Abstract
Bupropion is a promising candidate medication for methamphetamine use disorder. As such, we used a preclinical model of drug-taking to determine the effects of bupropion on the reinforcing effects of methamphetamine (0.025, 0.05 or 0.1 mg/kg/infusion). Specificity was determined by investigating the effects of bupropion on responding maintained by sucrose. In the self-administration study, rats were surgically prepared with indwelling jugular catheters and trained to self-administer methamphetamine under an FR5 schedule. A separate group of rats was trained to press a lever for sucrose. Once responding stabilized, rats were pretreated with bupropion (0, 10, 30 and 60 mg/kg IP) 5 min before chamber placement in a unique testing order. Following acute testing, rats were then repeatedly pretreated with 30 and 60 mg/kg bupropion. Acute treatments of bupropion dose dependently reduced drug intake for 0.025 to 0.1 mg/kg methamphetamine; sucrose deliveries were only reduced with the high bupropion dose. Repeated exposure to 60 mg/kg bupropion before the session resulted in a consistent decrease in methamphetamine intake (0.05 and 0.1 mg/kg) and sucrose deliveries. Considered together, this pattern of findings demonstrates that bupropion decreases responding for methamphetamine, but the effects are only somewhat specific.
Keywords: stimulant, substance use disorder, Wellbutrin, Zyban, reinforcement
1. Introduction
Methamphetamine is a highly potent, addictive drug that is widely abused in many countries around the world. In the United States, nearly 18 million people aged 12 and over (6% of the population) have tried methamphetamine in their lifetime and 700,000 of these individuals used some form of the drug within the last month (Substance Abuse and Mental Health Services Administration, 2007). Initially, methamphetamine produces a general state of well-being along with increased wakefulness, talkativeness, and physical activity and decreased appetite (NIDA, 2006). However, recreational use is often replaced with compulsive drug use, leading to addiction. Behavioral treatment programs have had some success in the treatment of methamphetamine addiction (Ling et al., 2006; Roll, 2007), yet many patients continue to relapse after repeatedly seeking treatment (NIDA, 2006). Thus, pharmacotherapies to compliment existing cognitive and behavioral treatments for methamphetamine addiction are being evaluated.
Numerous drugs from several different classes show promise as candidate medications for methamphetamine abuse (see Ling et al., 2006; Vocci and Appel, 2007 for reviews). One drug in particular, already approved by the U.S. Food and Drug Administration for use in humans is bupropion hydrochloride. Bupropion is an atypical antidepressant with stimulant properties used for the treatment of depression and smoking cessation (see Dwoskin et al., 2006 for a review). This drug has been used off-label to treat methamphetamine addicts, thus prompting the need for systematic investigations of its efficacy (Newton et al., 2006). The early clinical findings are promising. For example, bupropion treatment (twice daily, 150 mg, sustained release) was well tolerated in patients that received intravenous methamphetamine infusions (0, 15, or 30 mg) and by those abstaining from methamphetamine use (Elkashef et al., 2008; Newton et al., 2005). Additionally, bupropion reduced the subjective effects and cue-induced cravings of methamphetamine (Newton et al., 2006), and increased duration of abstinence in male participants classified as having “mild-to-moderate” methamphetamine dependence (Elkashef et al., 2008).
Preclinical behavioral and neurochemical studies support the theoretical bases and clinical findings for the use of bupropion to treat methamphetamine use disorder. Neurochemical studies demonstrated that bupropion treatment had neuroprotective effects against methamphetamine-induced neurotoxicity (Marek et al., 1990) and protected against the acute reduction of dopamine uptake in striatal synaptosomes (Kim et al., 2000). In locomotor activity studies with rodents, administration of intraperitoneal (IP) bupropion (12.5, 25, and 50 mg/kg) dose-dependently blocked methamphetamine-induced stereotypy in mice when methamphetamine followed bupropion (Muley et al., 1984). When methamphetamine preceded bupropion, methamphetamine-induced stereotypy was augmented (Muley et al., 1984). Additionally, a self-administration study with d-amphetamine (0.2 mg/kg/infusion) found that bupropion pretreatment decreased drug intake in rats (Rauhut et al., 2003). Most directly relevant to the present research is the finding from our laboratory that bupropion (60 mg/kg, IP) decreased the number of methamphetamine (0.025 mg/kg) infusions taken during acquisition and prevented rats from discriminating between the active and inactive levers (Reichel et al., 2008). Further, an acute administration of 30 mg/kg bupropion decreased the number of infusions in rats trained to self-administer 0.025 mg/kg/infusion methamphetamine. Taken together, these studies clearly indicate that bupropion and methamphetamine interact on a neurochemical and behavioral level.
There has been limited research concerning the impact of bupropion on methamphetamine self-administration given its consideration as a pharmacotherapy for methamphetamine abuse. Our previous research report demonstrated that bupropion can decrease drug intake (0.025 mg/kg); the goal of the present research was to extend this finding to various doses of self-administered methamphetamine to determine whether higher and presumably more rewarding doses would also be affected by bupropion pretreatment. In the first experiment, we evaluated bupropion’s impact during maintence of methamphetamine (0.025, 0.05, or 0.1 mg/kg/infusion) self-administration using acute and repeated bupropion pretreated protocols. In the second experiment, bupropion’s specificity for methamphetamine was assessed with rats whose responding was maintained by sucrose.
2. General Methods
2.1. Subjects
Thirty-six male, Sprague-Dawley rats weighing 275–300 g at the time of delivery from Harlan (Indianapolis, IN, USA) were housed individually in clear polycarbonate tubs lined with wood shavings in a temperature and humidity controlled room. Water was continuously available in the home cage; access to food was restricted as described later. All sessions were conducted during the light portion of a 12 h light:dark cycle. Experimental protocols were approved by the University of Nebraska-Lincoln Institutional Animal Care and Use Committee and followed the “Guide for the Care and Use of Laboratory Animals” (National Research Council, 1996).
2.2. Apparatus
Eight standard conditioning chambers (Med-Associates, Georgia, VT, USA) were used. Each chamber was housed in a PVC sound-attenuating cubicle fitted with a fan to provide airflow and mask noise and a house light to provide general illumination. Each chamber (30.5 × 24.1 × 21 cm; l × w × h) had side walls made of aluminum; the ceiling and front and back walls were clear polycarbonate. Located in the bottom center of one aluminum wall was an opening to a recessed dipper receptacle (5.2 × 5.2 × 3.8 cm; l × w × d). The dipper arm, when raised, allowed access to 0.1 ml of 26% sucrose solution (w/v). An infrared emitter/detector unit located 1.2 cm inside the receptacle and 3 cm above the floor recorded head entries. A second infrared emitter/detector unit was mounted on the center of the front and back polycarbonate wall, 4 cm above the rod floor, such that it bisected the chamber into two halves. The number of times this infrared beam was interrupted served as a measure of activity in the chamber. Retractable levers were located on either side of the dipper receptacle. Levers were set such that 147 nN of force was required for micro-switch closure and recording of a lever press. A white cue light (2.54 cm dia; 28 V, 100 mA) was centered 7 cm above each lever, 14.6 cm above the metal rod floor and 3.5 cm from the closest polycarbonate wall. Each chamber was equipped with a balanced metal arm and spring leash attached to a swivel. Tygon® tubing AAQ04103 (VWR, West Chester, PA, USA) extended through the leash and was connected to a 5-ml syringe mounted on an infusion pump (Med Associates, PMH-100VS) located outside of the sound-attenuating cubicle.
