Abstract
The Advanced Cognitive Training for Independent and Vital Elderly (ACTIVE) trial is a randomized, controlled, single-masked trial designed to determine whether cognitive training interventions (memory, reasoning, and speed of information processing), which have previously been found to be successful at improving mental abilities under laboratory or small-scale field conditions, can affect cognitively based measures of daily functioning. Enrollment began during 1998; 2-year follow-up will be completed by January 2002. Primary outcomes focus on measures of cognitively demanding everyday functioning, including financial management, food preparation, medication use, and driving. Secondary outcomes include health-related quality of life, mobility, and health-service utilization. Trial participants (n = 2832) are aged 65 and over, and at entry into the trial, did not have significant cognitive, physical, or functional decline. Because of its size and the carefully developed rigor, ACTIVE may serve as a guide for future behavioral medicine trials of this nature.
Keywords: Cognitive decline, activities of daily living, behavioral intervention
BACKGROUND AND CONTEXT OF THE TRIAL
Persons over the age of 65 account for almost half of all days of care in short stay hospitals, constitute the majority of residents of nursing homes, and account for over 75% of required formal home-based care supports [1–4]. Interventions that can postpone or prevent hospitalization or need for formal care, therefore, have much to contribute to both reducing morbidity and health-care costs and improving the quality of life among older people. This article describes a clinical trial designed to examine the effects of perceptual and cognitive interventions on the primary outcome of cognitively demanding tasks of daily living and secondary outcomes of health-related quality of life, mobility, and health-services utilization. This trial, called ACTIVE for Advanced Cognitive Training for Independent and Vital Elderly, is sponsored by the National Institute on Aging and the National Institute of Nursing Research at the National Institutes of Health. ACTIVE is a unique clinical trial in that it links specific cognitive and perceptual interventions to broader behavioral outcomes.
A number of studies have successfully used laboratory-based interventions to improve cognitive performance in older adults [5–10]. Other studies have identified a relationship between the cognitive and perceptual abilities that were the target of the interventions and performance on everyday activities such as driving and medication recall [11–13]. Reasoning ability has been shown to predict subsequent performance on cognitively demanding tasks of daily living, such as comprehension of medication labels, utilization of emergency telephone information, and understanding of transportation schedules [14, 15]. In addition, training on perceptual processing speed, an aspect of speed perceptual functioning that refers to how much information can be cognitively processed from the perceptual field during brief exposures, has been shown to impact aspects of driving performance [16–19].
Research has also demonstrated an association between decline in cognitive functioning and measures of hospitalization, and need for formal care, an association between quality of life and institutionalization; and an association between both mild and severe cognitive impairment and mortality in community-dwelling elderly [20–23].
The findings discussed above cite multiple studies and replications that demonstrate the success of certain cognitive interventions at improving cognitive performance on specific mental and perceptual abilities and some everyday cognitive activities [5–10, 12, 16]. Moreover, they illustrate clear links between decline in cognitive function and subsequent hospitalization, need for formal care, and mortality [20–23]. However, these data do not represent a clear consensus on the likelihood that cognitive interventions will improve or maintain performance on everyday cognitive activities, or that they will enhance well-being or mobility or reduce health-service utilization in the older population. The above studies differed in the outcome measures examined, in the interventions tested, and in the samples recruited, thus making generalizations across different studies impossible. Essentially no research has been conducted demonstrating training transfer to real-world functional outcomes in later adulthood. The present trial seeks to address these shortcomings by testing identical interventions at each of several sites using common inclusion-exclusion criteria and common outcome measures. Therefore, the trial will evaluate the applicability of the findings to a sample that is geographically, racially, and economically diverse.
ACTIVE formally began in September 1996, when 5-year cooperative agreements were awarded to six field centers: the University of Alabama at Birmingham, the Boston Hebrew Rehabilitation Center for Aged, the Indiana University School of Medicine, the Johns Hopkins University, the Pennsylvania State University, and Wayne State University. The data coordinating center was awarded to the New England Research Institutes.
PRIMARY OBJECTIVE AND HYPOTHESES
The primary objective of the trial is to test the effects of three distinct cognitive interventions, previously found to be successful in improving elders’ performance on basic measures of cognition under laboratory or small-scale field conditions on primary outcome measures of cognitively demanding daily activities related to living independently (e.g., food preparation, driving, medication use, financial management). Each of the three interventions is targeted at improving memory, or reasoning, or speed of information processing. A secondary objective of the trial is to investigate the process by which the interventions affect the primary outcomes, including exploring whether a set of proximal outcomes (measures of the basic abilities being trained) serve as mediators of the effects of cognitive training on the primary outcome measures.
Figure 1 consists of a schematic diagram of the major study hypotheses. Embedded in Figure 1 is the process assumption that the effects of cognitive training on primary outcomes will be largely mediated through the basic abilities being trained. That is, reasoning-trained participants will show greater and specific improvement on measures of reasoning relative to all other training and control groups. Likewise, memory-trained participants will show disproportionate and specific improvement on proximal memory measures, and speed-trained participants will show greater and specific improvement on tasks assessing attentional processing speed. Taking the example of reasoning training effects on the primary outcome construct labeled “everyday problem solving,” it is expected that a key component of the process by which enhancement of the primary outcome occurs will be via improvement in the proximal outcome of reasoning performance. This assumption is based on prior findings that reasoning and everyday problem solving are strongly related constructs [14, 15, 24].
Figure 1.
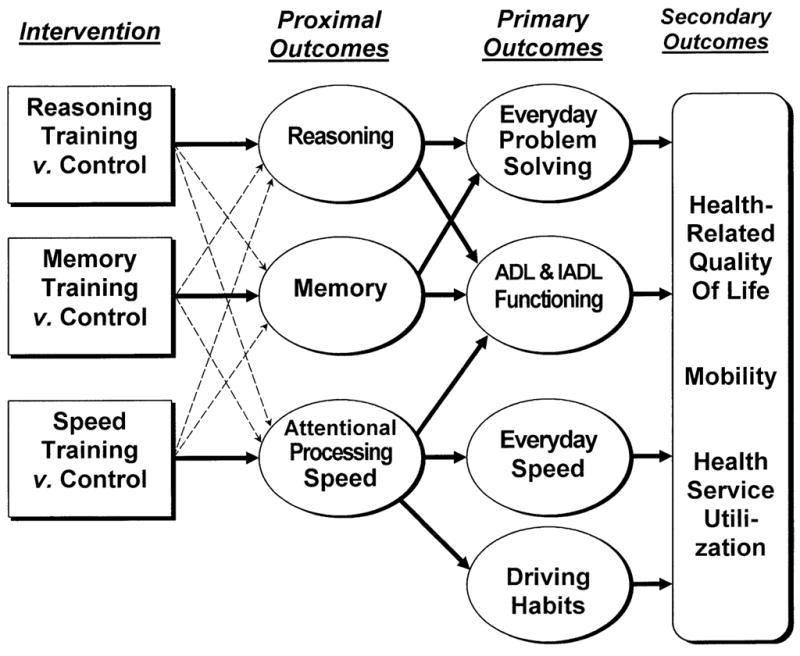
Hypothesized mode of effects in ACTIVE trial. Influence of intervention on primary and secondary outcomes is mediated through trained abilities. Bold lines represent specific effects of training. Dashed lines represent nonspecific effects of training on related abilities (e.g., through social contact or general cognitive arousal).
Drawing on the traditions of intent-to-treat analyses in clinical trials research, our primary study hypotheses address only the expected relations between study interventions and the primary outcomes. The process expectations (i.e., proximal outcomes as mediators of training effects) inform these hypotheses and will be examined in secondary analyses. The hypotheses refer to expected intervention group differences at each post-test occasion of measurement (immediate post-test, first annual post-test, second annual post-test). The hypotheses governing the primary intent-to-treat analyses can be generally stated as follows.
Ability-Specific Primary Outcome Hypotheses
Memory and reasoning training groups are expected to perform better than speed training or control groups on the primary outcome construct labeled everyday problem solving, based on previous research showing strong relationships between memory and reasoning and the measures represented by this composite [14, 15, 24].
The speed training group is expected to perform better than the memory and reasoning training groups and the control group on primary outcome constructs representing everyday speed and driving habits. Again, these hypotheses are informed by prior research showing strong, specific relationships between the speed of processing measures and these outcome domains [5, 16].
Effects of Booster Training Hypothesis
Each intervention group that receives booster training is expected to perform better than its corresponding intervention group not receiving booster training on primary outcomes represented in the first and second hypotheses.
Ability-General Primary Outcome Hypothesis
Participants in each intervention group are expected to perform better than participants in the control group on the primary outcome construct of activities of daily living and instrumental activities of daily living functioning, representing self-rated independence and difficulty with a variety of everyday self-care and home maintenance tasks.
