Abstract
Bupropion reduces the subjective effects and cue-induced craving for methamphetamine in humans. Given these effects of bupropion on methamphetamine in humans and its widespread clinical use, a preclinical model of drug-taking was used to determine if pretreatment with bupropion would alter the acquisition of methamphetamine self-administration. During acquisition, rats were given saline or bupropion (30 or 60 mg/kg, IP) 5 min before a 60-min session. For the first 8 days, each response on the active lever produced an infusion of methamphetamine (0.025 mg/kg). Responding on the inactive lever had no programmed consequence. This FR1 schedule was then increased to an FR3 for 4 more days. In a parallel study, the identical procedures were used to test the impact of bupropion on sucrose-maintained responding. Bupropion pretreatment decreased the number of methamphetamine infusions and sucrose deliveries earned on an FR1 and FR3. However, bupropion pretreatment only delayed discrimination between the active and inactive levers in the methamphetamine self-administration rats. Discrimination between active and inactive levers was acquired in all groups in the sucrose experiment regardless of pretreatment condition. Combined, these results suggest that bupropion has a more general effect within the appetitive/reward system of the brain rather than having complete specificity for methamphetamine.
1. Introduction
Methamphetamine is a highly potent, addictive drug that is widely abused in many countries around the world. In the United States, over 10.4 million people have tried methamphetamine, and 4.5 percent of high school seniors report using methamphetamine at least once (2005 National Survey on Drug Use and Health). In general, methamphetamine produces a general state of well-being along with increased wakefulness, talkativeness, and physical activity and decreased appetite (2005 National Survey on Drug Use and Health). Although behavioral treatment programs are useful in the treatment of methamphetamine addiction (Ling et al., 2006; Roll, 2007), many patients continue to relapse after repeatedly seeking treatment (NIDA, 2006). Thus, there is a pressing need for pharmacotherapies to compliment existing cognitive and behavioral treatments for methamphetamine addiction.
Several different drug classes show promise as candidate medications for methamphetamine abuse (see Ling et al., 2006; Vocci and Appel, 2007 for reviews). One drug in particular is bupropion. This drug is already being prescribed for smoking cessation, treatment of depression, obesity, and Attention Deficit Hyperactivity Disorder (see Dwoskin et al., 2007 for a review). Bupropion has been used off-label for methamphetamine dependence, thus prompting the need for systematic investigations on the efficacy of this drug as a candidate medication (Newton et al., 2006). To date, the clinical findings are promising. For example, bupropion treatment (twice daily, 150 mg SR) was well tolerated in patients that received intravenous methamphetamine infusions (0, 15, or 30 mg) and those abstaining from methamphetamine use (Elkashef et al., 2007; Newton et al., 2005). Additionally, bupropion reduced the subjective effects and cue-induced cravings of methamphetamine (Newton et al., 2006), and increased duration of abstinence in participants classified as having “mild-to-moderate” methamphetamine dependence (Elkashef et al., 2007).
Preclinical behavioral and neurochemical studies support these clinical findings. In behavioral studies with rodents, administration of bupropion (12.5, 25, and 50 mg/kg i.p.) dose-dependently antagonized methamphetamine-induced stereotypy in mice when methamphetamine followed bupropion (Muley et al., 1984). When methamphetamine preceded bupropion, methamphetamine-induced stereotypy was augmented (Muley et al., 1984). Perhaps the most compelling preclinical support comes from a d-amphetamine (0.2 mg/kg/infusion) self-administration study in which bupropion (52 and 78 mg/kg) pretreatment decreased amphetamine intake in rats (Rauhut et al., 2003). Further, in neurochemical studies bupropion pretreatment had neuroprotective effects against methamphetamine-induced neurotoxicity (Marek et al., 1990) and protected against the acute reduction of dopamine uptake in striatal synaptosomes (Kim et al., 2000).
In the current study, we investigated the effects of bupropion on the acquisition of methamphetamine self-administration. We chose to study the impact of bupropion on acquisition of self-administration for multiple reasons. First, bupropion is prescribed for treatment of multiple disorders in several clinical populations (see earlier). As such, this drug may already be a prescription medication for some individuals before the onset of methamphetamine use. For instance, with over 4.5 percent of high school seniors reportedly trying methamphetamine, a portion of these may also be taking stimulant medication (e.g., methylphenidate or bupropion) for the treatment of attentional disorders. Although individuals in this cohort may be tracked for correlation purposes and/or longitudinal studies, any direct manipulations to a clinical population during an acquisition period are beyond the scope of human clinical trials. Thus, acquisition studies with rodents are needed to examine the onset of methamphetamine use, develop preventative strategies, and implement translational approaches to the development of drug use. Additionally, we chose to study an acquisition period because monoamine reuptake inhibitors (e.g., bupropion and methylphenidate) have neuroprotective effects against methamphetamine-induced neurotoxicity (Marek et al., 1990; Sandoval et al., 2003). We sought to determine whether this protective property would also be expressed behaviorally.
2. General Methods
2.1. Subjects
Fifty-four male Sprague-Dawley rats (330 ± 21 g) obtained from Harlan (Indianapolis, IN, USA) were housed individually in clear polycarbonate tubs lined with wood shavings in a temperature and humidity controlled room. Water was continuously available in the home cage. Food access was restricted as described later. All sessions were conducted during the light portion of a 12 hour light/dark cycle. Experimental protocols were approved by the University of Nebraska-Lincoln Institutional Animal Care and Use Committee and followed the “Guide for the Care and Use of Laboratory Animals” (National Research Council, 1996).
2.2. Apparatus
Eight standard conditioning chambers (Med-Associates, Georgia, VT, USA) were used. Each chamber was housed in a PVC sound-attenuating cubicle fitted with a fan to provide airflow and masking noise and a house light to provide general illumination. Each chamber (30.5 × 24.1 × 21 cm; l × w × h) had side walls made of aluminum; the ceiling and front and back walls were clear polycarbonate. Located in the bottom center of one aluminum wall was an opening to a recessed dipper receptacle (5.2 × 5.2 × 3.8 cm; l × w × d). The dipper arm, when raised, allowed access to 0.1 ml of 26% sucrose solution (w/v). An infrared emitter/detector unit located 1.2 cm inside the receptacle and 3 cm above the floor recorded head entries. Retractable levers were located on either side of the dipper receptacle. Levers were set such that 15 mg of force was required for the bar press to register. Two white cue lights (2.54 cm dia; 28 V, 100 mA) were centered 7 cm above each lever, 14.6 cm above the metal rod floor and 3.5 cm from the closest polycarbonate wall. Each chamber contained a balanced metal arm with a spring leash attached to a swivel. Tygon® tubing AAQ04103 (VWR, West Chester, PA, USA) extended through the leash and was connected to a 5-ml syringe mounted on an infusion pump (Med Associates, PMH-100VS) located outside of the sound-attenuating cubicle.