2.3. Drugs
D-methamphetamine hydrochloride was purchased from Sigma (St. Louis, MO, USA). Bupropion hydrochloride was purchased from Toronto Research Chemicals (Toronto, ON, Canada). Methamphetamine was dissolved in 0.9% sterile saline (w/v). Bupropion was dissolved in sterile water. Bupropion was administered IP at a volume of 1 ml/kg. Methamphetamine was administered intravenously (IV) at a volume of 35.74 µl per infusion.
2.4. Catheter Surgery and Recovery
Rats were anesthetized with 1 ml/kg ketamine hydrochloride (100 mg/ml, IP) followed by 0.6 ml/kg xylazine hydrochloride (20 mg/ml, IP) (Midwestern Veterinary Supply, Des Moines, IA, USA). One end of a silastic catheter (CamCaths© IVSA28, Ely, Cambridgeshire, United Kingdom) was implanted into the left external jugular vein. The other end of the catheter went subcutaneous around the shoulder and exited via a polycarbonate back-plate implanted under the skin just below the scapula. This backmount allowed access to the catheter through a metal cannula embedded with a plastic bolt. Buprenorphine hydrochloride (0.1 mg/kg; Sigma) was injected subcutaneously immediately following surgery. For the evening of and day following surgery buprenorphine (0.5 mg/kg, mixed at a concentration of 0.175 mg/40 ml) was available in the drinking water to manage post-surgical pain. For the evening after surgery and the following 2 days (AM and PM), the catheter was flushed with 0.1 ml of streptokinase (ca. 8000 Units/ml; Sigma) dissolved in sterile heparinized saline (30 Units/ml; Midwest Veterinary Supply). The catheter was flushed twice a day for the remainder of the experiment with 0.2 ml of the heparinized saline. Rats were allowed 5 days of recovery before the start of the experiment. Catheter patency was assessed with a 0.05 ml IV infusion of xylazine (20 mg/ml) at pre-established points in the study. This concentration produces clear motor ataxia within 5 sec if the catheter is patent. Only rats with patent catheters were included in the data analyses.
3. Procedures
3.1. Experiment 1: Bupropion Pretreatment on Methamphetamine Self-administration
3.1.1. Preliminary Training
All rats were allowed to acclimate to the colony room and then handled for 2 min each on 3 separate days before the start of training. Rats were fed 20 g of chow per day during the initial training period. Following handling, rats were dipper trained. Dipper training entailed a 50-min automated session in which the probability of receiving 4-sec access to sucrose in any 4-sec interval was 0.1333 (ca. 3 deliveries per min). For the two days following dipper training, rats were autoshaped to lever press. On a given session only one retractable lever (right or left) was inserted into the chamber for 15 sec using a variable time 60-sec schedule. If a lever press occurred before the 15 sec, the lever was retracted and the dipper was raised allowing 4-sec access to 0.1-ml sucrose. If no lever press occurred after 15 sec, the lever was retracted and 4-sec access to sucrose was still provided. The order of lever presentation was counterbalanced such that the left lever was extended during the first session for half the rats and the right lever for the remaining rats. For all rats, each session ended after 60 sucrose deliveries; session length varied somewhat between rats depending upon when consistent lever pressing developed in the session (ca. 80-min). After concluding the last autoshaping session, rats were allowed free access to rat chow until the end of the surgical recovery period.
3.1.2. Methamphetamine Self-Administration
Twenty-seven (9 per group) rats were assigned to one of three methamphetamine doses (0.025, 0.05, or 0.1 mg/kg/infusion) in a manner that counterbalanced all experimental variables (e.g., which lever served as the active lever, chamber, etc) as much as the sample size allowed. Sessions were 60 min and the house light remained on throughout the session. Pressing the active lever resulted in a 1-sec illumination of the cue light above the active lever and a simultaneous 1-sec infusion of the assigned methamphetamine dose [i.e., a fixed ratio 1 (FR1) schedule of reinforcement]. Following an active lever press, both levers were retracted for 1 min. Pressing the inactive lever had no programmed consequence. The schedule of reinforcement was increased automatically from an FR1 (8 days) to an FR3 (5 days) then to an FR5 (5 days). However, rats were advanced to the next FR requirement early if they met the following criteria: number of infusions within 20% of each other for 2 consecutive days and 2:1 ratio of active to inactive lever presses.
3.1.3. Bupropion Acute Challenges
When responding stabilized on an FR5, rats entered the acute testing phase. Each rat was tested with a unique order of vehicle, 10, 30, and 60 mg/kg bupropion. Each solution was administered IP 5 min before placement in the chamber for a regular self-administration session and each test was separated by at least 2 maintenance days of methamphetamine self-administration without drug pretreatment. To meet test criteria, rats were required to have 2 consecutive days in which the number of infusions was within 20% of each other. After all doses of bupropion were tested, catheter patency was checked. Rats with patent catheters were then advanced to the next experimental phase.
3.1.4. Bupropion Repeated Testing
The repeated testing portion of the experiment consisted of 4 consecutive phases defined by the pre-injection of vehicle or bupropion. The following describes the specific phases. 1) The first 3 days were methamphetamine self-administration maintenance sessions that occurred following an injection of vehicle 5 min before chamber placement. 2) For the following 3 days rats were pretreated with 30 mg/kg bupropion 5 min before chamber placement for a regular self-administration session. 3) For the following 5 days rats were pretreated with 60 mg/kg bupropion 5 min before chamber placement for a regular self-administration session. 4) The last phase consisted of 5 maintenance days, in which vehicle was administered before the session. Following completion of this repeated testing schedule, a final patency check was conducted.
3.2. Experiment 2: Bupropion Pretreatment on Sucrose-Maintained Responding
3.2.1. Preliminary Training
A separate set of naïve rats (n=8) were used in this experiment. These rats also had 3 consecutive days of preliminary training consisting of one dipper training session and two autoshaping sessions as described previously. Following the last autoshaping session, rats were given free access to food and kept in their home cages for 5 days to simulate the surgical recovery period for the self-administration experiment. This experiment did not require surgery but all other experimental parameters (e.g., handling, start of acquisition, feeding, etc.) were matched with Experiment 1.
3.2.2. Sucrose-Maintained Responding
The procedures for the sucrose experiment were identical to those just described for the self-administration study except that instead of an infusion of methamphetamine, active lever presses resulted in 4-sec access to 0.1-ml sucrose. The exact protocol of Experiment 1 was followed for all phases of Experiment 2.
3.3 Dependent Measures and Data Analysis
The number of methamphetamine infusions (Experiment 1) or sucrose deliveries (Experiment 2) served as the main dependent measures. To assess non-specific effects of bupropion, the number of inactive lever presses and general chamber activity were also analyzed. Only data from rats that had patent catheters at the end of each experimental phase were used in the statistical analyses; the number of rats in each analysis is provided in the Results section. Some rats that were assigned to the 0.025 mg/kg/infusion group did not maintain responding on an FR5 schedule; schedules were adjusted for individual subjects until responding stabilized. In total, 3 rats stabilized on an FR1, 1 rat on an FR3, and 3 rats on an FR5.
