Abstract
In legumes, Ca2+/calmodulin-dependent protein kinase (CCaMK) is a component of the common symbiosis genes that are required for both root nodule (RN) and arbuscular mycorrhiza (AM) symbioses and is thought to be a decoder of Ca2+ spiking, one of the earliest cellular responses to microbial signals. A gain-of-function mutation of CCaMK has been shown to induce spontaneous nodulation without rhizobia, but the significance of CCaMK activation in bacterial and/or fungal infection processes is not fully understood. Here we show that a gain-of-function CCaMKT265D suppresses loss-of-function mutations of common symbiosis genes required for the generation of Ca2+ spiking, not only for nodule organogenesis but also for successful infection of rhizobia and AM fungi, demonstrating that the common symbiosis genes upstream of Ca2+ spiking are required solely to activate CCaMK. In RN symbiosis, however, CCaMKT265D induced nodule organogenesis, but not rhizobial infection, on Nod factor receptor (NFRs) mutants. We propose a model of symbiotic signaling in host legume plants, in which CCaMK plays a key role in the coordinated induction of infection thread formation and nodule organogenesis.
Keywords: arbuscular mycorrhizal symbiosis, CCaMK, common symbiosis genes, intracellular infection, root nodule symbiosis
Introduction
Recent studies have revealed the host legume genes that regulate RN and/or AM symbioses (Oldroyd and Downie, 2008; Parniske, 2008). RN symbiosis begins with the specific recognition of rhizobial Nod factors (NFs) (Ardourel et al., 1994; Lerouge et al., 1990; López-Lara et al., 1995; Niwa et al., 2001) by LysM receptor kinases of the compatible host plants. In Lotus japonicus, two LysM receptor kinases, NFR1 and NFR5 (Madsen et al., 2003; Radutoiu et al., 2003, 2007), are essential for perception of Nod factors secreted from Mesorhizobium loti (López-Lara et al., 1995; Niwa et al., 2001). This compatible recognition induces intracellular Ca2+ signals, i.e. Ca2+ influx at the tip of root hairs followed by Ca2+ spiking, an oscillation of cytosolic Ca2+ concentration around the peri-nuclear region of root hair cells (Ehrhardt et al., 1996; Miwa et al., 2006; Shaw and Long, 2003). Genetic and molecular studies have positioned NFR1 and NFR5 upstream of both Ca2+ signals, because either nfr1 or nfr5 mutants were defective in the generation of both Ca2+ influx and Ca2+ spiking upon Nod factor application. On the other hand, NIN (Marsh et al., 2007; Schauser et al., 1999), NSP1 and NSP2 (Heckmann et al., 2006; Kalóet al., 2005; Murakami et al., 2006; Smit et al., 2005), which are putative transcription factors, function downstream of both Ca2+ signals (Miwa et al., 2006). NSP1, NSP2 and NIN have been shown to be necessary for nodule organogenesis and rhizobial infection, which is accompanied by formation of infection threads (ITs). NFR1, NFR5, NSP1, NSP2 and NIN are only required for RN symbiosis, but not for AM symbiosis.
Among the genes required for both RN and AM symbioses (i.e. common symbiosis genes), SYMRK (Endre et al., 2002; Stracke et al., 2002), CASTOR and POLLUX (Anéet al., 2004; Imaizumi-Anraku et al., 2005), NUP85 (Saito et al., 2007) and NUP133 (Kanamori et al., 2006) are positioned upstream of Ca2+ spiking (Miwa et al., 2006) and believed to be required for generation of Ca2+ spiking in response to the infection signals released from symbiotic partners. However, mutations in these ‘upstream genes’ do not affect the elicitation of Ca2+ influx in response to Nod factors. The other common symbiosis gene, CCaMK (Gleason et al., 2006; Lévy et al., 2004; Tirichine et al., 2006) and CYCLOPS (Yano et al., 2008) lie downstream of Ca2+ spiking (Miwa et al., 2006) and act together as a signal transduction complex required for infection (Yano et al., 2008). CCaMK is a strong candidate for the decoder of Ca2+ spiking, on the basis of its domain structure, which is composed of a serine/threonine kinase domain, a calmodulin (CaM) binding domain and three EF-hand motifs that potentially trap Ca2+ ions (Lévy et al., 2004; Yang et al., 2007). In L. japonicus, a gain-of-function CCaMK mutant snf1, in which Thr at the autophosphorylation site of the kinase domain was substituted by Ile, developed spontaneous nodules in the absence of rhizobia (Tirichine et al., 2006). In addition, point or truncated mutations of CCaMK, which lead to loss of auto-inhibition, resulted in the formation of spontaneous nodules in Medicago truncatula (Gleason et al., 2006). These results indicate that activation of CCaMK is necessary and is also sufficient for nodule organogenesis (Gleason et al., 2006; Tirichine et al., 2006). Besides CCaMK, a gain-of-function LHK1 (Lotus histidine kinase 1) has been identified from the snf2 mutant, which also shows spontaneous nodulation (Tirichine et al., 2007). LHK1 encodes a cytokinin (CK) receptor kinase and substitution of Leu266 by Phe in the receptor domain confers CK-independent activity (Tirichine et al., 2007). Together with hit1, a loss-of-function mutant of LHK1 (Murray et al., 2007), these mutants indicate the involvement of CK signaling in nodule organogenesis.
In the RN symbiosis, coordinated regulation between rhizobial infection and nodule organogenesis is essential for the development of fully effective nodules (Frugier et al., 2008; Oldroyd and Downie, 2008). Since almost all symbiotic genes described above have been isolated from loss-of-function mutants (Crespi and Frugier, 2008), i.e. non-nodulating mutants, the roles of individual symbiotic genes in infection and/or nodule organogenesis processes remain elusive. Hitherto, a number of schemes have been proposed to explain the mechanism underlying the guidance and control system for rhizobial infection. These models were devised on the basis of symbiotic defects in nodulating mutants, i.e. loss-of-function of Ca2+ spiking, Ca2+ influx, root hair deformation, IT formation, cortical cell division and gene expression upon rhizobial infection or NF treatment (Ardourel et al., 1994; Geurts et al., 2005; Marsh et al., 2007; Miwa et al., 2006; Murray et al., 2007; Smit et al., 2007; Tirichine et al., 2007; Wais et al., 2000; Yano et al., 2008). Recent identification of gain-of-function mutants and of its causative genes prompted us to examine the epistatic relationships between symbiotic genes. However, recent models have focused on the regulation pathways for nodule organogenesis (Gleason et al., 2006; Marsh et al., 2007; Tirichine et al., 2006, 2007; Yano et al., 2008) and it remains unclear whether those symbiotic genes are involved in infection processes directly or indirectly. To understand the function of symbiotic genes in bacterial and/or fungal intracellular symbiotic processes, we examined the phenotypes of a diverse array of symbiotic gene mutants after transformation with a gain-of-function CCaMK (CCaMKT265D) (Gleason et al., 2006; Rasmussen and Rasmussen, 1994; Sheen, 1996; Waldmann et al., 1990), for nodule organogenesis and rhizobial and/or mycorrhizal infection. We also evaluated the epistatic interactions between a gain-of-function LHK1 (LHK1L266F) and the other symbiotic genes on the basis of their nodulation phenotypes. Our results indicate that activation of CCaMK through upstream genes is prerequisite to allow infection of rhizobia and AM fungi. Furthermore, intracellular infection of rhizobia through ITs requires another signaling pathway derived from NFR1 and NFR5 besides the pathway involving Ca2+ spiking mediated by common symbiosis genes. We show here the crucial roles of CCaMK in intracellular symbioses.
Results
CCaMKT265D induces spontaneous nodulation and fully complements CCaMK loss-of-function mutants
It has been reported that substitution of Thr at the autophosphorylation site in the kinase domain by Asp confers Ca2+ independent activation of CCaMKs and CaMKII (Gleason et al., 2006; Rasmussen and Rasmussen, 1994; Sheen, 1996; Waldmann et al., 1990). To evaluate the efficiency of CCaMKT265D in which Thr265 was substituted by Asp, it was expressed in the Lotus ccamk-3 mutant under the control of the CaMV 35S promoter by hairy root transformation. The transformed roots showed spontaneous nodulation under mock inoculation (Table 1; Figure S1c,d). To avoid the possibility that ectopic expression of CCaMK led to spontaneous nodulation, CaMV35S-CCaMKT265T (wild type CCaMK, denoted as wt-CCaMK hereafter) was also transformed into ccamk-3. No spontaneous nodulation was induced by ectopic wt-CCaMK expression (Table 1; Figure S1e,f). Furthermore, rhizobial and mycorrhizal infections were restored by CCaMKT265D as well as by wt-CCaMK transformation (Table 1; Figures 1a, 2a,f and S1a,b), indicating that CCaMKT265D is fully functional in the infection processes in Lotus roots.
Table 1.