Ability-General Secondary Outcome Hypotheses
In subsequent secondary outcome analyses, it is expected that health-related quality of life, everyday mobility, and health-services utilization will similarly show a general performance benefit for participants in each intervention group, relative to participants in the control group.
DESIGN
ACTIVE is a randomized, controlled, single-masked trial utilizing a four-group design (Figure 2). The four groups include three treatment arms and a no-contact control group. Each treatment arm consists of a ten-session training intervention for one of three cognitive abilities (memory, reasoning, and speed of processing). Testers are blind to participant treatment assignment. Training exposure and social contact have been standardized across the three interventions so that each intervention can serve as a contact control for the other interventions. Therefore, the design allows for testing of both social contact effects, as represented by the dashed lines in Figure 1 (via the contact control groups) and retest effects (via the no-contact control group) on outcomes.
Figure 2.
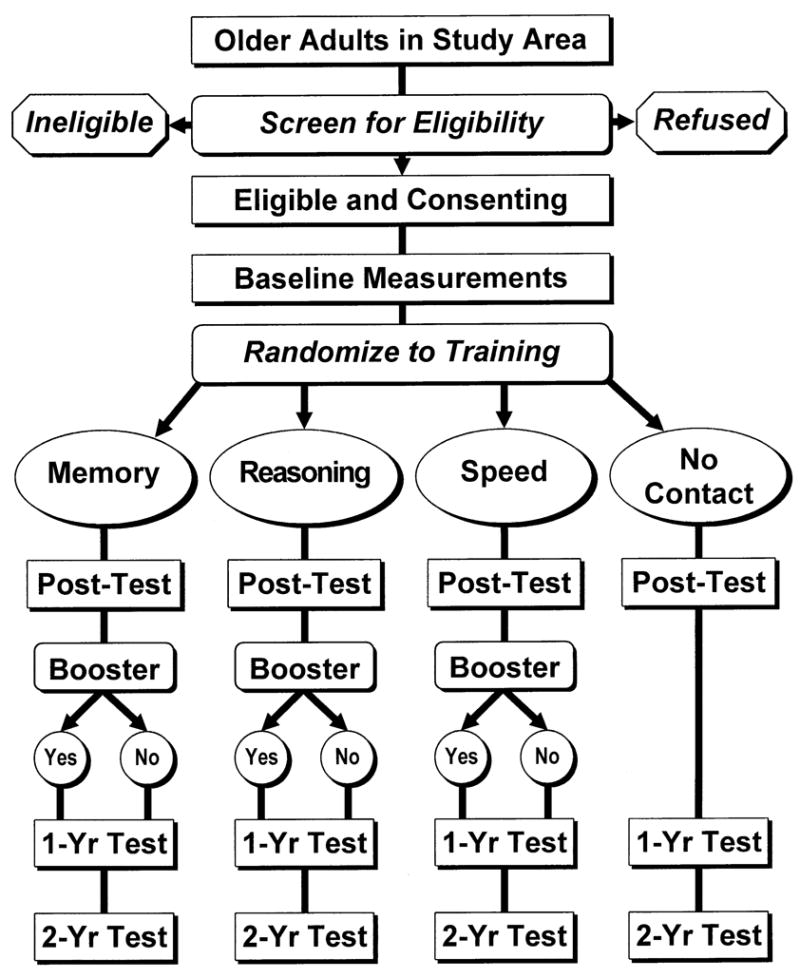
ACTIVE study design.
Selection of the treatment conditions (memory training, reasoning training, speed of processing training, and a no-contact control condition) was based on a number of considerations. First, the three targeted abilities, memory, reasoning, and speed of processing, have been shown in longitudinal research to exhibit relatively early age-related decline, beginning on average in the mid-sixties [25]. Second, performance on these abilities has been shown to be associated with performance of cognitively demanding instrumental activities of daily living, critical for independent living in our society [11, 14, 15, 18, 24]. Instrumental activities of daily living focus on the individual’s competence in taking medications, managing finances, shopping, telephone usage, household management, transportation, and meal preparation. Third, these interventions have been shown to be effective in training the targeted abilities and to be maintained by booster training. Memory training has been shown to affect memory ability more than control or placebo treatments, and booster training facilitates long-term remembering [8, 26–31]. Reasoning training has been shown in prior descriptive and intervention research to affect reasoning ability and to persist up to 7 years after initial training [32–36]. Speed of processing training has been shown to facilitate speed of processing performance [16, 37]. Fourth, prior descriptive and intervention research has shown that the training outcomes are specific to the ability trained, such that hypotheses regarding the pattern of training transfer can be clearly articulated [16, 32, 33, 37–39]. That is, reasoning training should transfer broadly to a number of reasoning measures, but not to measures of memory or speed of processing. Similarly, memory training should transfer to a number of memory measures, but not to reasoning or speed measures. The same pattern of training transfer is predicted for speed of processing.
STUDY POPULATION
Inclusion-Exclusion Criteria
The ACTIVE trial focuses on older adults who may be at risk for loss of independence from causes to which the variables under study contribute. A key element is to identify participants at risk for functional declines, but who have not yet experienced them. Thus, the ACTIVE investigators selected a population living largely independent of formal care at the point of entry into the study. Diversity in representation of older adults was another goal, with a particular emphasis on representation of African-American elders (who have been consistently under-represented in most previous cognitive training research with older adults).
Despite the broad sampling goals of ACTIVE, it also became necessary to identify criteria (see Table 1) that would exclude persons who: (a) were less than 65 years of age at initial screening; (b) had already experienced substantial cognitive decline (score of 23 or less on the Mini-Mental State Examination [40]; self-report diagnosis of Alzheimer’s Disease); (c) had experienced substantial functional decline (self-report of needing extensive assistance with dressing, personal hygiene, or bathing) [41]; (d) had medical conditions that would likely predispose participants to imminent functional decline (e.g., recent stroke) or that would be likely to result in mortality before the 2-year follow-up phase of the trial had been completed (e.g., certain cancers or currently undergoing chemotherapy/radiation treatment); (e) had severe sensory losses such that participants would be unlikely to be able to participate in the ACTIVE testing protocol, even with substantial accommodations (self-report of extreme difficulty reading ordinary newspaper print or a performance-tested vision test score of worse than 20/50) [42]; (f) had communicative difficulties so substantial that they would prevent persons from effectively participating in the highly interactive study protocol (based on an interviewer’s rating of a person’s ability to be understood and to understand others); (g) had recent cognitive training; or (h) were unavailable during the testing and training phases of the study (i.e., planning to move out of the area, anticipating being away from the study site for an extended period during the upcoming year, or having other commitments that would substantially limit availability).
Table 1.