Data from each experiment were analyzed with analyses of variance (ANOVAs). Depending upon the specific experimental phase, the between-subjects factor was methamphetamine dose and the within-subject factors were bupropion dose or session. For the repeated testing phase, an average of the 3 consecutive days (before repeated testing began) of methamphetamine self-administration or sucrose-maintained responding was used for a baseline. An average of the 5 days after repeated testing was calculated for comparison purposes to determine if responding returned to baseline after bupropion exposure ceased. In the first experiment, the number of methamphetamine infusions, inactive lever presses, and activity counts were analyzed with separate two-way (Methamphetamine × Bupropion) mixed ANOVAs for the acute bupropion tests. For the repeated bupropion testing, a two-way (Methamphetamine × Session) mixed ANOVA was conducted. A significant interaction was followed by separate one-way (Session) ANOVAs for each methamphetamine dose. In the second experiment, the number of sucrose deliveries, inactive lever presses, and activity counts were analyzed with separate one-way (Bupropion) ANOVAs for the acute bupropion tests. Repeated bupropion testing was analyzed by a one-way (Session) ANOVA. Only the significant effects are reported. In all cases, significant interactions were interpreted with Fisher’s least significant difference (LSD) tests for post-hoc comparisons. Statistical significance was set at p<0.05 (two-tailed) for all tests.
4. Results
4.1. Experiment 1: Methamphetamine Self-Administration
4.1.1 Bupropion Acute Testing
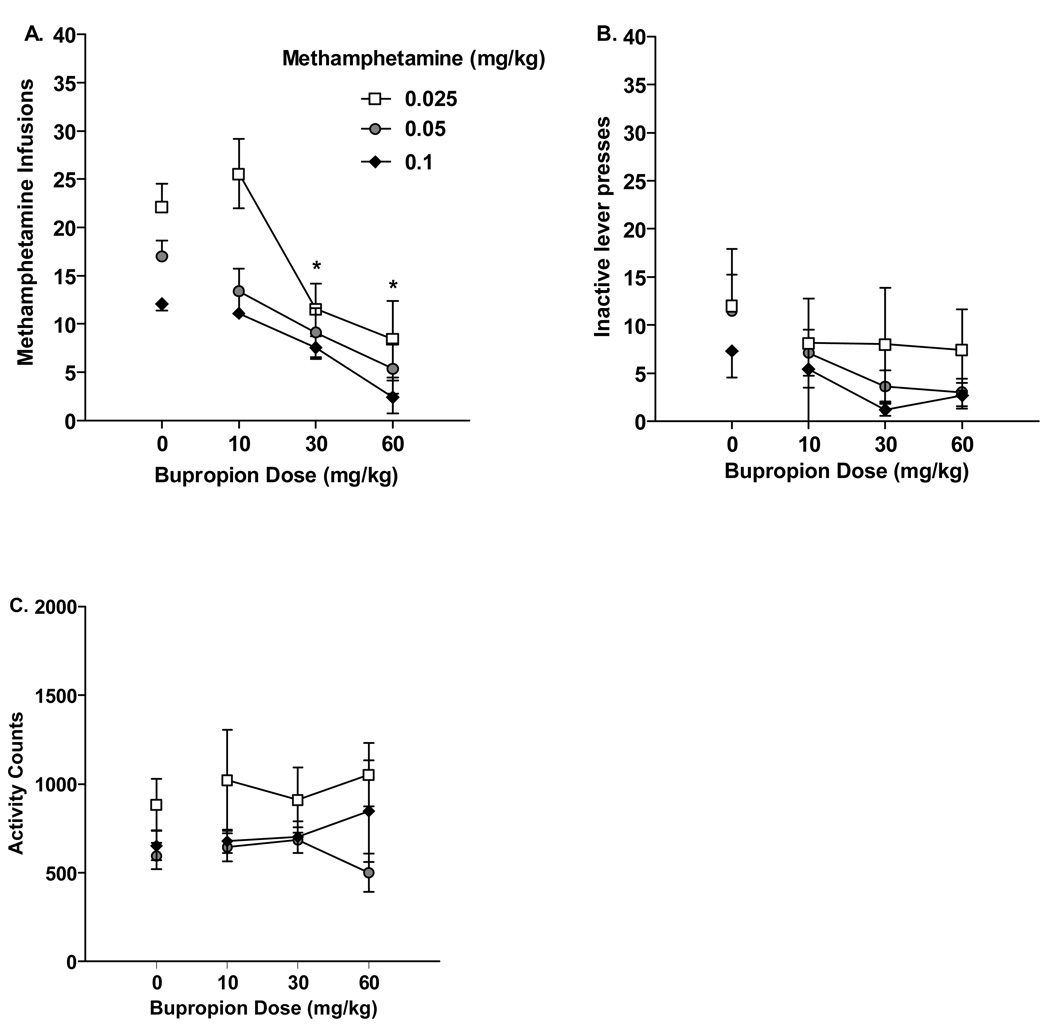
Bupropion reduced methamphetamine infusions regardless of the self-administration dose [Figure 1A, main effect of Bupropion, F(3,36)=28.73, p<.001]. Specifically, 30 and 60 mg/kg bupropion decreased the number of methamphetamine infusions compared to vehicle and 10 mg/kg bupropion (LSDmmd = 5.19). Figure 1B shows the impact of bupropion on inactive lever presses. Although the overall analysis suggested a reduction in responding [main effect of Bupropion, F(3,36)=4.10, p<0.01], differences did not emerge with the post-hoc analysis (LSDmmd = 6.79). Bupropion had no impact on general chamber activity during methamphetamine self-administration (Figure 1C). A total of 21 rats (0.025 n=7; 0.05 n=6; 0.1 n=8) were used in this analysis.
Figure 1.
A) Mean (±SEM) number of infusions for rats trained to self-administer 0.025, 0.05 and 0.1 mg/kg/infusion methamphetamine (ns= 7, 8, and 9, respectively) during acute testing with vehicle, 10, 30, and 60 mg/kg bupropion. Bupropion (30 and 60 mg/kg) decreased methamphetamine infusions. B) Mean (±SEM) responses on the inactive lever during acute testing. C) This graph illustrates the mean (±SEM) activity during the self-administration sessions. *Significant difference from vehicle and 10.
