SUMMARY
Stress mobilizes elements from the neuroendocrine system to modulate immune responses. Cholinergic stimulation via nicotinic receptor (nAchR) is a major neuroendocrine signaling axis associated with the stress response whose specific effects on the immune system are unknown. Here, we show that nAchR activation by topical agonist application or deletion of the nAChR antagonist catestatin (Chga−/−) reduced antimicrobial peptide (AMP) activity in skin extracts and increased susceptibility to methicillin-resistant Staphylococcus aureus and Group A Streptococcus infections. The adverse effects on AMP expression and infection were rescued by topical application of a nAChR antagonist. Stress-induced nAChR activation increased infection in wild-type, but not Chga−/− or cathelicidin-deficient, mice. These data identify a mechanism for the negative regulation of host-innate AMP response to infection through cholinergic activation and indicate nAChR-mediated cathelicidin dysregulation as a potential mechanism for increased susceptibility to infection following prolonged stress or nicotine use.
INTRODUCTION
Epithelia, such as the skin, prevent most exogenous pathogenic microbes from invasion and colonization through both a physical barrier, composed of epithelial cells, and by an outer chemical shield comprised of antimicrobial molecules that includes certain peptides, enzyme inhibitors, and reactive oxygen species (Aberg et al., 2008; Radek and Gallo, 2007). Antimicrobial peptides (AMPs) such as the cathelicidin (LL-37) (Dorschner et al., 2001; Nizet et al., 2001), human β-defensin 2 (hBD2) (Ganz et al., 1985; van Wetering et al., 1999), Psoriasin (Harder et al., 1997), and RNase7 (Harder and Schroder, 2002) are produced in epithelia, comprising an important innate barrier to infection. Although expressed at relatively low levels under basal conditions, AMPs are inducible by various stimuli such as pathogen challenge, 1,25 (OH)2 vitamin D (VD3), and/or external injury (Heilborn et al., 2003; Schauber et al., 2006; Weber et al., 2005). In addition to their direct and indirect antimicrobial activity, AMPs have multiple roles in modulating both the immune and wound healing responses (Lai and Gallo, 2009; Mookherjee and Hancock, 2007). AMPs can dampen the response to septic shock (Mookherjee et al., 2006), increase chemokines and chemokine receptor expression (Scott et al., 2002), promote adaptive immune responses (Davidson et al., 2004; Tani et al., 2000), and stimulate epithelial proliferation (Heilborn et al., 2003; Shaykhiev et al., 2005) and angiogenesis (Koczulla et al., 2003) during wound repair. The multifunctional capacity of these peptides orchestrates host immune responses by subtly enhancing innate immunity, while taming exacerbated inflammatory responses. Collectively, AMPs help to maintain the delicate inflammatory balance to combat infection and promote healing, without excessive inflammation. Thus, disrupting this tight balance via changes in AMP regulation can shift the balance to restrict the ability of epithelia to combat infection or initiate detrimental inflammatory processes.
Currently, the mechanisms responsible for the negative regulation of AMPs are incompletely understood, but recent studies have determined that factors such as psychological stress (PS) adversely affect production of AMPs in murine skin (Aberg et al., 2007). Substantial evidence now supports the notion of active crosstalk between the nervous, endocrine, and immune systems and the regulation of inflammatory processes across a multitude of cell types and tissues (Elenkov et al., 2000; Tracey, 2002). Stress mobilizes various elements from the neuroendocrine system to modulate immune responses. Acute stress was typically thought to be immunoenhancing as part of the “fight-or-flight” response, while sustained stress tends to be immunosuppressive (Aberg et al., 2007; Ashcraft and Bonneau, 2008; Dhabhar, 2009).