Induction of spontaneous nodulation and restoration of symbiotic defective phenotypes of non-nodulating mutants, transformed with wt-CCaMK (TT) or CCaMKT265D (TD) constructs
| Phenotypes |
||||
|---|---|---|---|---|
| Lotus lines | CCaMK construct | SpNa | Nodb | AMc |
| Gifu (B-129) | TT | 0/24 | 22/23 | 33/33 |
| Gifu (B-129) | TD | 29/43 | 21/21 | 25/25 |
| ccamk-3 | TT | 0/19 | 31/32 | 24/26 |
| ccamk-3 | TD | 57/77 | 32/35 | 21/27 |
| nfr1-4 | TT | 0/91 | 0/29 | nt |
| nfr1-4 | TD | 33/93 | 19/38d | nt |
| nfr5-2 | TT | 0/27 | 0/30 | nt |
| nfr5-2 | TD | 22/32 | 19/33d | nt |
| symrk-3 | TT | 0/5 | 0/20 | nt |
| symrk-3 | TD | 12/18 | 27/42 | nt |
| symrk-7 | TT | 0/36 | 0/40 | 0/24 |
| symrk-7 | TD | 23/54 | 37/41 | 37/38 |
| castor-4 | TT | 0/51 | 0/54 | 0/19 |
| castor-4 | TD | 51/67 | 58/67 | 19/25 |
| pollux-2 | TT | 0/58 | 0/46 | 0/16 |
| pollux-2 | TD | 44/66 | 46/52 | 14/17 |
| nup85-3 | TT | 0/38 | 0/41 | 0/20 |
| nup85-3 | TD | 25/26 | 41/46 | 28/31 |
| cyclops-4 | TT | –e | 15/15f | 0/9 |
| cyclops-4 | TD | –e | 23/27f | 4/17g |
| nsp2-1 | TT | 0/72 | 0/51 | nt |
| nsp2-1 | TD | 0/96 | 0/59 | nt |
| nin-2 | TT | 0/25 | 0/32 | nt |
| nin-2 | TD | 0/36 | 0/30 | nt |
| hit1-1 | TT | 0/64 | 6/22h | nt |
| hit1-1 | TD | 0/73 | 9/50h | nt |
Spontaneous nodulation in the absence of Mesorhizobium loti (scored at 6 weeks after transplantation).
Nodule formation under M. loti inoculation.
Mycorrhization with arbuscule formation.
Empty nodule without rhizobial invasion.
Previously reported by Yano et al. (2008).
Bump-like structure without rhizobial invasion.
Formation of few arbuscules.
Infected nodule with abnormal shape.
nt, not tested.
Number of plants with the phenotypes describe above (a–h) per number of transformed plants are listed. Infection phenotypes were examined 4 weeks after inoculation. Data were compiled from more than two independent experiments.
Figure 1.
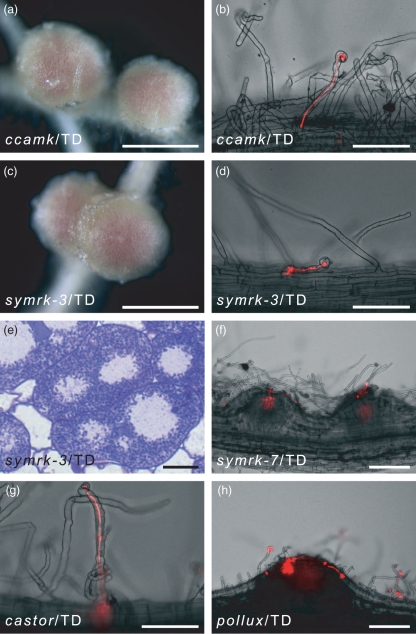
Complementation of rhizobial infection phenotypes of non-nodulating mutants by CCaMKT265D transformation. (a–h) Transformed plants were inoculated with DsRed-labeled Mesorhizobium loti. (a, c) Mature nodules formed on the roots of ccamk-3/CCaMKT265D (ccamk/TD) and symrk-3/CCaMKT265D (symrk-3/TD) after 4 weeks of inoculation. Scale bars are 1 mm. (b, d, g) Root hairs of ccamk/TD, symrk-3/TD and castor-4/CCaMKT265D (castor/TD) 2 weeks after inoculation, shown as merged images of bright-field and fluorescence images (DsRed). Infection threads can be seen inside the curled root hairs. Scale bars are 100 μm. (e) A mature nodule section of symrk-3/TD stained with toluidine blue. The nodule was filled with differentiated bacteroids. Scale bar is 20 μm. (f, h) Nodule primordia with rhizobial infection on the roots of symrk-7/CCaMKT265D (symrk-7/TD) and pollux-2/CCaMKT265D (pollux/TD), shown as merged images of bright-field and fluorescence images (DsRed). Scale bars are 200 μm.
Figure 2.
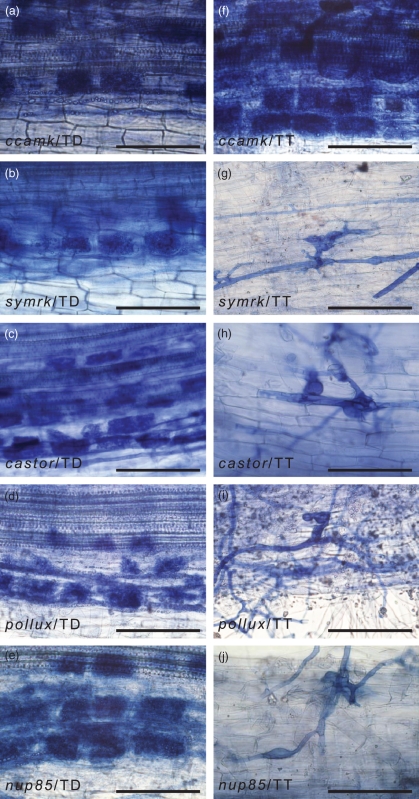
Complementation of mycorrhization phenotypes of ccamk-3 and upstream mutants by CCaMKT265D or wt-CCaMK transformation. (a–j) Symbiotic phenotypes of transformed plants were observed 4 weeks after inoculation with Glomus intraradices. (a–f) Roots of ccamk-3/CCaMKT265D (ccamk/TD), symrk-7/CCaMKT265D (symrk/TD), castor-4/CCaMKT265D (castor/TD), pollux-2/CCaMKT265D (pollux/TD) and nup85-3/CCaMKT265D (nup85/TD) as well as ccamk-3/wt-CCaMK (ccamk/TT) were filled with well developed arbuscules. (g–j) In the case of symrk-7/wt-CCaMK (symrk/TT), castor-4/wt-CCaMK (castor/TT), pollux-2/wt-CCaMK (pollux/TT) and nup85-3/wt-CCaMK (nup85/TT), mycorrhizal invasion was aborted in the epidermis and only running hyphae (g) and swollen appressoria (h, i) were observed. All scale bars are 100 μm.
CCaMKT265D dispenses the requirement of ‘upstream genes’ for not only nodule organogenesis but also for rhizobial infection through ITs
We examined the function of CCaMK in mutants that are defective in common symbiosis genes, viz: SYMRK, CASTOR, POLLUX and NUP85. As these common symbiosis genes have been shown to be required for the generation of Ca2+ spiking, they are supposed to function upstream of CCaMK and thus are denoted as ‘upstream genes.’ Under mock inoculation, expression of CCaMKT265D resulted in formation of spontaneous nodules on the roots of the upstream gene mutants (Table 1; Figure S2c–f), indicating that CCaMKT265D could obviate the requirement of the upstream genes for nodule organogenesis.
The upstream gene mutants transformed with CCaMKT265D formed fully mature and functional nodules upon M. loti inoculation (Figure 1). Using DsRed-labelled M. loti (Maekawa et al., 2009), we found occurrence of successful infection events comparable to those in wild type plants, including root hair curling with micro-colonies, ITs that were developed within curled root hairs and ramified infection thread networks towards the central zone of nodules (Figure 1b,d,f–h). Among upstream genes, SYMRK has been implicated to be involved in infection processes of rhizobia (Bersoult et al., 2005; Capoen et al., 2005; Limpens et al., 2005). SYMRK encodes protein kinase with leucine-rich repeat and its non-legume orthologs have been proved to be functional in RN and/or AM symbioses (Markmann et al., 2008). In M. truncatula, knockdown or ectopic expression of DMI2, a Medicago ortholog of SYMRK, resulted in the development of aberrant ITs within nodules (Capoen et al., 2005; Limpens et al., 2005). Analyses using DMI2-GFP fusion revealed that DMI2 is localized on the plasma membrane and infection thread membrane at the distal part of the infection zone, suggesting the involvement of DMI2 in symbiosome formation (Capoen et al., 2005; Limpens et al., 2005). In addition, the fact that MtHMGR1 (Kevei et al., 2007) and SIP1 (Zhu et al., 2008) interact with the kinase domain of SYMRK, suggests that a signaling pathway(s) other than the one mediated by common symbiosis genes is crucial for rhizobial infection (Holsters, 2008). To exclude the possibility of residual activity of symrk-7, which retains most of the kinase domain of SYMRK (Kistner et al., 2005; Stracke et al., 2002), we examined the phenotypes of symrk-3, which lacks the kinase domain completely (Kistner et al., 2005; Stracke et al., 2002), when transformed with CCaMKT265D. Spherical, pink nodules with differentiated bacteroids were formed on the roots of symrk-3/CCaMKT265D (Figure 1c,e), as well as on those of symrk-7/CCaMKT265D (Figure 1f), similar to wild type nodules. On those roots, neither white nodules nor nodules with aberrantly developed infection threads were observed. These results indicate that SYMRK is required solely for activation of CCaMK in rhizobial infection processes, as well as other upstream genes.
