Abstract
Cytochrome P450 1B1, expressed in vascular smooth muscle cells, can metabolize arachidonic acid in vitro into several products including 12- and 20-hydroxyeicosatetraenoic acids that stimulate vascular smooth muscle cell growth. This study was conducted to determine if cytochrome P450 1B1 contributes to angiotensin II-induced rat aortic smooth muscle cell migration, proliferation and protein synthesis. Ang II stimulated migration of these cells, measured by the wound healing approach, by 1.78 fold and DNA synthesis, measured by [3H]thymidine incorporation, by 1.44 fold after 24 hours, and protein synthesis, measured by [3H]leucine incorporation, by 1.40 fold after 48 hours. Treatment of vascular smooth muscle cells with the cytochrome P450 1B1 inhibitor, 2, 4, 3′, 5′-tetramethoxystilbene, or transduction of these cells with adenovirus cytochrome P450 1B1 shRNA, but not its scrambled control, reduced the activity of this enzyme and abolished angiotensin II- and arachidonic acid-induced cell migration, [3H]thymidine and [3H]leucine incorporation. Metabolism of arachidonic acid to 5-, 12-, 15- and 20-hydoxyeicosatetraenoic acids in these cells was not altered, but angiotensin II- and arachidonic acid-induced reactive oxygen species production and extracellular signal-regulated kinase 1/2, and p38 mitogen-activated protein kinase, activity were inhibited by 2, 4, 3′, 5′-tetramethoxystilbene and cytochrome P450 1B1 shRNA, and by tempol that inactivates reactive oxygen species. Tempol did not alter cytochrome P450 1B1 activity. These data suggest that angiotensin II-induced vascular smooth muscle cell migration and growth are mediated by reactive oxygen species generated from arachidonic acid by cytochrome P450 1B1 and activation of extracellular signal-regulated kinase 1/2, and p38 mitogen-activated protein kinase.
Keywords: angiotensin II, CYP1B1, vascular smooth muscle cell growth, ROS
Introduction
The renin-angiotensin system is one of the major components of the mechanisms that contribute to the regulation of blood volume and vascular resistance (1). Angiotensin II (Ang II), the main biologically active agent of this system, also stimulates vascular smooth muscle cell (VSMC) hypertrophy and/or hyperplasia and inflammation and contributes to the development of hypertension, atherosclerosis, heart failure and restenosis after vascular injury (1–6). The pathophysiological actions of Ang II are mediated by activation of one or more serine-threonine and tyrosine kinases, generation of oxygen radicals (7–9) and/or release of arachidonic acid (AA) by cytosolic phospholipase A2 and production of its metabolites, generated via lipoxygenase (LO), 12-hydroxyeicosatetraenoic acid (12-HETE), and/or cytochrome P450 (CYP) 4A, 20-HETE (10–18). Both 12- and 20-HETE promote VSMC migration, hyperplasia and/or hypertrophy (11, 19–22).
CYP enzymes that metabolize xenobiotics including polycyclic aromatic hydrocarbons and endobiotics such as fatty acids and retinoids are also expressed in extrahepatic tissues including the cardiovascular system (23–27). CYP1A1-encoded enzymes are expressed in vascular endothelium and smooth muscle cells, with much higher levels of activity in endothelial cells, whereas CYP1B1 is highly expressed in VSMCs and to a lesser degree in endothelial cells (28, 29), but shear stress upregulates mRNA and protein levels of CYP1A1 and CYP1B1 in endothelial cells (30). Whether CYP1A1 and CYP1B1 contribute to the vascular function is not known. Recombinant CYP1B1 has been shown to metabolize AA into mid-chain HETEs and terminal-HETEs (26). These observations and the demonstration that bioactivation of xenobiotics by CYP1B1 is associated with generation of reactive oxygen species (ROS) (31) led us to hypothesize that CYP1B1, through generation of AA metabolites, HETEs and/or ROS activate signaling molecules including extracellular signal-regulated kinase 1/2 (ERK1/2) and p38 mitogen-activated protein kinase (p38MAPK) that contribute to the action of Ang II on VSMC migration and growth. To test this hypothesis, we have examined the effect of the CYP1B1 inhibitor, 2, 4, 3′, 5′ tetramethoxystilbene (TMS) (32) and silencing the CYP1B1 gene with adenovirus (Ad) CYP1B1 shRNA on Ang II- and AA-induced rat VSMC migration, proliferation and protein synthesis and on HETEs and ROS production and ERK1/2 and p38MAPK activity. The results of this study indicate that Ang II-induced migration, proliferation and protein synthesis of VSMCs is mediated by CYP1B1-dependent generation of ROS from AA and activation of ERK1/2 and p38MAPK, independent of HETEs production.
Methods
Methods section, please see the online Data Supplement at http://hyper.ahajournals.org.
Results
Ang II-induced VSMC migration and DNA and protein syntheses are dependent on CYP1B1 activity
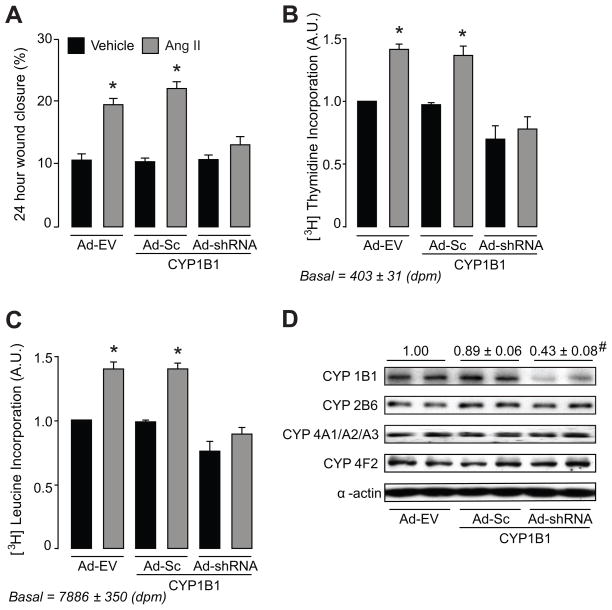
To determine the contribution of CYP1B1 to Ang II-induced VSMC migration, first we measured VSMC migration after exposure to Ang II for 24, 48 and 72 hours using wound healing approach. Since there was no significant difference in percentage of wound closure by Ang II at these time points, we used migration of VSMCs at 24-hour time point in our experiments. Treatment of VSMCs with TMS, a selective CYP1B1 inhibitor (32) (Figure S1A–C), or transduction with Ad-CYP1B1 shRNA, but not Ad-Sc CYP1B1 shRNA or empty virus (Ad-EV) (Figure 1A–C) attenuated Ang II-induced wound healing and [3H]thymidine and [3H]leucine incorporation in VSMCs.
Figure 1. Ang II-induced migration and DNA and protein synthesis depend on CYP1B1 in VSMCs.
A–C. Quiescent VSMCs were transduced with Ad CYP1B1 shRNA (Ad-shRNA), Ad-Sc CYP1B1 shRNA (Ad-Sc) or Ad-EV (200 MOI each) for 24 hours and Ang II (200 nmol/L) or its vehicle was added at time points in the presence of adenoviruses. Migration of VSMCs was determined by measuring the wounds perimeters as described in the Methods. Data shown are percentage of wound closure measured from 2–3 locations in each well from 6 different experiments (A). In the DNA synthesis assay, [3H]thymidine was added with Ang II, [3H]thymidine incorporation was measured after 24 hours and data is shown as fold increase in [3H]thymidine incorporation in VSMCs versus vehicle, as described in “Methods” (n=3) (B). In protein synthesis assay, [3H]leucine was added 24 hours after addition of Ang II, [3H]leucine incorporation was measured at 48th hour and data is presented as fold increase of [3H]leucine incorporation versus vehicle, as described in “Methods” (n=3). Values are means ± S.E. The asterisk denotes a value significantly different from the corresponding value obtained in the presence of vehicle of Ang II (p<0.05) (C). Cells were transduced with Ad-Sc CYP1B1 shRNA (Ad-Sc), Ad CYP1B1 shRNA (Ad-shRNA) or empty adenovirus (Ad-EV) (200 MOI) for 48 hours. Cells were harvested; lysed and equal amount of protein in each sample was subjected to SDS-PAGE and Western blot analysis, as described in “Methods”. The blots were probed with anti-CYP4A1/A2/A3, CYP2B6 and CYP4F2 antibodies. The density of detected bands were measured as described under “Methods” section. Values are the mean density for each band from 3 different experiments (p<0.05). # denotes a value significantly different from corresponding value obtained in transduced cells with Ad-Sc (D).