2.3. Drugs
D-methamphetamine hydrochloride was purchased from Sigma (St. Louis, MO, USA). Bupropion hydrochloride was purchased from Toronto Research Chemical (Toronto, ON, Canada). Methamphetamine was dissolved in 0.9% sterile saline (w/v). Bupropion was dissolved in sterile water. Bupropion was administered intraperitoneally (i.p.) at a volume of 1 ml/kg. Methamphetamine (0.025 mg/kg/infusion) was administered intravenously (i.v.).
2.4. Catheter Surgery and Recovery
Rats (n=30) were anesthetized with 1 ml/kg ketamine hydrochloride (100 mg/ml, i.p.) followed by 0.6 ml/kg xylazine hydrochloride (20 mg/ml, i.p.) (Midwestern Veterinary Supply, Des Moines, IA, USA). One end of a silastic catheter (CamCaths© IVSA28, Ely, Cambridgeshire, United Kingdom) was implanted into the left external jugular vein. The other end of the catheter went subcutaneous around the shoulder and exited via a backmount just below the scapula. The backmount allowed access to the catheter through a metal cannula. Buprenorphine hydrochloride (0.1 mg/kg; Sigma) was injected subcutaneously (s.c.). immediately following surgery. For the evening of and day following surgery, buprenorphine (0.5 mg/kg) was available in the drinking water to manage post-surgical pain. For the evening after surgery and the following 2 days (AM and PM), the catheter was flushed with 0.1 ml of streptokinase (ca. 8000 Units/ml; Sigma) dissolved in sterile heparinized saline (30 Units/ml; Midwest Veterinary Supply). The catheter was flushed twice a day for the remainder of the experiment with 0.2 ml of the heparinized saline. Rats were allowed 5 days of recovery before the start of the experiment. Catheter patency was assessed with a 0.05 ml i.v. infusion of xylazine (20 mg/ml) at pre-established points in the study. This concentration produces clear motor ataxia within 5 sec if the catheter is patent. Only rats with patent catheters were included in the data analyses.
3. Procedures
3.1. Experiment 1: Methamphetamine
3.1.1. Preliminary Training
All rats were allowed to acclimate to the colony room and then handled for 2 min each on 3 separate days before the start of training. Rats were fed 20 g of chow per day during the initial training period. Following handling, rats were dipper trained. That is, rats were placed in the chambers for three 50-min automated sessions in which the probability of receiving 4-sec access to sucrose in any 4-sec interval decreased from 0.1333 (ca. 2 deliveries per min) to 0.05 (ca. 3 deliveries per 4 min). For the two days following dipper training, rats were autoshaped to lever press. On a given session only 1 of the retractable levers (right or left) was inserted into the chamber for 15 sec using a variable time 60-sec schedule. If a lever press occurred before the 15 sec, the lever was retracted and the dipper was raised allowing 4-sec access to 0.1 ml sucrose. If no lever press occurred after 15 sec had elapsed the lever was retracted and sucrose was delivered for 4 sec. The order of lever presentation was counter-balanced such that the left lever was extended during the first session for half the rats and the right lever for the remaining rats. Session length varied for each rat depending upon whether lever presses occurred during the session. After conclusion of the last autoshaping session, rats were allowed free access to rat chow until the end of the recovery period.
3.1.2. Methamphetamine Self-administration
Following surgery and the recovery period, rats were randomly divided into three groups of 10 rats: saline or bupropion (30 or 60 mg/kg) pretreatment. During this time rats were fed 20 g of rat chow per day. Rats received an i.p. injection of its assigned solution 5 min before placement in the conditioning chamber. Self-administration sessions were 60 min and the house light remained on throughout the session. Each rat was assigned an active and inactive lever; which lever functioned as the active lever was counter-balanced. Pressing the active lever resulted in a 1-sec presentation of the cue light and a simultaneous 1-sec, 35.74 µl infusion of 0.025 mg/kg of methamphetamine (i.e., a fixed ratio 1 or FR1 schedule of reinforcement). Selection of this dose was based on pilot data from our laboratory indicating that this dose of methamphetamine would maintain reliably self-administration on an FR1. Following an active lever press, both levers were retracted for 1 min. Pressing the inactive lever had no programmed consequence.
After the 8th day of self-administration, the schedule of reinforcement was increased to a FR3 for 4 consecutive days. Thus, three presses on the active lever were required for a methamphetamine infusion (0.025 mg/kg). All other details were identical to the previous phase including the pretreatment solution.
3.1.3. Bupropion Pretreatment for Saline Controls
Although the main focus of this study was on the impact of repeated bupropion treatment during acquisition of methamphetamine self-administration, the saline Control group provided us with an opportunity to assess whether bupropion would alter established methamphetamine self-administration. Thus, for the following 4 days the Control group now received a 30 mg/kg bupropion injected 5 min before placement in the chamber. Session length remained 60 min and the FR3 schedule of reinforcement was in force.
3.2. Experiment 2: Sucrose
3.2.1 Preliminary Training
A separate set of naïve rats were used in this experiment. These rats also had five consecutive days of preliminary training consisting of three dipper training sessions and two autoshaping sessions. Following the last autoshaping session, rats were placed on free feed and kept in their home cages for 5 days. Handling, start of acquisition, feeding, etc. was matched with Experiment 1.
3.2.2. Sucrose-maintained Responding
Rats were randomly divided into 3 groups (n=8 per group): saline or bupropion (30 or 60 mg/kg) pretreatment. The handling, pretreatment, and procedural details were identical to those just described for the self-administration study except that active lever presses resulted in 4-sec access to 0.1 ml of sucrose. As in the self-administration study, after 8 days on an FR1 the schedule of reinforcement was increased to an FR3 for 4 days.
3.2.3. Bupropion Pretreatment for Saline Controls
Similar to Experiment 1, rats in the saline Control group now received a 30 mg/kg injection of bupropion 5 min before the start of the 60-min session; the FR3 schedule of reinforcement remained in force for the 4 days of this phase.
3.3. Dependent Measures and Data Analysis
The number of methamphetamine infusions and the number of sucrose deliveries served as the main dependent measure for both experiments. Additionally, presses on the active and inactive levers were analyzed. To conform to the homogeneity of variance assumptions of the statistical tests, a constant of one was added to all values before the data were log10 transformed. For comparison purposes, the mean number of methamphetamine infusions or sucrose deliveries (mean ± SEM) during the FR1 and FR3 are provided in the Results section.