Testing Battery
| Measures | Screening | Baseline | Immed. Post-Test | 1st Ann. Post-Test | 2nd Ann. Post-Test | Dimension | Mode | Reference |
|---|---|---|---|---|---|---|---|---|
| Eligibility measures | ||||||||
| Age | X | telephone interview | ||||||
| Mini-Mental State Examination | X | X | cognitive status | telephone interview | 40 | |||
| Activities of daily living: dressing, hygiene, bathing | X | in-person interview | 41 | |||||
| Selected health conditions | X | telephone interview | ||||||
| Current chemo/radiation | X | telephone interview | ||||||
| Vision (self-report) | X | telephone interview | 42 | |||||
| Vision acuity | X | X | physical measurement | |||||
| Communication | X | telephone interview | ||||||
| Prior cognitive training | X | telephone interview | ||||||
| Availability for study | X | telephone interview | ||||||
| Proximal outcomes | ||||||||
| Hopkins Verbal Learning Test | X | X | X | X | memory | paper/pencil | 43 | |
| Rey Auditory-Verbal Learning Test | X | X | X | X | memory | paper/pencil | 44 | |
| Rivermead Behavioral Memory Test | X | X | X | X | memory | paper/pencil | 45 | |
| Word series | X | X | X | X | reasoning | paper/pencil | 46 | |
| Letter series | X | X | X | X | reasoning | paper/pencil | 47 | |
| Letter sets | X | X | X | X | reasoning | paper/pencil | 48 | |
| Useful Field of View | X | X | X | X | speed | computer | 11, 13, 19 | |
| Digit Symbols Substitution | X | X | X | X | speed | paper/pencil | 49 | |
| Digit Symbols Copy | X | X | X | X | speed | paper/pencil | 49 | |
| Vocabulary | X | X | X | X | paper/pencil | 48 | ||
| Primary Outcomes | ||||||||
| Everyday Problems Test | X | X | X | X | everyday problem solving | paper/pencil | 56 | |
| Observed Tasks of Daily Living | X | X | X | everyday problem solving | observed | 14, 57 | ||
| Complex Reaction Time; 2 tests | X | X | X | X | everyday speed of processing | computer | 16 | |
| Timed Instrumental Activities of Daily Living | X | X | X | X | everyday speed of processing | observed | 58 | |
| Minimum Data Set—Home Care: 3 scores | X | X | X | general functional ability | in-person interview | 41 | ||
| Driving habits: difficulty, avoidance, space | X | X | X | driving habits | in-person interview | 59 | ||
| Secondary outcomes | ||||||||
| MOS 36-item Short Form (SF-36) | X | X | X | quality of life | self- administered | 60 | ||
| Falls | X | X | X | mobility | in-person interview | 61 | ||
| Lifespace | X | X | X | mobility | in-person interview | 61 | ||
| Motor vehicle crashes | X | mobility | state motor vehicle records | |||||
| Health-service utilization | X | X | X | health-care utilization | in-person interview; Medicare claims files | 62–63 | ||
| Covariates | ||||||||
| Chronic disease list | X | X | X | health | telephone interview/in-person interview | 64 | ||
| Health behaviors | X | X | X | health | in-person interview | 63 | ||
| Medication audit | X | X | health | transcription | 65 | |||
| Height and weight | X | X | physical | physical measurement | ||||
| Blood pressure and pulse | X | X | X | physical | physical measurement | |||
| Vision acuity | X | X | physical | physical measurement | 66 | |||
| 360° turn | X | X | physical | physical measurement | 67 | |||
| Grip strength | X | X | physical | physical measurement | 68 | |||
| Personality in Intellectual Contexts Inventory | X | X | X | X | psychosocial | self- administered | 69 | |
| Memory Functioning Questionnaire | X | X | X | X | psychosocial | self- administered | 70 | |
| Center for Epidemiological Studies–Depression-12 | X | X | X | psychosocial | self- administered | 71 | ||
| Sociodemographics | X | telephone interview | ||||||
| Mini-Mental State Examination | X | X | cognitive status | in-person interview | 40 | |||
| Hearing (self-report) | X | telephone interview | ||||||
Recruitment
Recruitment began in March 1998 and ended in October 1999. A total of 2832 persons were enrolled in the trial. Each field center had a specific study population and recruitment strategy. The University of Alabama at Birmingham (UAB) recruited participants from two population sources: (1) residents of Jefferson County, Alabama, who were licensed to drive or who had nondriver identification cards through the Alabama Department of Public Safety; and (2) patients of the UAB eye clinics. The Hebrew Rehabilitation Center for Aged in Boston recruited residents from congregate and senior housing sites, from senior centers, and from a registry of volunteers interested in participating in aging research at the Harvard Cooperative Program on Aging in Massachusetts. Indiana University recruited clients of the Community Centers of Indianapolis, a network of 14 facilities that provide activities and social services for older adults. Indiana University also recruited through local churches and senior citizens organizations. Johns Hopkins University recruited participants from senior centers, churches, senior housing, and senior community organizations in the Baltimore metropolitan area and in Cumberland, Maryland. Johns Hopkins University also recruited through the wellness and service programs offered by the Urban Medical Institute in Baltimore and in partnership with the Commission on Aging and Retirement Education, an organization that coordinates and funds services in Baltimore. Pennsylvania State University recruited from the enrollment files of a state-funded pharmaceutical assistance program for low-income elders, called PACE. Wayne State University recruited from a large range of community organizations, churches, hospital-based senior assessment centers, and senior housing sites in metropolitan Detroit as well as from driver registration lists. Recruitment strategies varied by site and included on-site presentations with letters to interested persons, newspaper advertisements, introductory letters, and follow-up telephone calls. Due to logistical considerations related to testing and training a large sample, recruitment and all subsequent field work were conducted in six replicates of approximately 8 weeks duration.
Sample Characteristics
Sociodemographic and functional characteristics of the 2832 participants are displayed in Table 2. The participants are predominantly female and range in age from 65–94 years with an average age of 73.6 years. The majority (72%) are white, 26% are African American, and 2% are from other minority groups. Participants are not likely to be married, and most report good to excellent health. As a result of the screening criteria, they are not likely to report functional or cognitive impairments. However, as indicated by the data in Table 2, showing the prevalence of chronic conditions, the sample includes persons at risk for functional decline.
Table 2.
Sample Characteristics (n=2832)
| % | Mean | S.D. | Range | |
|---|---|---|---|---|
| Gender | ||||
| Female | 75.8 | |||
| Age | 73.6 | 5.9 | 65–94 | |
| Years of education | 13.5 | 2.7 | 4–20 | |
| Race | ||||
| Caucasian | 72.1 | |||
| African American | 26.0 | |||
| Other | 1.7 | |||
| Unknown | 0.2 | |||
| Marital status | ||||
| Married | 35.9 | |||
| Self-reported health | ||||
| Excellent/very good | 43.9 | |||
| Good | 40.4 | |||
| Fair/poor | 15.7 | |||
| Chronic conditions | ||||
| Hypertension | 51.0 | |||
| Cataracts | 49.7 | |||
| Hypercholesterolemia | 44.0 | |||
| Ischemic heart disease | 19.2 | |||
| Osteoporosis | 17.9 | |||
| Chronic lung disease | 14.9 | |||
| Cognitive status | ||||
| Mini-Mental State Examination score | 27.3 | 2.0 | 23–30 | |
| Functional status | ||||
| IADLa total performance | 4.3 | 4.9 | 0–26 | |
| IADL difficulty | 1.4 | 2.4 | 0–20 | |
| ADLb total performance | 0.3 | 0.9 | 0–11 | |
Instrumental activities of daily living.
Activities of daily living.
OUTCOME MEASURES
In the ACTIVE trial, a large number of measurement approaches are used in the collection of data. These include telephone interviewing, face-to-face interviews, administration of standardized paper-and-pencil tests, computer-administered tests, observational measurement of activity performance, measurement of physical functioning, self-administered questionnaires, transcription of medications taken, collection of archival data from Medicare/Medicaid health-service utilization records, and collection of driving records from state departments of motor vehicles. Specific measures, along with the schedule and mode of administration and the dimension assessed, are displayed in Table 1.
Proximal Outcomes
Proximal outcomes refer to direct outcome measures of training effects. As explicated above, it was hypothesized that in order for training effects to generalize to real-world, or primary, study outcomes, it must first be shown that training is effective in modifying the specific cognitive abilities targeted for intervention (i.e., memory, reasoning, and speed of processing).
For memory training, three proximal outcomes are assessed: Hopkins Verbal Learning Test, Related Word Lists [43]; Rey Auditory-Verbal Learning Test, Unrelated Word Lists [44]; and the Rivermead Behavioral Memory Test Paragraph Recall task [45]). From each measure, several proximal outcome variables are derived (e.g., total recall, recognition, and learning gain from first to last trial). Because specific memories of lists or paragraphs may be recalled for long periods of time, parallel (not identical) memory stimuli are administered at each testing occasion. Scores represent the number of words or text propositions correctly recalled.
For reasoning training, three direct proximal outcomes are assessed (Word Series [46]; Letter Series [47]; and Letter Sets [48]). These measures are standardized, timed, paper-and-pencil assessments, and are administered at each testing occasion. Scores represent the number of correct responses in the time allowed.
For speed of processing training, the main proximal outcome is the Useful Field of View measure [11, 13, 19], which is computer-administered. The measure involves four tasks. For each task, the dependent variable is the minimum (shortest) presentation time needed by participants to correctly perform the task 75% of the time. The four tasks represent increments in difficulty, from a fairly simple identification task in which participants must determine which of two objects (a car or a truck) appears in a fixation box in the center of a 17-inch color computer touch screen, up to a fairly complex task in which participants must judge which configuration of objects (two cars, two trucks, a car and a truck) appears in a fixation box in the center of the screen, while simultaneously identifying the location of a peripheral target on the outside of a cluttered display. Other speed proximal outcome variables are the Digit Symbol Substitution [49] and Digit Symbols Copy [49]. Vocabulary [48] is a nonspecific proximal outcome.
Primary Outcomes
The trial’s primary outcomes include measures of everyday functioning, specifically cognitively demanding in the tradition of the instrumental activities of daily living [50, 51] and activities of daily living [52–55], as incorporated in the Minimum Data Set for Home Care [41]. The primary outcome measures were selected because they are directly relevant to the primary objectives of the trial. Of specific interest in this trial are those everyday activities that involve a cognitive component, such as financial management, food preparation, or medication use, or have been affected by cognitive training in previous research such as driving [16]. In addition, we are interested in multimode assessment of daily function, including self-report, paper-and-pencil test, and observed performance. Therefore, in contrast with most clinical trials that involve a single primary outcome, ACTIVE has multiple primary outcome measures, specifically 11 different measures, as displayed in Table 1. These measures cover four major domains of functional ability, that is, everyday problem solving, personal and instrumental activities of daily living, everyday speed of processing, and driving habits, as shown in Figure 1.