Upstream genes are only required for the activation of CCaMK in both rhizobial and mycorrhizal infection processes
Besides overcoming rhizobial infection defects, CCaMKT265D could also complement defects in mycorrhizal infection in all upstream mutants examined (Table 1; Figure 2). Mutant roots expressing CCaMKT265D were filled with well-developed arbuscules (Figure 2b–e), while no endosymbiotic structures were observed in the mutants/wt-CCaMK (Figure 2g–j). In symrk-7/wt-CCaMK roots, we found only running hyphae on the root surface (Figure 2g). Abnormally-shaped appressoria were formed on the roots of castor-4/wt-CCaMK, pollux-2/wt-CCaMK and nup85-3/wt-CCaMK (Figure 2h–j), indicating that wt-CCaMK did not suppress the epidermal block for mycorrhizal invasion in these mutants. These results suggest that CCaMKT265D could function in a similar way to wt-CCaMK activated by infection signals from rhizobia or mycorrhizae through the upstream genes and strengthen the idea that upstream genes are only required for the activation of CCaMK in rhizobial and mycorrhizal infection processes.
Rhizobial and mycorrhizal infection processes are CYCLOPS-dependent
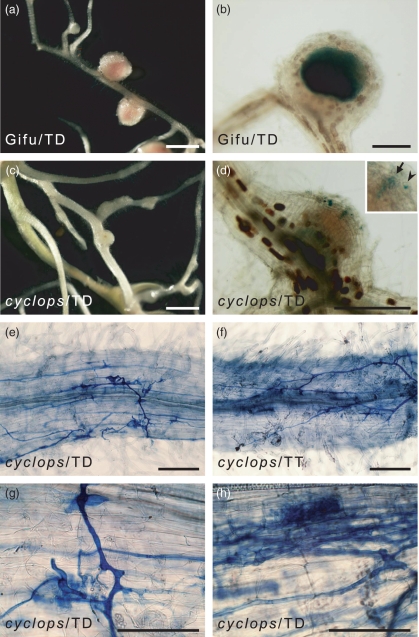
Among the common symbiosis genes identified so far, CYCLOPS is positioned downstream of Ca2+ spiking (Miwa et al., 2006). The cyclops mutants abort intracellular infection by rhizobia and AM fungi. IT development accompanied by rhizobial infection was arrested within curled root hairs, leading to formation of small bumps with no bacteria inside. For the AM symbiosis, hyphal penetration through the epidermis was blocked, although arbuscules were formed at a very low frequency (Kistner et al., 2005; Yano et al., 2006, 2008). At the molecular level, CYCLOPS has been shown to interact with CCaMK in planta and be phosphorylated by CCaMK in vitro, suggesting that CYCLOPS acts in concert with CCaMK to regulate intracellular symbioses (Yano et al., 2008). Yano et al. (2008) also reported that nodule organogenesis is independent of CYCLOPS, because spontaneous nodules were formed in cyclops-4/CCaMKT265D roots under mock inoculation. To examine whether CYCLOPS is involved in infection processes, CCaMKT265D was transformed into the cyclops-4 mutant. CCaMKT265D, as well as wt-CCaMK, did not restore rhizobial infection defects of cyclops-4 (Table 1). On the roots of cyclops-4/CCaMKT265D, bump formation was observed in which rhizobial invasion was aborted within root hairs (Figure 3c,d). In the case of AM symbiosis, hyphal penetration was aborted in epidermis or outer cortical cell layers, except for rare occasions where a few internal hyphae and/or arbuscules developed on the roots of cyclops-4/CCaMKT265D (Table 1; Figure 3e,g,h). These results are in contrast to those for upstream mutants/CCaMKT265D roots in which the cortical cell layer was filled with numerous arbuscules (Figure 2a–e). We conclude that CYCLOPS is epistatic to CCaMKT265D in respect to rhizobial and mycorrhizal infection processes, opposite to the case of nodule organogenesis.
Figure 3.
Complementation of rhizobial infection phenotypes and mycorrhization phenotypes of cyclops mutant by CCaMKT265D or wt-CCaMK transformation. Symbiotic phenotypes of transformed plants were observed 4 weeks after inoculation with lacZ-labelled Mesorhizobium loti (a–d) or Glomus intraradices (e–h). (a, c) Mature nodules on the roots of wild-type/CCaMKT265D (Gifu/TD) and bump-like structures on the roots of cyclops-4/CCaMKT265D (cyclops/TD) were formed. Scale bars are 1 mm. (b, d) Rhizobial infection was confirmed by lacZ staining. Effective nodules with rhizobial infection were formed on the roots of Gifu/TD (b), but rhizobial infection was aborted at the epidermis on bump-like structures on the roots of cyclops/TD (d). The inset shows magnified view of the aborted infection thread (arrow) and the micro-colony (arrowhead). Scale bars are 500 μm. (e–h) Roots of cyclops/TD were filled with arbuscules only occasionally (h), fungal invasion was aborted in the epidermis and only running hyphae (e) and swollen appressoria (g) were observed in the roots of cyclops-4/TD, as well as cyclops-4/wt-CCaMK (f). Scale bars are 200 μm (e,f) and 100 μm (g, h).
NFR1 and NFR5 are indispensable for rhizobial infection through root hair ITs
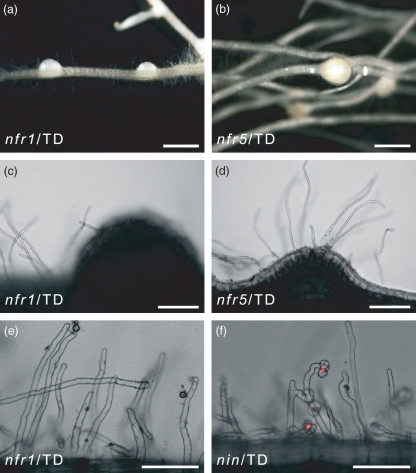
NFR1 and NFR5, putative NF receptors, are considered to be the starting point of the RN symbiosis in Lotus. Indeed, their corresponding mutants lack any symbiotic responses, including Ca2+ signals in response to M. loti NFs (Miwa et al., 2006). Introduction of CCaMKT265D in the nfr1-4 or nfr5-2 mutants resulted in spontaneous nodulation under mock inoculation (Table 1; Figure S2a,b). However, in contrast to the mutants of upstream genes described above, although nodule-like structures developed, neither bacterial colonization nor root hair ITs were found on the roots of both nfr1-4/CCaMKT265D and nfr5-2/CCaMKT265D upon inoculation of M. loti (Table 1; Figure 4a–e). In nfr1-4/CCaMKT265D roots, no root hair deformation occurred (Figure 4c,e), as well as in nfr5-2/CCaMKT265D roots. These results indicate that NFR1 and NFR5, upon perception of Nod factors, may generate a signal or signals other than the one mediated by the common symbiosis genes and that these signals are required for infection of rhizobia through root hair ITs.
Figure 4.
Complementation of rhizobial infection phenotypes of nfr1 and nfr5 mutants by CCaMKT265D transformation. (a–f) Transformed plants were inoculated with DsRed-labelled M. loti. (a, b) The empty nodules formed on the roots of nfr1-4/CCaMKT265D (nfr1/TD) and nfr5-2/CCaMKT265D (nfr5/TD) after 4 weeks of inoculation. Scale bars are 1 mm. (c–f) Bright-field and fluorescence (DsRed) images were merged into single images. (c, d) Nodule primordia without rhizobial infection on the roots of nfr1/TD and nfr5/TD. Scale bars are 200 μm. (e, f) Root hairs of nfr1/TD and nin-2/CCaMKT265D (nin/TD) 2 weeks after inoculation. Scale bars are 100 μm. (e) Neither bacterial colonization nor infection thread formation was observed on the roots of nfr1/TD. (f) Aberrant curled root hairs with micro-colonies were observed on the roots of nin/TD.
Nodule organogenesis and rhizobial infection processes are dependent on NSP2 and NIN
In contrast to spontaneous nodulation on the roots of nfr1-4/CCaMKT265D and nfr5-2/CCaMKT265D, no nodule structures were formed on the roots of nsp2-1/CCaMKT265D and nin-2/CCaMKT265D (Table 1) (Gleason et al., 2006; Marsh et al., 2007). Upon M. loti inoculation, infection defects in both nsp2-1 and nin-2 mutants were not restored by CCaMKT265D (Table 1). The root hair phenotype of nsp2-1/CCaMKT265D was the same as that of nsp2-1/wt-CCaMK, i.e. almost no micro-colonies and no ITs were formed (Heckmann et al., 2006; Murakami et al., 2006). Both nin-2/CCaMKT265D (Figure 4f) and nin-2/wt-CCaMK showed the nin infection phenotypes, with abnormally curled root hairs without ITs (Schauser et al., 1999). Taken together, we conclude that NSP2 and NIN both act downstream of CCaMK in both the infection process and nodule organogenesis.