4.1.2. Bupropion Repeated Testing
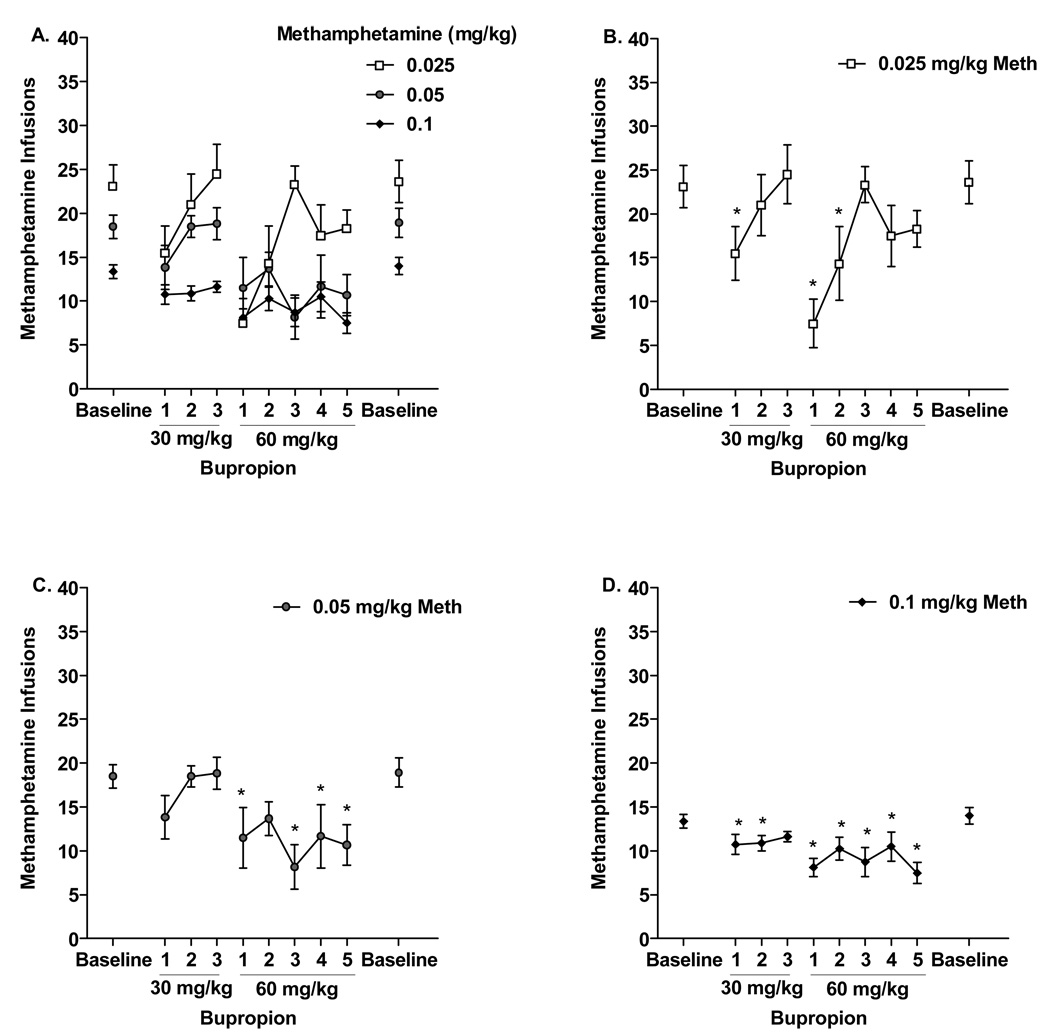
Repeated bupropion exposure produced a reduction in methamphetamine infusions depending upon the methamphetamine training dose and the bupropion test dose [Figure 2A, Methamphetamine × Session interaction, F(16,136)=2.75, p<0.001]. In particular, when 0.025 mg/kg methamphetamine (n=6) was the training dose, 30 mg/kg bupropion decreased infusions the first day of administration and 60 mg/kg bupropion had a similar effect the first and second day it was given [Figure 2B, F(8,40)=3.99, p<0.001; LSDmmd = 7.76]. In contrast, for the 0.05 mg/kg methamphetamine (n=6) group, only 60 mg/kg bupropion decreased infusions on days 1, 3, 4, and 5 relative to baseline [Figure 2C, F(8,40)=4.06, p<0.001; LSDmmd = 5.51]. Finally, 30 and 60 mg/kg bupropion decreased infusions throughout the repeated testing phase when 0.1 mg/kg methamphetamine (n=8) was the training dose [Figure 2D, F(8,56)=4.00, p<0.001]. Specifically, 30 mg/kg bupropion reduced infusions on days 1 and 2, and 60 mg/kg bupropion reduced infusions on all 5 days (LSDmmd = 2.61). For all methamphetamine doses, discontinuing bupropion pretreatment resulted in a return to baseline methamphetamine intake (individual paired t-tests between pre and post baseline responding, ts<1). Bupropion did not impact inactive lever responding during repeated treatment for any of the methamphetamine doses (see Table 1). There was a tendency for activity in all three methamphetamine groups to decline over the sessions [see Table 2, main effect of Session, F(8,136)=8.50, p<0.001]. Specifically, 60 mg/kg bupropion pretreatment on day 5 decreased activity relative to baseline [mean ± SEM: Baseline = 690.87 ± 56.36; Bupropion day 5 = 503.03 ± 61.43; LSDmmd = 181.0].
Figure 2.
A) Mean (±SEM) number of infusions for rats trained to self-administer 0.025, 0.05 and 0.1 mg/kg/infusion methamphetamine (ns= 6, 6, and 8, respectively) during repeated testing with 30, and 60 mg/kg bupropion. B) Repeated testing for rats in the 0.025 methamphetamine group, C) the 0.05 methamphetamine group, and D) the 0.1 methamphetamine group. *Significant difference from baseline.
Table 1.
Inactive lever presses for rats trained with methamphetamine or sucrose during repeated testing with bupropion.
| Methamphetamine (or Sucrose) Group Mean ± SEM inactive lever presses |
||||
|---|---|---|---|---|
| Bupropion Dose (mg/kg) |
0.025 | 0.05 | 0.1 | Sucrose |
| Baseline | 6.56 ± 1.59 | 11.17 ± 2.78 | 3.38 ± 1.17 | 9.21 ± 3.94 |
| 30 day 1 | 3.33 ± 2.03 | 4.67 ± 2.28 | 1.75 ± 1.146 | 60.62 ± 58.91 |
| 30 day 2 | 2.83 ± 1.30 | 4.00 ± 1.39 | 1.62 ± 1.34 | 15.75 ± 8.55 |
| 30 day 3 | 2.50 ± 1.28 | 2.83 ± 2.09 | 4.12 ± 1.59 | 4.00 ± 1.24 |
| 60 day 1 | 0.17 ± 0.17 | 3.50 ± 2.50 | 0.62 ± 0.42 | 4.12 ± 1.88 |
| 60 day 2 | 2.50 ± 1.57 | 18.00 ± 13.36 | 1.37 ± 0.62 | 3.12 ± 2.32 |
| 60 day 3 | 2.33 ± 1.23 | 16.17 ± 14.98 | 0.87 ± 0.64 | 3.13 ± 2.72 |
| 60 day 4 | 0.83 ± 0.40 | 4.83 ± 3.76 | 1.37 ± 0.65 | 3.63 ± 2.29 |
| 60 day 5 | 3.16 ± 1.68 | 9.83 ± 8.51 | 2.00 ± 1.08 | 4.87 ± 2.07 |
| Baseline | 5.67 ± 1.78 | 13.67 ± 4.86 | 4.10 ± 1.23 | 5.43 ± 2.48 |
Table 2.
Activity counts for rats trained with methamphetamine or sucrose during repeated testing with bupropion.
