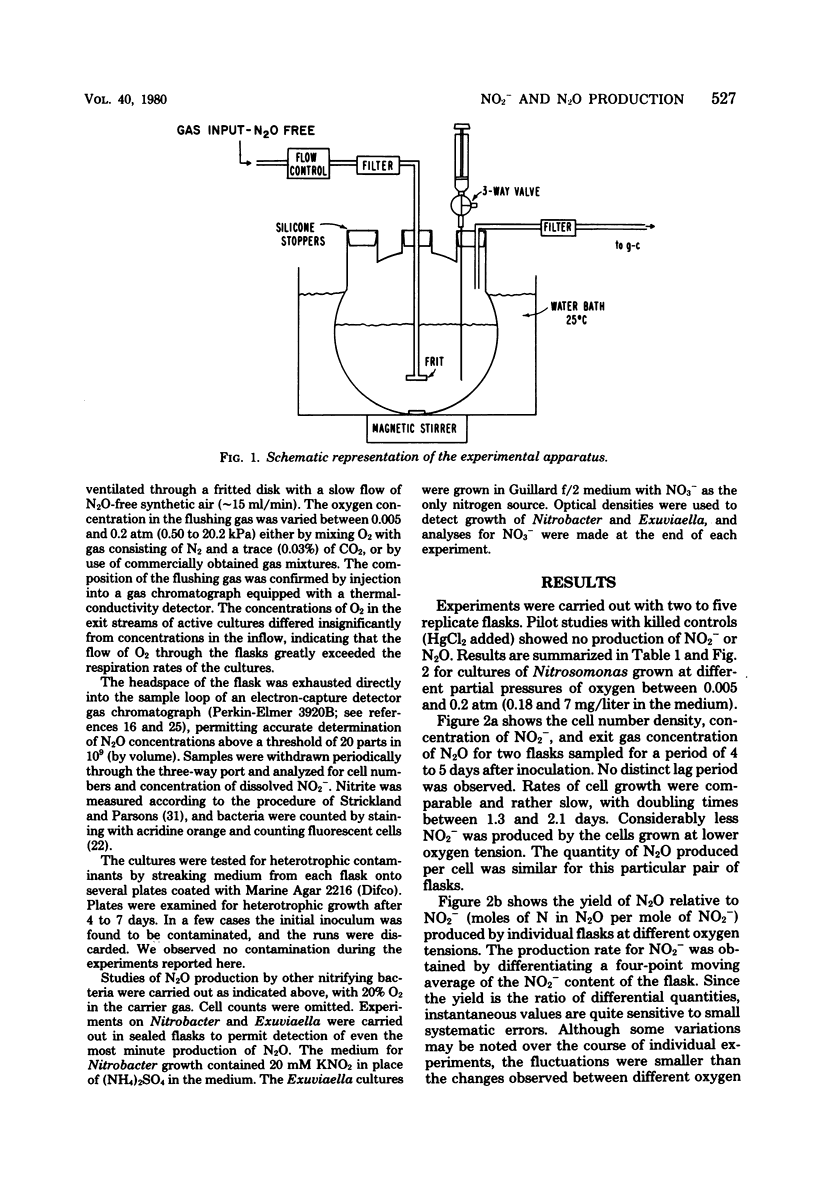
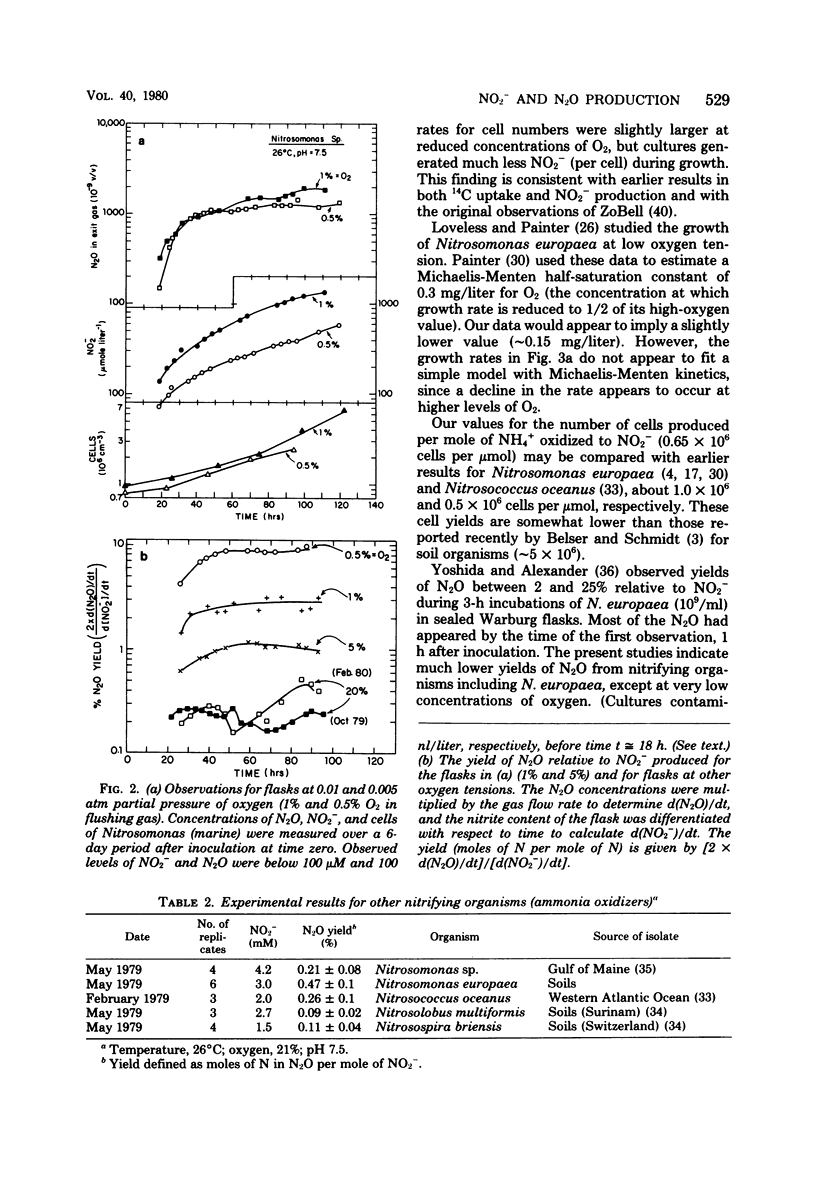
Abstract
Pure cultures of the marine ammonium-oxidizing bacterium Nitrosomonas sp. were grown in the laboratory at oxygen partial pressures between 0.005 and 0.2 atm (0.18 to 7 mg/liter). Low oxygen conditions induced a marked decrease in the rate for production of NO2-, from 3.6 × 10−10 to 0.5 × 10−10 mmol of NO2- per cell per day. In contrast, evolution of N2O increased from 1 × 10−12 to 4.3 × 10−12 mmol of N per cell per day. The yield of N2O relative to NO2- increased from 0.3% to nearly 10% (moles of N in N2O per mole of NO2-) as the oxygen level was reduced, although bacterial growth rates changed by less than 30%. Nitrifying bacteria from the genera Nitrosomonas, Nitrosolobus, Nitrosospira, and Nitrosococcus exhibited similar yields of N2O at atmospheric oxygen levels. Nitrite-oxidizing bacteria (Nitrobacter sp.) and the dinoflagellate Exuviaella sp. did not produce detectable quantities of N2O during growth. The results support the view that nitrification is an important source of N2O in the environment.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belser L. W., Schmidt E. L. Diversity in the ammonia-oxidizing nitrifier population of a soil. Appl Environ Microbiol. 1978 Oct;36(4):584–588. doi: 10.1128/aem.36.4.584-588.