developed capillary network, mild nuclear pleomorphism, no mitosis, no vascular endothelial hyperplasia, and no necrosis (H & E,×100). B and C : Photographs of immunohistochemical stainings demonstrating diffuse cytoplasmic expression for glial fibrillary acidic protien (B) and vimentin (C) (×200).
Immunohistochemically, the tumor cells showed diffuse expression of glial fibrillary acidic protein (GFAP), vimentin, S-100 protein, neuron-specific enolase (NSE), CD56, epithelial membrane antigen (EMA), and cytokeratin, also with platelet-derived growth factor receptor-alpha (PDGFR-alpha), and glucose transporter-1 (Glut-1) (Fig. 3B, C). But, expressions of neurofilament and synaptophysin were absent. Based on the pathological findings above, the tumor was diagnosed as chordoid glioma3,5,10,11,17,26,27).
DISCUSSION
Chordoid glioma of the third ventricle was incorporated into WHO classification in 2000. The name itself included the stereotypical location of the tumor. It seemed suitable to name it "chordoid glioma of the third ventricle" because, according to the literature at that time, all of the chordoid gliomas were located in the third ventricle, hypothalamus and suprasellae2,19,24,25). However, to our best knowledge, approximately 50 cases have been reported so far, which inevitably limits the scope of understanding this rare disease.
Radiological findings from neuroimaging studies of this tumor were also consistent and typical, in that they characteristically exhibit a well-circumscribed border, uniform contrast enhancement, and a fusiform gross morphology2,19,27,28). On the histological side, it was cautiously suggested that the tumor showed features of ependymal differentiation6,19,23,25). Taken altogether, it was even proposed that the tumor be renamed "chordoid ependymoma of the lamina terminalis"6,19,23,26). It was inferred likewise, not only due to the ultrastructural findings, but also due to its unique midline location.
However, there are some differences between our case and typical chordoid glioma of the third ventricle. This case is not associated with any of the midline anatomical landmarks that were mentioned above : third ventricle, hypothalamus, and suprasellae. It is also different from others in that the mass was not uniformly enhanced, rather only focally enhanced from a radiological standpoint. Due to the rarity of this tumor, it can be risky to form a hasty conclusion on the characteristics and on the origin of this tumor. Our case only suggests a good possibility that this tumor entity is composed of heterogeneous subgroups. Archiving more cases of the kind, and correctly identifying their location, histology, neuroimaging findings, and clinical course, are warranted to define this relatively new neoplasm entity.
previously described, despite the successful surgery and favourable recovery process, the patient had respiratory failure of unknown cause on the third day after the surgery. Such a clinical course is in line with previous reports. Vanhauwaert et al.28) reported overall immediately postoperative mortality rate was about 30% through the literature reviews. They were considered to have had a thromboembolism or to suffer from hypothalamic injury. Poor prognosis might be due to the tumor location in the deep structure of the brain, making it difficult to approach surgically. The possible cause of death and unfortunate poor prognosis of our case is consistent with previously reported cases9,11,17,25,28).
CONCLUSION
We have presented a case of a chordoid glioma that has unusual location and neuroradiological characteristics.
References
- 1.Baehring JM, Bannykh S. Chordoid glioma of the third ventricle. J Neurooncol. 2006;76:269. doi: 10.1007/s11060-006-6054-y. [DOI] [PubMed] [Google Scholar]
- 2.Brat DJ, Scheithauer BW, Staugaitis SM, Cortez SC, Brecher K, Burger PC. Third ventricular chordoid glioma : a distinct clinicopathologic entity. J Neuropathol Exp Neurol. 1998;57:283–290. doi: 10.1097/00005072-199803000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Buccoliero AM, Caldarella A, Gallina P, Di Lorenzo N, Taddei A, Taddei GL. Chordoid glioma : clinicopathologic profile and differential diagnosis of an uncommon tumor. Arch Pathol Lab Med. 2004;128:e141–e145. doi: 10.5858/2004-128-e141-CGCPAD. [DOI] [PubMed] [Google Scholar]
- 4.Carrasco R, Pascual JM, Reina T, Nieto S, Linera J, Sola RG. Chordoid glioma of the third ventricle attached to the optic chiasm. Successful removal through a trans-lamina terminalis approach. Clin Neurol Neurosurg. 2008;110:828–833. doi: 10.1016/j.clineuro.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 5.Castellano-Sanchez AA, Recine MA, Restrepo R, Howard LH, Robinson MJ. Chordoid glioma : a novel tumor of the third ventricle. Ann Diagn Pathol. 2000;4:373–378. doi: 10.1053/adpa.2000.19369. [DOI] [PubMed] [Google Scholar]
- 6.Cenacchi G, Roncaroli F, Cerasoli S, Ficarra G, Merli GA, Giangaspero F. Chordoid glioma of the third ventricle : an ultrastructural study of three cases with a histogenetic hypothesis. Am J Surg Pathol. 2001;25:401–405. doi: 10.1097/00000478-200103000-00016. [DOI] [PubMed] [Google Scholar]
