Abstract
Psychopathy is a personality disorder that is strongly linked to criminal behavior. Using [18F]fallypride PET and BOLD fMRI, we show that impulsive-antisocial psychopathic traits selectively predict nucleus accumbens dopamine release and reward anticipation-related neural activity in response to pharmacological and monetary reinforcers, respectively. These findings suggest that neurochemical and neurophysiological hyperreactivity of the dopaminergic reward system may comprise a neural substrate for impulsivity, antisocial behavior and substance abuse in psychopathy.
The net annual burden of crime in the United States has been estimated to exceed $1 trillion(1), making criminal behavior a costly large-scale social problem and a critical target for scientific investigation. While the risk architecture underlying criminality is complex, psychopathy has emerged as a particularly robust predictor of criminal behavior and recidivism. Psychopathy is a personality disorder characterized by a combination of superficial charm, persistent instrumental antisocial behavior, marked sensation-seeking and impulsivity, blunted empathy and punishment sensitivity, and shallow emotional experiences(2). Recent research on the neural substrates of psychopathy has focused on the profound emotional deficits seen in psychopaths, and has emphasized the possible contributions of amygdala and ventromedial prefrontal cortex dysfunction to deficits in fear processing and empathy(3). However, while emotional and interpersonal deficits are often considered to be core features of the disorder, the empirical linkage of such deficits to criminality (particularly, to risk for committing violent crimes) is mixed(4–6). By contrast, the impulsive and antisocial facets of the disorder more consistently predict many of the most socially problematic behaviors associated with psychopathy, including violent criminal offending(5–7). To date, the neural underpinnings of this impulsive–antisocial dimension remain essentially unknown.
Prior research has also shown that psychopathic individuals are at markedly increased risk for developing substance use problems(8). Such associations mirror preclinical work demonstrating that impulsive traits predict enhanced susceptibility to drug-seeking and relapse(9). Given the strong link between psychopathy and substance abuse, an extensive literature demonstrating that the mesolimbic dopamine (DA) system plays a critical role in the pathophysiology of substance use disorders, and evidence that individual differences in the mesolimbic DA system predispose the development of substance abuse(9) we hypothesized an association between psychopathic traits and dysfunction within mesolimbic DA reward circuitry. To test the hypothesis that individuals with psychopathic traits are characterized by alterations in mesolimbic DA neurochemistry and neurophysiology, we used positron emission tomography (PET) imaging of psychostimulant-induced DA release, in concert with a functional magnetic resonance imaging (fMRI) probe of the reward system. Psychopathic traits were measured with the Psychopathic Personality Inventory (PPI), a well-validated trait measure of psychopathy, in a sample of community volunteers with no prior history of substance abuse (see Supplementary Data and Supplementary Discussion). Previous studies have shown that the PPI is composed of two underlying latent factors: a “fearless dominance” (PPI-FD) factor indexing emotional-interpersonal facets of psychopathy, and an “impulsive antisociality” (PPI-IA) factor linked to socially deviant behavior (10). Prior work has demonstrated that the PPI-IA (but not the PPI-FD) factor is selectively associated with aggression, impulsivity, and substance abuse in both incarcerated and community samples(7, 10). Given that animal studies have linked impulsive and aggressive traits to substance abuse and NAcc DA function(9, 11), we hypothesized that IA factor scores would selectively predict amphetamine-induced DA release within the NAcc.