During the FR1 and FR3 training, direct comparisons between groups on active lever presses were conducted with separate two-way [Group × Session] mixed analysis of variance (ANOVAs) for the FR1 and FR3 schedules. Further, the number of infusions or sucrose deliveries was summed across all FR1 sessions to obtain a total that was then analyzed with separate one-way [Group] ANOVAs; a similar analysis was conducted for the FR3 sessions. Group differences in these analyses prompted an ensuing analysis independently for each group. The number of responses on the active and inactive levers were analyzed with separate two-way [Lever × Session] mixed ANOVAs for each pretreatment group. To assess the acute impact of bupropion on the control group, data were analyzed with separate 2 × 2 [Drug (bupropion and saline) × Lever] ANOVAs. Saline scores for this analysis were taken from the day before the acute challenge with bupropion. When the control group received bupropion repeatedly, separate 2-way [Lever × Session] repeated measures ANOVAs were used. Only data from rats that had patent catheters at the end of each experimental phase were used in the statistical analyses; the number of rats in each analysis is detailed in the results section. Tukey HSD tests were used for post-hoc comparisons. Statistical significance was set at p < 0.05 (two-tailed) for all tests.
4. Results
4.1. Experiment 1: Methamphetamine
4.1.1. Methamphetamine Self-administration
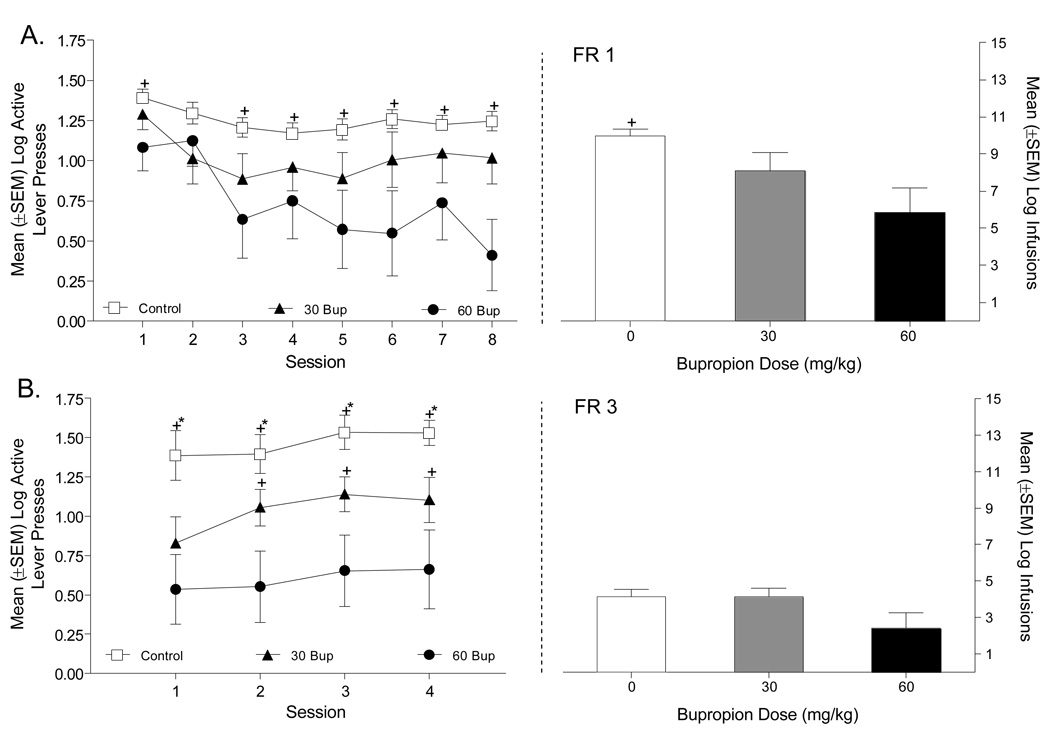
On the FR1 schedule of reinforcement, bupropion pretreatment appeared to decrease active lever responding (Figure 1A, upper left panel). Specifically, on an FR1 there was a significant main effect of Group [F(2,21)=4.34, p=0.026] and Session [F(7,147)=4.39, p=0.001], but not a Group × Session interaction [F(14,147)=1.18, p=0.3]. Rats pretreated with 60 mg/kg bupropion responded less on the active lever than Control rats (Tukey p<0.05). A similar analysis was conducted on the inactive lever and revealed a main effect of Session [F(7,147)=11.67, p=.001]. The main effect of Group [F(2, 21)=2.26, p=0.13] and the Group × Session interaction were not significant (F<1). Consistent with the active lever data, rats treated with 60 mg/kg bupropion took significantly fewer total methamphetamine infusions in comparison to the other 2 groups [Group main effect, F(2,21)=4.34, p=0.026; Tukey p<0.05; Figure 1A, upper right panel]. When data were combined across the 8 days of FR1 training, Control rats took a total of 147 ± 13.49 methamphetamine infusions; the rats treated with 30 or 60 mg/kg bupropion took 122 ± 78.74 and 70.83 ± 61.56 infusions, respectively.
Figure 1.
This figure shows the number of active lever presses (left graphs) and methamphetamine infusions (right graphs) between rats pretreated with saline, 30, or 60 mg/kg bupropion. Panel A depicts responding on an FR1 schedule and Panel B shows responding on an FR3 schedule. +Significant difference from rats pretreated with 60 mg/kg bupropion. *Significant difference from rats pretreated with 30 mg/kg bupropion.
Directly comparing the groups on the FR3 (Figure 1B, lower left panel) schedule of reinforcement for active lever presses revealed a significant main effect of Group [F(2,21)=7.51, p=0.005], but not of Session [F(3, 51)=2.35, p=0.084]; the Group × Session interaction was not significant [F<1]. Overall, rats pretreated with 60 mg/kg bupropion responded less on the active lever than Control and 30 mg/kg bupropion pretreated rats (Tukey p<0.05). For the inactive lever, neither main effect nor the Groups × Session interaction was significant (Fs<2.8, ps>0.1). Differences in the total number of infusions, however, were not statistically significant [F(2,17)=2.59, p=0.11; Figure 1B, lower right panel]. During the 4 days of FR3 training rats pretreated with saline or bupropion (30 or 60 mg/kg) took a total of 49.17 ± 12.15, 56.12 ± 15.68, and 27.33 ± 14.97 methamphetamine infusions, respectively.