CK signaling through LHK1 is required for nodule organogenesis, but is dispensable for rhizobial infection
In addition to CCaMK, the gain-of-function LHK1 (LHK1L266F) also has an ability to induce spontaneous nodulation (Tirichine et al., 2007). Introduction of the LHK1L266F construct into several symbiotic mutants revealed that LHK1L266F is epistatic to the symbiotic genes except for NIN and NSP2 in nodule organogenesis (Tirichine et al., 2007). To examine the involvement of LHK1 in the rhizobial infection process, LHK1L266F and LHK1L266L (wt-LHK1) under the control of its own promoter was introduced into symbiotic mutants. wt-LHK1 restored the infection defective phenotype of hit1, a loss-of-function mutant of LHK1 (Murray et al., 2007). In the roots of hit1-1/wt-LHK1, nodule organogenesis was accompanied by infection of rhizobia, resulting in formation of fully effective nodules (Figures S3b and S4b). In contrast, both empty and effective nodules were formed on the roots of hit1-1/LHK1L266F (Figures S3a and S4a), suggesting that LHK1L266F enables the restoration of infection defects in the hit1 mutants, while it also gives rise to a defect in the cooperative regulation of the symbiotic programs between epidermis and cortex, leading to the formation of empty nodules with no associated rhizobial infection.
In accordance with the results reported by Tirichine et al. (2007), spontaneous nodulation was induced on the roots of nfr1-4, symrk-7, castor-4, nup85-3, ccamk-3 and cyclops-4 transformed with LHK1L266F, indicating that LHK1L266F is epistatic, in regard to nodule organogenesis, to the genes noted above (Figure S3h and Table S1). Coincidently, no spontaneous nodulation occurred on the hit1-1/CCaMKT265D roots (Figure S3g). In contrast to nodule organogenesis, the defect in rhizobial infection of ccamk-3 was not restored by LHKL266F (Figure S3f). Moreover, LHK1L266F could not restore infection defective phenotypes of the upstream mutants, symrk-7, castor-4 and nup85-3 (Figure S3d,e and Table S1). Only empty nodules were found on those roots of upstream mutants/LHK1L266F, as well as nfr1-4/LHK1L266F roots (Figure S3c). These results demonstrate that the rhizobial infection process is independent of LHK1. The hit1 mutant showed distinct symbiotic defective phenotypes: the formation of an excessive numbers of ITs in the epidermis, while IT development was arrested at the cortex (Murray et al., 2007). Because of this, we also examined the effects of CCaMKT265D in the rhizobial infection processes of the hit1 mutant. On both hit1-1/CCaMKT265D and hit1-1/wt-CCaMK roots, abundant ITs, majority of which did not penetrate to the cortical layer, were observed (Figure S4c,d). Very occasionally, aberrantly-developed infected nodules were formed on both mutant roots (Table 1 and Figure S4e,f), similar to the results described by Murray et al. (2006), who reported that the hit1-1 mutant formed effective but irregularly shaped nodules on rare occasions. These data support the conclusion that CCaMKT265D does not modulate abnormal infection phenotypes of hit1-1, as well as wt-CCaMK. Collectively, our results suggest that symbiotic defective phenotype of hit1 is caused by decoupling of the infection events in the epidermis with nodule organogenesis, which is initiated in cortex (Figure S4c,d). In either rhizobial infection or nodule organogenesis, NSP2 and NIN are also positioned downstream of LHK1, because LHK1L266F constructs could not suppress the defects of nodule organogenesis in nsp2-1 and nin-2 mutants (Table S1).
Epistatic analysis shown here supports the idea that LHK1-dependent CK signaling is positioned downstream of CCaMK in nodule organogenesis (Murray et al., 2007; Tirichine et al., 2007). In addition, our studies provide evidence that LHKL266F could not obviate the requirement of upstream genes for rhizobial infection, indicating that rhizobial infection process is regulated by an LHK1-independent pathway. Taken together with the results of CCaMKT265D, we conclude that rhizobial infection process is regulated by cooperation of CCaMK and CYCLOPS, while both CCaMK and LHK1 are responsible for regulation of nodule organogenesis and both symbiotic processes are dependent on NSP2 and NIN (Figure 5a).
Figure 5.
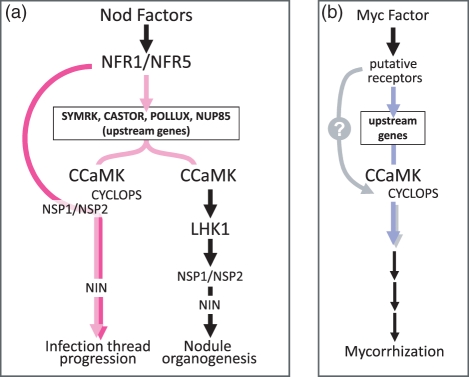
A model for regulation pathways responsible for RN and AM symbioses. (a) In response to Nod factors, the signal generated by NFR1/NFR5 splits into two pathways, one of these flows into the common symbiosis pathway (pink line). The input of another pathway (deep pink line) is prerequisite for successful infection of rhizobia. Epistasis between CYCLOPS and NSP2 on the pathway remains unclear. ITs were rarely, but initiated in cyclops (Yano et al., 2006, 2008), while no micro-colonies were observed in nsp2 (Murakami et al., 2006). Therefore, CYCLOPS appears to be downstream of NSP2 on the pathway leading to IT formation. One possible explanation is that NSP2 may be positioned on another pathway that originates from NFR1/NFR5. For nodule organogenesis, only one signal is sufficient for activation of the downstream pathway, in which LHK1, NSP1/2 and NIN are involved. CYCLOPS is not involved in nodule organogenesis. (b) In the AM symbiosis, a plausible AM pathway that bifurcates after putative receptors (grey arrow) might be converged with common symbiosis pathway (blue arrows).
Discussion
To accommodate their microsymbiotic partners properly, host plants have developed complex and highly organized signaling pathways, which perceive and process information from the symbionts and/or its own plant cell status, such as Ca2+ and CK signaling (Crespi and Frugier, 2008; Kosuta et al., 2008; Oldroyd and Downie, 2008; Parniske, 2008). In the present work, we investigated epistatic relationships of genes involved in the early symbiotic signaling pathways, by means of transformation of gene mutants with gain-of-function CCaMKT265D and LHK1L266F.
In the roots of the upstream gene mutants, introduction of CCaMKT265D allowed rhizobia to enter host plants through ITs as well as the initiation of nodule organogenesis and thus fully compensated for the gene mutant symbiotic defects (Figure 1c–h). Similarly, CCaMKT265D transformation resulted in suppression of the defects in AM symbiosis in these mutants (Figure 2b–e). These results provide conclusive evidence that the common symbiosis genes upstream of Ca2+ spiking are only required for the activation of CCaMK and its activation allows symbiotic interaction with rhizobia and AM fungi in L. japonicus. Ca2+ spiking is very likely to participate in activation of CCaMK, because upstream genes are essential for the generation of Ca2+ spiking (Miwa et al., 2006); there is already an analogy to mammal CaMKII, namely the autophosphorylation of CaMKII is sensitive to the frequency of Ca2+ spikes (Hudmon and Schulman, 2002). Our data indicate that CaMV35S-driven CCaMKT265D efficiently mimics the CCaMK that is activated in response to Ca2+ derived from the cytoplasm (Figures 1a,b and 2a).
In contrast to the upstream gene mutants above described, the infection defects of the NF receptor mutants, nfr1 and nfr5, were never restored by CCaMKT265D in our hairy root transformation system, although it could induce nodule organogenesis in these mutants irrespective of the presence or absence of M. loti (Figures 4a–d and S2a,b). This finding indicates that CCaMKT265D alone is sufficient for the induction of cortical cell division and successive nodule organogenesis, while intracellular accommodation of rhizobia through IT within root hairs absolutely requires NF perception by NFR1 and NFR5. Therefore, our results strongly suggest that the infection signal, elicited by Nod factor perception by NFR1/NFR5 receptors in L. japonicus, is split into two signaling pathways; one is through Ca2+ spiking and is mediated by the common symbiosis genes (Figure 5a, indicated by pink arrows) and the other is separately derived from the NF receptors (Figure 5a, indicated by the deep pink arrow). The former appears to be essential and sufficient for nodule organogenesis, but the progression of the infection process via ITs additionally requires the operation of the latter (Figure 5a).
In some cases, M. loti can infect L. japonicus independently of Nod factor perception by NFR1 and NFR5, as a gain-of-function mutation of CCaMK (snf1) under nfr1/nfr5 background was shown to be infected by M. loti, even though at a very low frequency (Madsen et al., 2010). This NF-independent infection is, however, not via root hair ITs and rhizobia enter the cortex intercellularly. In contrast, nodules formed on symrk-14 (Murray et al., 2006) was shown to be infected by rhizobia through a process similar to ‘crack entry’, even though there is no induction of Ca2+ spiking in symrk-14 (K. Szczyglowski, personal communication, 2009). Thus, the signal input from only one of the pathways may occasionally allow aberrant infection of rhizobia, but it is never accompanied by formation of root hair ITs. These observations strengthen the idea that the integration of two signaling pathways, one through Ca2+ spiking and another derived from NF receptors separately from the one mediated by the common symbiosis genes, is prerequisite for rhizobial infection through root hair ITs, which serve as the main route of rhizobial entry into host cells (Figure 5a).