Everyday problem solving is assessed with two measures of the ability to process everyday materials and answer questions about them. In the Everyday Problems Test [56], participants are presented with 14 everyday stimuli (e.g., medication labels, transportation schedules, telephone rate charts, Medicare benefit charts) and are asked to answer two questions about each stimulus. Scores represent the number of correct answers generated. In the Observed Tasks of Daily Living [14, 57], participants are presented with a number of tasks (e.g., examining medication bottles to determine which might be associated with particular side effects, balancing a checkbook). Their performance is observed and recorded by data collectors and is later scored for correctness by independent scorers. Based on prior research, the Everyday Problems Test and the Observed Tasks of Daily Living measures are expected to be most closely related to memory and reasoning and should be most positively affected by those interventions.
Everyday Speed was assessed with the Complex Reaction Time task [16] and the Timed Instrumental Activities of Daily Living tasks [58]. In the Complex Reaction Time test (a computer-administered task), the time taken for participants to perform particular motor behaviors (e.g., clicking a mouse, moving a mouse) following the screen presentation of an instructional road sign is recorded. Of primary interest is the time taken (as recorded via stopwatch) to successfully complete each task. Accuracy of response is also recorded. The Timed Instrumental Activities of Daily Living measure presents participants with five tasks (finding a telephone number in a phone book, making change, reading food can ingredients, finding items on a shelf, and reading directions on medicine containers).
Activities of daily living and instrumental activities of daily living functioning are assessed with a self-report measure, drawn from the Minimum Data Set methodology [41]. Instrumental activities of daily living functioning questions assessed participants’ self-report performance and capacity for seven instrumental activities of daily living areas: preparing meals, housework, managing finances, managing health care, phone use, shopping, and travel. Participants are asked to describe their performance in all of the related tasks over the last 7 days. A score that describes their degree of independence is selected from a five-point scale. A second score, which describes their perceived degree of difficulty for each task, is also selected. Activities of daily living functioning is assessed through a series of self-report questions in which participants are asked to rate their independent performance in tasks related to bathing, dressing, and personal hygiene.
Driving habits are assessed with three measures: Total driving space, total exposure to difficult driving, and total driving avoidance score [59]. Each of these scores is weighted equally in the driving habits composite. Total driving space is a score reflecting the extent of travel (e.g., restricted driving to one’s own neighborhood versus frequently driving across state lines). Total exposure to difficult driving is a measure of the degree of difficulty experienced across a number of driving tasks (e.g., merging, turning left across traffic). Total driving avoidance is a score reflecting the number of driving situations a driver avoids (e.g., avoids driving in the rain, avoids driving alone). Thus those drivers who will drive only in their own neighborhood during daylight with a friend would have a very low driving habits composite score, while those driving at any time and in all situations with no difficulty would have a high score.
Secondary Outcomes and Covariates
Secondary outcomes of the trial include the impact of illness on quality of life/well-being, everyday mobility (including self-reports of incidence and frequency of falls, life space, and abstracted archival driving record information on crashes), and health-service utilization [60–63]. These variables are included to determine whether cognitive interventions influence long-term health-care utilization and independent living.
Covariates are assessed in several domains including presence of chronic disease [64], health behaviors (smoking, alcohol consumption, medication usage) [63], medications taken [65], physical characteristics (height, weight, blood pressure, pulse rate, visual acuity [66], 360-degree turn [67], grip strength [68]), and psychosocial variables (locus of control using the Personality in Intellectual Aging Contexts Inventory [69], memory self-efficacy using the Memory Functioning Questionnaire [70], depressive symptoms using the Center for Epidemiological Studies-Depression-12 Scale [71]), demographics (gender, age, education), cognitive impairment [40], and hearing self-report.
FIELD METHODS
Enrollment and Baseline Assessment
A general overview of the field design is shown in Figure 2. At all field centers, potential participants were first screened by telephone, during which all but two of the study eligibility assessments were made. Individuals who met eligibility criteria and were interested were asked to participate in an in-person assessment. The two eligibility tests that required in-person administration, the vision test and the test of cognitive function (Mini-Mental State Examination), were administered at the beginning of individual assessment part I. After administration of these two measures and if the participant was eligible and willing, assessment continued with baseline measures. The baseline assessment was completed by two additional testing sessions, individual assessment part II and group assessment, as well as a take-home questionnaire of self-report psychosocial measures that was completed and returned at the last testing session. Total time required for screening and baseline assessment averaged 4–5 hours and was scheduled over three visits. The schedule of test administration is displayed in Table 1.
Informed consent followed the requirements of each institution’s human subjects review committee. Consent to participate was obtained for three points of assessment: telephone screening (verbal consent), in-person assessment (written consent), and completion of baseline assessment/full-study participation prior to randomization (written consent).
Randomization
The field centers used a computer randomization program in the study data management system to randomize participants to one of four treatment groups. Participants were randomized only after completing all baseline testing. Centers randomized participants as close to the start of training as was feasible to minimize study dropout/deactivation between the point of randomization and the start of treatment (training) due to changes in participants’ personal circumstances.
Follow-up Assessments
Post-testing occurs at three points in time (Table 1). All participants are being followed for 24 months. The immediate post-test was conducted < 10 days following completion of training and averaged 2.5 hours for completion (June 1998–January 2000). It consisted of tests to measure the proximal (cognitive) outcomes and selected primary outcomes and was completed in individual and group sessions with a self-administered questionnaire completed between the sessions. The first annual post-test was conducted at 12 months (May 1999–January 2001) and the second annual post-test will be conducted at 24 months (May 2000–January 2002) following completion of training. The length of these sessions is similar to that of baseline assessment (that is, about 4 hours). Both consist of individual and group sessions with self-administered questionnaires and include primary and secondary outcome measures (function and health-service utilization) as well as key health covariates. On average, annual post-tests are completed within 4 weeks of the date of the anniversary that a participant completed training. Specific test intervals are measured and included as an individual differences covariate. Specific measures collected at each post-test are listed in Table 1.
Standardization of Assessment
Standardization of data collection procedures is ensured through a variety of training and quality-control procedures. Data collectors are masked with regard to treatment group assignment. All data collectors from all field centers participated in an intensive 4-day central training workshop at the Coordinating Center in March 1998. The training sessions included didactic presentations regarding study design, recruitment issues, and general research interviewing principles; detailed instruction in the administration of each test or measurement procedure; demonstrations of each test/measurement; and practice sessions with other data collectors and then with older volunteers from the community.
Uniform certification requirements were established for telephone interviewers (for telephone screening only) and testers (all data collection activities). The requirements include review of the training manuals, attendance at central training, and demonstrated competence in administration of all tests and measurements, including coding and scoring. All data collectors had to complete the requirements and be certified by the Coordinating Center prior to any data collection activities at the field center. The Coordinating Center maintained responsibility for training and certification of telephone interviewers conducted via conference calls, local observation of certified staff, and review of audiotaped interviews.
At the central workshop, staff from each field center were further trained as local testers and certifiers after practice with the measurement protocol and observation by qualified, experienced testers. Local certifiers are responsible for subsequent training and certification of locally hired field testing staff at each of the field centers. Regular quality-control monitoring is conducted for all field staff: at least every 6 months, local certifiers observe and complete a standardized assessment checklist for each data collector and conduct debriefing sessions with each of the field staff members. Principal investigators, certified site coordinators, and central site visit personnel also participate in this quality-control monitoring.
TREATMENTS
Common Features of the Treatments
Initial Training Phase
The intensity and duration of the ACTIVE cognitive interventions are based on the effects found in previous research and considerations of participant burden and are approximately equal for each intervention. The interventions are provided in small-group settings by a certified trainer in ten 60- to 75-minute sessions. The optimum group size was three to four participants per group, with a maximum number of five participants per group. Participants received all ten training sessions in a specified order within a 6-week interval. Two or three sessions were administered during each week of training. However, due to special circumstances, a small number of participants needed to receive training in less than 6 weeks. The minimum interval for compressed training was 2 weeks. Participants who attended at least 80% of the training sessions (eight out of ten) were considered compliant with the intervention program. Make-up sessions were scheduled when sessions (or parts of sessions) were missed; these make-up sessions involved small groups of participants whenever possible.