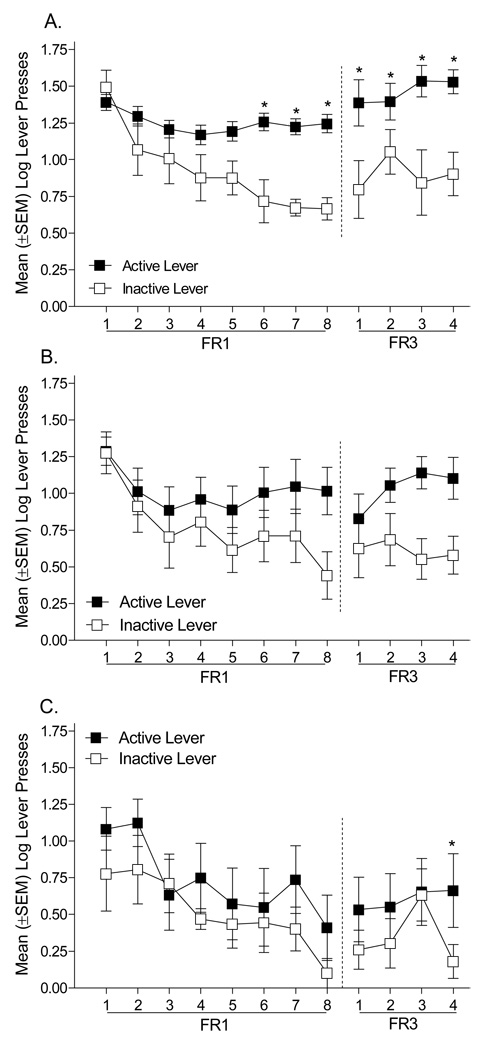
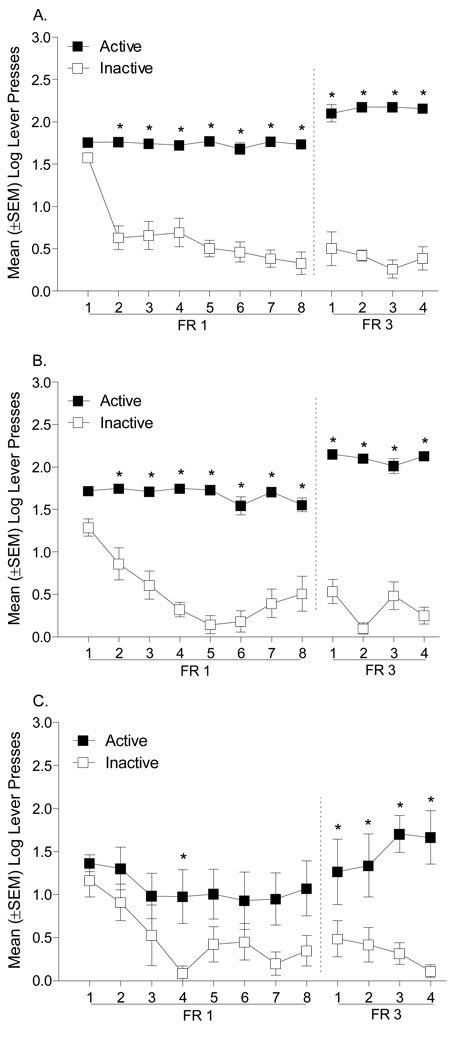
The group differences in active lever responding and number of infusions prompted us to examine each group independently to determine whether bupropion altered the discrimination between the active and inactive levers. As shown in Figure 2, rats pretreated with bupropion (30 or 60 mg/kg) did not learn to discriminate between the active and inactive levers on an FR1 schedule of reinforcement; rats pretreated with saline readily learned the discrimination. For the saline Controls (n=8; see Figure 2A), there was a significant main effect of Lever [F(1,7)=18.20, p=0.004], a main effect of Session [F(7,49)=8.07, p=.001], and a Lever × Session interaction [F(7,49)=4.33, p=0.001]. Specifically, on sessions 6 to 8 responding on the active lever was significantly higher than responding on the inactive lever (Tukey p<0.05). In contrast, for rats pretreated with 30 mg/kg bupropion (n=10; Figure 2B) there was only a main effect of Session [F(7,63)=4.61, p=0.001]; the main effect of Lever [F(1,9)=2.17, p=0.18] and the Lever × Session interaction [F(7,63)=1.86, p=0.092] were not significant. Similarly, rats pretreated with 60 mg/kg bupropion (n=6; Figure 2C) had a significant main effect of Session [F(7,35)=2.96, p=0.015]; the main effect of Lever [F(1,5)=4.98, p=0.08] and the Lever × Session interaction [F<1] were not significant.
Figure 2.
This figure shows the number of presses on the active and inactive levers for rats pretreated with saline (A), 30 mg/kg bupropion (B), and 60 mg/kg bupropion (C). The dashed line represents the change of the response requirement from an FR1 to an FR3 schedule of reinforcement. *Significant difference from the inactive lever.
Control rats maintained the discrimination when the schedule was shifted to an FR3 (see Figure 2). Control rats (n=6) responded more on the active than inactive lever across the 4 days of the FR3 such that there was a significant main effect of Lever [F(1,5)=97.53, p=0.001], but not a main effect of Session [F<1] nor a significant Lever × Session interaction [F(3,15)=1.65, p=0.22]. Rats pretreated with 30 mg/kg bupropion (n=8) also responded more on the active than the inactive lever; however, the main effect of Lever approached, but did not reach, statistical significance [F(1,7)=5.34, p=0.054]. More so, there was not a main effect of Session [F<1], nor a Lever × Session interaction [F(3,21)=2.11, p=0.13]. Rats pretreated with 60 mg/kg bupropion did not have a main effect of Lever [F(1,5)=5.25, p=0.07] or Session [F(3,15)=2.75, p=0.08]; however, there was a significant Lever × Session interaction [F(3,15)=4.42, p=0.02]. On the last day of FR3 training, rats pretreated with 60 mg/kg bupropion (n=6) had more lever presses on the active than the inactive lever (Tukey p<0.05).