Three major pathways of the stress response include catecholamines via adrenergic stimulation, glucocorticoids (GCs) via activation of the hypothalamic-pituitary-adrenal axis, and cholinergic stimulation via acetylcholine (Ach) (Brogden et al., 2005). Early evidence for catecholamine effects on AMP regulation was seen in frogs, where pharmacologic doses of noradrenaline promoted the release of fully processed active AMPs from the highly innervated granular glands at the skin surface (Benson and Hadley, 1969; Zasloff, 1987). In mammals, recent studies identified that both PS (i.e., insomnia) and topical GC applications reduced the expression of both cathelicidin (CAMP) and β-defensin 3 (mBD-3) in murine skin, resulting in more severe cutaneous bacterial lesions (Aberg et al., 2007). These studies revealed that PS reduced AMPs in a GC-dependent fashion: use of the GC receptor antagonist, RU-486, blocked PS-induced suppression of AMP expression, suggesting a direct role for GCs on this regulatory pathway. Although Ach is another major signaling molecule involved in the stress response, the specific effects of Ach on AMP regulation either in the skin or in extracutaneous tissues have not yet been elucidated.
The discovery of a nonneuronal cholinergic system in epidermis emphasizes the intricate relationship between the epidermis and nervous systems, because both of these tissues evolve embryologically from the ectoderm. The epidermis contains several molecules, multiple neurotransmitters, receptors, and ion channels that are typically present within the nervous system, and nonneuronal cholinergic activation regulates a multitude of functions in various cell types to maintain normal homeostasis. For example, keratinocytes synthesize and degrade Ach to allow for functions including cell proliferation, apoptosis, migration, and permeability barrier homeostasis (Denda et al., 2000; Grando et al., 1993). Binding of Ach to the cell membrane elicits these diverse effects through receptor-dependent mechanisms involving both muscarinic acetylcholine (mAChRs) and nicotinic acetylcholine receptors (nAChRs), which are classified by the specificity of their respective agonists, muscarine and nicotine (Grando et al., 2006). Furthermore, we recently identified the presence of the nicotinic antagonist catestatin (Cst) in the epidermis (Radek et al., 2008). This observation suggests that Cst could counteract the effects of cholinergic activation in a manner important for epidermal homeostasis.
Understanding the mechanisms that regulate AMPs and epithelial homeostasis is critical for the prevention and treatment of skin infections. Over 25% of all nosocomial infections that involve the skin or adjacent tissue have methicillin-resistant S. aureus (MRSA) as the primary agent (Lipsky et al., 2007). Recent evidence also indicates that the incidence of postoperative wound infections is higher in smokers than in nonsmokers (Sorensen et al., 2003), whereas nicotinic activation has been shown to impair host defense against pneumococcal pneumonia (Giebelen et al., 2009). Cholinergic activation was shown to inhibit local proinflammatory production of cytokines and modulate lymphocyte activation, since the vagus nerve innervates major organs involved in the response to endotoxin (De Rosa et al., 2009; Pavlov et al., 2006). This response was later termed the “cholinergic anti-inflammatory pathway” and indicates the promiscuous nature of nAChR activation on immune function (Bernik et al., 2002). Although the immunosuppressive effect of nicotinic stimulation has been extensively studied in immune cells and other epithelia, the influence of nAChR stimulation on keratinocyte innate immune function, specifically AMP activity, has not yet been examined. Hence, we hypothesized that the immunosuppression and susceptibility to infection induced by nAChR signaling could be linked to altered AMP activity, and that Cst could function within this pathway to regulate these effects. In this study, we explored the interrelationship between nAChR signaling and AMP activity in epidermis and report here that control of nicotinic activation dramatically alters susceptibility to infection through alterations of epidermal AMP production.