To determine the selectivity of Ad-CYP1B1 shRNA, we transuded VSMCs with Ad-EV, Ad-Sc CYP1B1 shRNA or Ad-CYP1B1 shRNA and CYP1B1, CYP4A1/A2/A3, CYP2B6 and CYP4F2 protein levels were determined by Western blot analysis as described in “Methods”. Ad-CYP1B1 shRNA, but not Ad-EV or Ad-Sc CYP1B1 shRNA mutants decreased CYP1B1 protein levels. Ad-CYP1B1 shRNA did not alter the protein levels of CYP4A1/A2/A3, CYP2B6 or CYP4F2 indicating the selectivity of the Ad-CYP1B1 shRNA in reducing CYP1B1 protein levels (Figure 1D). Also TMS or Ad-CYP1B1 shRNA did not alter the expression of Ang II (AT1) receptor expression (Fig S2 A–B) or its function as indicated by the effect of Ang II to increase protein kinase Cα (PKCα) activity as indicated by its increased phosphorylation (Figure S2 C–D).
Ang II-induced VSMC migration, DNA and protein syntheses are mediated by cPLA2 activation
To determine if AA released by Ang II stimulates VSMC migration, proliferation and protein synthesis, we examined the effect of cPLA2 inhibitor, BMPD on the action of Ang II and AA. BMPD (200 nmol/L) inhibited Ang II-, but not AA-induced VSMC wound healing, and [3H]thymidine and [3H]leucine incorporation in VSMCs (Figure S3 A–C).
AA-induced VSMC migration and DNA and protein synthesis are mediated by CYP1B1
CYP1B1 metabolizes AA into mid-chain and terminal HETEs in vitro (26) and HETEs are involved in VSMC migration, proliferation and/or hypertrophy (11, 19–22). Therefore, we investigated the contribution of CYP1B1 in AA-induced wound healing and [3H]thymidine and [3H]leucine incorporation in VSMCs. TMS (100 nmol/L) (Figure S4 A–C) and Ad-CYP1B1 shRNA, but not Ad-Sc CYP1B1 shRNA or Ad-EV (Figure S4 D–F), inhibited AA-induced wound healing and [3H]thymidine and [3H]leucine incorporation in VSMCs.
Ang II, AA and cPLA2 inhibitor BMPD do not alter CYP1B1 activity or expression
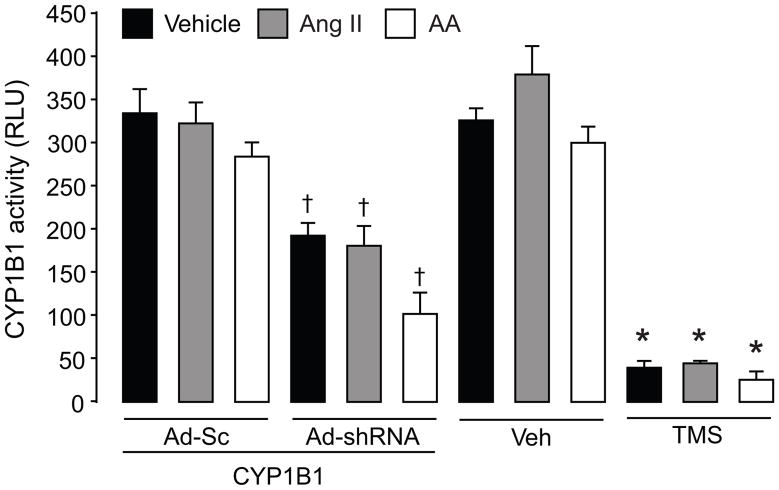
Ang II, AA or cPLA2 inhibitor, BMPD did not alter basal CYP1B1 activity, measured by P450 Glo™ assay, as described in “Methods” (Figure 2, S5) or its expression in VSMCs (Figure S6 A–B). CYP1B1 inducer, benzo(a)pyrene (BZP), but not H2O2 increased CYP1B1 expression (Figure S6 A–B). CYP1B1 activity was inhibited in VSMCs treated with TMS or transduced with Ad-CYP1B1 shRNA but not Ad-Sc CYP1B1 shRNA or Ad-EV (Figure 2).
Figure 2. Effect of Ang II and AA on CYP1B1 activity.
To measure the activity of CYP1B1, VSMCs were transduced with Ad-Sc CYP1B1 shRNA (Ad-Sc), Ad CYP1B1 shRNA (Ad-shRNA), or Ad-EV for 48 hours or pretreated with TMS (100 nmol/L) or its vehicle (Veh) for 30 minutes before adding Ang II (200 nmol/L) or AA (30 μmol/L) or their vehicle for 20 minutes. CYP1B1 activity was measured using luciferin detection agent (LDR) and luminescence was measured as described in “Methods”. Values are means ± S.E. * denotes a value significantly different from the corresponding value obtained in cells transduced with Ad-Sc (p<0.05). *denotes a value significantly different from the corresponding value obtained in the presence of Veh of TMS (p<0.05).
Metabolism of AA in VSMCs into HETEs is independent of CYP1B1 activity
AA increased the production of 5-, 12-, 15-, and 20-HETE in VSMCs, which was not affected by either treatment with TMS or transduction with Ad-CYP1B1 shRNA (Table S1).
CYP1B1 contributes to Ang II- and AA-induced ROS production in VSMCs
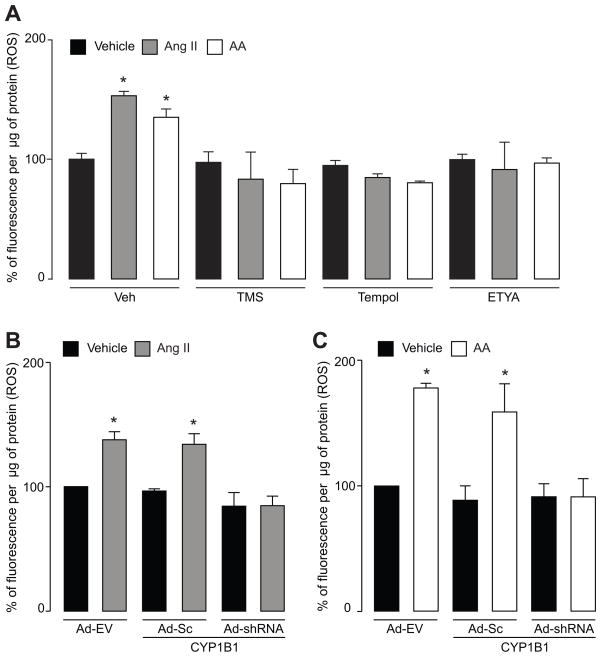
Ang II and AA are known to stimulate ROS production in VSMCs (7–9, 33) and metabolism of AA is associated with ROS generation (34). To determine if CYP1B1 is involved in Ang II- and AA-induced ROS production in VSMCs, we determined the effect of TMS and Ad-CYP1B1 shRNA and its controls on superoxide production. TMS and Ad-CYP1B1 shRNA, but not Ad-Sc CYP1B1 shRNA or Ad-EV diminished Ang II- and AA-induced ROS production (Figure 3A–C), measured by the fluorescence of oxyethidium generation from DHE, as described in “Methods”. cPLA2 inhibitor, BMPD blocked Ang II- but not AA-induced ROS production in VSMCs (Figure S7 A). We also determined the effect of tempol that is capable of inactivating superoxides as well as H2O2 (35) on ROS production in VSMCs. ETYA, an inhibitor of AA metabolism, also reduced Ang II- and AA-induced ROS production in VSMCs (Figure 3A). Oleic acid did not alter production of ROS in VSMCs (Figure S7 B). Tempol inhibited Ang II-and AA-induced ROS production in VSMCs (Figure 3A) and did not alter CYP1B1 activity (Figure S8). These data suggest that CYP1B1 activity is required for generation of ROS in response to Ang II and AA and that its activity is independent of ROS production.