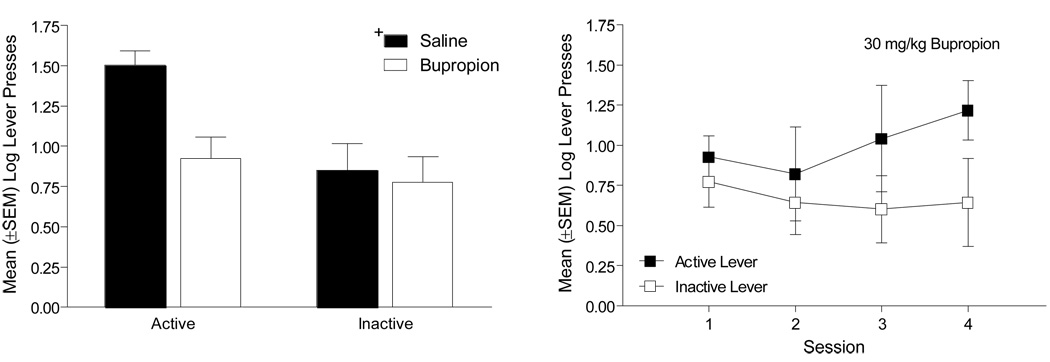
4.1.2. Bupropion Pretreatment for Saline Controls
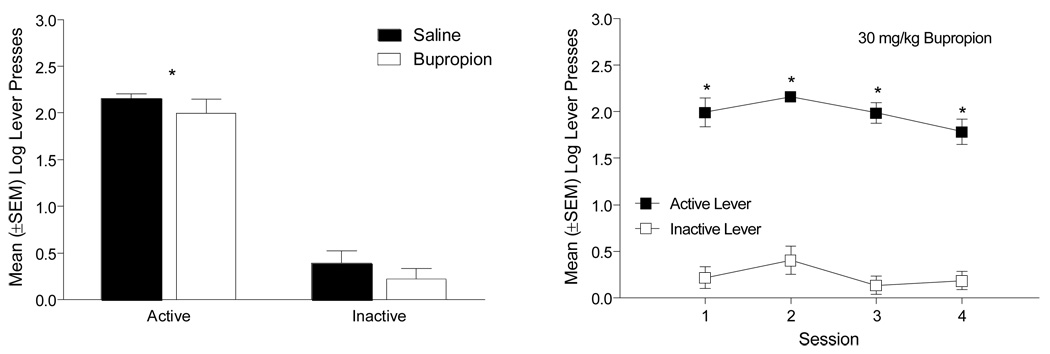
When Control rats (n=6) (i.e., those pretreated with saline in the previous phase) were given an acute injection of 30 mg/kg bupropion, lever pressing decreased (Figure 3, left graph). Specifically, there was a main effect of Drug pretreatment [F(1,5)=9.18, p=0.029], but not of Lever [F(1,5)=6.40, p=0.053]; the Drug × Lever interaction was not significant [F(1,5)=3.45, p=0.12]. Additionally, the number of infusions after pretreatment with saline (11.17 ± 2.33) was significantly decreased when rats were pretreated with 30 mg/kg bupropion (2.83 ± 1.05) [paired t(5)=3.37, p=0.02]. When bupropion pretreatment continued, active lever responding was higher than inactive lever responding across the four sessions (Figure 3, right graph). There was a main effect of Lever [F(1,5)=14.83, p=0.012], but not a main effect of Session or a Lever × Session interaction [Fs<1]. Combined, these analyses suggest that 30 mg/kg bupropion diminished active lever pressing acutely and with repeated exposure, but that the discrimination was maintained between the active and inactive levers across the 4 days.
Figure 3.
The left graph shows the number of active and inactive lever presses for control rats when given an acute injection of 30 mg/kg bupropion. The right graph depicts the active and inactive lever presses when bupropion pretreatment continued for these rats. +Significant main effect of Drug pretreatment.
4.2. Experiment 2: Sucrose
4.2.1. Sucrose-maintained Responding
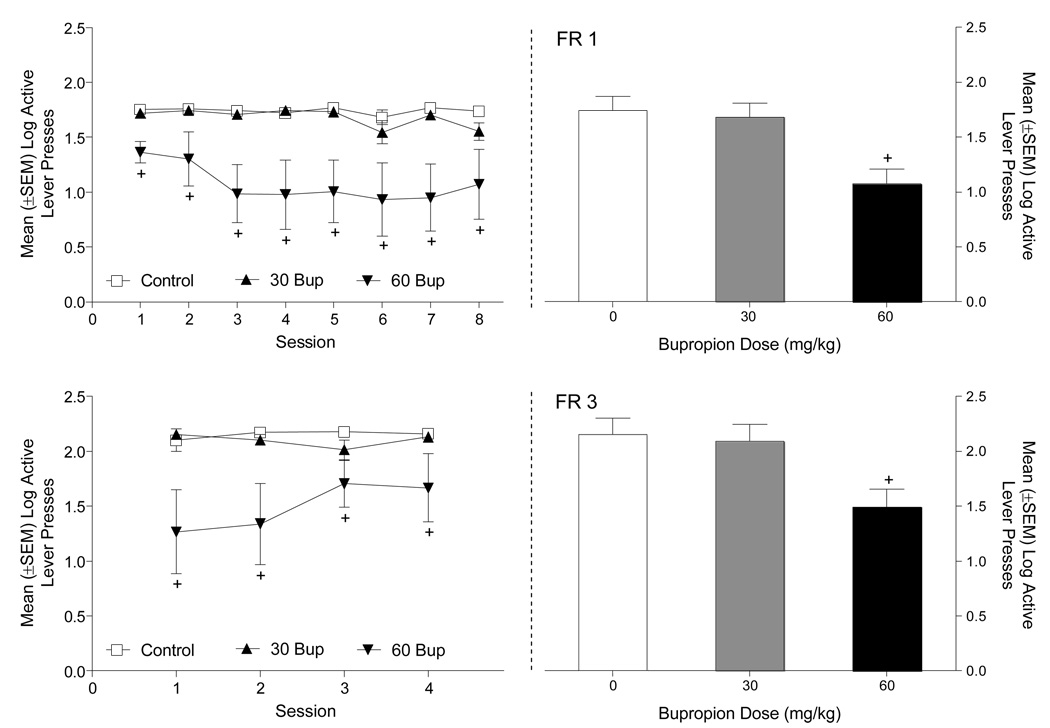
When groups were directly compared, bupropion reduced responding on the active lever (Figure 4). On an FR1 schedule of reinforcement (Figure 4A, upper left graph), there was a significant main effect of Group [F(2,20)=7.95, p=0.003] and Session [F(7,140)=2.37, p=0.025], but not a Group × Session interaction [F(14,140)=1.24, p=0.25]. Rats pretreated with 60 mg/kg bupropion responded significantly less on the active lever than Control and 30 mg/kg bupropion pretreated rats (Tukey p<0.05). A similar analysis was conducted on the inactive lever and revealed a main effect of Session [F(7,140)=20.68, p=.001]. The main effect of Group (F<1) and the Group × Session interaction were not significant [F(14,140)=1.61, p=0.08]. Consistent with active lever data, rats pretreated with 60 mg/kg bupropion received significantly fewer sucrose deliveries in comparison to the other two groups [Group main effect, F(2,20)=7.95, p=0.003; Tukey p<0.05; Figure 4A, upper right graph]. During the 8 days of the FR1 training, Control rats received a total of 448.88 ± 10.75 sucrose deliveries; the rats treated with 30 or 60 mg/kg bupropion had 401.62 ± 16.42 and 212.57 ± 58.44 deliveries, respectively.
Figure 4.
This figure shows the number of active lever presses (left graphs) and sucrose deliveries (right graphs) between rats pretreated with saline, 30, or 60 mg/kg bupropion. Panel A depicts responding on an FR1 schedule and Panel B shows responding on an FR3 schedule. +Significant difference from rats pretreated with saline and 30 mg/kg bupropion.