RESULTS
Nicotinic Stimulation Decreases Antimicrobial Activity from Skin
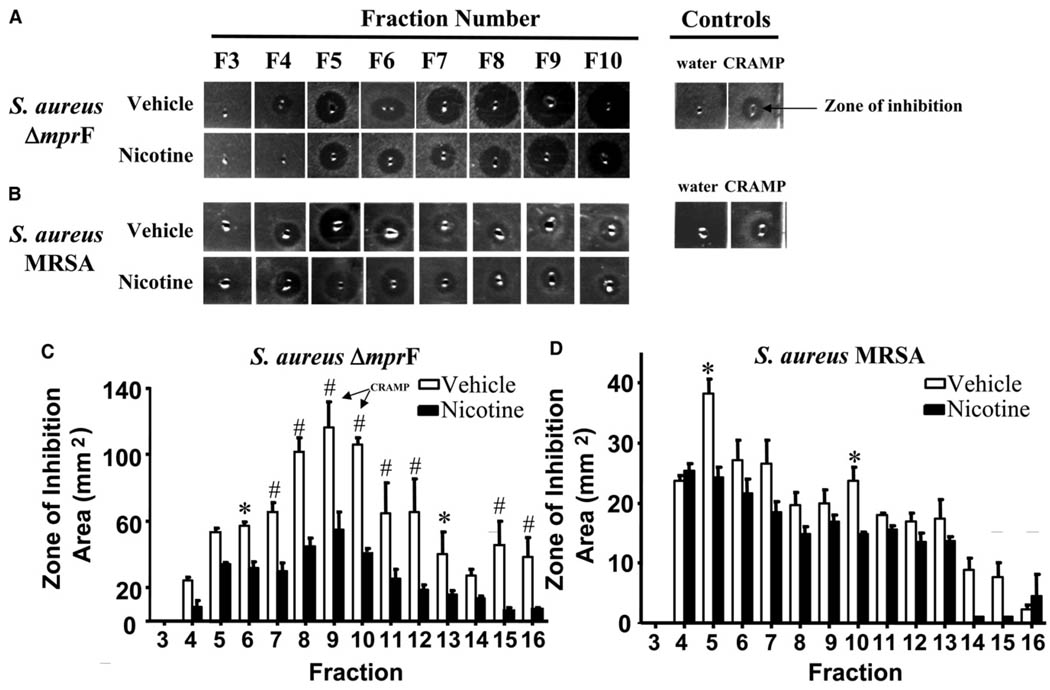
Nicotine increases the incidence of skin and lung infections, but the mechanism responsible for this increase in susceptibility is unknown (Giebelen et al., 2009; Sorensen et al., 2003). Because microbial survival is inhibited by AMPs, we questioned whether the increased susceptibility to infection could be due to a decrease in AMP production. First, to directly evaluate the effects of nicotine on epithelial AMP production, mice were treated topically with nicotine and their skin was subsequently isolated for analysis of extractable AMPs by radial diffusion assay (Lehrer et al., 1991). Peptide extracts were partially purified by high-pressure liquid chromatography (HPLC) prior to analysis of antimicrobial action in order to link activity within different fractions with specific AMP populations. Extract fractions from nicotine-treated mice exhibited much smaller zones of inhibition compared with vehicle-treated extracts when assayed against the AMP-sensitive Staphylococcus aureus mutant ΔmprF, consistent with reduced antimicrobial activity in epidermis of nicotine-treated mice (Figure 1A). When fractions were also analyzed against a S. aureus strain that is relatively resistant to AMP killing (methicillin-resistant S. aureus Sanger 252 MRSA), the differences between nicotine-treated and vehicle-treated skin extracts were less apparent (Figure 1B), although there was a trend toward a decrease in AMP activity across all fractions, indicating a global suppression of antimicrobial activity. Measurement of the area of inhibition of S aureus ΔmprF revealed a significant reduction (ca. 50% reduction) in overall diffusible antimicrobial activity across multiple fractions with nicotine (fractions 6–12 and 15–16, Figure 1C). The elution fraction of cathelicidin-related antimicrobial peptide (CRAMP) is indicated within fractions 9 and 10. Fractions from nicotine-treated mice assayed against S. aureus Sanger 252 MRSA exhibited significantly reduced activity only in fractions 5 and 10 (Figure 1D).
Figure 1. Nicotine Reduces Extractable AMPs from Normal Mouse Skin.
(A) Vehicle or nicotine-treated mouse skin extracts incubated with agar containing S. aureus ΔmprF. HPLC fractions 3–10 are shown (F3–F10). Right side depicts water and CRAMP peptide as negative and positive controls, respectively. Zone of inhibition refers to the zone of bacterial clearance surrounding the central well.
(B) Vehicle or nicotine-treated mouse skin extracts incubated with agarose containing S. aureus MRSA. Same representation as in (A).
(C) The zone of inhibition (area in mm2) against S. aureus ΔmprF for fractions 3–16 was quantified. *p < 0.05, #p < 0.01 versus vehicle by two-way ANOVA. Data are represented as mean ± SEM.
(D) The zone of inhibition (area in mm2) against S. aureus MRSA for fractions 3–16 was quantified. Same representation as in (C).