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner J. M., Blackmer A. M. Nitrous oxide: emission from soils during nitrification of fertilizer nitrogen. Science. 1978 Jan 20;199(4326):295–296. doi: 10.1126/science.199.4326.295. [DOI] [PubMed] [Google Scholar]
- Delwiche C. C. The nitrogen cycle. Sci Am. 1970 Sep;223(3):137–146. [PubMed] [Google Scholar]
- ENGEL M. S., ALEXANDER M. Growth and autotrophic metabolism of Nitrosomonas europaea. J Bacteriol. 1958 Aug;76(2):217–222. doi: 10.1128/jb.76.2.217-222.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen K., Carlucci A. F., Boström K. Growth of some chemoautotrophic bacteria at different oxygen tensions. Experientia. 1966 Apr 15;22(4):229–230. doi: 10.1007/BF01900925. [DOI] [PubMed] [Google Scholar]
- Hooper A. B., Terry K. R. Hydroxylamine oxidoreductase of Nitrosomonas. Production of nitric oxide from hydroxylamine. Biochim Biophys Acta. 1979 Nov 9;571(1):12–20. doi: 10.1016/0005-2744(79)90220-1. [DOI] [PubMed] [Google Scholar]
- Watson S. W. Reisolation of Nitrosospira briensis S. Winogradsky and H. Winogradsky 1933. Arch Mikrobiol. 1971;75(3):179–188. doi: 10.1007/BF00408979. [DOI] [PubMed] [Google Scholar]
- Yoshinari T. N2O reduction by Vibrio succinogenes. Appl Environ Microbiol. 1980 Jan;39(1):81–84. doi: 10.1128/aem.39.1.81-84.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]