Directly comparing the groups on an FR3 (Figure 4B, lower left graph) schedule of reinforcement there was a significant main effect of Group [F(2,20)=5.23, p=0.015). The main effect of Session [F(3,60)=1.55, p=0.21] and the Group × Session interaction [F(6,60)=2.03, p=0.075] were not significant. Overall, rats pretreated with 60 mg/kg bupropion responded less on the active lever than Control and 30 mg/kg bupropion pretreated rats (Tukey p<0.05). For the inactive lever, neither main effect nor the Groups × Session interaction was significant (Fs<2.7, ps>0.06). Further, the 60 mg/kg bupropion group received fewer sucrose deliveries than the other groups [F(2,20)=4.07, p=0.033; Tukey p<0.05; Figure 4B, lower right graph]. During the 4 days of training on an FR3 rats pretreated with saline or bupropion (30 or 60 mg/kg) received a total of 202.13 ± 17.74, 179.88 ± 15.54, and 110.86 ± 34.68 sucrose deliveries, respectively.
Rats pretreated with saline or bupropion (30 or 60 mg/kg) learned to discriminate between the active and inactive levers when trained on an FR1 schedule of reinforcement. For the Control group (Figure 5A), there was a significant main effect of Lever [F(1,7)=137.3, p=0.001], a main effect of Session [F(7,49)=19.3, p=0.001], and a Lever × Session interaction [F(7,49)=14.6, p=0.001]. Specifically, on sessions 2 to 8 responding on the active lever was significantly higher than responding on the inactive lever (Tukey p<0.05). Similarly, for rats pretreated with 30 mg/kg bupropion (Figure 5B) there was a main effect of Session [F(1,7)=196.8, p=0.001], Lever [F(7,49)=7.78, p=0.001], and a Lever × Session interaction [F(7,49)=7.86, p=0.001]. On sessions 2 to 8 responding on the active lever was greater than on the inactive lever (Tukey p<0.05). Rats pretreated with 60 mg/kg bupropion (Figure 5C) had a main effect of Lever [F(1,6)=7.26, p=0.036] and Session [F(7,42)=5.13, p=0.001]; the Lever × Session interaction was not significant [F<1].
Figure 5.
This figure shows the number of presses on the active and inactive levers for rats pretreated with saline (A), 30 mg/kg bupropion (B), and 60 mg/kg bupropion (C). The dashed line represents the change of the response requirement from an FR1 to an FR3 schedule of reinforcement. *Significant difference from the inactive lever.
Regardless of pretreatment condition, the discrimination was maintained when the schedule was shifted to an FR3 (see Figure 5). Control rats responded more on the active lever than the inactive lever across the 4 days such that there was a significant main effect of Lever [F(1,7)=453.83, p=0.001]; there was not a main effect of Session [F<1] or a Lever × Session interaction [F(3,15)=1.29, p=0.30]. Rats pretreated with 30 mg/kg bupropion also responded more on the active than the inactive lever. Specifically, there was a main effect of Lever [F(1,7)=868.9, p=0.001], and a significant Lever × Session interaction [F(3,21)=5.12, p=0.008]. The main effect of Session [F(3,21)=2.66, p=0.08] was not significant. Responding on the active lever was higher on sessions 1 to 4 (Tukey p<0.05). Consistently, rats pretreated with 60 mg/kg bupropion had a main effect of Lever [F(1,7)=25.72, p=0.002], but not of Session [F<1]. The Lever × Session interaction [F(3,15)=4.42, p=0.02] was significant; responding on the active lever was higher on sessions 1 to 4 (Tukey p<0.05).
4.2.2. Bupropion Pretreatment for Saline Controls
Sucrose-maintained responding did not change when rats in the Control group were given an acute injection of 30 mg/kg bupropion (Figure 6, left graph). Specifically, there was a main effect of Lever [F(1,7)=187.42, p=0.001]. The was no main effect of Drug [F(1,7)=4.74, p=0.066] and no Drug × Lever interaction [F<1]. Thus, an acute challenge with 30 mg/kg bupropion did not impact well-established sucrose-maintained responding. Additionally, the number of sucrose deliveries on saline (50.13 ± 4.34) did not differ from bupropion (43.25 ± 7.22) [paired t(7)=2.08, p=0.076]. When bupropion pretreatment was continued, the discrimination between the active and inactive lever was maintained (Figure 6, right graph). There was a main effect of Lever [F(1,7)=240.60, p=0.001]. But, there was no a main effect of Session [F(3,21)=2.54, p=0.08] or a Lever × Session interaction [F<1].
Figure 6.
The left graph shows the number of active and inactive lever presses for Control rats when given an acute injection of 30 mg/kg bupropion. The right graph depicts the active and inactive lever presses when bupropion pretreatment continued for these rats. *Significant difference from the inactive lever.
4. Discussion
Bupropion has been shown to be a promising option in human participants to treat methamphetamine dependence (Elkashef et al., 2007; Newton et al., 2005, 2006). This pre-clinical study with rats supports their conclusions that bupropion may be a candidate medication for methamphetamine use disorders. Further, this study found some interesting similarities and differences between bupropion’s impact on the acquisition of methamphetamine self-administration and sucrose-maintained responding. For example, 60 mg/kg bupropion reduced overall intake of methamphetamine infusions and sucrose deliveries. Yet, this dose of bupropion (as well as 30 mg/kg) prevented learning of the discrimination between the active and inactive lever only in the methamphetamine study. Additionally, methamphetamine infusions were reduced when previously saline-treated controls were challenged with 30 mg/kg bupropion in the self-administration study; sucrose-maintained responding remained the same.
Several pieces of evidence suggest to us that differences in responding between the two reinforcers were not due to the non-specific effects of bupropion. First, such an account might predict that inactive lever pressing would be differentially altered by bupropion dose. This did not occur in either experiment. More so, our chambers are equipped with an infrared emitter/detector unit that bisects the chamber into two halves. The number of times this infrared beam is interrupted serves as a measure of activity in the chamber [see Reichel et al., (2007) for more detail]. Comparisons of these activity counts reveal no differences between experiments. Specifically, the self-administration and sucrose rats pretreated with 30 mg/kg bupropion had similar daily mean activity counts (self-administration = 703.91 ± 56.51 and sucrose = 670.89 ± 31.13; t<1). Likewise, rats pretreated with 60 mg/kg bupropion had similar activity counts (self-administration = 776.30 ± 107.40 and sucrose = 721.96 ± 137.13; t<1). This finding, combined with the lack of differences on inactive lever presses, diminishes our enthusiasm for the notion that non-specific motor effects of bupropion are responsible for the effects observed in the present study. Nonetheless, sucrose engendered a higher rate of responding than methamphetamine in the present research. Thus, the differential impact of bupropion on responding maintained by methamphetamine versus sucrose may be partially influenced by differences in response rate. There is no way to avoid this difference with an FR5 schedule of reinforcement unless one varies some other behavioral parameter along with the reinforcer such as forcing a different pattern of responding and access to the reinforcer by imposing longer timeouts in the sucrose experiment (cf. Paterson et al., 2003). We decided against varying more than the reinforcer.