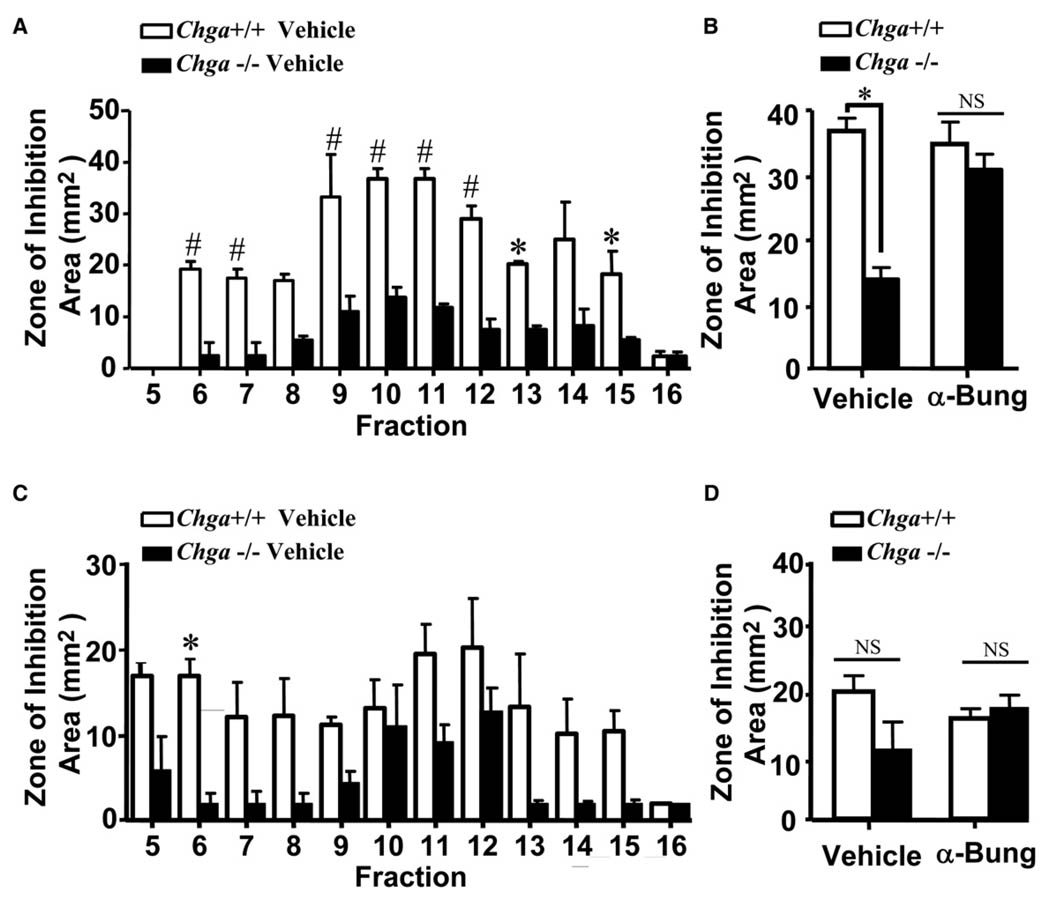
Next, a genetic mouse model was used to evaluate whether unopposed endogenous nAChR activation can influence epidermal antimicrobial activity. For these experiments, we assessed cutaneous antimicrobial defense in Chga−/− mice that lack chromogranin A and its derived peptide fragment catestatin (Cst), which acts as an endogenous antagonist of the nAChR (Mahapatra et al., 2005). Similar to exogenous nicotine application, extracts from Chga−/− mice showed significantly smaller zones of inhibition than did wild-type mice (Figure 2A), and area quantification revealed a similar reduction (~60% decrease) of total antimicrobial activity in multiple fractions (fractions 6–7, 9–15).
Figure 2. Unopposed Nicotinic Activation in Chga−/− Mice Reduces Extractable AMPs and Is Reversed by Topical nAChR Antagonist.