Figure 3. CYP1B1 contributes to intracellular superoxide production by Ang II and AA in VSMCs.
A. VSMCs were pretreated with TMS (100 nmol/L), tempol (1 mmol/L), ETYA (20 μmol/L) or their vehicles (Veh) for 30 minutes before addition of Ang II (200 nmol/L), AA (30 μmol/L) or their vehicle for another 20 minutes. B and C. VSMCs were transduced with Ad-Sc CYP1B1 shRNA (Ad-Sc), Ad CYP1B1 shRNA (Ad-shRNA) or Ad-EV for 48 hours. Cells were then exposed to Ang II (200 nmol/L), AA (30 μmol/L) or their vehicle for 20 minutes, respectively. After addition of stimulants in these experiments, cells were incubated with dihydroethidium (5 μmol/L) for another 20 minutes and fluorescent oxyethidium, an indicator of intracellular superoxide production, was measured as described in “Methods”. The values of 3 experiments are shown as means ± S.E. * denotes a value significantly different from the corresponding value obtained in the presence of vehicle of Ang II or AA (p<0.05).
Metabolism of AA by CYP1B1 supersomes results in superoxide production
We determined superoxide production in a reconstituted system in the presence of AA (30 μmol/L), oleic acid (30 μmol/L) or their vehicle, as described in “Methods”. Incubation of AA but not oleic acid with CYP1B1 supersomes increased superoxide production measured by oxyethidium fluorescence. Inhibitor of AA metabolism ETYA (20 μmol/L) or CYPY1B1 TMS (100 nmol/L) blocked this effect of AA (Figure S9).
Contribution of ROS in Ang II- and AA-induced VSMC migration and DNA and protein syntheses
To confirm that Ang II- or AA-induced VSMC migration, proliferation and protein synthesis mediated by CYP1B1 is due to ROS production, we examined the effect of tempol on wound healing and [3H]thymidine and [3H]leucine incorporation, elicited by Ang II or AA in VSMCs. Tempol blocked Ang II- and AA-induced wound healing and [3H]thymidine and [3H]leucine incorporation in VSMCs (Figure S10 A–F).
Ang II stimulates ERK1/2 and p38MAPK via activation of cPLA2 and CYP1B1 contributes to Ang II- and AA-induced ERK1/2 and p38MAPK activation
Ang II is known to release AA by activation of cPLA2 and Ang II is known to activate ERK1/2 and p38MAPK, which have been implicated in VSMC migration, proliferation and/or hypertrophy (11, 36, 37). Therefore, we examined the effect of cPLA2 inhibitor BMPD, TMS and Ad-CYP1B1 shRNA on Ang II- and AA-induced ERK1/2 and p38MAPK phosphorylation in VSMCs. cPLA2 inhibitor BMPD attenuated Ang II-, but not AA-induced ERK1/2 and p38MAPK phosphorylation (Figure S11 AB). TMS and Ad-CYP1B1 shRNA, but not Ad-Sc CYP1B1 shRNA or Ad-EV, diminished Ang II- or AA-induced phosphorylation of ERK1/2 and p38MAPK, measured by Western blot analysis (Figure S11 C–F). Tempol also inhibited Ang II- and AA-induced ERK1/2 and p38MAPK phosphorylation (Fig S11 G–H) suggesting that CYP1B1 dependent ROS production is involved in the activation of these kinases.
Discussion
This is the first study to demonstrate that Ang II promotes VSMC migration, proliferation and protein synthesis via CYP1B1-dependent generation of ROS and activation of ERK1/2 and p38MAPK, independent of HETEs production. This conclusion is based on our findings that Ang II-induced migration of VSMCs, measured by wound healing approach, was abolished by TMS, an inhibitor of CYP1B1 activity (32). TMS also inhibited Ang II-induced VSMC proliferation and protein synthesis, as measured by [3H]thymidine and [3H]leucine incorporation, respectively, in VSMCs. These observations, together with the effect of TMS in inhibiting CYP1B1 activity in VSMCs, suggest that Ang II-induced migration, proliferation and protein synthesis are dependent upon CYP1B1 activity in VSMCs. Further supporting this view was our demonstration that transduction of VSMCs with Ad CYP1B1 shRNA but not Ad-EV or Ad-Sc shRNA diminished CYP1B1 protein levels, blocked Ang II- as well as AA-induced wound closure, [3H]thymidine and [3H]leucine incorporation in VSMCs. The effect of Ad-CYP1B1shRNA to decrease CYP1B1 protein levels in VSMCs was selective because it did not reduce the protein levels of CYP2B6, 4A1/A2/A3 or 4F2, as shown in our study. Moreover, the effect of TMS or Ad-shRNA was not due to alteration in the expression or coupling of AT1 receptor with G-protein because treatment of VSMC with these agents did not alter AT1 receptor expression or Ang II-induced PKCα activity as measured by its phosphorylation, respectively. That Ang II stimulates release of AA by activating cPLA2 (38) together with our demonstration that Ang II-, but not AA-induced VSMC migration, proliferation and protein synthesis was attenuated by cPLA2 inhibitor suggest that Ang II produces these effects via release of AA.
The effect of TMS and Ad CYP1B1 shRNA to inhibit Ang II- and AA-induced migration, proliferation and protein syntheses in VSMCs was most likely due to decreased CYP1B1 activity because in VSMCs treated with TMS or transduced with Ad CYP1B1 shRNA but not its scrambled control, basal CYP1B1 activity was inhibited. Since CYP1B1 is constitutively active and Ang II or AA did not increase CYP1B1 activity or its expression and that cPLA2 inhibitor, BMPD blocked VSMC migration, DNA and protein synthesis, it appears that Ang II produces these effects by releasing AA release from tissue phospholipids. AA could be metabolized by CYP1B1 to generate HETEs that are known to stimulate migration, proliferation and/or protein synthesis VSMCs by activating one or more signaling molecules (11, 19–22, 39, 40). However, this is unlikely because conversion of AA into HETEs was not altered in VSMCs treated with TMS or transuded with Ad CYP1B1 shRNA. Since Ang II is known to stimulate VSMC growth via production of ROS (7–9) and AA increases ROS generation and noncyclooxygenase inhibitors of AA metabolism attenuate Ang II-induced VSMC growth, it has been suggested that AA metabolites via generation of ROS stimulate VSMC growth (33). Our demonstration that Ang II- and AA-induced ROS production was inhibited in VSMCs treated with TMS or transuded with Ad CYP1B1 shRNA suggest that ROS generated via CYP1B1 from AA mediates the effect of Ang II to stimulate VSMC migration, DNA and protein syntheses. That Ang II- and AA-induced increase fluorescence generated from oxyethidium, product of dihydroethidium oxidation by superoxide production, was confirmed by loss of fluorescence using tempol, a ROS inactivator (35). Moreover, tempol also blocked Ang II- and AA-induced migration, proliferation and hypertrophy of VSMCs. Since tempol did not alter CYP1B1 activity, it appears that ROS are generated most likely by CYP1B1 from Ang II-induced AA release. Supporting this conclusion was our demonstration that Ang II-, but not AA-induced ROS production, was blocked by cPLA2 inhibitor, BMPD. Furthermore, our finding that the inhibitor of AA metabolism, ETYA blocked the generation of ROS elicited by Ang II and AA supports this view. Our demonstration that incubation of CYP1B1 super some with AA but not oleic acid resulted in generation of ROS that was inhibited by TMS and ETYA, suggest that ROS are generated directly from AA during its metabolism by CYP1B1. Whether one or more AA metabolite(s) other than HETEs generated via CYP1B1 or other unsaturated fatty acids also contribute to the generation of ROS remains to be determined. Since Ang II and AA are known to stimulate production of ROS by activation of NAD(P)H oxidase in VSMCs, which is attenuated by ETYA or antisense p22phox (33), and Ang II- and AA-induced ROS is blocked by inhibitors of CYP1B1, it raises the possibility that ROS and/or metabolite(s) of AA generated by CYP1B1 results in activation of NAD(P)H oxidase. Supporting this view is our preliminary work that the inhibitor of CYP1B1, TMS, and Ad-CYP1B1shRNA reduced Ang II- and AA-induced increase in NAD(P)H oxidase activity and Nox-1 level, whereas the NAD(P)H oxidase inhibitor, apocynin, attenuated NAD(P)H oxidase activity and Nox-1 protein level but not CYP1B1 activity (Chi Yong Song, Fariboz A. Yaghini, Hafiz U. B. Ghafoor, Xia-R. Fang and Kafait U. Malik, our unpublished work). It has been proposed that ROS can amplify their own production by activation of NAD(P)H oxidases, xanthine oxidase, increase intercellular uptake of iron and/or uncoupling of endothelial nitric oxide synthase (41). Further studies would establish the relationship and the underlying mechanism of interaction between CYP1B1 and NAD(P)H oxidase and other ROS generating systems.