The ACTIVE cognitive intervention conditions of memory, reasoning, and speed of processing training shared a number of key elements, including (1) focusing on strategies for solving problems, remembering, or responding quickly to information; (2) modeling and demonstrating of strategy usage by trainers; (3) practicing on exemplar problems; (4) individual and group exercises; (5) feedback on performance; (6) fostering of self-efficacy with regard to performance; (7) applying strategies to real-world tasks; (8) individualized training experiences; and (9) activities focusing on social interaction. In all three conditions, Sessions 1–5 focused on strategy instruction and exercises to practice the strategy. Sessions 6–10 provided additional practice exercises, but no new strategies were introduced. Content for each of the ten sessions was scripted in a trainer’s manual. Initial training was conducted between May 1998 and December 1999.
Booster Training
For each of the three intervention conditions, booster training was provided to a subset of participants approximately 11 months after the end of the primary training. To determine the effect of booster training, participants in each intervention condition were randomly selected, with the restriction that they had to have completed 80% of the initial training sessions to actually receive booster training. It was estimated that 10% of intervention participants would refuse or not be eligible to receive booster training; therefore, a 60% random subsample was drawn from each training sample to ensure that analysis of the booster training would have close to 50% of participants in the booster and nonbooster group for each training condition.
The booster training was delivered in four 75-minute sessions over a 3-week period. The structure and content of the sessions were similar to those used in the primary training. The goal was to help participants maintain the gains made from the initial training and to further improve their perceptual and cognitive skills. Booster training was conducted from May 1999 through December 2000.
Training of the Intervention Trainers
Training, certification, and quality-control monitoring of the intervention trainers paralleled that for data collectors. Prior to the implementation of the ACTIVE intervention trial, all trainers from all field centers participated in an intensive 6-day central training workshop at the Coordinating Center. For interventionists hired after April 1998, central workshops were held as needed at Pennsylvania State University for reasoning trainers, at Johns Hopkins University for memory trainers, and at the University of Alabama Birmingham or Western Kentucky University for speed trainers. Training was conducted by the principal investigators, who were responsible for developing the interventions and supervising intervention delivery. Over the course of the workshop, trainers received detailed instruction in and observed demonstrations of the training procedures, and they led practice sessions with other trainers and with older volunteers from the community. Each trainer also attended didactic sessions on topics such as the rationale for the multisite intervention trial, strategies for handling participant and group problems, addressing health and medical issues, self-efficacy concerns involving aging and cognition, and environmental issues.
Trainers were not allowed to be cross-trained in the other intervention conditions if they served as primary trainers for the intervention trial. Trainers who backed up the primary trainers could be cross-trained. However, back-up trainers could serve as a primary trainer in only one training condition during any given replicate of the trial. This was done to reduce the potential for contamination of training conditions.
Uniform certification requirements for trainers in all three intervention conditions were established. They included reviewing the training manuals and participant materials for a given intervention condition, attending central training, practicing all ten training sessions with at least one older volunteer at the local field centers, being observed by a certifier, and completing a learning worksheet and environmental checklist. All trainers had to complete the certification requirements and be certified by the Coordinating Center prior to the beginning of training for a replicate. Conference calls led by the principal investigators expert in each training condition were held with individual field centers and with all field centers together to monitor trainers and training activities. At least every 6 months, each trainer and back-up trainer was observed by another certified trainer at the field center. A standardized assessment checklist was completed and a debriefing session was held with each trainer. In addition, back-up trainers were required to participate in training activities to ensure that they maintained their proficiency in a training condition. Thus, the interventions were delivered in a standardized fashion across training sites, both within a field center and across field centers.
Memory Training
Several components of the memory training were designed to promote active transfer of the mnemonic training to everyday cognitive tasks. These included instruction and extensive practice in multiple mnemonic strategies, specifically, strategies for organizing stimulus materials into meaningful categories (e.g., grocery shopping lists), organizing main ideas and details for remembering everyday text-based information (e.g., medication labels), and visualizing and associating items to be remembered (e.g., list of errands).
After a brief introduction and overview of the main memory ability targeted by the training session, participants received instruction in a strategy or mnemonic rule, exercises to practice the rule, individual and group feedback on performance, self-efficacy enhancement, and a practice test. In strategy instruction the trainer described the rule or strategy in relation to four basic memory principles (meaningfulness, organization, visualization, and association) and then modeled strategy usage with concrete examples. Participants practiced the strategy during both individual and group exercises involving laboratory-like memory tasks as well as memory tasks that are related to activities of everyday life.
Reasoning Training
Reasoning training focused on improving the ability to solve problems that require linear thinking and that follow a serial pattern or sequence. Such problems involved identifying the pattern in a series of letters or numbers, or understanding the pattern in an everyday activity such as the dosing for a prescription drug or a travel schedule. Participants were taught strategies to identify the pattern or sequence required to solve the problem and how to apply these strategies to determine the next item in the pattern. Participants were given an opportunity to practice the strategies in both individual and group exercises. The exercises involved abstract reasoning tasks (e.g., letter series) as well as reasoning problems that are related to activities of daily living (e.g., identifying medication dosing pattern and filling in a pill reminder case). Participants were given an opportunity to practice the strategies in both individual and group exercises.
Due to the large individual differences in baseline performance by ACTIVE participants on reasoning measures during the ACTIVE pilot study, we decided to develop and utilize two levels of reasoning training that differed in three ways: (1) difficulty/complexity of tasks presented during the early training sessions; (2) pacing and amount of instructional time on tasks; and (3) relative emphasis on the trainer’s modeling and demonstration of strategy usage. The two levels were similar in the focus on strategies, practice on problems, feedback, and fostering of self-efficacy.
Speed of Processing Training
Speed of processing training focused on improving the speed of visual search and the ability to perform one or more attentional tasks quickly. Speed of processing was trained by systematically reducing the stimulus duration in a series of progressively more difficult information processing tasks presented via computer. In the simplest task (Task 1), participants were asked to identify objects at increasingly brief exposures. Once this ability was mastered at the shortest possible stimulus duration, participants were asked to divide their attention between two tasks: stimulus identification in the center of a large computer monitor and localization of another target presented somewhere in peripheral vision (Task 2). Again task difficulty was increased by either decreasing stimulus duration, expanding the area within which targets can be localized, or increasing the difficulty of the central identification task. Once this task was mastered for the most difficult condition and the minimum stimulus exposure, Task 3 added visual distractors to the stimulus display. Stimulus duration was then reduced systematically once again, in response to improving performance, alternating with increasing task difficulty as in Task 2. Finally, in the most difficult training conditions (Task 4), task demand was increased even further by superimposing an auditory identification component over the visual tasks. Although this individualized computer-based training has traditionally been conducted one-on-one with a trainer, it was modified for the purpose of this trial to accommodate a group training approach.
ANALYTICAL APPROACHES
Based on observed effect sizes in previous cognitive training research, an effect size of 0.20 has been established as the minimum criterion for judging treatment effectiveness. Power calculations were based on the assumptions of six Bonferroni-corrected two-sided comparisons with an overall alpha error of 0.05 (0.0042 for each comparison), a correlation of 0.7 between baseline and follow-up (based on pilot data), and an 80% completion rate. Following the methods of Cohen, the sample size of 2832 will yield 95% power to detect an effect size of 0.20 [72]. Based on the same assumptions, there will be 90% power to detect booster training effects in comparison of the subgroup receiving booster training in a training condition versus the control group.
To use all of the data collected over time, the analysis will utilize mixed effects models, also known as random effects models, Laird-Ware models, and hierarchical linear models [73]. In terms of the model, the study hypotheses translate into hypotheses about model parameters that carry the effect of the interaction between time and intervention group. All of the main analyses will be on an intent-to-treat (as randomized) basis. The booster training will be assessed by including additional covariates in the mixed effects model. Corrections for multiple tests are not planned among the four primary outcomes.
A complication of the ACTIVE study design is that multiple scores are collected that are designed to assess the same underlying domain of functional ability. In order to simplify the analysis, each primary outcome measure will be standardized to its baseline values. A single score for each of the four primary outcome domains will then be created as a linear combination of these standardized measures. The single scores are referred to as “composite” outcomes. The advantage of this technique is that it is relatively simple to explain and discuss. It also maintains the overall type I error rate within domain.
One problem anticipated with the analysis is missing data. As in any longitudinal trial, participants may drop out of the study before follow-ups are complete. In addition, participants may fail to complete all of the measures even on testing occasions when they are available for follow-up. In many studies, it may be plausible to assume that data are missing at random in the sense of Little and Rubin [74]. If the data are missing at random, then analyses based on maximum likelihood, such as the mixed effects models proposed here, will be unbiased. In the ACTIVE trial, it seems plausible that individual measures that are missing at a testing occasion when some measures are observed are missing at random. However, when a whole visit is missing, it seems much more likely that the missing data will be nonignorable nonresponse [74]. Unfortunately, there is no way of diagnosing nonignorable nonresponse, and no single way to correct the problem. A careful consideration must be made as to whether it is a plausible cause of the missing data, and if so, the best approach is to test the sensitivity of the results to a range of proposed nonignorable non-response mechanisms that might plausibly have caused the missing data.