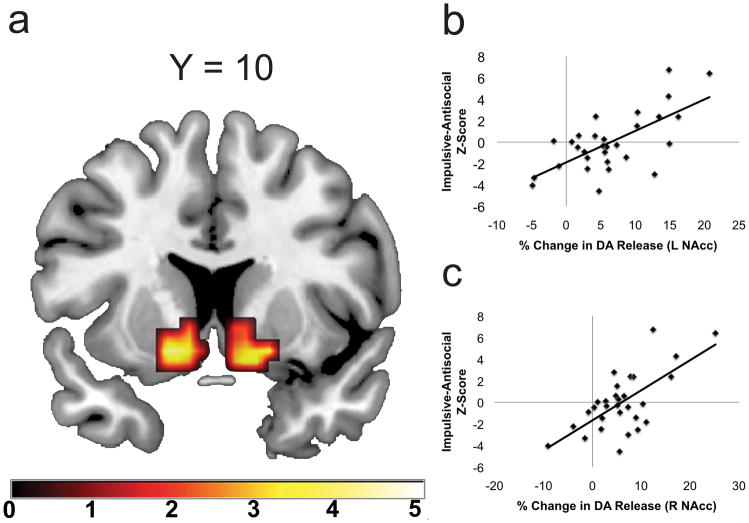
To examine the relationship between psychopathic traits and DA release, we performed voxelwise correlation analyses between PPI factor scores and maps of percent-change in [18F]fallypride binding potential (BP) between placebo and amphetamine within an anatomical NAcc region of interest (0.43 mg/kg, two-day, single-blind protocol, n = 30; See Supplementary Methods and Supplementary Figs. 1 and 2). PPI-IA scores were strongly correlated with amphetamine-induced DA release in bilateral NAcc (Fig. 1, see Supplementary Methods and Data). However, no relationship was observed between participants’ FD factor scores and amphetamine-induced NAcc DA release, even at a liberal statistical threshold of p < 0.1, uncorrected for multiple comparisons. Tests for differences in dependent correlations confirmed that the correlation between NAcc release and PPI-IA is significantly stronger than the correlation between NAcc DA release and PPI-FD (left NAcc: t27 =3.87; right NAcc: t27 = 2.86; p’s < 0,05; calculated using per-subject mean DA release values from entire anatomical NAcc ROI; Supplementary Fig. 3). Thus, trait psychopathy is associated with NAcc DA hyper-reactivity, and this relationship is selective for the impulsive-antisocial dimension of this construct.
Figure 1.
Impulsive-Antisocial traits predict nucleus accumbens DA release. A. Statistical Parametric Map (SPM) demonstrating that PPI-IA factor scores selectively predict increased amphetamine-induced DA release in bilateral NAcc (A; left NAcc: −16, 10, −10, pFDR = 0.003, Z = 3.74, k = 56; right NAcc: 16, 18, −6; pFDR = 0.002, Z = 4.21, k = 44). All coordinates reference MNI space. SPM thresholded at p < 0.05 uncorrected for visualization purposes. B–C. Scatter plot depicts the relationship between PPI-IA factor scores and amphetamine-induced DA release in left (B) and right (C) NAcc. DA release values extracted from clusters defined by a pFDR < 0.05 threshold. C. Left = Left, Right = Right. Color bar indicates t-statistic value.
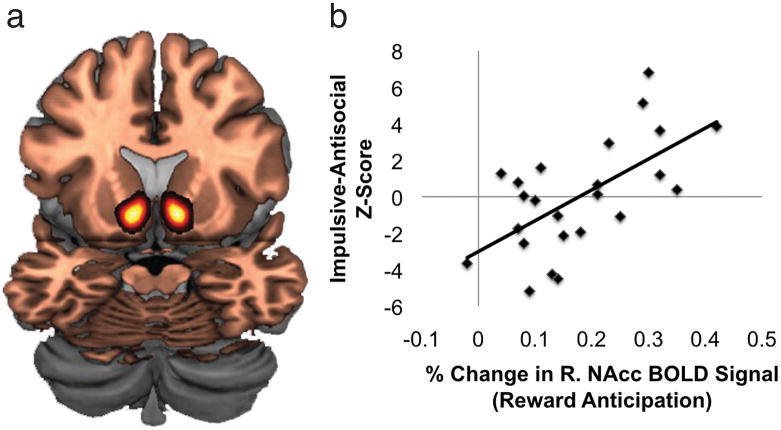
The observed trait-associated hypersensitivity to pharmacologically induced DA release in the NAcc raised the possibility that impulsive-antisocial traits might be linked to reward-related information processing biases in this region. Specifically, in light of recent findings that have demonstrated coupling between NAcc DA release and NAcc blood oxygen level-dependent (BOLD) signal(12), we predicted that individuals with high levels of impulsive-antisocial traits would show enhanced NAcc recruitment in response to monetary reinforcement. To test this possibility, we scanned 24 subjects with fMRI during the Monetary Incentive Delay task, which allows assessment of the extent of ventral striatal (NAcc) engagement by reward (see Supplementary Methods). To examine the relationship between psychopathic traits and reward-related NAcc BOLD signal, we calculated percent-change in NAcc BOLD signal for each subject (mean percent-change value within the anatomical ROI mentioned above) during reward anticipation trials (reward vs. no reward contrast). In a striking parallel to the PET data, we found a strong correlation between PPI-IA factor scores and BOLD signal in the right NAcc during reward anticipation (r = 0.63, p = 0.001; Figure 2; Supplementary Figs. 4 and 5; see Supplementary Data). The correlation between left NAcc BOLD signal and PPI-IA was also nominally significant [r = 0.44, p = 0.03] but did not survive correction for multiple correlation tests (see Supplementary Methods). Further mirroring the PET data, we did not find any relationship between PPI-FD factor scores and NAcc BOLD signal during reward anticipation (right NAcc: r = −0.02, p = 0.94; left NAcc: r = −0.18, p = 0.41; Fig. 2). A test for differences in dependent correlations revealed a significant difference in the correlations between right NAcc BOLD and PPI-IA vs. PPI-FD scores (t21 = 2.68, p < 0.05; Supplementary Fig. 6). Of note, no significant relationships were found between either PPI-IA or PPI-FD and BOLD signal during the reward feedback phase of the task in either the NAcc or in the medial prefrontal cortex (see Supplementary Data, Supplementary Fig. 7). These data indicate that NAcc information processing is selectively biased toward the anticipation of (i.e., the motivated preparation to obtain) potential rewards in individuals with high levels of IA traits, but not FD traits.