| Methamphetamine (or Sucrose) Group Mean ± SEM chamber activity |
||||
|---|---|---|---|---|
| Bupropion Dose (mg/kg) |
0.025 | 0.05 | 0.1 | Sucrose |
| Baseline | 760.06 ± 145.39 | 667.50 ± 64.33 | 645.04 ± 73.45 | 433.00 ± 37.08 |
| 30 day 1 | 964.16 ± 195.86 | 835.66 ± 98.45 | 725.50 ± 48.54 | 604.75 ± 68.92 |
| 30 day 2 | 981.16 ± 150.61 | 705.00 ± 80.35 | 716.75 ± 83.17 | 614.87 ± 47.69 |
| 30 day 3 | 913.00 ± 160.74 | 629.83 ± 45.72 | 633.25 ± 81.72 | 556.37 ± 36.37 |
| 60 day 1 | 759.50 ± 213.23 | 505.66 ± 71.63 | 509.37 ± 86.48 | 483.50 ± 27.33 |
| 60 day 2 | 713.00 ± 118.21 | 553.83 ± 56.90 | 619.87 ± 99.67 | 513.25 ± 69.06 |
| 60 day 3 | 753.16 ± 124.75 | 499.66 ± 59.75 | 533.75 ± 83.77 | 489.25 ± 60.94 |
| 60 day 4 | 789.16 ± 157.86 | 486.66 ± 64.22 | 514.00 ± 56.93 | 533.00 ± 76.66 |
| 60 day 5 | 643.83 ± 154.18 | 400.00 ± 103.71 | 465.25 ± 63.19 | 432.12 ± 34.65 |
| Baseline | 643.10 ± 83.02 | 554.83 ± 36.59 | 622.93 ± 45.22 | 381.65 ± 34.48 |
4.2 Experiment 2: Sucrose-Maintained Responding
4.2.1 Bupropion Acute Testing
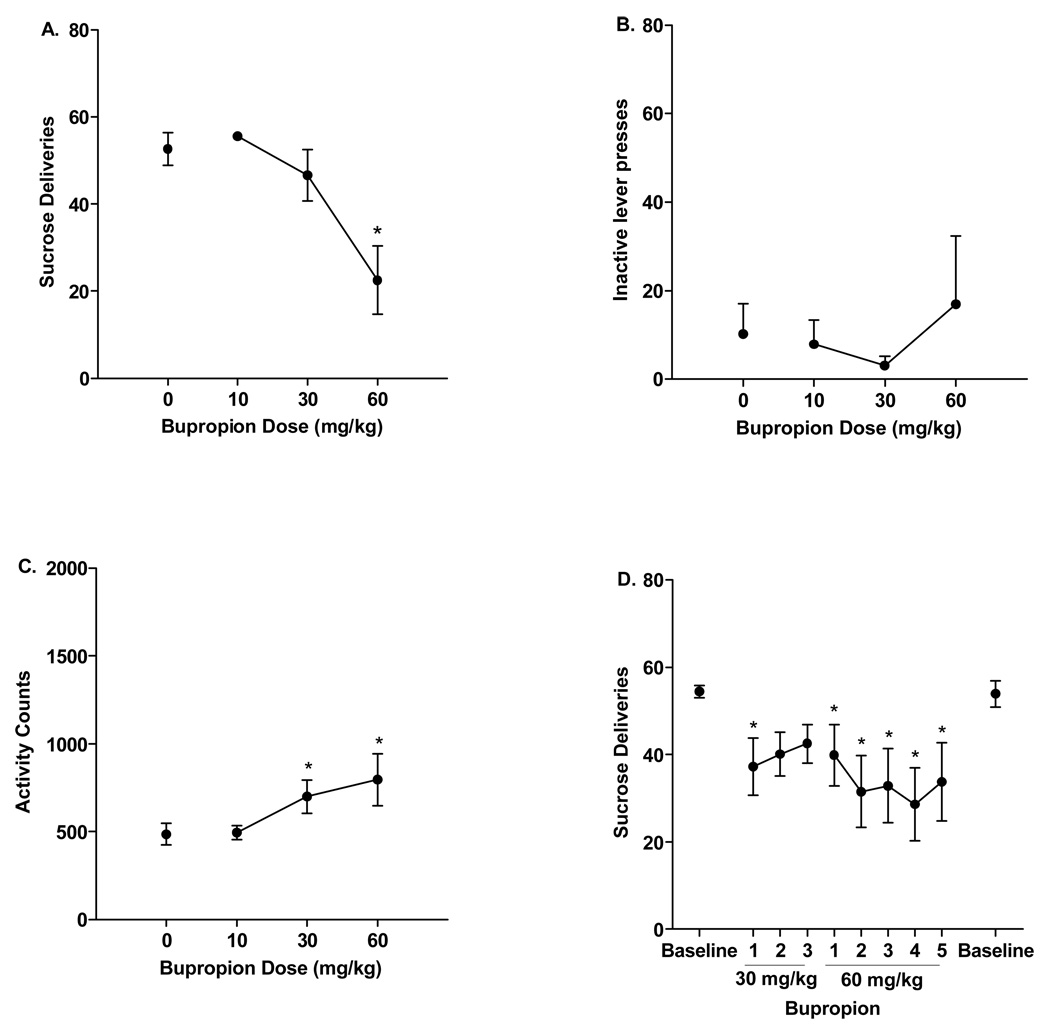
The high dose of bupropion, 60 mg/kg, produced a decrease in sucrose deliveries relative to vehicle, 10, and 30 bupropion [Figure 3A, main effect of Bupropion, F(3,21)=11.58, p<0.001; LSDmmd = 13.01]. Bupropion did not, however, impact inactive lever presses (Figure 3B). One rat pressed the inactive lever over 1100 times; over 2 standard deviations from the mean. As such, this value was excluded from the inactive lever analysis. Bupropion increased activity during the session [Figure 3C, Bupropion effect, F(3,21)=5.52, p<0.006]. Specifically, 30 and 60 mg/kg bupropion resulted in higher activity counts relative to vehicle and 10 bupropion (LSDmmd = 193.29).
Figure 3.
Data from rats trained to lever press for sucrose reinforcement (n=8) during testing with bupropion. A) Mean (±SEM) number of sucrose deliveries during acute testing with vehicle, 10, 30, and 60 mg/kg. Reinforcers earned were decreased with 60 mg/kg bupropion. B) Mean (±SEM) responses on the inactive lever during acute testing. C) Mean (±SEM) activity during the self-administration sessions; bupropion (30 and 60 mg/kg) increased activity relative to vehicle. D) Mean (±SEM) number of sucrose deliveries during the repeated testing phase. *Significant difference from vehicle or baseline.
2.2.2 Bupropion Repeated Testing
Bupropion reduced the number of sucrose deliveries [Figure 3D, Session effect, F(8,56)=2.28, p<0.05]. Specifically, 30 mg/kg bupropion reduced deliveries on the first day of administration whereas, 60 mg/kg bupropion decreased deliveries on days 1 through 5 (LSDmmd =14.55). Discontinuing bupropion pretreatment resulted in a return to baseline sucrose deliveries (paired t-test between pre and post baseline responding, t<1). Bupropion did not impact inactive lever responding during repeated treatment (see Table 1), nor did it not significantly affect chamber activity during repeated testing (see Table 2).
Discussion
In the present study we investigated the effects of acute (vehicle, 10, 30, and 60 mg/kg) and repeated (30 and 60 mg/kg) bupropion treatment on 3 different doses of methamphetamine self-administration and responding for sucrose. We found that acute administration of 30 and 60 mg/kg bupropion decreased intake of 0.025, 0.05, and 0.1 mg/kg/infusion of methamphetamine. More so, the highest dose of bupropion also decreased sucrose deliveries. Repeated administrations of 30 and 60 mg/kg bupropion reduced methamphetamine infusions differentially across session depending upon the training dose. Lever pressing for sucrose was also decreased across test sessions with 30 and 60 mg/kg bupropion pretreatment. Importantly, in all instances these reductions in response to methamphetamine and sucrose as reinforcers were independent of changes in inactive lever presses and mostly unaffected by activity during the session.
Earlier reports show that bupropion reduced intake of nicotine, amphetamine, and methamphetamine (Buijnzeel and Markou, 2003; Rauhut et al., 2003; Reichel et al., 2008). We extend these findings to include multiple doses of methamphetamine during acute and repeated exposure. That bupropion decreased intake of various stimulant drugs indicates some level of non-specificity in its action on abused drugs. Bupropion has been shown to be an antagonist at nicotinic acetylcholine receptors (Slemmer et al., 2000), and blockade of these receptors prevents the induction of behavioral sensitization to amphetamine (Schoffelmeer et al., 2002) and decreases nicotine and methamphetamine intake (Hiranita et al., 2006; Watkins et al., 1999). These studies used the potent and nonspecific nicotinic antagonist mecamylamine. In contrast, bupropion is a relatively weak antagonist particularly at α7-containing nicotinic acetylcholine receptors (Slemmer et al., 2000). For this reason, we believe that nicotinic acetylcholine receptor antagonism seems an unlikely candidate to explain bupropion mediated decrease in methamphetamine self-administration in the present study. Alternatively, methamphetamine and bupropion increase dopaminergic neurotransmission in brain reward pathways (Kuczenski et al., 1995; Melega et al., 1995; Nomikos et al., 1989; 1992). This similar mechanism results in substitution of the drug effects (Munzar and Goldberg, 2000; Reichel et al., 2007) that may reduce drug intake (Munzar et al., 1999). This dopaminergic mechanism is also appealing because it also provides a potential common process to explain our sucrose findings. That is, blockade of nicotinic acetylcholine receptors does not typically decrease sucrose-maintained responding (Nadal, et al., 1998; Pratt and Kelly, 2004), but blockade of dopamine receptors does (Beninger et al., 1987, Ikemoto and Panksepp, 1996; Johnston et al., 2001).