The approach will be to use the method of multiple imputation to deal with missing measures within a domain when other measures within the domain are observed at a given visit [75]. This will result in the maximum number of composite scores without introducing bias, assuming the missing-at-random assumption is valid and the multiple imputation is appropriate. A mixed-effects model will then be used on this data set as a basis for comparisons. Finally, a series of different multiple imputation schemes will be used, each incorporating a different nonignorable nonresponse mechanism, to assess the sensitivity of the basic model.
DISCUSSION
The ACTIVE trial is a randomized, controlled, single-masked trial designed to determine whether cognitive interventions, previously found to be successful at improving mental abilities under laboratory or small-scale field conditions, can affect cognitive-based measures of daily functioning. Primary outcomes focus on measures of cognitively demanding everyday functioning, including financial management, food preparation, medication use, and driving. Secondary outcomes include quality of life and health-service utilization; these variables are included to determine whether cognitive interventions influence long-term health-care utilization and independent living. Trial participants are aged 65 and over, and may be at risk for, but at entry into the trial did not have significant, cognitive, physical, or functional decline.
ACTIVE is unique in that it links specific cognitive and perceptual interventions to broader behavioral outcomes that are basic to living independently, have a strong cognitive component, and whose decline has been associated with loss of independence. The strengths of ACTIVE are the common protocols, the common screening batteries, the large number of participants (2832 enrolled), and the diversity of participants on racial, economic, and geographical variables. If successful, these interventions may reduce morbidity, reduce health-care costs, and improve the quality of life for older adults.
The racial diversity of the study population, which includes 26% African-American participants (Table 2), is important. African Americans have been underrepresented in cognitive aging studies; therefore, a significant aspect of this trial is whether these interventions are effective with this population. It is, however, unfortunate that developing the measurement instruments and training in languages other than English was beyond the resources available for this trial. Therefore, any ethnic group analysis will be restricted to English-speaking participants.
A key issue in the development of the protocol for this trial was what type of control group to use. Control groups normally used in clinical trials include placebo, sham treatment, social contact, usual care, and no-contact. There is no pharmacologic treatment or other usual care for normal older adults for cognitive performance. Therefore, usual care could be considered to be no care and thus no-contact was considered to be the equivalent control group to usual care. Because no pharmacologic treatment exists, no pharmacologic placebo could be used in this trial. Moreover, previous investigations of cognitive training and occupational therapy interventions to improve functioning have shown that a no-contact control group does not differ from a placebo social-contact group [34, 76]. Most importantly, the design of the trial allows multiple estimates of the nontreatment effects on proximal study outcomes by estimating the influence of each training intervention (e.g., Reasoning) on nontrained abilities relevant to the other two interventions (e.g., Speed of Processing, Memory). In this manner, the trial allows the estimation of effects such as attention, social contact, and general cognitive arousal.
Another key issue in the development of the protocol for the trial was whether to give some, all, or none of the participants booster training. Prior research has shown that booster training is effective in maintaining long-term improvement in cognitive performance [30, 31]. However, because the effectiveness of booster training is an empirical question, it was decided to provide booster training to half of the trained participants and determine analytically the effectiveness of the booster training in this protocol. To ensure that approximately half of the participants received booster training, 60% were invited to attend booster training. The as-treated analysis is important here because the assignment to booster training was not deemed appropriate unless the subject had complied with the intervention, and therefore this experiment could not be generalized to the wider population.
In ACTIVE we restricted the primary trainers to training only on one intervention. As mentioned previously, this was done to reduce the potential for contamination of training conditions. It should be noted, however, that a consequence of restricting the trainers in this manner confounds training and trainers. Therefore, at an individual field center, we cannot separate the effects of training and each individual trainer. Theoretically, confounding should be less of a problem with a multisite study than with a single site. Moreover, at some field centers, different trainers were used in different replicates, so the effects of trainers can be separated from training at those field centers. The ideal solution would be to have multiple trainers for each intervention at each site. However, this solution would be very costly.
This study has several limitations. First, it is limited by the specific training interventions designed. For example, there are other memory intervention programs and other cognitive and perceptual programs in the extant literature that could have been used. The study is limited by the intensity and duration of the interventions used. The study is limited by the confounding of trainers and interventions; that is, at each site each interventionist trained only one intervention. However, multiple interventionists were used across sites and occasionally, within sites. Finally, although the study population was geographically, economically, and somewhat ethnically diverse, the study is limited by its restriction to English-speaking participants.
ACTIVE is one of the largest purely behavioral treatment trials. Because of its size and the carefully developed rigor, it may serve as a guide to future alternative/complementary medicine trials of this nature, such as comparing these behavioral interventions to cognitively enhancing drugs, or both behavioral and pharmacological interventions, or delivering the interventions by community groups.
Acknowledgments
This study is being conducted by cooperative agreements (U01AG14260; U01AG14263; U01AG14276; U01AG14282; U01AG14289; U01NR04507; U01NR04508) from the National Institute on Aging and the National Institute of Nursing Research, National Institutes of Health.
APPENDIX: ACTIVE RESEARCH INVESTIGATORS
The committees and investigators at the field centers for ACTIVE are listed below.
ACTIVE Steering and Publications Committees
Karlene Ball, PhD, Karin F. Helmers, PhD, Jared B. Jobe, PhD, Michael Marsiske, PhD, John N. Morris, PhD, George W. Rebok, PhD, David M. Smith, MD, Sharon L. Tennstedt, PhD, RN, and Sherry L. Willis, PhD
ACTIVE Advisory Committee and Data Safety Monitoring Board
Laurence G. Branch, PhD, Duke University Medical School; Sara T. Fry, PhD, RN, FAAN, Boston College School of Nursing; John J. McArdle, PhD, Department of Psychology, University of Virginia; Stephanie A. Studenski, MD, Center on Aging, University of Kansas Medical Center; Timothy Salthouse, PhD, School of Psychology, Georgia Institute of Technology; Barbara Tilley, PhD, Department of Biometry & Epidemiology, College of Medicine, Medical University of South Carolina; and May L. Wykle, PhD, RN, FAAN, University Center on Aging and Health, Frances Payne Bolton School of Nursing, Case Western Reserve University.
ACTIVE Coinvestigators and Locations
Frank Ahern, PhD, Department of Biobehavioral Health, Pennsylvania State University (PSU); Jason C. Allaire, MA, Institute of Gerontology and Department of Psychology, Wayne State University (WSU); Jason Brandt, PhD, School of Medicine, Johns Hopkins University (JHU); Gretchen Cornwell, PhD, Department of Sociology, PSU; Mary Kay Cresci, RN, PhD, School of Nursing, JHU; Ada Davis, RN, PhD, School of Nursing, JHU; Manfred Diehl, PhD, Department of Psychology, University of Colorado, Colorado Springs; Linda Duke, PhD, Department of Psychology, University of Alabama Birmingham (UAB); Karen S. Dunn, RN, MS, Institute of Gerontology and College of Nursing, WSU; Jeffrey W. Dwyer, PhD, Institute of Aging, University of Florida; R. Darin Ellis, PhD, Institute of Gerontology and Department of Industrial and Manufacturing Engineering, WSU; Melissa M. Franks, PhD, Institute of Gerontology and Department of Psychology, WSU; Joseph Gallo, MD, MPH, School of Medicine, University of Pennsylvania; Pearl German, ScD, School of Public Health, JHU; Ann L. Horgas, RN, PhD, Institute of Gerontology and College of Nursing, WSU; Kathy E. Johnson, PhD, Department of Psychology, Indiana University-Purdue University at Indianapolis; Richard N. Jones, ScD, Hebrew Rehabilitation Center for Aged (HRCA), Boston; Gail Jensen, PhD, Institute of Gerontology and Department of Economics, WSU; Kenneth Kleinman, ScD, New England Research Institutes; Gisela Labouvie-Vief, PhD, Department of Psychology, WSU; Paul F. Malloy, PhD, Butler Hospital, Providence, RI; Gerald McGwin, PhD, School of Public Health, UAB; Cynthia Owsley, PhD MPH, Department of Ophthalmology, UAB; George W. Reed, PhD, New England Research Institutes; Jeffery Roseman, MD, PhD, School of Public Health, UAB; Adrienne L. Rosenberg, MS, HRCA; Michael Sloane, PhD, Department of Psychology, UAB; Cynthia Steele, RN, PhD (Expected), MPH, School of Medicine, JHU; Fredrick W. Unverzagt, PhD, Department of Psychiatry, Indiana University School of Medicine; Virginia Wadley, PhD, Center for Research in Applied Gerontology, UAB; Morris Weinberger, PhD, Regenstrief Institute for Health Care and HSR&D of Indianapolis VAMC; Keith E. Whitfield, PhD, Department of Biobehavioral Health, PSU; Frederic D. Wolinsky, PhD, Department of Health Services Research, School of Public Health, Saint Louis University; Elizabeth Wright, PhD, New England Research Institutes; Susan Yutz, PhD, School of Nursing, PSU; Steven Zarit, PhD, Department of Human Development and Family Studies, PSU.