Figure 2.
Impulsive-Antisocial factor scores are selectively associated with NAcc BOLD signal during monetary reward anticipation. A. Image depicts the Harvard-Oxford Nucleus Accumbens anatomical region of interest from which BOLD signal estimates were obtained. B. Scatter plot depicts the relationships between PPI-IA and PPI-FD factor scores and reward anticipation-related BOLD signal in the right NAcc. Left = Left, Right = Right.
The remarkable correspondence between trait correlations for the PET and fMRI measures suggested that NAcc DA release and BOLD signal might themselves be linked. Eighteen subjects were available with both fMRI and PET data. For these subjects we examined the relationship between amphetamine-induced NAcc DA release and reward anticipation-related BOLD signal by extracting signal estimates from the NAcc region of interest used above. Correlation analysis confirmed a significant positive relationship between right NAcc DA release and right NAcc BOLD signal during reward anticipation (r = .49, p = 0.02; Supplementary Fig. 8), consistent with recent reports that NAcc engagement during reward anticipation may be at least partially driven by DA release (12).
Taken together, our PET and fMRI data indicate that one specific factor within the larger psychopathy trait construct – impulsive-antisociality – is associated with the neurochemistry and function of the NAcc. However, we were concerned that the present results might be due not to our principal construct of interest (the impulsive–antisocial dimension of psychopathy), but rather to related – but more general – personality constructs that do not necessarily access antisocial behavior or aggression. We therefore used multiple-regression analyses to control for the potentially confounding effects of individual differences in attentional/cognitive impulsivity (measured using the Barratt Impulsiveness Scale), Novelty-Seeking (measured using the Novelty Seeking scale from the Tridimensional Personality Questionnaire) and Extraversion (measured using the Extraversion scale from the NEO-PI-R). PPI-IA remained a significant predictor of NAcc amphetamine-induced DA release and reward-related BOLD signal even after adjusting for these scores (all p-values < 0.05; see Supplementary Data). On the whole, the pattern of these correlations suggests a unique link between impulsive-antisocial temperament and mesolimbic DA system hypersensitivity to pharmacological and monetary rewards that is at least partially independent from the effects of “purely” attentional or cognitive impulsivity, and from the effects of other higher-order or cardinal personality traits that are conceptually or empirically related to dopamine.
These data implicate DA as an important neurochemical modulator of individual differences in human antisocial personality traits, with impulsive-antisocial temperament predicting excess neurochemical and functional engagement of the mesolimbic DA system in response to reward. While a generation of clinical work has outlined a clear role for serotonin in impulsive violence, the role of DA in human antisociality has been largely overlooked. However, mounting preclinical research in rodents suggests that mesolimbic DA is critical for the expression of aggression: DA is released during aggressive episodes(13), NAcc DA blockade attenuates aggressive responding(14), trait differences in aggression are associated with NAcc DA levels(11), and genetic manipulations that reduce striatal DA clearance increase aggressive behavior(15). While aggression and antisociality are not synonymous, aggression is a strong behavioral correlate of impulsive-antisocial traits: PPI-IA scores significantly predict aggressive behavior in both incarcerated and community samples(7, 10). Further, insofar as we have demonstrated that antisocial traits are tied to alterations in a brain system that is compromised in addiction, these results suggest a neurobiological mechanism that mediates the linkage between substance abuse and personality disorders characterized by antisocial behavior.