(A) Topically treated vehicle or α-bungarotoxin (100nM) Chga+/+ and Chga−/− mouse skin extracts skin extracts against S. aureus ΔmprF. The zone of inhibition (area in mm2) against S. aureus ΔmprF for fractions 5–16 from Chga+/+ and Chga−/− vehicle-treated skin extracts was quantified. *p < 0.05, #p < 0.01 versus vehicle by two-way ANOVA. Data are represented as mean ± SEM.
(B) β-Bungarotoxin rescued antimicrobial activity in Chga−/− mouse skin against S. aureus ΔmprF. HPLC fraction 11 is shown from vehicle and α-bungarotoxin-treated Chga+/+ and Chga−/− mice. NS, not significant.
(C) Topically treated vehicle or α-bungarotoxin (100 nM) Chga+/+ and Chga−/− mouse skin extracts against S. aureus MRSA. The zone of inhibition (area in mm2) against S. aureus MRSA for fractions 5–16 from Chga+/+ and Chga−/− vehicle-treated skin extracts was quantified. Same representation as in (A). NS, not significant.
(D) α-Bungarotoxin effects on Chga−/− mouse skin against S. aureus MRSA. HPLC fraction 11 is shown from vehicle and α-bungarotoxin-treated Chga+/+ and Chga−/− mice.
To determine if reduced antimicrobial activity in Chga−/− mice was indeed due to nAChR activation, we attempted to rescue Chga−/− mice with topical applications of α-bungarotoxin, a pharmacologic nAChR antagonist. This treatment completely restored antimicrobial activity against S. aureus ΔmprF in all epidermal fractions from Chga−/− mice (Figure 2B). Similar to nicotine-treated wild-type mouse skin, low levels of antimicrobial activity were seen when wild-type or Chga−/− mouse skin was assayed against an AMP-resistant strain of MRSA (Figures 2C and 2D), which is attributed to the relative AMP resistance of this strain. The remarkable differences between extracts assayed against the antimicrobial-susceptible mutant S. aureus ΔmprF compared with wild-type S. aureus MRSA indicate that the effects of nAChR activation are greatly directed toward antimicrobial molecules in the skin, which may also influence other aspects of inflammation in response to bacterial infection. Together with the results seen with topical nicotine application, these results indicate that increased nAChR activation in skin decreases the potency of innate antimicrobials to inhibit growth of AMP-susceptible S. aureus.