References
- 1.Graves EJ, Kozak LJ. National hospital discharge survey: Annual summary, 1996. Vital Health Stat. 1999 Jan;(140):i–iv. 1–46. Series 13. [PubMed] [Google Scholar]
- 2.Strahan GW. Advance Data from Vital and Health Statistics. 280. Hyattsville, MD: National Center for Health Statistics; 1997. An overview of nursing homes and their current residents: Data from the 1995 National Nursing Home Survey. [PubMed] [Google Scholar]
- 3.General Accounting Office. Report to the Chairman, Special Committee on Aging, US Senate. GAO/HEHS; 1996. Medicare: Home Health Utilization Expands While Program Controls Deteriorate; pp. 96–16. [Google Scholar]
- 4.Levit KR, Lazenby HC, Braden BR, et al. National health expenditures, 1996. Health Care Financ Rev. 1997;19:161–200. [PMC free article] [PubMed] [Google Scholar]
- 5.Ball K, Beard BL, Roenker DL, Miller RL, Griggs DS. Age and visual search: Expanding the useful field of view. J Opt Soc Am. 1998;5:2210–2219. doi: 10.1364/josaa.5.002210. [DOI] [PubMed] [Google Scholar]
- 6.Baltes PB, Willis SL. Plasticity and enhancement of intellectual functioning in old age: Penn State’s Adult Enrichment Project (ADEPT) In: Craik FIM, Trehub S, editors. Aging and Cognitive Processes. New York: Plenum Press; 1982. [Google Scholar]
- 7.Greenberg C, Powers SM. Memory improvement among adult learners. Educ Gerontol. 1987;12:385–394. [Google Scholar]
- 8.Rebok GW, Balcerak LJ. Memory self-efficacy and performance differences in young and old adults: Effects of mnemonic training. Dev Psychol. 1989;25:714–721. [Google Scholar]
- 9.Willis SL. Cognitive training and everyday competence. In: Schaie KW, editor. Annual Review of Gerontology and Geriatrics. Vol. 7. New York: Springer; 1987. [PubMed] [Google Scholar]
- 10.Yesavage JA. Nonpharmacologic treatments for memory losses with normal aging. Am J Psychiatry. 1985;142:600–605. doi: 10.1176/ajp.142.5.600. [DOI] [PubMed] [Google Scholar]
- 11.Ball K, Owsley C, Sloane ME, Roenker DL, Bruni JR. Visual attention problems as a predictor of vehicle crashes in older drivers. Invest Ophthalmol Vis Sci. 1993;34:3110–3123. [PubMed] [Google Scholar]
- 12.Leirer VO, Morrow DG, Pariante GM, Sheikh JI. Elders’ nonadherence, its assessment, and computer assisted instruction for medication recall training. J Am Ger Soc. 1988;36:877–884. doi: 10.1111/j.1532-5415.1988.tb05779.x. [DOI] [PubMed] [Google Scholar]
- 13.Owsley C, Ball K, Sloane ME, Roenker DL, Bruni JR. Visual/cognitive correlates of vehicle accidents in older drivers. Psychol Aging. 1991;6:403–415. doi: 10.1037//0882-7974.6.3.403. [DOI] [PubMed] [Google Scholar]
- 14.Diehl M, Willis SL, Schaie KW. Everyday problem solving in older adults: Observational assessment and cognitive correlates. Psychol Aging. 1995;10:478–491. doi: 10.1037//0882-7974.10.3.478. [DOI] [PubMed] [Google Scholar]
- 15.Willis SL, Jay GM, Diehl M, Marsiske M. Longitudinal change and prediction of everyday task performance in the elderly. Res Aging. 1992;14:68–91. doi: 10.1177/0164027592141004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ball K, Owsley C. Increasing mobility and reducing accidents in older drivers. In: Schaie KW, editor. Societal Impacts on Mobility in the Elderly. New York: Springer; 2000. [Google Scholar]
- 17.Owsley C, McGwin G, Ball K. Vision impairment, eye disease, and injurious motor vehicle crashes in the elderly. Ophthalmic Epidemiol. 1998;5:101–113. doi: 10.1076/opep.5.2.101.1574. [DOI] [PubMed] [Google Scholar]
- 18.Ball K, Owsley C, Stalvey B, et al. Driving avoidance and functional impairment in older drivers. Accident Analysis and Prevention. 1998;30:313–322. doi: 10.1016/s0001-4575(97)00102-4. [DOI] [PubMed] [Google Scholar]
- 19.Owsley C, Ball K, McGwin G, et al. Visual processing impairment and risk of motor vehicle crash among older adults. JAMA. 1998;279:1083–1088. doi: 10.1001/jama.279.14.1083. [DOI] [PubMed] [Google Scholar]
- 20.Wolinsky FR, Johnson RJ. The use of health services by older adults. J Gerontol: Soc Sci. 1991;46:S345–S357. doi: 10.1093/geronj/46.6.s345. [DOI] [PubMed] [Google Scholar]
- 21.Branch LG, Jette AM. A prospective study of long-term care institutionalization among the aged. Am J Public Health. 1982;72:137–139. doi: 10.2105/ajph.72.12.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelman HR, Thomas C, Kennedy GJ, Cheng J. Cognitive impairment and mortality in older community residents. Am J Public Health. 1994;84:1255–1260. doi: 10.2105/ajph.84.8.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swan GE, Carmelli D, LaRue A. Performance on the digit-symbol substitution test and 5-year mortality in the Western Collaborative Group Study. Am J Epidemiol. 1995;141:32–40. doi: 10.1093/oxfordjournals.aje.a117342. [DOI] [PubMed] [Google Scholar]
- 24.Willis SL. Everyday cognitive competence in elderly persons: Conceptual issues and empirical findings. Gerontologist. 1996;36:595–601. doi: 10.1093/geront/36.5.595. [DOI] [PubMed] [Google Scholar]
- 25.Schaie KW. The Seattle Longitudinal Study. New York: Cambridge University Press; 1996. Intellectual Development in Adulthood. [Google Scholar]
- 26.Kliegl R, Smith J, Baltes PB. On the locus and process of magnification of age differences during mnemonic training. Dev Psychol. 1990;26:894–904. [Google Scholar]
- 27.Lachman ME, Weaver SL, Bandura M, Elliot E, Lewkowicz C. Improving memory and control beliefs through cognitive restructuring and self generated strategies. J Gerontol: Psychol Sci. 1992;47:P293–P298. doi: 10.1093/geronj/47.5.p293. [DOI] [PubMed] [Google Scholar]
- 28.Mohs RC, Ashman TA, Jatzen K, et al. A study of the efficacy of a comprehensive memory enhancement program in healthy elderly persons. Psychiatry Res. 1998;77:183–195. doi: 10.1016/s0165-1781(98)00003-1. [DOI] [PubMed] [Google Scholar]
- 29.Rasmusson DX, Rebok GW, Bylsma FW, Brandt J. Effects of three types of memory training in normal elderly. Aging Neuropsychol Cognit. 1999;6:56–66. [Google Scholar]
- 30.McDougal GJ. Cognitive interventions among older adults. In: Fitzpatrick JJ, editor. Annual Review of Nursing Research. New York: Springer Publishing; 1999. [PMC free article] [PubMed] [Google Scholar]
- 31.Oswald WD, Rupprecht R, Gunzelmann T, Tritt K. The SIMA-project: Effects of 1 year cognitive and psychomotor training on cognitive abilities of the elderly. Behav Brain Res. 1996;78:67–72. doi: 10.1016/0166-4328(95)00219-7. [DOI] [PubMed] [Google Scholar]
- 32.Willis SL. Current issues in cognitive training research. In: Lovelace EA, editor. Aging and Cognition: Mental Processes, Self Awareness, and Interventions. Amsterdam: Elsevier; 1990. [Google Scholar]
- 33.Willis SL, Schaie KW. Training the elderly on the ability factors of spatial orientation and inductive reasoning. Psychol Aging. 1986;1:239–247. doi: 10.1037//0882-7974.1.3.239. [DOI] [PubMed] [Google Scholar]
- 34.Willis SL, Cornelius SW, Blow FC, Baltes PB. Training in research in aging: Attentional processes. J Educ Psychol. 1983;75:257–270. [Google Scholar]
- 35.Willis SL, Nesselroade CS. Long term effects of fluid ability training in old-old age. Dev Psychol. 1990;26:905–910. [Google Scholar]
- 36.Willis SL, Schaie KW. Cognitive training in the normal elderly. In: Forette F, Christen Y, Boller F, editors. Plasticité Cérébrale et Stimulation Cognitiv. Paris: Foundation National de Gérontologie; 1994. [Google Scholar]