A similar model including two signaling pathways for nodulation and IT formation has been proposed for another model legume, M. truncatula (Smit et al., 2007). However, the framework of NF perception in Lotus (NFR1/NFR5) and in Medicago (LYK3/NFP) appears not to be exactly the same. In L. japonicus, NFR1 and NFR5 are required for the generation of both Ca2+ influx and Ca2+ spiking (Miwa et al., 2006). Co-transformation of NFR1 and NFR5 allows M. truncatula to be infected by M. loti, suggesting that they form a receptor complex that is responsible for specific recognition of NFs derived from M. loti (Radutoiu et al., 2003, 2007). In M. truncatula, NFP, a putative ortholog of NFR5 (Arrighi et al., 2006; Lohmann et al., 2010), is positioned upstream of both Ca2+ signals (Amor et al., 2003). Neither root hair swelling (Has) nor root hair deformation (Had) were observed in the roots of nfp mutant (Amor et al., 2003), as well as in the roots of nfr5 and nfr1 in Lotus (Radutoiu et al., 2003). Although, LYK3 has been proposed to be an ortholog of NFR1 (Arrighi et al., 2006; Lohmann et al., 2010), hcl mutants retain the ability to induce Ca2+ spiking, Ca2+ influx, Has and Had in response to Sinorhizobium meliloti infection (Catoira et al., 2001; Smit et al., 2007; Wais et al., 2000), indicating that these symbiotic responses are LYK3 independent. Phenotypic divergence between nfr1 and hcl implies that the position of NFR1 and LYK3 within symbiotic signaling pathway is not identical. Because of the highly strict structural requirement of LYK3 for S. meliloti NFs, LYK3 is proposed to be an ‘entry receptor’ that is responsible for IT formation rather than nodule primordium initiation and to be independent of the pathway mediated by the common symbiosis genes (Smit et al., 2007). While another receptor complex with a lower requirement toward NF structures is postulated as a ‘signaling receptor’, which is responsible for nodule initiation through the pathway mediated by the common symbiosis genes (Smit et al., 2007). In our model for L. japonicus, a receptor complex putatively composed of NFR1 and NFR5 is responsible for processing two signaling pathways leading to not only nodule organogenesis, but IT formation. It should be noted, however, that in silico searches of genome databases of L. japonicus and M. truncatula have revealed the presence of a number of LysM receptor kinases in their genome (Arrighi et al., 2006; Lohmann et al., 2010). In addition, M. loti has been shown to produce Nod factors with diverse side-chain modifications and acyl moieties (Shibata et al., 2005). Thus, although applicability of the ‘signaling/entry receptor model’ to the Lotus NF signaling pathway(s) is still an open question, NF signaling, from the first contact of rhizobia on root hairs to the development of ITs towards the cortex, might be mediated by complex combinations of multiple LysM receptor kinases including those other than NFR1 and NFR5. Nevertheless, our data demonstrate that NFR1 and NFR5 are both essential for initiating two signaling pathways for nodule primordium formation and IT formation.
It has recently been proposed that the symbiotic signal transduction pathway(s) is bifurcated at or just downstream of CCaMK; one is a CYCLOPS-dependent pathway required for initiation of ITs and the other is a CYCLOPS-independent pathway leading to nodule organogenesis (Yano et al., 2008). In accordance with this proposal, we have shown that the CYCLOPS-dependent pathway regulates both rhizobial and AM fungal infection processes (Figure 3c–e,g,h). It has been reported that the size of spontaneous nodules formed on the roots of cyclops-4/CCaMKT265D did not differ from those formed on wt/CCaMKT265D roots under mock inoculation (Yano et al., 2008). However, restoration of nodule organogenesis appeared to be impaired in response to rhizobial inoculation, i.e. nodule organogenesis on cyclops-4/CCaMKT265D roots remained at the stage of small bumps when inoculated with M. loti (Figure 3c). A similar phenotype has been described for the roots of cerberus/CCaMKT265D (Yano et al., 2009). CERBERUS encodes a novel U-box protein containing WD-40 repeats and is shown to be essential for the development of ITs. On both cyclops/CCaMKT265D and cerberus/CCaMKT265D roots, bump formation were induced by M. loti inoculation, while spontaneous nodules with genuine nodule structure were developed under mock inoculation (Yano et al., 2008, 2009). In the case of nfr1-4/CCaMKT265D and nfr5-2/CCaMKT265D, the deficiency in the ability to recognize NFs resulted in complete loss of infection events in epidermis even in the presence of M. loti. Taken together, developmental arrest of ITs in the epidermis appears to affect adversely the progression of nodule organogenesis in the cortex.
In this study, we showed that the introduction of LHK1L266F could not rescue the infection defective phenotypes of the upstream gene mutants, in contrast to CCaMKT265D. Lohar et al. (2004) has demonstrated that CK signaling is activated in response to NFs in L. japonicus; CK-responsive Arabidopsis response regulator (ARR5) promoter-GUS expression was induced along symbiosis with M. loti, in deformed root hairs, dividing cortical cells and nodule primordia. Although the role of CK in rhizobial infection processes remains to be proven, CK signaling through LHK1 appears to be not necessary for IT formation in the RN symbiosis. It is believed that coordinated regulation of rhizobial infection and nodule organogenesis is essential for the development of effective nodules (Frugier et al., 2008; Gonzalez-Rizzo et al., 2006; Murray et al., 2007; Oldroyd, 2007). The phenotype of hit1-1/LHK1L266F appeared to be due to cortical cell division which could not coupled appropriately with IT development within root hairs, thus leading to the formation of a large number of empty nodules, even though it also formed effective nodules on much rarer occasions (Figure S4a and Table S1). IT formation program functions first in the epidermis, while nodule primordium formation, which involves LHK1-mediated CK signaling, occurs in root cortical cells. Our results, together with the infection defective phenotype of cyclops/CCaMKT265D, indicate that disturbance of nodule organogenesis programs by LHK1L266F adversely affects the rhizobial infection process, suggesting that coordinated regulation of symbiotic signaling cascades between epidermis and cortex is essential for the establishment of successful symbiosis.
The RN symbiosis is assumed to have evolved by recruiting the pre-existing common symbiosis genes for the AM symbiosis (Markmann and Parniske, 2009; Parniske, 2008). Indeed, non-leguminous orthologs of common symbiosis genes have been isolated and their involvement in the AM symbiosis has been proven in rice (Banba et al., 2008; Chen et al., 2007, 2008, 2009; Gutjahr et al., 2008; Markmann et al., 2008; Yano et al., 2008; Zhu et al., 2006). Leguminous plants have an ability to interact with rhizobia in addition to AM fungi. This means that leguminous plants can distinguish different symbionts and regulate respective pathways appropriately. Although RN and AM symbioses share common symbiosis genes that play roles in signal transduction mediated by Ca2+ spiking, leguminous plants can open different gates for different symbionts. It has been shown that Ca2+ spiking has different signatures depending on RN or AM symbiotic interactions, leading to the transmission of RN- or AM-specific information to the downstream pathways (Kosuta et al., 2008). However, in the roots of the upstream gene mutants/CCaMKT265D, the gain-of-function status of CCaMKT265D is apparently identical regardless of whether the roots are infected by rhizobia or AM fungi, implying that a specific signal(s), other than those mediated by common symbiosis genes, plays a role in determination of downstream pathways responsible for each of the symbioses. In the case of the upstream mutants/CCaMKT265D, the gain-of-function status of CCaMKT265D driven by CaMV35S promoter is presumed to far exceed the threshold of the CCaMK activity required for intracellular infections of both rhizobia and AM fungi. During evolution of the RN symbiosis, leguminous plants were likely to have acquired not only the competence to transmit RN-specific signals through the common symbiosis genes, but also another RN-specific signaling pathway directly originated from the NF receptors. Based on cross-species complementation analyses of leguminous common symbiosis mutants with corresponding rice ortholog genes, most of the common symbiosis genes show functional conservation in AM and RN symbioses (Chen et al., 2007; Banba et al., 2008; Yano et al., 2008), while only SYMRK has the distinctive position as the adaptive factor that confers the RN symbiosis on leguminous plants (Markmann et al., 2008). It is of great interest to analyze the correlation between the domain composition of SYMRK and the signal intensity of Ca2+ spiking induced by respective SYMRK orthologs.
Although it remains unclear whether CCaMKT265D only represents CCaMK activated by cytosolic Ca2+ derived from Ca2+ spiking, our results suggest that CCaMKT265D is sufficient for intracellular rhizobial infection when it has received an input from another signaling cascade(s), which is derived directly from NFR1 and NFR5 separately from that involving Ca2+ spiking. Among well characterized physiological reactions of host cells in response to NF application, Ca2+ influx may be a good candidate for another signal derived from Nod factor perception (Geurts et al., 2005; Miwa et al., 2006; Shaw and Long, 2003). In fact, NFR1/NFR5 are required for induction of both Ca2+ influx and Ca2+ spiking, while the mutants of upstream genes are able to induce Ca2+ influx (Miwa et al., 2006). Although the convergence of these two Ca2+ signals in the symbiotic signal transduction cascades remains to be elucidated, candidates responsible for signal integration are likely to be capable of binding Ca2+. Kinase-only CCaMK, which lacks both the CaM-binding domain and EF hands, induces spontaneous nodulation, while neither rhizobial colonization nor IT initiation are observed on the roots of dmi3/kinase-only DMI3 in M. truncatula (Gleason et al., 2006). This finding indicates that the Ca2+ binding capacity of CCaMK is necessary for rhizobial infection and a possible function of CCaMK is as the acceptor of the two Ca2+ signals. This idea is consistent with the proposal of Miwa et al. (2006) that accumulation of NF caused by M. loti colonization within curled root hairs leads to Ca2+ influx, which may drive infection thread growth in Lotus.