Mice Lacking Endogenous nAChR Antagonist Are More Susceptible to Cutaneous Infection
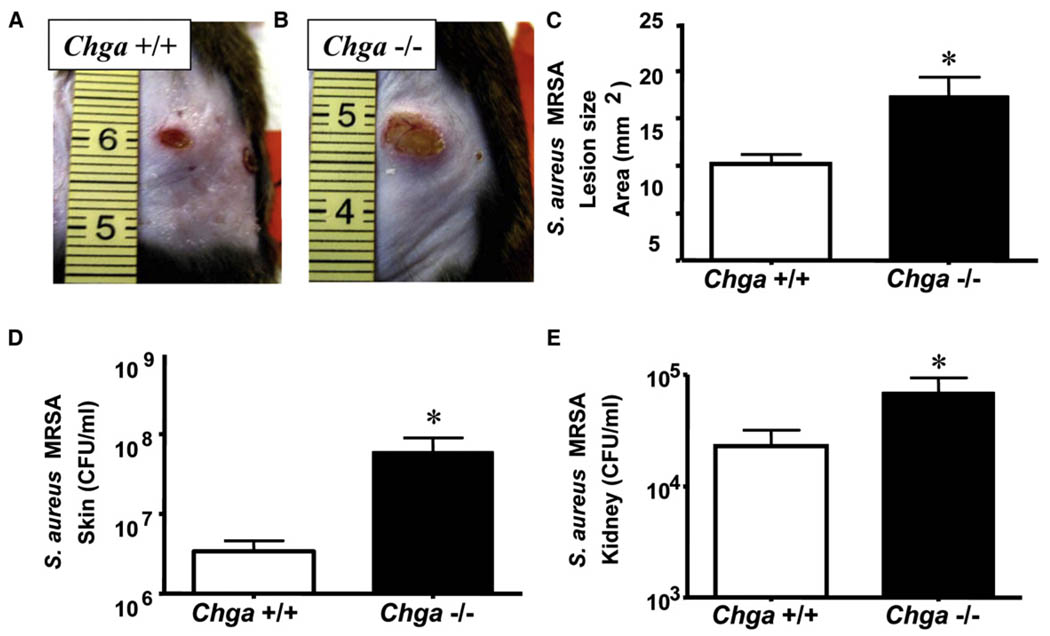
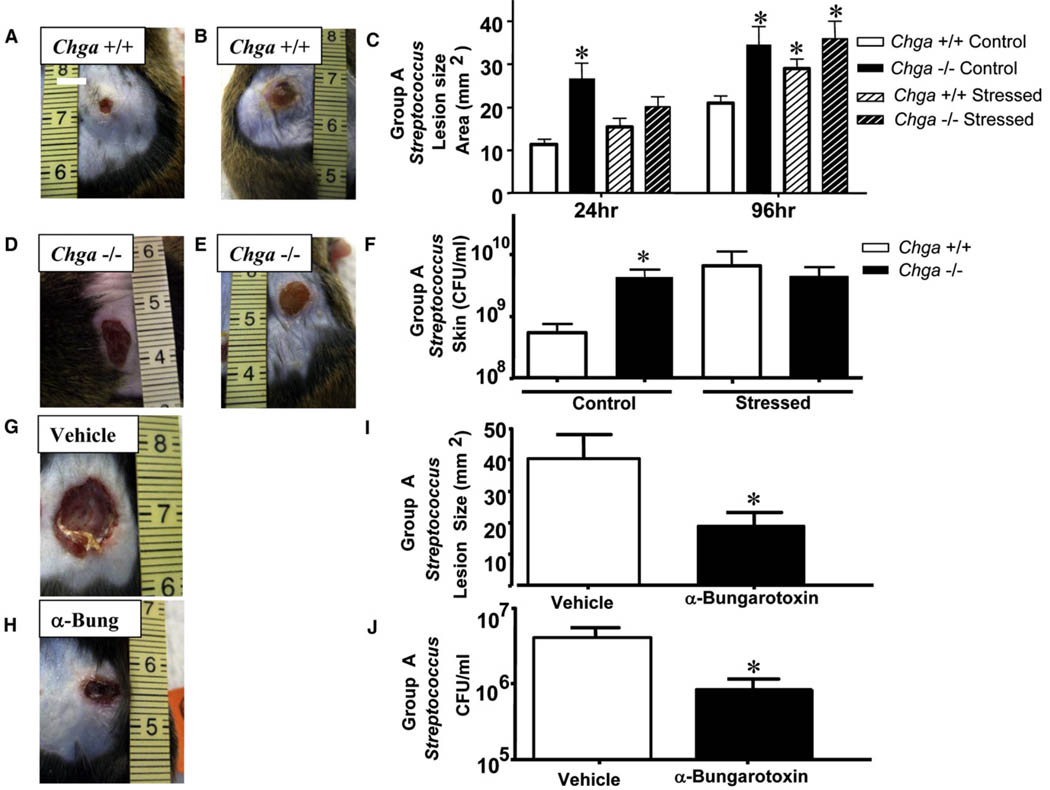
We next examined the in vivo functional relevance of AMP suppression using the more relevant skin pathogen MRSA, because this strain was more resistant to growth inhibition by skin fractions from nicotine-treated or Chga−/− mice. Chga−/− mice were challenged by intradermal injection of MRSA and exhibited larger necrotic skin lesions (~81% increase) compared with wild-type animals (Figures 3A and 3B). Culture of tissue taken from surrounding skin and kidney showed increase in bacterial counts both locally and systemically in Chga−/− mice (Figures 3C and 3D). Wild-type and Chga−/− mice were also challenged with Group A Streptococcus (GAS) to determine whether susceptibility to infection in a setting of nAChR activation could also predispose the host to other invasive skin pathogens. Similar to findings with MRSA, Chga−/− mice were more susceptible to GAS infection than wild-type mice (Figures 4A and 4D) and exhibited larger necrotic skin lesions (~67% increase) (Figure 4C) and greater bacterial burden in skin (Figure 4F). As previously reported (Aberg et al., 2007), exposure of wild-type mice to stress increased infection by GAS, however, stressed Chga−/− mice did not show a further susceptibility in comparison to stressed wild-type mice (~50% decrease) (Figures 4B, 4C, and 4E). Furthermore, when Chga−/− mice were compared with each other, they showed similar susceptibility to infection with or without stress (Figures 4C, 4D, and 4E). Skin culture and local bacterial counts showed results similar to lesion size (Figure 4F).