- 37.Ball K. Enhancing mobility in the elderly: Attentional interventions for driving. In: Dollinger C, DiLalla LF, editors. Assessment and Intervention Issues across the Lifespan. Mahwah, NJ: Lawrence Erlbaum Associates; 1997. [Google Scholar]
- 38.Neely AS, Backman L. Effects of multifactorial memory training in old age: Generalizability across tasks and individuals. J Gerontol: Psychol Sci. 1995;50B:P134–P140. doi: 10.1093/geronb/50b.3.p134. [DOI] [PubMed] [Google Scholar]
- 39.Schaie KW, Willis SL, Hertzog C, Schulenberg JE. Effects of cognitive training on primary mental ability structure. Psychol Aging. 1987;2:233–242. doi: 10.1037//0882-7974.2.3.233. [DOI] [PubMed] [Google Scholar]
- 40.Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 41.Morris JN, Fries BE, Steel K, et al. Comprehensive clinical assessment in community setting: Applicability of the MDS-HC. J Am Ger Soc. 1997;45:1017–1024. doi: 10.1111/j.1532-5415.1997.tb02975.x. [DOI] [PubMed] [Google Scholar]
- 42.Mangione CM, Phillips RS, Seddon JM, et al. Development of the “Activities of Daily Vision Scale.” A measure of visual functional status. Med Care. 1992;30:111–126. doi: 10.1097/00005650-199212000-00004. [DOI] [PubMed] [Google Scholar]
- 43.Brandt J. The Hopkins Verbal Learning Test: Development of a new memory test with six equivalent forms. Clin Neuropsychol. 1991;5:125–142. [Google Scholar]
- 44.Rey A. L’examen psychologique dans les cas d’encephalopathie tramatique. Archives de Psychologie. 1941;28:21. [Google Scholar]
- 45.Wilson BA, Cockburn J, Baddeley A. The Rivermead Behavioral Memory Test. Reading, England: Thames Valley Test Co; Gaylord, MI: National Rehabilitation Services; 1985. [Google Scholar]
- 46.Gonda J, Schaie KW. Schaie-Thurstone Mental Abilities Test: Word Series Test. Palo Alto, CA: Consulting Psychologists Press; 1985. [Google Scholar]
- 47.Thurstone LL, Thurstone TG. Examiner Manual for the SRA Primary Mental Abilities Test (Form 10–14) Chicago: Science Research Associates; 1949. [Google Scholar]
- 48.Ekstrom RB, French JW, Harman H, Derman D. Kit of Factor-Referenced Cognitive Tests. Princeton, NJ: Educational Testing Service; 1976. rev. ed. [Google Scholar]
- 49.Wechsler D. Manual for the Wechsler Adult Intelligence Scale—Revised. New York: The Psychological Corporation; 1981. [Google Scholar]
- 50.Lawton MP, Brody E. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 51.Myers AM. The clinical Swiss army knife: Empirical evidence on the validity of IADL functional status measures. Med Care. 1992;30:MS96–MS111. [PubMed] [Google Scholar]
- 52.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The Index of ADL: A standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 53.Mahoney FI, Barthel DW. Functional evaluation: The Barthel Index. Maryland State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 54.Morris JN, Morris SA. ADL assessment measures for use with frail elders. In: Teresi JA, Lawton MP, Holmes D, Ory M, editors. Measurement in Elderly Chronic Care Populations. New York: Springer Publishing Company; 1997. [Google Scholar]
- 55.Morris JN, Fries BE, Morris SA. Scaling ADLs within the MDS. J Gerontol: Med Sci. 1999;54A:M546–M553. doi: 10.1093/gerona/54.11.m546. [DOI] [PubMed] [Google Scholar]
- 56.Willis SL, Marsiske M. Manual for the Everyday Problems Test. University Park, PA: Pennsylvania State University; 1993. [Google Scholar]
- 57.Diehl M, Marsiske M, Horgas AL, Saczynski J. Psychometric Properties of the Revised Observed Tasks of Daily Living (OTDL-R). Poster session presented at annual meeting of the Gerontological Society of America; Philadelphia. November 1998. [Google Scholar]
- 58.Owsley C, McGwin G, Jr, Sloane ME, Stalvey BT, Wells J. Timed instrumental activities of daily living tasks: Relationship to visual function in older adults. Optom Vis Sci. 2001;78:350–359. doi: 10.1097/00006324-200105000-00019. [DOI] [PubMed] [Google Scholar]
- 59.Owsley C, Stalvey B, Wells J, Sloane ME. Older drivers and cataract: Driving habits and crash risk. J Gerontol: Med Sci. 1999;54A:M203–M211. doi: 10.1093/gerona/54.4.m203. [DOI] [PubMed] [Google Scholar]
- 60.Ware JE, Jr, Sherbourne CD. The MOS 36-item short form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 61.Stalvey B, Owsley C, Sloane ME, Ball K. The Life Space Questionnaire: A measure of the extent of mobility of older adults. J Appl Gerontol. 1999;18:479–498. [Google Scholar]
- 62.Fitti JE, Kovar MG. The supplement on aging to the 1984 National Health Interview Survey. Vital Health Stat. 1987;21:1–115. [PubMed] [Google Scholar]
- 63.Myers GC, Juster FT, Suzman RM. Asset and health dynamics among the oldest old (AHEAD): Initial results from the longitudinal study. Introduction. J Gerontol: Psychol Sci. 1997;52:v–viii. [PubMed] [Google Scholar]
- 64.Fillenbaum GG. Multidimensional Functional Assessment of Older Adults: The Duke Older Americans Resources and Services Procedures. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- 65.Horgas AL. Dissertation. Department of Human Development and Family Studies, Pennsylvania State University; 1992. Prescription Drug Use and Drug-Drug Interactions in Nursing Homes. Prevalence, Predictors, and Health Outcomes. [Google Scholar]
- 66.Rubin G, Salive M. Vision and hearing. In: Guralnik JM, Fried LP, Simonsick EM, Kasper JD, Lafferty ME, editors. The Women’s Health and Aging Study: Health and Social Characteristics of Older Women with Disability. Bethesda, MD: National Institute on Aging; 1995. [Google Scholar]
- 67.Steinhagen-Thiessen E, Borchelt M. Morbidity, medication, and functional limitations in very old age. In: Baltes PB, Mayer KU, editors. The Berlin Aging Study: Aging from 70 to 100. Cambridge, UK: Cambridge University Press; 1999. [Google Scholar]
- 68.Ferrucci L, Guralnik JM, Bandeen-Roche KJ, et al. Physical performance measures. In: Guralnik JM, Fried LP, Simonsick EM, Kasper JD, Lafferty ME, editors. The Women’s Health and Aging Study: Health and Social Characteristics of Older Women with Disability. Bethesda, MD: National Institute on Aging; 1995. [Google Scholar]
- 69.Lachman ME, Baltes PB, Nesselroade JR, Willis SL. Examination of personality-ability relationships in the elderly: The role of the contextual (interface) model. J Res Pers. 1982;16:485–501. [Google Scholar]
- 70.Zelinski EM, Gilewski MJ, Thompson LW. Do laboratory tests relate to self-assessment of memory ability in the young and old? In: Poon LW, Fozard JL, Cermak LS, Arenberg D, Thompson LW, editors. New Directions in Memory and Aging: Proceedings of the George A. Talland Memorial Conference. Hillsdale, NJ: Lawrence Erlbaum Associates; 1980. [Google Scholar]
- 71.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 72.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Mahwah, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- 73.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 74.Little RJA, Rubin DB. Statistical Analysis with Missing Data. New York: J. Wiley and Sons; 1987. [Google Scholar]
- 75.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: J. Wiley and Sons; 1987. [Google Scholar]
- 76.Clark F, Azen SP, Zemke R, et al. Occupational therapy for independent-living older adults. JAMA. 1997;278:1321–1326. [PubMed] [Google Scholar]