The finding that 60 mg/kg bupropion reduced sucrose deliveries and methamphetamine infusions suggests that bupropion acts on a generalized reward system (Panksepp et al., 2004) rather than having complete specificity for methamphetamine. This interpretation is not surprising given that bupropion increases dopamine neurotransmission in brain areas (e.g., nucleus accumbens) typically associated with reward processes (Ascher et al., 1995; Nomikos, et a., 1992). Notably, bupropion is prescribed for the treatment of obesity in humans (Gadde et al., 2001) and reduces food intake in laboratory animals (Zarrindast and Hosseini-Nia, 1988). Thus, repeatedly administering 60 mg/kg bupropion may have had an anorexic effect for rats in the sucrose experiment. Consistent with this possibility, rats in the high dose group inserted their heads into the dipper receptacle during sucrose deliveries 64% of the time, whereas rats pretreated with saline or 30 mg/kg bupropion retrieved sucrose 99% of the time. The question remains as to whether the bupropion-induced decrease in sucrose deliveries and retrieval are independent of bupropion’s effects on the reward system. Parsing apart this distinction may prove challenging because an anorexic account is clearly not mutually exclusive from the “reward system” account.
Although there was a similar effect of bupropion on reducing the total number of delivered reinforcers, there was also an interesting dissociation in performance when rats were administered 30 and 60 mg/kg bupropion during an FR1 and FR3 schedule. Rats in the sucrose experiment pretreated with saline, 30, or 60 mg/kg bupropion all acquired the discrimination between the active and inactive levers. In contrast, only saline pretreated rats learned to discriminate between the levers in the self-administration experiment. One reason for such contrasting findings between experiments may be that the stimulant properties of bupropion interfered with the salience of the methamphetamine infusion. Such interference would not be evident in the sucrose experiment because the sucrose reinforcement is delivered externally; methamphetamine reinforcement, in contrast, is internal. That is, bupropion and methamphetamine share stimulus properties through similar pharmacological mechanisms (Munzar and Goldberg, 2000; Reichel et al., 2007; Suzuki et al., 2004). Namely, these similar mechanisms are increased dopaminergic neurotransmission (Ascher et al., 1995; Barr et al., 2006; Nomikos et al., 1992). Thus, bupropion in the central nervous system before lever access may “blur” or “overshadow” detection of a methamphetamine infusion. Indeed, an ordinal relation was revealed, as the bupropion dose increased methamphetamine infusions decreased. Accordingly, bupropion pretreatment prevented learning the contingency between active lever responding and methamphetamine because rats were experiencing the interoceptive effects of bupropion before methamphetamine, with the impact of bupropion being of higher magnitude in the 60 mg/kg group. Data also suggests acquisition of methamphetamine self-administration is transiently delayed and not completely blocked. As such, rats pretreated with 30 mg/kg bupropion began to discriminate between levers toward the end of training, and, albeit speculative, continued training may have yielded a statistical difference (see Figure 2B). A similar point could be made for rats pretreated with 60 mg/kg bupropion; they had more active than inactive lever presses on the final day (see Figure 2C). Even so, the current data still demonstrates delayed acquisition of lever discrimination in the bupropion treatment groups given that rats pretreated with saline had the discrimination from day 5.
A marked dissociation between methamphetamine and sucrose also occurred when Control rats were given 30 mg/kg bupropion for the first time. In the self-administration experiment, an acute challenge of bupropion decreased both active lever presses and the number of infusions earned. In the sucrose experiment, bupropion had no effect. A similar dissociation was previously reported by Bruijnzeel and Markou (2003). They demonstrated that an acute injection of bupropion (40 mg/kg i.p.) reduced the number of nicotine infusions (0.03 mg/kg) self-administered by rats; this same bupropion dose, however, did not decrease the number of sucrose deliveries on a schedule of reinforcement designed to promote comparable levels of responding as nicotine. Conversely, Rauhut et al. (2003) reported that acute bupropion (26, 52, and 78 mg/kg i.p.) injections decreased the number of sucrose pellets earned on an FR5 schedule of reinforcement. When taken together, these studies suggest the extent to which bupropion reduces sucrose-maintained responding may be partially dependent on drug dose and schedule of reinforcement.
The interplay between the pharmacological actions of methamphetamine and bupropion at pre- and post-synaptic dopaminergic terminals may also have an important role in the observed acute blockade of methamphetamine intake. On the pre-synaptic membrane, bupropion binds to dopamine transporters preventing the removal of dopamine from the synaptic cleft (Ascher et al., 1995; Nomikos et al., 1992). When administered before methamphetamine, this action inhibits methamphetamine-induced reverse transport of the reuptake pump (Sulzer et al., 2005), which attenuates increases in synaptic dopamine levels associated with methamphetamine. Blockade of transport mechanisms also increases endogenous neurotransmitter levels that can potentially enhance dopaminergic neurotransmission at the post-synaptic membrane (Ascher et al., 1995; Nomikos et al., 1992). That is, pharmacological substitution may result from activation of post-synaptic dopamine receptors. Indeed, in drug discrimination studies, bupropion substitutes for the interoceptive stimulus effects of methamphetamine indicating shared pharmacological mechanisms (Munzar and Goldberg, 2000; Reichel et al., 2007; Suzuki et al., 2004).
The use of bupropion to treat methamphetamine addiction may be categorized as a treatment approach termed “agonist substitution” therapy (Grabowski et al., 2004; Rothman et al., 2002). This pharmacotherapy approach stabilizes deregulated neurotransmitter systems by using a purportedly less potent and less addictive agonist to activate similar receptor sites. Although agonist substitution treatment has had some success with cocaine addicts, the greatest success has been with methadone maintenance programs for heroin abusers or nicotine replacement therapies for tobacco dependence (see Grabowski et al., 2004 for a review). The success of these treatments prompts the exploration of substitution drugs for the treatment of psychostimulant, and more specifically, methamphetamine addiction. With this in mind, the findings from the present study combined with earlier research with methamphetamine addicts (Elkashef et al., 2007; Newton et al., 2005; 2006) indicate that bupropion’s use as a candidate medication for methamphetamine use disorders are worth pursuing.
Acknowledgments
We thank Scott Sanderson for help in conducting the experiments. R. A. Bevins was in part supported by United States Public Health Service grant DA018114 while developing this research and writing this report. C. M. Reichel was supported by DA023283 while preparing this manuscript for publication. This research was partially funded by Promunne Inc.