Figure 3. Mice Lacking Endogenous nAChR Antagonist Are More Susceptible to Cutaneous MRSA Infection.
(A) Wild-type (Chga+/+) mice were injected intradermally with MRSA. A representative photograph of day 4 lesions is shown.
(B) Chga−/− mice were injected intradermally with MRSA and are represented as described in (A).
(C) Lesion size in mm2 was calculated for day 4 lesions. *p < 0.05 by unpaired t test.
(D) Day 4 lesions were excised from Chga+/+ and Chga−/− mice for quantitation of bacteria. The CFU/mlMRSA in day 4 lesions is shown. *p < 0.05 by unpaired t test.
(E) Kidneys were excised 4 days following injection of MRSA. The CFU/ml MRSA on day 4 is shown. *p < 0.05 by unpaired t test. All data are represented as mean ± SEM.
Figure 4. Stress Activates Nicotinic Signaling to Increase Susceptibility to Infection and Mimics Phenotype of Chga−/− Mice.
(A) Control Chga+/+ mice were injected intradermally with GAS. A representative photograph of day 4 lesions is shown.
(B) Control Chga−/− mice were injected intradermally with GAS and are represented as in (A).
(C) Lesion size in mm2 was calculated in each group for day 1 and day 4 lesions. *p < 0.05 by unpaired t test.
(D) Psychological stressed Chga+/+ mice mimic phenotype of control Chga−/− mice. Mice were injected intradermally with GAS and are represented as in (A).
(E) Susceptibility to GAS infection in Chga−/− mice is not exacerbated by stress. Psychological stressed Chga−/− mice were injected intradermally with GAS and are represented as in (A).
(F) Day 4 lesions were excised from control or stressed Chga+/+ and Chga−/− mice for quantitation of bacteria. The CFU/ml of GAS in day 4 lesions is shown. *p < 0.05 by unpaired t test.
(G) Wild-type mice were subjected to PS and simultaneously treated topically with vehicle for 3 days and then injected with GAS. A representative photograph of day 4 lesions is shown.
(H) Wild-type mice were subjected to PS and simultaneously treated topically with 100 nM α-bungarotoxin for 3 days and then injected with GAS. Lesions are represented as in (G).
(I) Lesion size in mm2 was calculated for day 4 lesions. *p < 0.05 by unpaired t test.
(J) Day 4 lesions were excised for quantitation of bacteria. The CFU/ml of GAS in day 4 lesions is shown. *p < 0.05 by unpaired t test. All data are represented as mean ± SEM.
Stress Activates Nicotinic Signaling to Increase Susceptibility to Infection
To further confirm and extend the physiologic relevance of infections in the setting of nAChR activation, we next studied the response to PS. Prior work has shown that levels of Ach increase during stress (Schlereth et al., 2007), and stressed individuals appear to be more susceptible to a wide range of opportunistic infections (Ashcraft and Bonneau, 2008; Wu et al., 2005). The elements responsible for increasing infection after stress are incompletely understood. To further establish the role of nAChR activation in stress, wild-type stressed mice were also treated with α-bungarotoxin. This treatment with a topical Ach receptor antagonist, selective for the α-7 nAChR, rescued the increase in susceptibility to GAS infection induced by stress as seen by a reduction in lesion size (Figures 4G, 4H, and 4I) and bacterial burden in skin (Figure 4J).