The mechanism by which CYP1B1 mediates the effect of Ang II on VSMC migration, proliferation and protein synthesis via generation of ROS could involve activation of one or more signaling molecules (7–9). ERK1/2 and p38MAPK have been implicated in Ang II-induced VSMC migration, proliferation and hypertrophy (11, 36, 37) and Ang II-induced activation of p38MAPK but not ERK1/2 in VSMCs has been shown to be dependent on NAD(P)H oxidase activity (42, 43). However, in glomerular mesengial cells, Ang II-induced activation of ERK1/2 and protein synthesis are dependent on NAD(P)H oxidase activity (44). Our findings that cPLA2 inhibitor, BMPD, attenuated the effect of Ang II but not AA and that TMS and Ad-CYP1B1 shRNA inhibited the effect of both these agents to increase ERK1/2 and p38MAPK activity, suggest that ROS generated by CYP1B1 from AA released by Ang II, via activation of ERK1/2 and p38MAPK stimulate VSMC migration, proliferation and protein synthesis. Supporting this view was our finding that tempol inhibited Ang II- or AA-induced increase in ERK1/2 and p38MAPK activity and VSMC migration, proliferation and protein synthesis.
In conclusion, this study demonstrates that Ang II-induced VSMC migration, proliferation and protein synthesis are mediated via ROS generated by CYP1B1 most likely from AA released by cPLA2 activation (Figure 4). Although in blood vessels CYP1B1 is expressed primarily in smooth muscle cells and CYP1A1 in endothelial cells, CYP1B1 has also been found to be present in low levels in endothelial cells and steady shear stress increases mRNA, protein levels and the enzymatic activities of both CYP1A1 and CYP1B1, which are diminished by reversing shear stress (30). Since increased CYP1A1 immunostaining and nuclear localization of aryl hydrocarbon receptor was observed in the descending mouse aorta and low levels of aryl hydrocarbon receptor and expression of CYP1A1 in the lesser curvature of the aortic arch (levels of CYP1B1 were not examined), it has been proposed that increased expression of CYP1A1 and CYP1B1 by shear stress may reflect an anti-atherogenic endothelial cell type (30). However, the results of the present study that CYP1B1 is required for Ang II-induced VSMC migration, proliferation and protein synthesis together with our recent finding that TMS and Ad CYP1B1 shRNA reduces neointimal growth after rat carotid artery injury (45) suggests that CYP1B1 in VSMCs may function as pro-athrogenic rather than anti-atherogenic.
Figure 4. Schematic pathway of CYP1B1 dependent Ang II-induced migration, proliferation and hypertrophy of VSMCs.
ROS generated via CYP1B1 from AA released consequent to activation of cPLA2, stimulate one or more signaling molecules (ERK 1/2 and p38MAPK) that in turn mediates the action of Ang II on VSMC migration and DNA and protein syntheses in VSMCs.
Perspectives
Increased activity of the renin-angiotensin system promotes VSMC migration, proliferation and hypertrophy that contribute to one or more vascular disease including hypertension, restenosis and atherosclerosis. Understanding the cellular mechanisms by which Ang II produces these actions are important in development of new therapeutic approaches toward treatment of vascular diseases. The present study provides a novel insight into the mechanism by which Ang II promotes VSMC migration and growth. We have found that CYP1B1, which is highly expressed in VSMC, mediates Ang II-induced migration, proliferation and protein synthesis via generation of ROS, probably from AA released consequent to cPLA2 activation. These findings suggest that CYP1B1 could serve as a novel target for the development of agents for the treatment of vascular diseases including hypertension, stroke, restenosis and atherosclerosis. Our recent work that TMS prevents the development and/or maintenance of Ang II- and DOCA/Salt-induced hypertension and spontaneous hypertension and neointimal growth caused by balloon angioplasty in rats (communicated at the 63rd Meeting of the Council for High Blood Pressure, 2009) highlights TMS as a promising agent in the treatment of vascular diseases.
Supplementary Material
Table S1. Metabolism of AA into HETEs in VSMCs. Cells were incubated with TMS (100 nmol/L) along with AA (30 μmol/L) or its vehicle overnight (A), or subconfluent cells were transduced with Ad CYP1B1 shRNA or Ad-EV for 24 h, and then AA (30 μmol/L) or its vehicle was added and incubated overnight (B). The cells and the media were collected and subjected to lipid extraction, liquid chromatography, and mass spectrometric analysis of HETEs, as described in Methods, and the average values of HETEs (pg/mg of protein) from two experiments are shown.
Figure S1. Contribution of CYP1B1 in Ang II-induced VSMC migration and DNA and protein syntheses. Subconfluent cells were pretreated with TMS (50 or 100 nmol/L) for 30 min, and Ang II (200 nmol/L) or its vehicle was added at time points, as described in Methods. A. Images of the outline of wounds were captured at the initial time of wounding and after 24 h with 10X magnification. Migration of VSMCs was assessed by measuring the wound perimeters at these two time points. Data are presented as percentage of wound closure, as described in Methods, from two to three locations in each well from six different experiments. B. In the DNA synthesis assay, [3H]thymidine was added with Ang II; [3H]thymidine incorporation was measured after 24 h, and data are shown as x-fold increase in [3H]thymidine incorporation (A.U. = arbitrary unit) in VSMCs versus vehicle, as described in Methods (n=3). C. In the protein synthesis assay, [3H]leucine was added 24 h after adding Ang II, [3H]leucine incorporation was measured at 48 h, and the data are presented (A.U.) as x-fold increase of [3H]leucine incorporation versus vehicle, as described in Methods (n=3). Values are means ± S.E. * denotes a value significantly different from the corresponding value obtained in the presence of vehicle of Ang II (p<0.05).
Figure S2. Effect of Ang II on AT1R, and PKC α expression and phosphorylation. VSMCs were pretreated with TMS (100 nmol/L) or its vehicle; transduced with Ad -Sc CYP1B1 shRNA, Ad-CYP1B1shRNA or Ad-EV (200 MOI) over night. Cells were treated with Ang II (200 nmol/L) for 10 min. Cell lysates were subjected to SDS-PAGE and Western blot analysis, as described previously. The blots were probed with anti-AT1R (A and B), PKC α, and phospho-PKC α(C and D) antibodies. The intensity of bands was measured and values are shown as mean density for each band from 3 different experiments. * denotes a value significantly different from the corresponding value obtained in the absence of Ang II(p<0.05).