How might mesolimbic DA hyperreactivity lead to the development of a psychopathic personality style? We propose a model that treats heightened stimulated NAcc DA release as a stable trait, which in turn leads to excessive recruitment of mesolimbic reward circuitry by behaviorally relevant environmental reinforcers (see Supplementary Discussion). This hypersensitivity could be due either to an intrinsic hyper-reactivity of midbrain DA neurons (i.e., heightened phasic midbrain DA neuron burst firing to potentially rewarding stimuli) or to diminished regulatory control of NAcc DAergic function stemming from a more widespread failure of inhibitory mechanisms (e.g. dysregulation of inhibitory afferents to the ventral tegmental area). Whatever the proximal mechanism, magnified brain responses during reward anticipation might lead to enhanced motivation to obtain reward; this predisposition, in concert with reduced sensitivity in brain regions responsible for detecting the emotions of others and involved in learning from aversive outcomes(3), could lead to the instrumental style of aggression that is common in psychopaths. By the same token, given evidence that aggression may itself have reinforcing properties that are similar in some ways to drugs of abuse(14), neurochemical and neurophysiological hypersensitivity to reinforcers within the mesolimbic DA system may promote the development of persistent aggression in parallel with its putative influence on substance abuse susceptibility.
Supplementary Material
Acknowledgments
The authors wish to thank Dr. Brian Knutson for kindly making available the MID task, and Candice Weiner and Maureen McHugo for assistance with fMRI scanning and data analysis. This research was funded by the National Institute on Drug Abuse (R01DA019670-04).
Footnotes
Author Contributions
JWB, RMK, and DHZ designed the study. ESS and ANS recruited participants into the study and collected PET and personality data. JWB collected fMRI data, with assistance from ESS and ANS. RL, NDW, and RMK performed single-subject PET data analysis and quality control. JWB performed group level PET data analysis, with assistance from MTT. JWB analyzed fMRI data at all stages. MSA and RB synthesized radiolabeled fallypride for PET scanning. SDB provided conceptual advice, statistical support, and supplementary analyses for the PPI data. RLC oversaw all medical aspects of the protocol. CES and RMK provided medical support for PET scanning. DC provided conceptual support and statistical advice for the study. JWB, MTT, and DHZ wrote the manuscript, with assistance from RLC.
References
- 1.Anderson DA. Journal of Law and Economics. 1999;42:611. [Google Scholar]
- 2.Hare RD. Psychiatr Clin North Am. 2006 Sep;29:709. doi: 10.1016/j.psc.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 3.Blair RJ. Philos Trans R Soc Lond B Biol Sci. 2008 Aug 12;363:2557. doi: 10.1098/rstb.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neumann CS, Hare RD. J Consult Clin Psychol. 2008 Oct;76:893. doi: 10.1037/0022-006X.76.5.893. [DOI] [PubMed] [Google Scholar]
- 5.Walters GD. Law Hum Behav. 2003 Oct;27:541. doi: 10.1023/a:1025490207678. [DOI] [PubMed] [Google Scholar]
- 6.Skeem JL, Mulvey EP. J Consult Clin Psychol. 2001 Jun;69:358. [PubMed] [Google Scholar]
- 7.Edens JF, Poythress NG, Jr, Lilienfeld SO, Patrick CJ. Behav Sci Law. 2008;26:529. doi: 10.1002/bsl.823. [DOI] [PubMed] [Google Scholar]
- 8.Smith SS, Newman JP. J Abnorm Psychol. 1990 Nov;99:430. doi: 10.1037//0021-843x.99.4.430. [DOI] [PubMed] [Google Scholar]
- 9.Everitt BJ, et al. Philos Trans R Soc Lond B Biol Sci. 2008 Oct 12;363:3125. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benning SD, Patrick CJ, Hicks BM, Blonigen DM, Krueger RF. Psychol Assess. 2003 Sep;15:340. doi: 10.1037/1040-3590.15.3.340. [DOI] [PubMed] [Google Scholar]
- 11.Lewis MH, Gariepy JL, Gendreau P, Nichols DE, Mailman RB. Neuropsychopharmacology. 1994 Apr;10:115. doi: 10.1038/npp.1994.13. [DOI] [PubMed] [Google Scholar]
- 12.Schott BH, et al. J Neurosci. 2008 Dec 24;28:14311. doi: 10.1523/JNEUROSCI.2058-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrari PF, van Erp AM, Tornatzky W, Miczek KA. Eur J Neurosci. 2003 Jan;17:371. doi: 10.1046/j.1460-9568.2003.02447.x. [DOI] [PubMed] [Google Scholar]
- 14.Couppis MH, Kennedy CH. Psychopharmacology (Berl) 2008 Apr;197:449. doi: 10.1007/s00213-007-1054-y. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguiz RM, Chu R, Caron MG, Wetsel WC. Behav Brain Res. 2004 Jan 5;148:185. doi: 10.1016/s0166-4328(03)00187-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.