Mycorrhizal infection might also require another specific signal derived from AM specific pathway (Figure 5b) in a similar way to the RN symbiosis. Indeed, it has been reported that MtENOD11 expression in response to mycorrhizal infection is independent of DMI genes (Kosuta et al., 2003). These results suggest the presence of an AM specific pathway which might determine downstream AM specific pathway(s) to be activated (Figure 5b).
The function of symbiotic genes to different cellular responses, leading to rhizobial infection and to nodule organogenesis, is a complex biological problem. To further elucidate the regulation pathways responsible for RN symbiosis, we focused on evaluating the possible involvement of a number of symbiotic genes in the infection process, by analyzing the infection phenotypes of corresponding mutants with expression of gain-of-function CCaMK and LHK1. Taken together with epistatic analyses on the basis of spontaneous nodulation and infection phenotypes, we demonstrate that the compositions of gene sets responsible for the infection process (IT formation) and nodule organogenesis are not equal, even though they share the same components in part (Figure 5a). In addition, our results clearly indicate the key role(s) of CCaMK in both rhizobial infection and the nodule organogenesis program.
The common symbiosis genes are considered to be a conserved genetic pathway for the AM symbiosis and to act as a generator of symbiotic signals, i.e. Ca2+ spiking, in response to rhizobia and AM fungi interactions (Kosuta et al., 2008; Markmann and Parniske, 2009). We propose that activation of CCaMK through the common symbiosis genes confers competence for the accommodation of rhizobia or AM fungi intracellularly. The study presented herein reveals dominant roles for CCaMK in endosymbioses and also raises the question of how CCaMK is activated differently by bacterial and fungal symbionts in leguminous plants.
Experimental procedures
Biological materials
Detailed information of L. japonicus used in this study is provided in Data S1. To visualize the infection processes of rhizobia, M. loti MAFF303099 constitutively expressing DsRed (Maekawa et al., 2009) or MAFF303099 derivative ML001 constitutively expressing the β-galactosidase (lacZ) (Tansengco et al., 2003) were inoculated onto hairy roots of L. japonicus. To examine mycorrhization phenotype, Glomus intraradices DAOM 197198 (Premier Tech, http://www.premiertech.com/) was used (Banba et al., 2008).
Plasmid construction
Detailed information is provided in Data S1.
Transformation of CCaMK and LHK1 constructs
wt-CCaMK, CCaMKT265D, LHK1 and LHK1L266F constructs were introduced into the L. japonicus mutants by hairy root transformation with Agrobacterium rhizogenesLBA1334 as described previously (Maeda et al., 2006). Plants with GFP-positive hairy roots were selected by GFP fluorescence using a Leica MZFLIII stereomicroscope (Leica, http://www.leica-microsystems.com/).
Examination of spontaneously nodulated plants
To examine the extent of spontaneous nodulation, transformants were transplanted into vermiculite pots supplied with B&D medium supplemented with 0.5 μm ammonium nitrate (Banba et al., 2008). Four weeks after transplantation, GFP-positive roots were selected again and the spontaneous nodulation phenotype was observed with a Leica MZFLIII stereomicroscope.
Inoculation tests with rhizobial or mycorrhizal strains
For characterization of infection phenotypes, transformants were transplanted into vermiculite pots supplied with B&D medium supplemented with 0.5 μm ammonium nitrate. Three days after transplantation, M. loti strains were inoculated. For mycorrhizal inoculation, transformants were transplanted into an autoclaved 1:1 mixture of lawn soil (Shibametsuchi) and a nutrient-rich commercial horticulture soil (Kureha, http://www.kureha.co.jp/), as described previously (Banba et al., 2008). Glomus intraradices was inoculated using approx. 200 spores per plant. The plants were grown in a growth cabinet with a 16 h-day/8 h-night cycle at 24°C. Four weeks after inoculation, plants with GFP-positive hairy roots were selected using a Leica MZFLIII stereomicroscope.
Histological examination of rhizobial infection phenotypes
To examine the extent of rhizobial infection and nodule organogenesis, lacZ-expressing M. loti was visualized with a chemical staining method as described previously (Tansengco et al., 2003) and observed using a Leica MZFLIII stereomicroscope. DsRed-expressing M. loti was observed using a 565/595 nm bandpass filter and a CCD camera system (Penguin 600CL; Pixera, http://www.pixera.com/) attached to the Leica MZFLIII stereomicroscope. For observation of ITs, samples were analyzed under an epifluorescence microscope (BZ-9000; Keyence, http://www.keyence.co.jp/) using a filter set (excitation BP560–600, dichroic 595, emission BP630–690).
Histological observations of mycorrhization phenotypes
Glomus intraradices-inoculated roots were stained with trypan blue as described previously (Saito et al., 2007; Banba et al., 2008). Hyphal or arbuscule colonization was observed under a bright-field microscope (Leitz DMRB, Leica) with a CCD camera system (Penguin 600CL; Pixera).
Acknowledgments
We thank Jens Stougaard (University of Aarhus) and Krzysztof Szczyglowski (Agriculture and Agri-food Canada) for critical reading of this manuscript and providing nfr5-2, symrk-3 and symrk-7, hit1-1, respectively. We also thank Yosuke Umehara (NIAS) for providing nfr1-4 and Koji Yano (NIAS) for discussion. We are grateful to Robert Ridge (International Christian University) for English editing of this manuscript. This work was supported by the Program of Basic Research Activities for Innovative Biosciences (to H. I.-A.) and the Ministry of Agriculture, Forestry and Fisheries of Japan (Rice Genome Project Grant PMI-0001, to M. H. and H. I.-A.).
Author Contributions
H.I-A., M.H. and H.K. designed research; T.H., M.B., Y.S., H.K. and H.I-A. performed research; T.H., M.B., H.I-A. and M.H. analyzed data; H.I-A., M.H. and H.K. wrote the paper. The authors declare no conflict of interest.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article:
Data S1. Experimental procedures.
Figure S1. Transformation of wt-CCaMK or CCaMKT265D in the ccamk-3 mutant.
Figure S2. Transformation of CCaMKT265D in non-nodulating mutants.
Figure S3. Complementation of rhizobial infection phenotypes of non-nodulating mutants by LHK1L266F transformation.
Figure S4. Transformation of LHK1L266F, wt-LHK1, CCaMKT265D or wt-CCaMK in hit1-1 mutant.
Table S1. Induction of spontaneous nodulation and restoration of symbiotic defective phenotypes of non-nodulating mutants, transformed with LHK1L266L (LHK1) or gain of function LHK1L266F (gof- LHK1) constructs.