Figure S3. Contribution of cPLA2 in Ang II -induced VSMC migration and DNA and protein syntheses. Subconfluent cells were pretreated with cPLA2 inhibitor BMPD ( 200 nmol/L) for 30 min, and Ang II (200 nmol/L) or its vehicle was added at time points, as described in “Methods”. A. The images of the outline of wounds were captured at initial time of wounding and after 24 hours with 10X magnification. Migration of VSMCs was assessed and data presented as described in Figure S1 legend. Data are presented from two to three locations in each well from six different experiments. B. In the DNA synthesis assay, [3H]thymidine was added with Ang II; [3H]thymidine incorporation was measured after 24 h, and data are shown as x-fold increase in [3H]thymidine incorporation (A.U. = arbitrary unit) in VSMCs versus vehicle, as described in “Methods”(n=3). C. In the protein synthesis assay, [3H]leucine was added 24 h after adding Ang II, [ 3H]leucine incorporation was measured at 48 h, and the data are presented (A.U.) as x-fold increase of [3H]leucine incorporation versus vehicle, as described in Methods (n=3). Values are means ± S.E. * denotes a value significantly different from the corresponding value obtained in the presence of vehicle of Ang II (p<0.05).
Figure S4. Contribution of CYP1B1 in AA-induced VSMC migration and DNA and protein synthesis. VSMCs were treated with TMS (50 or 100 nmol/L) for 30 min or transduced with Ad-Sc CYP1B1 shRNA, Ad-CYP1B1shRNA or Ad-EV (200 MOI) over night before adding AA (10 μmol/L) or its vehicle in each well. A and D. The images of the outline of wounds were captured at initial time of wounding and after 24 hours with 10X magnification. Migration of VSMCs was assessed and data presented as described in Figure S1 legend (n=6). B and E. In the DNA synthesis assay, [3H]thymidine was added with AA (10 μmol/L); [3H]thymidine incorporation was measured after 24 h, and data are shown as x-fold increase in [3H]thymidine incorporation (A.U.) in VSMCs versus vehicle (n=3). C and F. In the protein synthesis assay, [3H]leucine was added 24 h after adding AA (10 μmol/L); [3H]leucine incorporation was measured at 48 h, and data are presented as x-fold increase of [3H]leucine incorporation (A.U.) versus vehicle (n=3). Values are the means ± S.E. * denotes a value significantly different from the corresponding value obtained in the presence of vehicle of AA (p<0.05).
Figure S5. Effect of cPLA2 inhibitor BMPD on CYP1B1 activity. To measure the activity of CYP1B1, VSMCs were treated with BMPD (200 nmol/L)or its vehicle (Veh ) for 30 minutes before adding Ang II (200 nmol/L) or AA (30 μmol/L) or their vehicle for 20 minutes. CYP1B1 activity was measured using luciferin detection agent (LDR) and luminescence was measured as described in “Methods”. Values are means ± S.E.
Figure S6. Effect of Ang II or AA on CYP1B1 expression. Confluent VSMCs were incubated with Ang II (200 nmol/L), AA (10 μmol/L), Benzo(a)pyrene (BZP, 40 μmol/L) or H2O2( 100 μmol/L) for 24 (A) or 48 (B) hours. Equal amount of protein in cells lysates were subjected to SDS-PAGE and Western blot analysis. The blots were probed with antibodies against CYP1B1 and α-actin and density of the bands was measured, as described in “Methods”. Values are the means ± S.E. of at least 3 different sets of experiments. * denotes a value significantly different from the corresponding value obtained in the presence of vehicle (Veh) (p<0.05).
Figure S7. Effect of cPLA2 inhibitor BMPD on Ang II -and AA -induced ROS production. A. VSMCs were treated with BMPD (200 nmol/L) or its vehicle (Veh) for 30 minutes before addition of Ang II (200 nmol/L), AA (30 μmol/L) or their vehicles for another 20 minutes. B. The cells were treated with oleic acid (30 μmol/L) or its vehicle for 20 minutes. After addition of stimulants in these experiments, cells were incubated with dihydroethidium (5 μmol/L) for another 20 minutes and fluorescent oxyethidium, was measured as described in “Methods”. The values of 3 experiments are shown as means ± S.E. * denotes a value significantly different from the corresponding value obtained in the presence of vehicle of Ang II or AA (p<0.05).
Figure S8. Effect of tempol on CYP1B1 activity. VSMCs were treated with a ROS inactivator, tempol (1 mmol/L) or its vehicle(Veh) for 30 min and then Ang II (200 nmol/L) or AA (30 μmol/L) or their vehicle for 20 minutes, according to manufacturer’s instruction. Cells were incubated with 250 μL of LCEE (100 μmol/L) and NADPH (100 μmol/L) in K2PO4 (0.1 mol/L) for 150 minutes. Equal amount of luciferin detection reagent (250 μL, LDR) was added and cells were incubated for an additional 20 minutes. Luminescence was measured using a 20/20 luminometer (n=3 for each treatment), as described in “Methods”. Values are means ± S.E.
Figure S9. Superoxide production from AA by CYP1B1 supersomes in vitro. In a recombinant system containing CYP1B1 supersome (150 mmol/L), NADPH (0.5 mmol/L) and DHE (5 μmol/L) in presence of AA (30 μmol/L), oleic acid (30 μmol/L)or their vehicle, along with TMS (100 nmol/L) or ETYA (20 μmol/L) or their vehicle(Veh), we measured the oxyethidium fluorescence, as described in “Methods”. Values are the means ± S.E. * denotes a value significantly different from the corresponding value obtained in the presence of vehicle of AA (p<0.05).
Figure S10. Contribution of ROS to Ang II-and AA induced VSMCs migration and DNA and protein syntheses. Subconfluent cells were pretreated with tempol (0.5 and 1 mmol/L) for 30 min, and Ang II (200 nmol/L), AA (10 μmol/L), or their vehicle was added at the time points, described in Methods section. A and D. Images of the outline of wounds were captured at the initial time of wounding and after 24 h with 10X magnification. Migration of VSMCs was assessed and the data presented as described in Figure S1 legend (n=6). B and E. [3H]Thymidine was added with Ang II, AA, or their vehicle; [3H]thymidine incorporation was measured after 24 h and the data presented as described in Figure S1 legend. (n=3). C and F. [3H]Leucine incorporation was determined and data presented as described in Figure S1 legend (n=3). Values are means ± S.E. * denotes a value significantly different from the corresponding value obtained in the presence of vehicle of Ang II or AA (p<0.05).
Figure S11. Ang II-and AA-induced MAPKs activity is dependent on CYP1B1. Quiescent cells were pretreated with cPLA2 inhibitor BMPD (200 nmol/L, A and B), TMS (100 nmol/L, C and D), Tempol (1 μmol/L, G and H) or their vehicle (Veh) for 30 minutes. Subconfluent VSMCs were transduced with Ad-Sc CYP1B, Ad CYP1B1 shRNA or Ad-EV (each 200 MOI) for 48 hours (E and F). Ang II (200 nmol/L, A, C, E and G) or AA (30 μmol/L, B, D, F and H) or their vehicles was added for 10 minutes. Equal amount of protein in cells lysates were subjected to SDS-PAGE and Western blot analysis. The blots were probed with antibodies against phospho-ERK 1/2, ERK 1/2, phospho-p38 MAPK and p38 MAPK and density of the bands was measured. Values are the means ± S.E. of at least 3 different sets of experiments (p<0.05). * denotes a value significantly different from the corresponding value obtained in the presence of vehicle of Ang II or AA (p<0.05).
Acknowledgments
We thank Dr. David L. Armbruster for his excellent editorial assistance and Dr. Brett L. Jennings for his valuable comments.
Sources of Funding
The described project was supported by the Grants R01-19134 (K.U.M.) and R01-51055 (W.B.C.) from “The National Institutes of Health, Heart, Lung and Blood Institutes (HLBI)”. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of HBLI.