- 7.Chung SB, Park SH, Kim JE. Chordoid glioma of the third ventricle with unusual MRI features. J Korean Neurosurg Soc. 2007;42:224–227. [Google Scholar]
- 8.Gallina P, Pansini G, Mouchaty H, Mura R, Buccoliero AM, Di Lorenzo N. An incidentally detected third ventricle chordoid glioma. Neurol India. 2007;55:406–407. doi: 10.4103/0028-3886.33301. [DOI] [PubMed] [Google Scholar]
- 9.Galloway M, Afshar F, Geddes JF. Chordoid glioma : an uncommon tumour of the third ventricle. Br J Neurosurg. 2001;15:147–150. doi: 10.1080/02688690120036865. [DOI] [PubMed] [Google Scholar]
- 10.Grand S, Pasquier B, Gay E, Kremer S, Remy C, Le Bas JF. Chordoid glioma of the third ventricle : CT and MRI, including perfusion data. Neuroradiology. 2002;44:842–846. doi: 10.1007/s00234-002-0820-0. [DOI] [PubMed] [Google Scholar]
- 11.Hanbali F, Fuller GN, Leeds NE, Sawaya R. Choroid plexus cyst and chordoid glioma. Report of two cases. Neurosurg Focus. 2001;10:E5. doi: 10.3171/foc.2001.10.6.6. [DOI] [PubMed] [Google Scholar]
- 12.Horbinski C, Dacic S, McLendon RE, Cieply K, Datto M, Brat DJ, et al. Chordoid glioma : a case report and molecular characterization of five cases. Brain Pathol. 2009;19:439–448. doi: 10.1111/j.1750-3639.2008.00196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iwami K, Arima T, Oooka F, Fukumoto M, Takagi T, Takayasu M. Chordoid glioma with calcification and neurofilament expression : case report and review of the literature. Surg Neurol. 2009;71:115–120. doi: 10.1016/j.surneu.2007.07.032. discussion 120. [DOI] [PubMed] [Google Scholar]
- 14.Jain D, Sharma MC, Sarkar C, Suri V, Rishi A, Garg A, et al. Chordoid glioma : report of two rare examples with unusual features. Acta Neurochir (Wien) 2008;150:295–300. doi: 10.1007/s00701-008-1420-x. discussion 300. [DOI] [PubMed] [Google Scholar]
- 15.Kawasaki K, Kohno M, Inenaga C, Sato A, Hondo H, Miwa A, et al. Chordoid glioma of the third ventricle : a report of two cases, one with ultrastructural findings. Neuropathology. 2009;29:85–90. doi: 10.1111/j.1440-1789.2008.00925.x. [DOI] [PubMed] [Google Scholar]
- 16.Koh EJ, Choi HY. Chordoid glioma in the third ventricle : case report. J Korean Neurosurg Soc. 2003;33:501–504. [Google Scholar]
- 17.Kurian KM, Summers DM, Statham PF, Smith C, Bell JE, Ironside JW. Third ventricular chordoid glioma : clinicopathological study of two cases with evidence for a poor clinical outcome despite low grade histological features. Neuropathol Appl Neurobiol. 2005;31:354–361. doi: 10.1111/j.1365-2990.2005.00551.x. [DOI] [PubMed] [Google Scholar]
- 18.Lee HW, Lee SB, Kim JH, Suh YL. Suprasellar chordoid glioma combined with Rathke's cleft cyst : case report. J Korean Neurosurg Soc. 2002;32:376–379. [Google Scholar]
- 19.Leeds NE, Lang FF, Ribalta T, Sawaya R, Fuller GN. Origin of chordoid glioma of the third ventricle. Arch Pathol Lab Med. 2006;130:460–464. doi: 10.5858/2006-130-460-OOCGOT. [DOI] [PubMed] [Google Scholar]
- 20.Nakajima M, Nakasu S, Hatsuda N, Takeichi Y, Watanabe K, Matsuda M. Third ventricular chordoid glioma : case report and review of the literature. Surg Neurol. 2003;59:424–428. doi: 10.1016/s0090-3019(03)00066-1. [DOI] [PubMed] [Google Scholar]
- 21.Nga ME, Tan KB, Laporte JP, Takano A. Test and teach A. recurrent third ventricular brain tumour. Diagnosis : Chordoid glioma of the third ventricle. Pathology. 2006;38:254–257. doi: 10.1080/00313020600699151. [DOI] [PubMed] [Google Scholar]
- 22.Park SH, Hwang JH. Chordoid glioma : an uncommon tumor of the third ventricle. J Korean Neurosurg Soc. 2006;40:40–43. [Google Scholar]
- 23.Pasquier B, Péoc'h M, Morrison AL, Gay E, Pasquier D, Grand S, et al. Chordoid glioma of the third ventricle : a report of two new cases, with further evidence supporting an ependymal differentiation, and review of the literature. Am J Surg Pathol. 2002;26:1330–1342. doi: 10.1097/00000478-200210000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Pomper MG, Passe TJ, Burger PC, Scheithauer BW, Brat DJ. Chordoid glioma : a neoplasm unique to the hypothalamus and anterior third ventricle. AJNR Am J Neuroradiol. 2001;22:464–469. [PMC free article] [PubMed] [Google Scholar]
- 25.Raizer JJ, Shetty T, Gutin PH, Obbens EA, Holodny AI, Antonescu CR, et al. Chordoid glioma : report of a case with unusual histologic features, ultrastructural study and review of the literature. J Neurooncol. 2003;63:39–47. doi: 10.1023/a:1023752717042. [DOI] [PubMed] [Google Scholar]
- 26.Ricoy JR, Lobato RD, Báez B, Cabello A, Martínez MA, Rodríguez G. Suprasellar chordoid glioma. Acta Neuropathol. 2000;99:699–703. doi: 10.1007/s004010051183. [DOI] [PubMed] [Google Scholar]
- 27.Vajtai I, Varga Z, Scheithauer BW, Bodosi M. Chordoid glioma of the third ventricle : confirmatory report of a new entity. Hum Pathol. 1999;30:723–726. doi: 10.1016/s0046-8177(99)90102-8. [DOI] [PubMed] [Google Scholar]
- 28.Vanhauwaert DJ, Clement F, Van Dorpe J, Deruytter MJ. Chordoid glioma of the third ventricle. Acta Neurochir (Wien) 2008;150:1183–1191. doi: 10.1007/s00701-008-0014-6. [DOI] [PubMed] [Google Scholar]