Repeated bupropion testing also decreased methamphetamine and sucrose-maintained responding. Bupropion (30 mg/kg) only transiently reduced methamphetamine intake and sucrose deliveries suggesting decreased effectiveness across administration. This decrease also developed with 60 mg/kg bupropion for the 0.025 mg/kg methamphetamine group. In contrast, 60 mg/kg bupropion also decreased methamphetamine (0.05 and 0.1 mg/kg) infusions and sucrose deliveries earned throughout the repeated testing sessions. Notably, reductions in sucrose deliveries resulting from repeated bupropion pretreatment have been reported previously (Rauhut et al., 2005). Importantly, any bupropion-induced changes in sucrose or food maintained responding must consider the anorexic effects of the drug. Bupropion is prescribed for the treatment of obesity in humans (Gadde et al., 2001) and reduces food intake in laboratory animals (Zarrindast and Hosseini-Nia, 1988). Thus, repeatedly administering 60 mg/kg bupropion may have had an anorexic effect for rats in the sucrose experiment. The question remains as to whether the bupropion-induced decrease in sucrose deliveries is independent of bupropion’s effects on the reward system.
Responding on the inactive lever was not altered whether methamphetamine or sucrose served as the reinforcer. Further, inactive lever responding was relatively stable in both experiments even when bupropion pretreatment occurred across consecutive days. This finding contrasts with Rauhut and colleagues (2003) in which bupropion (15–78 mg/kg) reduced inactive lever presses, but is in accordance with Bruijnzeel and Markou (2003) where bupropion did not change inactive lever presses. Nevertheless, the current findings question any account of the bupropion-induced decrease in responding maintained by methamphetamine or sucrose that relies on a generalized alteration of lever pressing.
Acute administration of bupropion increases locomotor activity in rodents (Cooper et al., 1980; Nomikos et al., 1992; Wilkinson & Bevins, 2007). More so, locomotor stimulation is maintained and sometimes enhanced with repeated administration (Cooper et al., 1980; Nielsen et al., 1986). The ability of a locomotor stimulating drug, in our case bupropion, to reduce responding for a reinforcer raises concerns about the competitive nature of the concurrent behaviors (Robbins and Sahakian, 1979). That is, with more time spent engaged in locomotion, there is less time available to lever press and complete the FR requirements. This competing locomotor account can explain the reduction of sucrose deliveries observed with 60 mg/kg bupropion because this dose simultaneously increased chamber activity. However, a competing locomotor account cannot wholly explain the reductions in sucrose deliveries for two specific reasons. First, during the acute testing with 30 mg/kg bupropion the number of sucrose deliveries were not affected but activity increased. Second, repeated bupropion treatment decreased sucrose responding but did not significantly impact activity.
The attenuation of methamphetamine self-administration during the acute challenges was not likely due to bupropion-induced increases in chamber activity because activity did not differ with bupropion pretreatment dose. As such, the combined effects of bupropion and self-administered methamphetamine produced similar locomotor activating effects. There was, however, an interesting pattern of the activity with repeated testing. Daily pretreatment with 60 mg/kg bupropion in combination with methamphetamine produced a decline in chamber activity. One possible reason for this decrease in general chamber activity is that the repeated exposure to bupropion preferentially induced stereotyped behaviors in a manner consistent with other stimulant drugs (Bhattacharyya and Pradhan, 1979; Kuczenski and Segal, 1989; Nomikos et al., 1989). Although stereotypy could explain the decrease in active lever responding, such behaviors were not measured in the present experiment. Thus, the exact mechanisms responsible for decreased activity and methamphetamine self-administration in this 60 mg/kg condition will require further investigation.
Any comparisons between the self-administration and sucrose experiment must take into account that sucrose engendered a higher rate of responding than methamphetamine (see Dews, 1958). Thus, the similar impact of 60 mg/kg bupropion on responding maintained by methamphetamine and sucrose may be partially influenced by differences in response rate. There is no way to avoid this difference with an FR5 schedule of reinforcement except by varying some other behavioral parameter (e.g., limiting the number of reinforcers available with a shorter session or longer timeout) along with the reinforcer. Some studies impose longer timeouts (cf. Neugebauer et al., 2007; Paterson et al., 2003), increase the response requirement, and/or manipulate the food deprivation schedule (Stairs and Dworkin, 2008) in the sucrose experiments meant to parallel drug self-administration studies. Unfortunately, making such a change in reinforcement schedule forces other factors to vary such as temporal pattern of responding and access to the reinforcer. The importance of these changes and how they interact with drug effects is unclear. With these considerations in mind, we examined the initial 15 min of the session as a way of assessing treatment as a function of a similar number of reinforcer deliveries in the test session. That analysis found the same bupropion-induced decline in reinforcers earned whether rate was adjusted for a similar number of reinforcer deliveries in the test session or left unadjusted. Additionally, any comparisons between the self-administration and sucrose experiment must take into consideration differences in the surgery procedures such as brief exposure to anesthesia and pain medication (i.e., sucrose rats did not undergo surgery) or the repeated stimulant exposure in the methamphetamine-taking rats.
The use of bupropion to treat methamphetamine addiction fits a treatment approach termed “agonist substitution” therapy (see Gorelick, 1998; Grabowski et al., 2004; Rothman et al., 2002). The most notable successes of this approach have been with methadone maintenance programs for heroin abusers or nicotine replacement therapies for tobacco users (see Grabowski et al., 2004; Lile 2006 for reviews). The potential value of these treatments prompts the exploration of substitution drugs for the treatment of methamphetamine use disorder. Typically, agonist medications share pharmacological mechanisms of action with the abused drug (Grabowski et al., 2004). As previously mentioned, bupropion and methamphetamine share interoceptive stimulus properties (Munzar and Goldberg, 2000; Reichel et al., 2007; Rush, et al., 1998) and they increase synaptic dopamine concentrations (Kuczenski et al., 1995; Melega et al., 1995; Nomikos et al., 1989; 1992). Bupropion does so by blocking reuptake of dopamine; interestingly drugs with this mechanism [e.g., GBR 12909 and 2-beta-propanoyl-3beta-(4_tolyl)-tropane] produced sustained and selective reductions in cocaine intake (Grabowski et al., 2004). Combined, the shared stimulus properties of methamphetamine and bupropion with the ability of dopamine reuptake inhibitors to reduce self-administered cocaine provide theoretical support that bupropion might be a viable choice as a substitution drug for methamphetamine. In fact, addicts often report using methamphetamine to “make them feel normal” (Bungay et al., 2006). Albeit speculative, bupropion may provide some aspects of this “normalization” to methamphetamine addicts.
To date our laboratory has demonstrated that bupropion impacts methamphetamine self-administration during acquisition (Reichel et al., 2008) and now during a maintenance phase (current study). While this information combined with results of early clinical reports indicates that bupropion may be useful as a treatment for methamphetamine use disorder, several questions remain about bupropion’s impact during withdrawal, abstinence, and relapse in a preclinical setting. Research investigating these aspects of addiction will help provide a clearer picture on the effects of bupropion on methamphetamine self-administration in laboratory animals and methamphetamine abuse in humans.