Footnotes
Disclosures
None
References
- 1.Kobori H, Nangaku M, Navar LG, Nishiyama A. The internal renin-angiotensin system: From physiology to the pathophysiology of hypertension and kidney disease. Pharmacol Rev. 2007;59:251–287. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 2.Gibbons GH, Pratt RE, Dzau VJ. Vascular smooth muscle cell hypertrophy vs. hyperplasia: Autocrine transforming growth factor-beta 1 expression determines growth response to angiotensin II. J Clin Invest. 1992;90:456–461. doi: 10.1172/JCI115881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suzuki Y, Ruiz-Ortega M, Lorenzo O, Ruperez M, Esteban V, Egido J. Inflammation and angiotensin II. Int J Biochem Cell Biol. 2003;35:881–900. doi: 10.1016/s1357-2725(02)00271-6. [DOI] [PubMed] [Google Scholar]
- 4.Weiss D, Sorescu D, Taylor WR. Angiotensin II and atherosclerosis. Am J Cardiol. 2001;87:25C–32C. doi: 10.1016/s0002-9149(01)01539-9. [DOI] [PubMed] [Google Scholar]
- 5.Rakugi H, Wang DS, Dzau VJ, Pratt RE. Potential importance of tissue angiotensin-converting enzyme inhibition in preventing neointima formation. Circulation. 1994;90:449–455. doi: 10.1161/01.cir.90.1.449. [DOI] [PubMed] [Google Scholar]
- 6.Feng TC, Ying WY, Hua RJ, Ji YY, de Gasparo M. Effect of valsartan and captopril in rabbit carotid injury. Possible involvement of bradykinin in the antiproliferative action of the renin-angiotensin blockade. J Renin Angiotensin Aldosterone Syst. 2001;2:19–24. doi: 10.3317/jraas.2001.003. [DOI] [PubMed] [Google Scholar]
- 7.Touyz RM, Schiffrin EL. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol Rev. 2000;52:639–672. [PubMed] [Google Scholar]
- 8.Griendling KK, Ushio-Fukai M. Reactive oxygen species as mediators of angiotensin II signaling. Regul Pept. 2000;91:21–27. doi: 10.1016/s0167-0115(00)00136-1. [DOI] [PubMed] [Google Scholar]
- 9.Touyz RM. Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: What is the clinical significance? Hypertension. 2004;44:248–252. doi: 10.1161/01.HYP.0000138070.47616.9d. [DOI] [PubMed] [Google Scholar]
- 10.Nasjletti A. The role of eicosanoids in angiotensin-dependent hypertension. Hypertension. 1998;31:194–200. doi: 10.1161/01.hyp.31.1.194. [DOI] [PubMed] [Google Scholar]
- 11.Reddy MA, Thimmalapura PR, Lanting L, Nadler JL, Fatima S, Natarajan R. The oxidized lipid and lipoxygenase product 12(S)-hydroxyeicosatetraenoic acid induces hypertrophy and fibronectin transcription in vascular smooth muscle cells via p38MAPK and cAMP response element-binding protein activation: Mediation of angiotensin II effects. J Biol Chem. 2002;277:9920–9928. doi: 10.1074/jbc.M111305200. [DOI] [PubMed] [Google Scholar]
- 12.Reddy MA, Kim YS, Lanting L, Natarajan R. Reduced growth factor responses in vascular smooth muscle cells derived from 12/15-lipoxygenase-deficient mice. Hypertension. 2003;41:1294–1300. doi: 10.1161/01.HYP.0000069011.18333.08. [DOI] [PubMed] [Google Scholar]
- 13.Kuhn H, Chaitidis P, Roffeis J, Walther M. Arachidonic Acid metabolites in the cardiovascular system: The role of lipoxygenase isoforms in atherogenesis with particular emphasis on vascular remodeling. J Cardiovasc Pharmacol. 2007;50:609–620. doi: 10.1097/FJC.0b013e318159f177. [DOI] [PubMed] [Google Scholar]
- 14.Yaghini FA, Zhang C, Parmentier J-H, Estes AM, Jafari N, Schaefer SA, Malik KU. Contribution of arachidonic acid metabolites derived via cytochrome P4504A to angiotensin II-induced neointimal growth. Hypertension. 2005;45:1182–1187. doi: 10.1161/01.HYP.0000168051.04275.ea. [DOI] [PubMed] [Google Scholar]
- 15.Schwartzman ML, McGiff JC. Renal cytochrome P450. J Lipid Mediat Cell Signal. 1995;12:229–242. doi: 10.1016/0929-7855(95)00021-h. [DOI] [PubMed] [Google Scholar]
- 16.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- 17.Capdevila JH, Falck JR, Imig JD. Roles of the cytochrome P450 arachidonic acid monooxygenases in the control of systemic blood pressure and experimental hypertension. Kidney Int. 2007;72:683–689. doi: 10.1038/sj.ki.5002394. [DOI] [PubMed] [Google Scholar]
- 18.Muthalif MM, Karzoun NA, Gaber L, Khandekar Z, Benter IF, Saeed AE, Parmentier J-H, Estes A, Malik KU. Angiotensin II-induced hypertension: Contribution of RasGTPase/mitogen-activated protein kinase and cytochrome P450 metabolites. Hypertension. 2000;36:604–609. doi: 10.1161/01.hyp.36.4.604. [DOI] [PubMed] [Google Scholar]
- 19.Nakao J, Ooyama T, Ito H, Chang W-C, Murota S. Comparative effects of lipoxygenase products of arachidonic acid on rat aortic smooth muscle cell migration. Atherosclerosis. 1982;44:339–342. doi: 10.1016/0021-9150(82)90008-9. [DOI] [PubMed] [Google Scholar]
- 20.Natarajan R, Gonzales N, Lanting L, Nadler J. Role of the lipoxygenase pathway in angiotensin II-induced vascular smooth muscle cell hypertrophy. Hypertension. 1994;23:I142–I147. doi: 10.1161/01.hyp.23.1_suppl.i142. [DOI] [PubMed] [Google Scholar]
- 21.Uddin MR, Muthalif MM, Karzoun NA, Benter IF, Malik KU. Cytochrome P-450 metabolites mediate norepinephrine-induced mitogenic signaling. Hypertension. 1998;31:242–247. doi: 10.1161/01.hyp.31.1.242. [DOI] [PubMed] [Google Scholar]
- 22.Stec DE, Gannon KP, Beaird JS, Drummond HA. 20-Hydroxyeicosatetraenoic acid (20-HETE) stimulates migration of vascular smooth muscle cells. Cell Physiol Biochem. 2007;19:121–128. doi: 10.1159/000099200. [DOI] [PubMed] [Google Scholar]
- 23.Shimada T, Hayes CL, Yamazaki H, Amin S, Hecht SS, Guengerich FP, Sutter TR. Activation of chemically diverse procaricinogens by human cytochrome P-450 1B1. Cancer Res. 1996;56:2979–2984. [PubMed] [Google Scholar]
- 24.Shimada T, Fuji-Kuriyama Y. Metabolic activation of polycyclic aromatic hydrocarbons to carcinogens by cytochromes P450 1A1 and 1B1. Cancer Sci. 2004;95:1–6. doi: 10.1111/j.1349-7006.2004.tb03162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayes CL, Spink DC, Spink BC, Cao JQ, Walker NJ, Sutter TR. 17-β-estradiol hydroxylation catalysed by human cytochrome P450 1B1. Proc Natl Acad Sci USA. 1996;93:9776–9781. doi: 10.1073/pnas.93.18.9776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choudhary D, Jansson I, Stoilov I, Sarfarazi M, Schenkman JB. Metabolism of retinoids and arachidonic acid by human and mouse cytochrome P450 1B1. Drug Metabol Disp. 2004;32:840–847. doi: 10.1124/dmd.32.8.840. [DOI] [PubMed] [Google Scholar]
- 27.Korashy HM, El-Kadi AOS. The role of aryl hydrocarbon receptor in the pathogenesis of cardiovascular diseases. Drug Metab Rev. 2006;38:411–450. doi: 10.1080/03602530600632063. [DOI] [PubMed] [Google Scholar]
- 28.Zhao W, Parrish AR, Ramos KS. Constitutive and inducible expression of cytochrome P450IA1 and P450IB1 in human vascular endothelial and smooth muscle cells. In Vitro Cell Dev Biol Anim. 1998;34:671–673. doi: 10.1007/s11626-998-0060-7. [DOI] [PubMed] [Google Scholar]