Please note: As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
References
- Amor BB, Shaw SL, Oldroyd GE, Maillet F, Penmetsa RV, Cook D, Long SR, Dénarié J, Gough C. The NFP locus of Medicago truncatula controls an early step of Nod factor signal transduction upstream of a rapid calcium flux and root hair deformation. Plant J. 2003;34:495–506. doi: 10.1046/j.1365-313x.2003.01743.x. [DOI] [PubMed] [Google Scholar]
- Ané JM, Kiss GB, Riely BK, et al. Medicago truncatula DMI1 required for bacterial and fungal symbioses in legumes. Science. 2004;303:1364–1367. doi: 10.1126/science.1092986. [DOI] [PubMed] [Google Scholar]
- Ardourel M, Demont N, Debellé F, Maillet F, de Billy F, Promé JC, Dénarié J, Truchet G. Rhizobium meliloti lipooligosaccharide nodulation factors, different structural requirements for bacterial entry into target root hair cells and induction of plant symbiotic developmental responses. Plant Cell. 1994;6:1357–1374. doi: 10.1105/tpc.6.10.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrighi JF, Barre A, Ben Amor B, et al. The Medicago truncatula lysine motif-receptor-like kinase gene family includes NFP and new nodule-expressed genes. Plant Physiol. 2006;142:265–279. doi: 10.1104/pp.106.084657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banba M, Gutjahr C, Miyao A, Hirochika H, Paszkowski U, Kouchi H, Imaizumi-Anraku H. Divergence of evolutionary ways among common sym genes: CASTOR and CCaMK show functional conservation between two symbiosis systems and constitute the root of a common signaling pathway. Plant Cell Physiol. 2008;49:1659–1671. doi: 10.1093/pcp/pcn153. [DOI] [PubMed] [Google Scholar]
- Bersoult A, Camut S, Perhald A, Kereszt A, Kiss GB, Cullimore JV. Expression of the Medicago truncatula DMI2 gene suggests roles of the symbiotic nodulation receptor kinase in nodules and during early nodule development. Mol. Plant Microbe Interact. 2005;18:869–876. doi: 10.1094/MPMI-18-0869. [DOI] [PubMed] [Google Scholar]
- Capoen W, Goormachtig S, De Rycke R, Schroeyers K, Holsters M. SrSymRK, a plant receptor essential for symbiosome formation. Proc. Natl Acad. Sci. USA. 2005;102:10369–10374. doi: 10.1073/pnas.0504250102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catoira R, Timmers AC, Maillet F, Galera C, Penmetsa RV, Cook D, Dénarié J, Gough C. The HCL gene of Medicago truncatula controls Rhizobium-induced root hair curling. Development. 2001;128:1507–1518. doi: 10.1242/dev.128.9.1507. [DOI] [PubMed] [Google Scholar]
- Chen C, Gao M, Liu J, Zhu H. Fungal symbiosis in rice requires an ortholog of a legume common symbiosis gene encoding a Ca2+/calmodulin-dependent protein kinase. Plant Physiol. 2007;145:1619–1628. doi: 10.1104/pp.107.109876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Ané JM, Zhu H. OsIPD3, an ortholog of the Medicago truncatula DMI3 interacting protein IPD3, is required for mycorrhizal symbiosis in rice. New Phytol. 2008;180:311–315. doi: 10.1111/j.1469-8137.2008.02612.x. [DOI] [PubMed] [Google Scholar]
- Chen C, Fan C, Gao M, Zhu H. Antiquity and function of CASTOR and POLLUX, the twin ion channel-encoding genes key to the evolution of root symbioses in plants. Plant Physiol. 2009;149:306–317. doi: 10.1104/pp.108.131540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespi M, Frugier F. De novo organ formation form differentiated cells: root nodule organogenesis. Sci. Signal. 2008;1:re11. doi: 10.1126/scisignal.149re11. [DOI] [PubMed] [Google Scholar]
- Ehrhardt DW, Wais R, Long SR. Calcium spiking in plant root hairs responding to Rhizobium nodulation signals. Cell. 1996;85:673–681. doi: 10.1016/s0092-8674(00)81234-9. [DOI] [PubMed] [Google Scholar]
- Endre G, Kereszt A, Kevei Z, Mihacea S, Kalo P, Kiss GB. A receptor kinase gene regulating symbiotic nodule development. Nature. 2002;417:962–966. doi: 10.1038/nature00842. [DOI] [PubMed] [Google Scholar]
- Frugier F, Kosuta S, Murray JD, Crespi M, Szczyglowski K. Cytokinin: secret agent of symbiosis. Trends Plant Sci. 2008;13:115–120. doi: 10.1016/j.tplants.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Geurts R, Fedorova E, Bisseling T. Nod factor signaling genes and their function in the early stages of Rhizobium infection. Curr. Opin. Plant Biol. 2005;8:346–352. doi: 10.1016/j.pbi.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Gleason C, Chaudhuri S, Yang T, Muñoz A, Poovaiah BW, Oldroyd GE. Nodulation independent of rhizobia induced by a calcium-activated kinase lacking autoinhibition. Nature. 2006;441:1149–1152. doi: 10.1038/nature04812. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rizzo S, Crespi M, Frugier F. The Medicago truncatula CRE1 cytokinin receptor regulates lateral root development and early symbiotic interaction with Sinorhizobium meliloti. Plant Cell. 2006;18:2680–2693. doi: 10.1105/tpc.106.043778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutjahr C, Banba M, Croset V, An K, Miyao A, An G, Hirochika H, Imaizumi-Anraku H, Paszkowski U. Arbuscular mycorrhiza-specific signaling in rice transcends the common symbiosis signaling pathway. Plant Cell. 2008;20:2989–3005. doi: 10.1105/tpc.108.062414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckmann AB, Lombardo F, Miwa H, Perry JA, Bunnewell S, Parniske M, Wang TL, Downie JA. Lotus japonicus nodulation requires two GRAS domain regulators, one of which is functionally conserved in a non-legume. Plant Physiol. 2006;142:1739–1750. doi: 10.1104/pp.106.089508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsters M. SYMRK, an enigmatic receptor guarding and guiding microbial endosymbioses with plant roots. Proc. Natl Acad. Sci. USA. 2008;105:4537–4538. doi: 10.1073/pnas.0801270105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudmon A, Schulman H. Structure-function of the multifunctional Ca2+/calmodulin-dependent protein kinase II. Biochem. J. 2002;364:593–611. doi: 10.1042/BJ20020228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi-Anraku H, Takeda N, Charpentier M, et al. Plastid proteins crucial for symbiotic fungal and bacterial entry into plant roots. Nature. 2005;433:527–531. doi: 10.1038/nature03237. [DOI] [PubMed] [Google Scholar]
- Kaló P, Gleason C, Edwards A, et al. Nodulation signaling in legumes requires NSP2, a member of the GRAS family of transcriptional regulators. Science. 2005;308:1786–1789. doi: 10.1126/science.1110951. [DOI] [PubMed] [Google Scholar]
- Kanamori N, Madsen LH, Radutoiu S, et al. A nucleoporin is required for induction of Ca2+ spiking in legume nodule development and essential for rhizobial and fungal symbiosis. Proc. Natl Acad. Sci. USA. 2006;103:359–364. doi: 10.1073/pnas.0508883103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevei Z, Lougnon G, Mergaert P, et al. 3-hydroxy-3-methylglutaryl coenzyme a reductase 1 interacts with NORK and is crucial for nodulation in Medicago truncatula. Plant Cell. 2007;19:3974–3989. doi: 10.1105/tpc.107.053975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kistner C, Winzer T, Pitzschke A, et al. Seven Lotus japonicus genes required for transcriptional reprogramming of the root during fungal and bacterial symbiosis. Plant Cell. 2005;17:2217–2229. doi: 10.1105/tpc.105.032714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosuta S, Chabaud M, Lougnon G, Gough C, Dénarié J, Barker DG, Bécard G. A diffusible factor from arbuscular mycorrhizal fungi induces symbiosis-specific MtENOD11 expression in roots of Medicago truncatula. Plant Physiol. 2003;131:952–962. doi: 10.1104/pp.011882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosuta S, Hazledine S, Sun J, Miwa H, Morris RJ, Downie JA, Oldroyd GE. Differential and chaotic calcium signatures in the symbiosis signaling pathway of legumes. Proc. Natl Acad. Sci. USA. 2008;105:9823–9828. doi: 10.1073/pnas.0803499105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerouge P, Roche P, Faucher C, Maillet F, Truchet G, Promé JC, Dénarié J. Symbiotic host-specificity of Rhizobium meliloti is determined by a sulphated and acylated glucosamine oligosaccharide signal. Nature. 1990;344:781–784. doi: 10.1038/344781a0. [DOI] [PubMed] [Google Scholar]
- Lévy J, Bres C, Geurts R, et al. A putative Ca2+ and calmodulin-dependent protein kinase required for bacterial and fungal symbioses. Science. 2004;303:1361–1364. doi: 10.1126/science.1093038. [DOI] [PubMed] [Google Scholar]
- Limpens E, Mirabella R, Fedorova E, Franken C, Franssen H, Bisseling T, Geurts R. Formation of organelle-like N2-fixing symbiosomes in legume root nodules is controlled by DMI2. Proc. Natl Acad. Sci. USA. 2005;102:10375–10380. doi: 10.1073/pnas.0504284102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohar DP, Schaff JE, Laskey JG, Kieber JJ, Bilyeu KD, Bird DM. Cytokinins play opposite roles in lateral root formation and nematode and rhizobial symbioses. Plant J. 2004;38:203–214. doi: 10.1111/j.1365-313X.2004.02038.x. [DOI] [PubMed] [Google Scholar]
- Lohmann GV, Shimoda Y, Nielsen MW, et al. Evolution and regulation of the Lotus japonicus LysM receptor gene family. Mol. Plant Microbe Interact. 2010;23:510–521. doi: 10.1094/MPMI-23-4-0510. [DOI] [PubMed] [Google Scholar]
- López-Lara IM, van den Berg JD, Thomas-Oates JE, Glushka J, Lugtenberg BJ, Spaink HP. Structural identification of the lipo-chitin oligosaccharide nodulation signals of Rhizobium loti. Mol. Microbiol. 1995;15:627–638. doi: 10.1111/j.1365-2958.1995.tb02372.x. [DOI] [PubMed] [Google Scholar]