Contributor Information
Carmela M. Reichel, Department of Psychology, University of Nebraska-Lincoln, Lincoln NE 68588-0308
Jennifer E. Murray, Department of Psychology, University of Nebraska-Lincoln, Lincoln NE 68588-0308
Kathleen M. Grant, Department of Internal Medicine, University of Nebraska Medical Center, Omaha NE 68198-5300
Rick A. Bevins, Department of Psychology, University of Nebraska-Lincoln, Lincoln NE 68588-0308.
References
- Beninger RJ, Cheng M, Hahn BL, Hoffman DC, Mazurski EJ, Morency MA, Ramm P, Stewart RJ. Effects of extinction, pimozide, SCH23390, and metoclopramide on food-rewarded operant responding of rats. Psychopharmacol. 1987;92:343–349. doi: 10.1007/BF00210842. [DOI] [PubMed] [Google Scholar]
- Bergman J, Kamien JB, Spealman RD. Antagonism of cocaine self-administration by selective dopamine D(1) and D(2) antagonists. Behav Pharmacol. 1990;1:355–363. doi: 10.1097/00008877-199000140-00009. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya AK, Pradhan SN. Interactions between motor activity and stereotypy in cocaine-treated rats. Psychopharmacol. 1979;63:311–312. doi: 10.1007/BF00433569. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Markou A. Characterization of the effects of bupropion on the reinforcing properties of nicotine and food in rats. Synapse. 2003;50:20–28. doi: 10.1002/syn.10242. [DOI] [PubMed] [Google Scholar]
- Bungay V, Malchy L, Buxton J, Johnson J, Macpherson D, Rosenfeld T. Life with jib: a snapshot study of street youth’s use of crystal methamphetamine. Addiction Theory and Research. 2006;14 235-151. [Google Scholar]
- Cooper BR, Hester TJ, Maxwell RA. Behavioral and biochemical effects of the antidepressant bupropion (Wellbutrin): evidence for selective blockade of dopamine uptake in vivo. J Pharmacol Exp Ther. 1980;215:127–134. [PubMed] [Google Scholar]
- Depoortere RY, Li DH, Lane JD, Emmett-Oglesby MW. Parameters of self-administration of cocaine in rats under a progressive-ratio schedule. Pharmacol Biochem Behav. 1993;45:539–548. doi: 10.1016/0091-3057(93)90503-l. [DOI] [PubMed] [Google Scholar]
- Dews PB. Studies on Behavior. IV. Stimulant action of Methamphetamine. J Pharmacol Exp Ther. 1958;122:137–147. [PubMed] [Google Scholar]
- Dwoskin LP, Rauhut AS, King-Pospisil KA, Bardo MT. Review of the pharmacology and clinical profile of bupropionropion, an antidepressant and tobacco use cessation agent. CNS Drug Reviews. 2006;12:178–207. doi: 10.1111/j.1527-3458.2006.00178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkashef AM, Rawson RA, Anderson AL, Li S, Holmes T, Smith EV, Chiang N, Kahn R, Vocci F, Ling W, Pearce VJ, McCann M, Campbell J, Gorodetzky C, Haning W, Carlton B, Mawhinney J, Weis D. Bupropion for the Treatment of Methamphetamine Dependence. Neuropsychopharmacol. 2008;33:1162–1170. doi: 10.1038/sj.npp.1301481. [DOI] [PubMed] [Google Scholar]
- Gadde KM, Parker CB, Maner LG, Wagner HR, II, Loque EJ, Drezner MK, Krishnan KR. Bupropion for weight loss: an investigation of efficacy and tolerability in overweight and obese women. Obes Res. 2001;9:544–551. doi: 10.1038/oby.2001.71. [DOI] [PubMed] [Google Scholar]
- Gorelick DA, Gardner EL, Xi ZX. Agents in development for the management of cocaine abuse. Drugs. 2004;64:1547–1573. doi: 10.2165/00003495-200464140-00004. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Shearer J, Merrill J, Negus S. Agonist-like, replacement pharmacotherapy for stimulant abuse and dependence. Addict Behav. 2004;29:1439–1464. doi: 10.1016/j.addbeh.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Hiranita T, Nawata Y, Sakimura K, Anggadiredja K, Yamamoto T. Suppression of methamphetamine-seeking behavior by nicotinic agonists. Proc Natl Acad Sci. 2006;103:8523–8527. doi: 10.1073/pnas.0600347103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. Dissociations between appetitive and consummatory responses by pharmacological manipulation of reward-relevant brain regions. Behav Neurosci. 1996;110:331–345. doi: 10.1037//0735-7044.110.2.331. [DOI] [PubMed] [Google Scholar]
- Johnston LD, Beninger RJ, Olmstead MC. Pimozide, like extinction, devalues stimuli associated with sucrose taking. Pharmacol Biochem Behav. 2001;68:583–590. doi: 10.1016/s0091-3057(01)00460-9. [DOI] [PubMed] [Google Scholar]
- Kim S, Westphalen R, Callahan B, Hatzidimitriou G, Yuan J, Ricaurte GA. Toward development of an in vitro model of methamphetamine-induced dopamine nerve terminal toxicity. J Pharmacol Exp Ther. 2000;293:625–633. [PubMed] [Google Scholar]
- Kuczenski R, Segal D. Concomitant characterization of behavioral and striatal neurotransmitter response to amphetamine using in vivo microdialysis. J Neurosci. 1989;9:2051–2065. doi: 10.1523/JNEUROSCI.09-06-02051.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczenski R, Segal D, Cho A, Melega W. Hippocampus norepinephrine, caudate dopamine and serotonin, and behavioral responses to the stereoisomers of amphetamine and methamphetamine. J Neurosci. 1995;15:1308–1317. doi: 10.1523/JNEUROSCI.15-02-01308.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lile JA. Pharmacological determinants of the reinforcing effects of psychostimulants: Relation to agonist substitution treatment. J Exp Clin Pharmacol. 2006;14:20–33. doi: 10.1037/1064-1297.14.1.20. [DOI] [PubMed] [Google Scholar]
- Ling W, Rawson R, Shoptaw S, Ling W. Management of methamphetamine abuse and dependence. Curr Psychiatry Rep. 2006;8:345–354. doi: 10.1007/s11920-006-0035-x. [DOI] [PubMed] [Google Scholar]
- Marek GJ, Vosmer G, Seiden LS. Dopamine uptake inhibitors block long-term neurotoxic effects of methamphetamine upon dopaminergic neurons. Brain Res. 1990;513:274–279. doi: 10.1016/0006-8993(90)90467-p. [DOI] [PubMed] [Google Scholar]
- Melega W, Williams A, Schmitz D, DiStefano E, Cho A. Pharmacokinetic and pharmacodynamic analysis of the actions of D-amphetamine and D-methamphetamine on the dopamine terminal. J Pharmacol Exp Ther. 1995;274:90–96. [PubMed] [Google Scholar]
- Muley MP, Joshi MA, Manekar MS. Effect of bupropion on dopamine and 5-hydroxytryptamine-mediated behavior in mice. J Pharm Pharmacol. 1984;36:208–210. doi: 10.1111/j.2042-7158.1984.tb06944.x. [DOI] [PubMed] [Google Scholar]
- Munzar P, Baumann MH, Shoaib M, Goldberg SR. Effects of dopamine and serotonin-releasing agents on methamphetamine discrimination and self-administration in rats. Psychopharmacol. 1999;141:287–296. doi: 10.1007/s002130050836. [DOI] [PubMed] [Google Scholar]
- Munzar P, Goldberg S. Dopaminergic involvement in the discriminative-stimulus effects of methamphetamine in rats. Psychopharmacol. 2000;148:209–217. doi: 10.1007/s002130050044. [DOI] [PubMed] [Google Scholar]