- 29.Tang Y, Sheef EA, Wang S, Sorenson CM, Marcus CB, Jefcoate CR, Sheibani N. CYP1B1 expression promotes the proangiogenic phenotype of endothelium through decreased intracellular oxidative stress and thrombospondin-2 expression. Blood. 2009;113:744–54. doi: 10.1182/blood-2008-03-145219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conway DE, Sakurai Y, Weiss D, Vega JD, Taylor WR, Jo H, Eskin SG, Marcus CB, McIntire LV. Expression of CYP1A1 and CYP1B1 in human endothelial cells: regulation by fluid shear stress. Cardiovas Res. 2009;81:669–677. doi: 10.1093/cvr/cvn360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Green RM, Hodges NJ, Chipman JK, O’Donovan MR, Graham M. Reactive oxygen species from the uncoupling of human cytochrome P450 1B1 may contribute to the carcinogenicity of dioxin-like polychlorinated biphenyls. Mutagenesis. 2008;23:457–463. doi: 10.1093/mutage/gen035. [DOI] [PubMed] [Google Scholar]
- 32.Chun Y-J, Kim S, Kim D, Lee S-K, Guengerich FP. A new selective and potent inhibitor of human cytochrome P450 1B1 and its application to antimutagenesis. Cancer Res. 2001;61:8164–8170. [PubMed] [Google Scholar]
- 33.Zafari AM, Ushio-Fukai, Minieri CA, Akers M, Lassegue B, Griendling KK. Aracidonic acid metabolites mediate angiotensin II-induced NADH/NADPH oxidase activity and hypertrophy in vascular smooth muscle cells. Antioxid Redox Signal. 1999;1:167–179. doi: 10.1089/ars.1999.1.2-167. [DOI] [PubMed] [Google Scholar]
- 34.Smith RL, Weidemann MJ. Reactive oxygen production associated with arachidonic acid metabolism by peritoneal macrophages. Biochem Biophys Res Commun. 1980;97:973–980. doi: 10.1016/0006-291x(80)91472-2. [DOI] [PubMed] [Google Scholar]
- 35.Wilcox CS, Pearlman A. Chemistry and antihypertensive effects of tempol and other nitroxides. Pharmacol Rev. 2008;60:418–469. doi: 10.1124/pr.108.000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mugabe BE, Yaghini FA, Song CY, Buharalioglu CK, Waters CM, Malik KU. Angiotensin II-induced migration of vascular smooth muscle cells is mediated by p38MAPK activated c-Src through spleen tyrosine kinase and EGFR transactivation. J Pharmacol Exp Ther. 2010;332:116–124. doi: 10.1124/jpet.109.157552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mabrouk ME, Touyz RM, Schriffrin EL. Differential Ang II-induced growth activation pathways in mesenteric artery smooth muscle cells from SHR. Am J Physiol Heart Circ Physiol. 2001;281:H30–H39. doi: 10.1152/ajpheart.2001.281.1.H30. [DOI] [PubMed] [Google Scholar]
- 38.Muthalif MM, Benter IF, Uddin MR, Harper JL, Malik KU. Signal transduction mechanisms involved in angiotensin-(1–7) stimulated arachidonic acid release and prostanoid synthesis in rabbit aortic smooth muscle cells. J Pharmacol Exp Ther. 1998;284:388–398. [PubMed] [Google Scholar]
- 39.Reddy MA, Sahar S, Villeneuve LM, Lanting L, Natarajan R. Role of Src tyrosine kinase in the atherogenic effects of the 12/15-lipoxygenase pathways in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2009;29:387–393. doi: 10.1161/ATVBAHA.108.179150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chava KR, Karpuapu M, Wang D, Bhanoori M, Kundumani-Sridharan V, Zhang Q, Ichiki T, Glasgoq WC, Rao GN. CREB-mediated IL-6 expression is required for 15(S)-hydroxyeicosatetraenoic acid-induced vascular smooth muscle cell migration. Arterioscler Thromb Vasc Biol. 2009;29:809–815. doi: 10.1161/ATVBAHA.109.185777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cai H. NAD(P)H oxidase-dependent self-propagation of hydroge peroxide and vascular disease. Circ Res. 2005;96:818–822. doi: 10.1161/01.RES.0000163631.07205.fb. [DOI] [PubMed] [Google Scholar]
- 42.Touyz RM, Cruzado M, Tabet F, Yao G, Salomon S, Schiffrin EL. Redox-dependent MAP kinase signaling by Ang II in vascular smooth muscle cells: role of receptor tyrosine kinase transactivation. Can J Physiol Pharmacol. 2003;81:159–167. doi: 10.1139/y02-164. [DOI] [PubMed] [Google Scholar]
- 43.Viedt C, Soto U, Krieger-Brauer HI, Fei J, Elsing C, Kubler W, Kreuzer J. Differential activation of mitogen-activated protein kinases in smooth muscle cells by angiotensin II: Involvement of p22phox and reactive oxygen species. Arterioscler Thromb Vasc Biol. 2000;20:940–948. doi: 10.1161/01.atv.20.4.940. [DOI] [PubMed] [Google Scholar]
- 44.Gorin Y, Ricono JM, Wagner B, Kim N-H, Bhandari B, Choudhury GG, Abboud HE. Angiotensin II-induced ERK1/ERK2 activation and protein synthesis are redox-dependent in glomerular mesangial cells. Biochem J. 2004;381:231–239. doi: 10.1042/BJ20031614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song CY, Yaghini FA, Tolkachjov SN, Estes AM, Fang XR, Malik KU. Angiotensin II-induced neointimal growth in balloon injured rat carotid artery is dependent upon cytochrome P450 1B1. Hypertension. 2009;54:e122. (Abstract) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Metabolism of AA into HETEs in VSMCs. Cells were incubated with TMS (100 nmol/L) along with AA (30 μmol/L) or its vehicle overnight (A), or subconfluent cells were transduced with Ad CYP1B1 shRNA or Ad-EV for 24 h, and then AA (30 μmol/L) or its vehicle was added and incubated overnight (B). The cells and the media were collected and subjected to lipid extraction, liquid chromatography, and mass spectrometric analysis of HETEs, as described in Methods, and the average values of HETEs (pg/mg of protein) from two experiments are shown.
Figure S1. Contribution of CYP1B1 in Ang II-induced VSMC migration and DNA and protein syntheses. Subconfluent cells were pretreated with TMS (50 or 100 nmol/L) for 30 min, and Ang II (200 nmol/L) or its vehicle was added at time points, as described in Methods. A. Images of the outline of wounds were captured at the initial time of wounding and after 24 h with 10X magnification. Migration of VSMCs was assessed by measuring the wound perimeters at these two time points. Data are presented as percentage of wound closure, as described in Methods, from two to three locations in each well from six different experiments. B. In the DNA synthesis assay, [3H]thymidine was added with Ang II; [3H]thymidine incorporation was measured after 24 h, and data are shown as x-fold increase in [3H]thymidine incorporation (A.U. = arbitrary unit) in VSMCs versus vehicle, as described in Methods (n=3). C. In the protein synthesis assay, [3H]leucine was added 24 h after adding Ang II, [3H]leucine incorporation was measured at 48 h, and the data are presented (A.U.) as x-fold increase of [3H]leucine incorporation versus vehicle, as described in Methods (n=3). Values are means ± S.E. * denotes a value significantly different from the corresponding value obtained in the presence of vehicle of Ang II (p<0.05).