- Madsen EB, Madsen LH, Radutoiu S, et al. A receptor kinase gene of the LysM type is involved in legume perception of rhizobial signals. Nature. 2003;425:637–640. doi: 10.1038/nature02045. [DOI] [PubMed] [Google Scholar]
- Madsen LH, Tirichine L, Jurkiewicz A, Sullivan JT, Heckmann AB, Bek AS, Ronson CW, James EK, Stougaard J. The molecular network governing nodule organogenesis and infection in the model legume Lotus japonicus. Nat. Commun. 2010;1:10. doi: 10.1038/ncomms1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda D, Ashida K, Iguchi K, Chechetka SA, Hijikata A, Okusako Y, Deguchi Y, Izui K, Hata S. Knockdown of an arbuscular mycorrhiza-inducible phosphate transporter gene of Lotus japonicus suppresses mutualistic symbiosis. Plant Cell Physiol. 2006;47:807–817. doi: 10.1093/pcp/pcj069. [DOI] [PubMed] [Google Scholar]
- Maekawa T, Maekawa-Yoshikawa M, Takeda N, Imaizumi-Anraku H, Murooka Y, Hayashi M. Gibberellin controls the nodulation signaling pathway in Lotus japonicus. Plant J. 2009;58:183–194. doi: 10.1111/j.1365-313X.2008.03774.x. [DOI] [PubMed] [Google Scholar]
- Markmann K, Parniske M. Evolution of root endosymbiosis with bacteria: how novel are nodules? Trends Plant Sci. 2009;14:77–86. doi: 10.1016/j.tplants.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Markmann K, Giczey G, Parniske M. Functional adaptation of a plant receptor-kinase paved the way for the evolution of intracellular root symbioses with bacteria. PLoS Biol. 2008;6:e68. doi: 10.1371/journal.pbio.0060068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh JF, Rakocevic A, Mitra RM, Brocard L, Sun J, Eschstruth A, Long SR, Schultze M, Ratet P, Oldroyd GE. Medicago truncatula NIN is essential for rhizobial-independent nodule organogenesis induced by autoactive calcium/calmodulin-dependent protein kinase. Plant Physiol. 2007;144:324–335. doi: 10.1104/pp.106.093021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa H, Sun J, Oldroyd GE, Downie JA. Analysis of Nod-factor-induced calcium signaling in root hairs of symbiotically defective mutants of Lotus japonicus. Mol. Plant Microbe Interact. 2006;19:914–923. doi: 10.1094/MPMI-19-0914. [DOI] [PubMed] [Google Scholar]
- Murakami Y, Miwa H, Imaizumi-Anraku H, Kouchi H, Downie JA, Kawaguchi M, Kawasaki S. Positional cloning identifies Lotus japonicus NSP2, a putative transcription factor of the GRAS family, required for NIN and ENOD40 gene expression in nodule initiation. DNA Res. 2006;13:255–265. doi: 10.1093/dnares/dsl017. [DOI] [PubMed] [Google Scholar]
- Murray J, Karas B, Ross L, et al. Genetic suppressors of the Lotus japonicus har1-1 hypernodulation phenotype. Mol. Plant Microbe Interact. 2006;19:1082–1091. doi: 10.1094/MPMI-19-1082. [DOI] [PubMed] [Google Scholar]
- Murray JD, Karas BJ, Sato S, Tabata S, Amyot L, Szczyglowski K. A cytokinin perception mutant colonized by Rhizobium in the absence of nodule organogenesis. Science. 2007;315:101–104. doi: 10.1126/science.1132514. [DOI] [PubMed] [Google Scholar]
- Niwa S, Kawaguchi M, Imazumi-Anraku H, Chechetka SA, Ishizaka M, Ikuta A, Kouchi H. Responses of a model legume Lotus japonicus to lipochitin oligosaccharide nodulation factors purified from Mesorhizobium loti JRL501. Mol. Plant Microbe Interact. 2001;14:848–856. doi: 10.1094/MPMI.2001.14.7.848. [DOI] [PubMed] [Google Scholar]
- Oldroyd GE. Plant science. Nodules and hormones. Science. 2007;315:52–53. doi: 10.1126/science.1137588. [DOI] [PubMed] [Google Scholar]
- Oldroyd GE, Downie A. Coordinating nodule morphogenesis with rhizobial infection in legume. Annu. Rev. Plant Biol. 2008;59:519–546. doi: 10.1146/annurev.arplant.59.032607.092839. [DOI] [PubMed] [Google Scholar]
- Parniske M. Arbuscular mycorrhiza: the mother of plant root endosymbioses. Nat. Rev. Microbiol. 2008;6:763–775. doi: 10.1038/nrmicro1987. [DOI] [PubMed] [Google Scholar]
- Radutoiu S, Madsen LH, Madsen EB, et al. Plant recognition of symbiotic bacteria requires two LysM receptor-like kinases. Nature. 2003;425:585–592. doi: 10.1038/nature02039. [DOI] [PubMed] [Google Scholar]
- Radutoiu S, Madsen LH, Madsen EB, Jurkiewicz A, Fukai E, Quistgaard EMH, Albrektsen AS, James EK, Thirup S, Stougaard J. LysM domains mediate lipochitin-oligosaccharide recognition and Nfr genes extend the symbiotic host range. EMBO J. 2007;26:3923–3935. doi: 10.1038/sj.emboj.7601826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen C, Rasmussen G. Inhibition of G2/M progression in Schizosaccharomyces pombe by a mutant calmodulin kinase II with constitutive activity. Mol. Biol. Cell. 1994;5:785–795. doi: 10.1091/mbc.5.7.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K, Yoshikawa M, Yano K, et al. NUCLEOPORIN85 is required for calcium spiking, fungal and bacterial symbioses and seed production in Lotus japonicus. Plant Cell. 2007;19:610–624. doi: 10.1105/tpc.106.046938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauser L, Roussis A, Stiller J, Stougaard J. A plant regulator controlling development of symbiotic root nodules. Nature. 1999;402:191–195. doi: 10.1038/46058. [DOI] [PubMed] [Google Scholar]
- Shaw SL, Long SR. Nod factor elicits two separable calcium responses in Medicago truncatula root hair cells. Plant Physiol. 2003;131:976–984. doi: 10.1104/pp.005546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen J. Ca2+-dependent protein kinases and stress signal transduction in plants. Science. 1996;274:1900–1902. doi: 10.1126/science.274.5294.1900. [DOI] [PubMed] [Google Scholar]
- Shibata S, Mitsui H, Kouchi H. Acetylation of a fucosyl residue at the reducing end of Mesorhizobium loti nod factors is not essential for nodulation of Lotus japonicus. Plant Cell Physiol. 2005;46:1016–1020. doi: 10.1093/pcp/pci099. [DOI] [PubMed] [Google Scholar]
- Smit P, Raedts J, Portyanko V, Debellé F, Gough C, Bisseling T, Geurts R. NSP1 of the GRAS protein family is essential for rhizobial Nod factor-induced transcription. Science. 2005;308:1789–1791. doi: 10.1126/science.1111025. [DOI] [PubMed] [Google Scholar]
- Smit P, Limpens E, Geurts R, Fedorova E, Dolgikh E, Gough C, Bisseling T. Medicago LYK3, an entry receptor in rhizobial nod factor signaling. Plant Physiol. 2007;145:183–191. doi: 10.1104/pp.107.100495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stracke S, Kistner C, Yoshida S, et al. A plant receptor-like kinase required for both bacterial and fungal symbiosis. Nature. 2002;417:959–962. doi: 10.1038/nature00841. [DOI] [PubMed] [Google Scholar]
- Tansengco ML, Hayashi M, Kawaguchi M, Imaizumi-Anraku H, Murooka Y. Crinkle, a novel symbiotic mutant that affects the infection thread growth and alters the root hair, trichome and seed development in Lotus japonicus. Plant Physiol. 2003;131:1054–1063. doi: 10.1104/pp.102.017020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirichine L, Imaizumi-Anraku H, Yoshida S, et al. Deregulation of a Ca2+/calmodulin-dependent kinase leads to spontaneous nodule development. Nature. 2006;441:1153–1156. doi: 10.1038/nature04862. [DOI] [PubMed] [Google Scholar]
- Tirichine L, Sandal N, Madsen LH, Radutoiu S, Albrektsen AS, Sato S, Asamizu E, Tabata S, Stougaard J. A gain-of-function mutation in a cytokinin receptor triggers spontaneous root nodule organogenesis. Science. 2007;315:104–107. doi: 10.1126/science.1132397. [DOI] [PubMed] [Google Scholar]
- Wais RJ, Galera C, Oldroyd G, Catoira R, Penmetsa RV, Cook D, Gough C, Denarié J, Long SR. Genetic analysis of calcium spiking responses in nodulation mutants of Medicago truncatula. Proc. Natl Acad. Sci. USA. 2000;97:13407–13412. doi: 10.1073/pnas.230439797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann R, Hanson PI, Schulman H. Multifunctional Ca2+/calmodulin-dependent protein kinase made Ca2+ independent for functional studies. Biochemistry. 1990;29:1679–1684. doi: 10.1021/bi00459a002. [DOI] [PubMed] [Google Scholar]
- Yang T, Du L, Poovaiah BW. Concept of redesigning proteins by manipulating calcium/calmodulin-binding domains to engineer plants with altered traits. Funct. Plant Biol. 2007;34:343–352. doi: 10.1071/FP06293. [DOI] [PubMed] [Google Scholar]
- Yano K, Tansengco ML, Hio T, Higashi K, Murooka Y, Imaizumi-Anraku H, Kawaguchi M, Hayashi M. New nodulation mutants responsible for infection thread development in Lotus japonicus. Mol. Plant Microbe Interact. 2006;19:801–810. doi: 10.1094/MPMI-19-0801. [DOI] [PubMed] [Google Scholar]
- Yano K, Yoshida S, Müller J, et al. CYCLOPS, a mediator of symbiotic intracellular accommodation. Proc. Natl Acad. Sci. USA. 2008;105:20540–20545. doi: 10.1073/pnas.0806858105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano K, Shibata S, Chen WL, et al. CERBERUS, a novel U-box protein containing WD-40 repeats, is required for formation of the infection thread and nodule development in the legume-Rhizobium symbiosis. Plant J. 2009;60:168–180. doi: 10.1111/j.1365-313X.2009.03943.x. [DOI] [PubMed] [Google Scholar]
- Zhu H, Riely BK, Burns NJ, Ané JM. Tracing nonlegume orthologs of legume genes required for nodulation and arbuscular mycorrhizal symbioses. Genetics. 2006;172:2491–2499. doi: 10.1534/genetics.105.051185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Chen T, Zhu M, Fang Q, Kang H, Hong Z, Zhang Z. A novel ARID DNA-binding protein interacts with SymRK and is expressed during early nodule development in Lotus japonicus. Plant Physiol. 2008;148:337–347. doi: 10.1104/pp.108.119164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.