- Nadal R, Chappell AM, Samson HH. Effects of nicotine and mecamylamine microinjections into the nucleus accumbens on ethanol and sucrose self-administration. Alcohol Clin Exp Res. 1998;22:1190–1198. [PubMed] [Google Scholar]
- National Research Council. Guide for the care and use of laboratory animals. Washington, DC: National Academy Press; 1996. [Google Scholar]
- Neugebauer NM, Harrod SB, Stairs DJ, Crooks PA, Dwoskin LP, Bardo MT. Lobelane decreases methamphetamine self-administration in rats. European Journal of Pharmacology. 2007;571:33–38. doi: 10.1016/j.ejphar.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton TF, Roache JD, De La Garza R, 2nd, Fong T, Wallace CL, Li SH, Elkashef A, Chiang N, Kahn R. Safety of intravenous methamphetamine administration during treatment with bupropion. Psychopharmacol. 2005;182:426–435. doi: 10.1007/s00213-005-0102-8. [DOI] [PubMed] [Google Scholar]
- Newton TF, Roache JD, De La Garza R, 2nd, Fong T, Wallace CL, Li SH, Elkashef A, Chiang N, Kahn R. Bupropion reduces methamphetamine-induced subjective effects and cue-induced craving. Neuropsychopharmacol. 2006;31:1537–1544. doi: 10.1038/sj.npp.1300979. [DOI] [PubMed] [Google Scholar]
- NIDA Research Report Series, Methamphetamine Abuse and Addiction. NIH Publication number 06-4210. 2006
- Nielsen JA, Shannon NJ, Bero L, Moore KE. Effects of acute and chronic bupropion on locomotor activity and dopaminergic neurons. Pharmacol Biochem Behav. 1986;24:795–799. doi: 10.1016/0091-3057(86)90413-2. [DOI] [PubMed] [Google Scholar]
- Nomikos GG, Damsma G, Wenkstern D, Fibiger HC. Acute effects of bupropion on extracellular dopamine concentration in rat striatum and nucleus accumbens studied by in vivo microdialysis. Neuropsychopharmacol. 1989;7:7–14. doi: 10.1016/0893-133x(89)90031-6. [DOI] [PubMed] [Google Scholar]
- Nomikos GG, Damsma G, Wenkstern D, Fibiger HC. Effects of chronic bupropion on interstitial concentrations of dopamine in rat nucleus accumbens and striatum. Neuropsychopharmacol. 1992;7:7–14. [PubMed] [Google Scholar]
- Paterson NE, Semenova S, Gasparini F, Markou A. The mGluR5 antagonist MPEP decreases nicotine self-administration in rats and mice. Psychopharmacol. 2003;167:257–264. doi: 10.1007/s00213-003-1432-z. [DOI] [PubMed] [Google Scholar]
- Pratt WE, Kelley AE. Nucleus accumbens acetylcholine regulates appetitive learning and motivation for food via activation of muscarinic receptors. Behav Neurosci. 2004;118:730–739. doi: 10.1037/0735-7044.118.4.730. [DOI] [PubMed] [Google Scholar]
- Rauhut AS, Dwoskin LP, Bardo MT. Tolerance does not develop to the decrease in nicotine self-administration produced by repeated bupropion administration. Nicotine Tob Res. 2005;7:901–907. doi: 10.1080/14622200500381384. [DOI] [PubMed] [Google Scholar]
- Rauhut AS, Neugebauer N, Dwoskin LP, Bardo MT. Effect of bupropion on nicotine self-administration in rats. Psychopharmacol. 2003;169:1–9. doi: 10.1007/s00213-003-1450-x. [DOI] [PubMed] [Google Scholar]
- Reichel CM, Linkugel JD, Bevins RA. Bupropion differentially impacts acquisition of methamphetamine self-administration and sucrose maintained behavior. Pharmacol Biochem Behav. 2008;89:463–472. doi: 10.1016/j.pbb.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Wilkinson JL, Bevins RA. Methamphetamine functions as a positive and negative drug feature in a Pavlovian appetitive discrimination task. Behav Pharmacol. 2007;18:755–765. doi: 10.1097/FBP.0b013e3282f14efc. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Sahakian BJ. “Paradoxical” effects of psychomotor stimulant drugs in hyperactive children from the standpoint of behavioural pharmacology. Neuropharmacol. 1979;18:931–950. doi: 10.1016/0028-3908(79)90157-6. [DOI] [PubMed] [Google Scholar]
- Roll JM. Contingency management: an evidence-based component of methamphetamine use disorder treatments. Addiction. 2007;102:114–120. doi: 10.1111/j.1360-0443.2006.01774.x. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Blough BE, Baumann MH. Appetite suppressants as agonist substitution therapies for stimulant dependence. Ann NY Acad Sci. 2002;965:109–126. doi: 10.1111/j.1749-6632.2002.tb04155.x. [DOI] [PubMed] [Google Scholar]
- Rush CR, Kollins SH, Pazzaglia PJ. Discriminative-stimulus and participant-rated effects of methylphenidate, bupropionropion, and triazolam in d-amphetamine-trained humans. Exp Clin Psychopharmacol. 1998;6:32–44. doi: 10.1037//1064-1297.6.1.32. [DOI] [PubMed] [Google Scholar]
- Schoffelmeer AM, De Vries TJ, Wardeh G, van de Ven HW, Vanderschuren LJ. Psycostimulant-induced behavioral sensitization depends on nicotinic receptor activation. J Neurosci. 2002;22:3269–3276. doi: 10.1523/JNEUROSCI.22-08-03269.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slemmer JE, Martin BR, Damaj ME. Bupropion is a nicotinic antagonist. J Pharmacol Exp Ther. 2000;295:321–327. [PubMed] [Google Scholar]
- Stairs DJ, Dworkin SI. Rate-dependent effects of bupropion on nicotine self-administration and food-maintained responding in rats. Pharm Biochem Behav. 2008 doi: 10.1016/j.pbb.2008.05.014. doi:10.1016/j.pbb.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2006 National Survey on Drug Use and Health: National Findings (Office of Applied Studies, NSDUH Series H-32, DHHS Publication No. SMA 07-4293) Rockville, MD: 2007. [Google Scholar]
- Watkins SS, Epping-Jordon MP, Koob GF, Markou A. Blockade of nicotine self-administration with nicotinic antagonists in rats. Pharmacol Biochem Behav. 1999;62:743–751. doi: 10.1016/s0091-3057(98)00226-3. [DOI] [PubMed] [Google Scholar]
- Wee S, Wang Z, Woolverton WL, Pulvirenti L, Koob GF. Effect of aripiprazole, a partial dopamine D2 receptor agonist, on increased rate of methamphetamine self-administration in rats with prolonged session duration. Neuropsychopharmacol. 2007;32:2238–2247. doi: 10.1038/sj.npp.1301353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson JL, Bevins RA. Bupropion hydrochloride produces conditioned hyperactivity in rats. Physiol Behav. 2007;90:790–796. doi: 10.1016/j.physbeh.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Vocci FJ, Appel NM. Approaches to the development of medications for the treatment of methamphetamine dependence. Addiction. 2007;102:96–106. doi: 10.1111/j.1360-0443.2007.01772.x. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Hosseini-Nia T. Anorectic and behavioural effects of bupropion. Gen Pharmac. 1988;19:201–204. doi: 10.1016/0306-3623(88)90061-4. [DOI] [PubMed] [Google Scholar]