References
- Ascher JA, Cole JO, Colin JN, Feighner JP, Ferris RM, Fibiger HC, et al. Bupropion: A review of its mechanism of antidepressant activity. J of Clin Psychiatry. 1995;56:395–401. [PubMed] [Google Scholar]
- Barr AM, Panenka WJ, MacEwan W, Thornton AE, Lang DJ, Honer WG, et al. The need for speed: An update on methamphetamine addiction. J Psychiatry Neurosci. 2006;31:301–313. [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW, Markou A. Characterization of the effects of bupropion on the reinforcing properties of nicotine and food in rats. Synapse. 2003;50:20–28. doi: 10.1002/syn.10242. [DOI] [PubMed] [Google Scholar]
- Dwoskin LP, Rauhut AS, King-Pospisil KA, Bardo MT. Review of the pharmacology and clinical profile of bupropion, an antidepressant and tobacco use cessation agent. CNS Drug Rev. 2007;12:178–207. doi: 10.1111/j.1527-3458.2006.00178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkashef AM, Rawson RA, Anderson AL, Li S, Holmes T, Smith EV, et al. Bupropion for the Treatment of Methamphetamine Dependence. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301481. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Gadde KM, Parker CB, Maner LG, Wagner HR, II, Loque EJ, Drezner MK, Krishnan KR. Bupropion for weight loss: an investigation of efficacy and tolerability in overweight and obese women. Obes Res. 2001;9:554–551. doi: 10.1038/oby.2001.71. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Shearer J, Merrill J, Negus S. Agonist-like, replacement pharmacotherapy for stimulant abuse and dependence. Addict Behav. 2004;29:1439–1464. doi: 10.1016/j.addbeh.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Kim S, Westphalen R, Callahan B, Hatzidimitriou G, Yuan J, Ricaurte GA. Toward development of an in vitro model of methamphetamine-induced dopamine nerve terminal toxicity. J Pharmacol Exp Ther. 2000;293:625–633. [PubMed] [Google Scholar]
- Ling W, Rawson R, Shoptaw S. Management of methamphetamine abuse and dependence. Curr Psychiatry Rep. 2006;8:345–354. doi: 10.1007/s11920-006-0035-x. [DOI] [PubMed] [Google Scholar]
- Muley MP, Joshi MA, Manekar MS. Effect of bupropion on dopamine and 5-hydroxytryptamine-mediated behavior in mice. J Pharm Pharmacol. 1984;36:208–210. doi: 10.1111/j.2042-7158.1984.tb06944.x. [DOI] [PubMed] [Google Scholar]
- Marek GJ, Vosmer G, Seiden LS. Dopamine uptake inhibitors block long-term neurotoxic effects of methamphetamine upon dopaminergic neurons. Brain Res. 1990;513:274–279. doi: 10.1016/0006-8993(90)90467-p. [DOI] [PubMed] [Google Scholar]
- Munzar P, Goldberg S. Dopaminergic involvement in the discriminative-stimulus effects of methamphetamine in rats. Psychopharmacol. 2000;148:209–217. doi: 10.1007/s002130050044. [DOI] [PubMed] [Google Scholar]
- NIDA Research Report Series, Methamphetamine Abuse and Addiction. NIH Publication number 06-4210. 2006. [Google Scholar]
- National Research Council. Guide for the care and use of laboratory animals. Washington, DC: National Academy Press; 1996. [Google Scholar]
- Newton TF, Roach JD, De La Garza R, II, Fong T, Wallace CL, Li S, et al. Safety of intravenous methamphetamine administration during treatment with bupropion. Psychopharmacology. 2005;182:426–435. doi: 10.1007/s00213-005-0102-8. [DOI] [PubMed] [Google Scholar]
- Newton TF, Roach JD, De La Garza R, II, Fong T, Wallace CL, Li S, et al. Bupropion reduces methamphetamine-induced subjective effects and cue-induced craving. Neuropsychopharmacology. 2006:1537–1544. doi: 10.1038/sj.npp.1300979. [DOI] [PubMed] [Google Scholar]
- Nomikos GG, Damsma G, Wenkstern D, Fibiger HC. Effects of chronic bupropion on interstitial concentrations of dopamine in rat nucleus accumbens and striatum. Neuropsychopharmacology. 1992;7:7–14. [PubMed] [Google Scholar]
- Panksepp J, Nocjar C, Burgdorf J, Panksepp JB, Huber R. The role of emotional systems in addiction: A neuroethological perspective. In: Bevins RA, Bardo MT, editors. Nebraska Symposium on Motivation: Vol. 50. Motivational factors in the etiology of drug abuse. Lincoln, NE: University of Nebraska Press; 2004. pp. 85–126. [PubMed] [Google Scholar]
- Paterson NE, Semenova S, Gasparini F, Markou A. The mGluR5 antagonist MPEP decreases nicotine self-administration in rats and mice. Psychopharmacology. 2003;167:257–264. doi: 10.1007/s00213-003-1432-z. [DOI] [PubMed] [Google Scholar]
- Rauhut AS, Neugebauer N, Dwoskin LP, Bardo MT. Effect of bupropion on nicotine self-administration in rats. Psychopharmacology. 2003;169:1–9. doi: 10.1007/s00213-003-1450-x. [DOI] [PubMed] [Google Scholar]
- Reichel CM, Wilkinson JL, Bevins RA. Methamphetamine functions as a positive and negative drug feature in a Pavlovian appetitive discrimination task. Behav Pharmacol. 2007 doi: 10.1097/FBP.0b013e3282f14efc. in press. [DOI] [PubMed] [Google Scholar]
- Roll JM. Contigency management: An evidence-based component of methamphetamine use disorder treatments. 2007;102 Suppl. 1:114–120. doi: 10.1111/j.1360-0443.2006.01774.x. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Blough BE, Baumann MH. Appetite suppressants as agonist substitution therapies for stimulant dependence. Ann N Y Acad Sci. 2002;1965:109–126. doi: 10.1111/j.1749-6632.2002.tb04155.x. [DOI] [PubMed] [Google Scholar]
- Sandoval V, Riddle EL, Hanson GR, Fleckenstein AE. Methylphenidate alters vesicular monoamine transport and prevents methamphetamine-induced dopamine deficits. J Pharmacol Exp Ther. 2003;304:1181–1187. doi: 10.1124/jpet.102.045005. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) Results from the 2005 National Survey on Drug Use and Health. NSDUH Series H-30. DHHS Pub No. SMA 06-4194. Rockville, MD: DHHS; 2006. [Google Scholar]
- Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: A review. Prog Neurobiol. 2005;75:406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Fukuoka Y, Mori T, Miyatake M, Narita M. Behavioral sensitization to the discriminative stimulus effects of methamphetamine in rats. Eur J Pharmacol. 2004;498:157–161. doi: 10.1016/j.ejphar.2004.07.064. [DOI] [PubMed] [Google Scholar]
- Vocci FJ, Appel NM. Approaches to the development of medications for the treatment of methamphetamine dependence. Addiction. 2007;102 Suppl. 1:96–106. doi: 10.1111/j.1360-0443.2007.01772.x. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Hosseini-Nia T. Anorectic and behavioural effects of bupropion. Gen Pharmac. 1988;19:201–204. doi: 10.1016/0306-3623(88)90061-4. [DOI] [PubMed] [Google Scholar]