Abstract
A Limulus amoebocyte lysate gel-clotting method for the determination of endotoxin in a small-volume parenteral product has been described. Sample dilution with 0.1 M potassium phosphate monobasic buffer (pH 8.0) effectively eliminated assay interference, whereas dilution with water did not. The threshold pyrogenic dose for Escherichia coli EC-2 and O127:B8 endotoxins was determined to be 1.0 ng of endotoxin per kg of body weight. Not more than 1.0 ng of endotoxin (the threshold pyrogenic dose) per the highest recommended human dose or the USP pyrogen test dose per kg of body weight, whichever dose is more stringent, is a logical limit for the quantity of bacterial endotoxin in small-volume parenteral products. Excellent correlation was attained when this criterion was used to compare the Limulus amoebocyte lysate assay with the USP pyrogen test.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Greisman S. E., Hornick R. B. Comparative pyrogenic reactivity of rabbit and man to bacterial endotoxin. Proc Soc Exp Biol Med. 1969 Sep;131(4):1154–1158. doi: 10.3181/00379727-131-34059. [DOI] [PubMed] [Google Scholar]
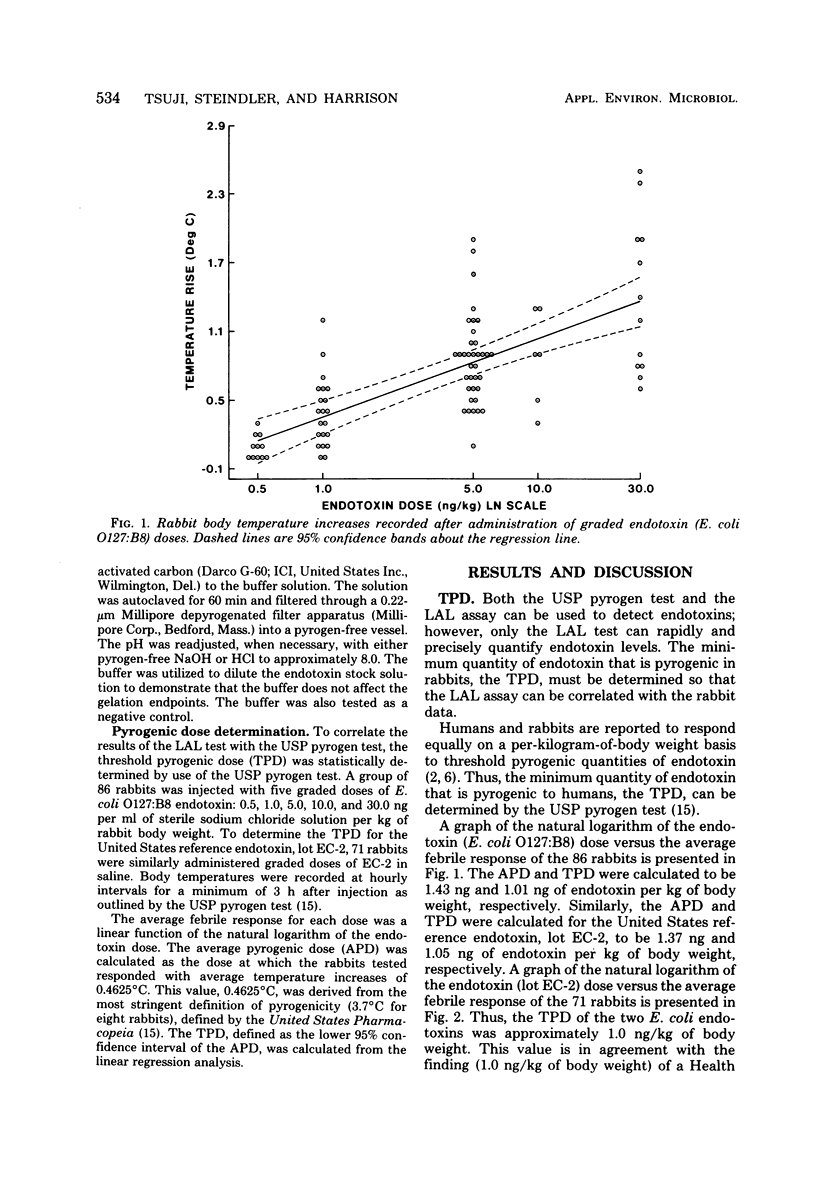
- Harrison S. J., Tsuji K., Enzinger R. M. Application of LAL for detection of endotoxin in antibiotic preparations. Prog Clin Biol Res. 1979;29:353–365. [PubMed] [Google Scholar]
- Hochstein H. D., Elfin R. J., Cooper J. F., Seligmann E. B., Jr, Wolff S. M. Further developments of Limulus Amebocyte Lysate test. Bull Parenter Drug Assoc. 1973 May-Jun;27(3):139–148. [PubMed] [Google Scholar]
- KEENE W. R., SILBERMAN H. R., LANDY M. Observations on the pyrogenic response and its application to the bioassay of endotoxin. J Clin Invest. 1961 Feb;40:295–301. doi: 10.1172/JCI104256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVIN J., BANG F. B. THE ROLE OF ENDOTOXIN IN THE EXTRACELLULAR COAGULATION OF LIMULUS BLOOD. Bull Johns Hopkins Hosp. 1964 Sep;115:265–274. [PubMed] [Google Scholar]
- Mascoli C. C., Weary M. E. Applications and advantages of the Limulus amebocyte lysate (LAL) pyrogen test for parenteral injectable products. Prog Clin Biol Res. 1979;29:387–402. [PubMed] [Google Scholar]
- Sullivan J. D., Jr, Watson S. W. Factors affecting the sensitivity of Limulus lysate. Appl Microbiol. 1974 Dec;28(6):1023–1026. doi: 10.1128/am.28.6.1023-1026.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan J. D., Jr, Watson S. W. Inhibitory effect of heparin on the Limulus test for endotoxin. J Clin Microbiol. 1976 Aug;2(2):151–151. [PMC free article] [PubMed] [Google Scholar]
- Wachtel R. E., Tsuji K. Comparison of limulus amebocyte lysates and correlation with the United States Pharmacopeial pyrogen test. Appl Environ Microbiol. 1977 Jun;33(6):1265–1269. doi: 10.1128/aem.33.6.1265-1269.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]