Figure S2. Effect of Ang II on AT1R, and PKC α expression and phosphorylation. VSMCs were pretreated with TMS (100 nmol/L) or its vehicle; transduced with Ad -Sc CYP1B1 shRNA, Ad-CYP1B1shRNA or Ad-EV (200 MOI) over night. Cells were treated with Ang II (200 nmol/L) for 10 min. Cell lysates were subjected to SDS-PAGE and Western blot analysis, as described previously. The blots were probed with anti-AT1R (A and B), PKC α, and phospho-PKC α(C and D) antibodies. The intensity of bands was measured and values are shown as mean density for each band from 3 different experiments. * denotes a value significantly different from the corresponding value obtained in the absence of Ang II(p<0.05).
Figure S3. Contribution of cPLA2 in Ang II -induced VSMC migration and DNA and protein syntheses. Subconfluent cells were pretreated with cPLA2 inhibitor BMPD ( 200 nmol/L) for 30 min, and Ang II (200 nmol/L) or its vehicle was added at time points, as described in “Methods”. A. The images of the outline of wounds were captured at initial time of wounding and after 24 hours with 10X magnification. Migration of VSMCs was assessed and data presented as described in Figure S1 legend. Data are presented from two to three locations in each well from six different experiments. B. In the DNA synthesis assay, [3H]thymidine was added with Ang II; [3H]thymidine incorporation was measured after 24 h, and data are shown as x-fold increase in [3H]thymidine incorporation (A.U. = arbitrary unit) in VSMCs versus vehicle, as described in “Methods”(n=3). C. In the protein synthesis assay, [3H]leucine was added 24 h after adding Ang II, [ 3H]leucine incorporation was measured at 48 h, and the data are presented (A.U.) as x-fold increase of [3H]leucine incorporation versus vehicle, as described in Methods (n=3). Values are means ± S.E. * denotes a value significantly different from the corresponding value obtained in the presence of vehicle of Ang II (p<0.05).
Figure S4. Contribution of CYP1B1 in AA-induced VSMC migration and DNA and protein synthesis. VSMCs were treated with TMS (50 or 100 nmol/L) for 30 min or transduced with Ad-Sc CYP1B1 shRNA, Ad-CYP1B1shRNA or Ad-EV (200 MOI) over night before adding AA (10 μmol/L) or its vehicle in each well. A and D. The images of the outline of wounds were captured at initial time of wounding and after 24 hours with 10X magnification. Migration of VSMCs was assessed and data presented as described in Figure S1 legend (n=6). B and E. In the DNA synthesis assay, [3H]thymidine was added with AA (10 μmol/L); [3H]thymidine incorporation was measured after 24 h, and data are shown as x-fold increase in [3H]thymidine incorporation (A.U.) in VSMCs versus vehicle (n=3). C and F. In the protein synthesis assay, [3H]leucine was added 24 h after adding AA (10 μmol/L); [3H]leucine incorporation was measured at 48 h, and data are presented as x-fold increase of [3H]leucine incorporation (A.U.) versus vehicle (n=3). Values are the means ± S.E. * denotes a value significantly different from the corresponding value obtained in the presence of vehicle of AA (p<0.05).
Figure S5. Effect of cPLA2 inhibitor BMPD on CYP1B1 activity. To measure the activity of CYP1B1, VSMCs were treated with BMPD (200 nmol/L)or its vehicle (Veh ) for 30 minutes before adding Ang II (200 nmol/L) or AA (30 μmol/L) or their vehicle for 20 minutes. CYP1B1 activity was measured using luciferin detection agent (LDR) and luminescence was measured as described in “Methods”. Values are means ± S.E.
Figure S6. Effect of Ang II or AA on CYP1B1 expression. Confluent VSMCs were incubated with Ang II (200 nmol/L), AA (10 μmol/L), Benzo(a)pyrene (BZP, 40 μmol/L) or H2O2( 100 μmol/L) for 24 (A) or 48 (B) hours. Equal amount of protein in cells lysates were subjected to SDS-PAGE and Western blot analysis. The blots were probed with antibodies against CYP1B1 and α-actin and density of the bands was measured, as described in “Methods”. Values are the means ± S.E. of at least 3 different sets of experiments. * denotes a value significantly different from the corresponding value obtained in the presence of vehicle (Veh) (p<0.05).
Figure S7. Effect of cPLA2 inhibitor BMPD on Ang II -and AA -induced ROS production. A. VSMCs were treated with BMPD (200 nmol/L) or its vehicle (Veh) for 30 minutes before addition of Ang II (200 nmol/L), AA (30 μmol/L) or their vehicles for another 20 minutes. B. The cells were treated with oleic acid (30 μmol/L) or its vehicle for 20 minutes. After addition of stimulants in these experiments, cells were incubated with dihydroethidium (5 μmol/L) for another 20 minutes and fluorescent oxyethidium, was measured as described in “Methods”. The values of 3 experiments are shown as means ± S.E. * denotes a value significantly different from the corresponding value obtained in the presence of vehicle of Ang II or AA (p<0.05).
Figure S8. Effect of tempol on CYP1B1 activity. VSMCs were treated with a ROS inactivator, tempol (1 mmol/L) or its vehicle(Veh) for 30 min and then Ang II (200 nmol/L) or AA (30 μmol/L) or their vehicle for 20 minutes, according to manufacturer’s instruction. Cells were incubated with 250 μL of LCEE (100 μmol/L) and NADPH (100 μmol/L) in K2PO4 (0.1 mol/L) for 150 minutes. Equal amount of luciferin detection reagent (250 μL, LDR) was added and cells were incubated for an additional 20 minutes. Luminescence was measured using a 20/20 luminometer (n=3 for each treatment), as described in “Methods”. Values are means ± S.E.
Figure S9. Superoxide production from AA by CYP1B1 supersomes in vitro. In a recombinant system containing CYP1B1 supersome (150 mmol/L), NADPH (0.5 mmol/L) and DHE (5 μmol/L) in presence of AA (30 μmol/L), oleic acid (30 μmol/L)or their vehicle, along with TMS (100 nmol/L) or ETYA (20 μmol/L) or their vehicle(Veh), we measured the oxyethidium fluorescence, as described in “Methods”. Values are the means ± S.E. * denotes a value significantly different from the corresponding value obtained in the presence of vehicle of AA (p<0.05).
Figure S10. Contribution of ROS to Ang II-and AA induced VSMCs migration and DNA and protein syntheses. Subconfluent cells were pretreated with tempol (0.5 and 1 mmol/L) for 30 min, and Ang II (200 nmol/L), AA (10 μmol/L), or their vehicle was added at the time points, described in Methods section. A and D. Images of the outline of wounds were captured at the initial time of wounding and after 24 h with 10X magnification. Migration of VSMCs was assessed and the data presented as described in Figure S1 legend (n=6). B and E. [3H]Thymidine was added with Ang II, AA, or their vehicle; [3H]thymidine incorporation was measured after 24 h and the data presented as described in Figure S1 legend. (n=3). C and F. [3H]Leucine incorporation was determined and data presented as described in Figure S1 legend (n=3). Values are means ± S.E. * denotes a value significantly different from the corresponding value obtained in the presence of vehicle of Ang II or AA (p<0.05).
Figure S11. Ang II-and AA-induced MAPKs activity is dependent on CYP1B1. Quiescent cells were pretreated with cPLA2 inhibitor BMPD (200 nmol/L, A and B), TMS (100 nmol/L, C and D), Tempol (1 μmol/L, G and H) or their vehicle (Veh) for 30 minutes. Subconfluent VSMCs were transduced with Ad-Sc CYP1B, Ad CYP1B1 shRNA or Ad-EV (each 200 MOI) for 48 hours (E and F). Ang II (200 nmol/L, A, C, E and G) or AA (30 μmol/L, B, D, F and H) or their vehicles was added for 10 minutes. Equal amount of protein in cells lysates were subjected to SDS-PAGE and Western blot analysis. The blots were probed with antibodies against phospho-ERK 1/2, ERK 1/2, phospho-p38 MAPK and p38 MAPK and density of the bands was measured. Values are the means ± S.E. of at least 3 different sets of experiments (p<0.05). * denotes a value significantly different from the corresponding value obtained in the presence of vehicle of Ang II or AA (p<0.05).