AMP Expression Decreases following Increased Nicotinic Activation
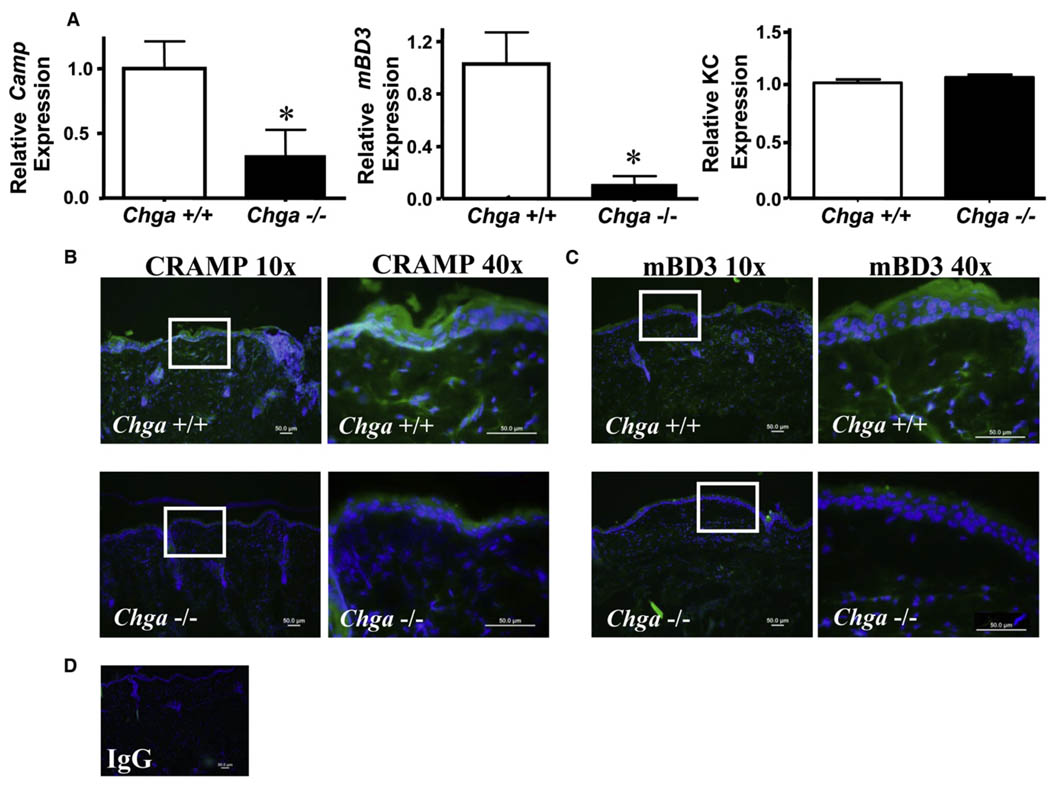
To identify potential candidate AMPs suppressed by nicotinic activation, we selectively analyzed the gene expression of the two major classes of cutaneous AMPs, cathelicidin and β-defensins, inChga−/− versus wild-type mice. In normal, uninfected skin, a significant reduction (~50%) in the mRNA abundance of Camp was also seen in Chga−/− mice (data not shown). During infection with GAS, Chga−/− mice exhibited a further significant reduction in the expression of the mouse cathelicidin gene Camp (~55% reduction) and mouse β-defensin 4 (mBD4) (~70% reduction), but not KC gene expression, which is the murine homolog of human IL-8 (Figure 5A). No change in the gene expression of two additional proinflammatory cytokines, IL-6 and IL-8, was observed between Chga+/+ or Chga−/−mice (data not shown). A slight decrease in mouse β-defensin 14 (mBD14) was also seen, but the difference did not reach significance (data not shown). Reductions in AMP gene expression were accompanied by a concomitant reduction in the abundance of protein as detected by immunostaining. GAS lesions fromChga−/−mice showed less immunofluorescent staining for both CRAMP (Figure 5B) and mBD3 compared with wild-type lesions (Figure 5C), but this does not exclude the contribution of other AMPs to this response. No staining was observed with IgG control (Figure 5D).Further studies are ongoing to identify alterations in the expression of additional cutaneous AMPs or cytokines following nAChR activation.
Figure 5. Cathelicidin and Defensin AMPs’ Expression Decreases following Increased Nicotinic Activation.
(A) Chga+/+ and Chga−/− mice were injected intradermally with of GAS. Lesions were excised after 24 hr for analysis of AMP gene expression. The relative expression of cathelicidin (Camp) and human beta-defensins 4 and 14 (DefB4 and DefB14) was calculated by qPCR and normalized to GAPDH.*p < 0.05 by unpaired t test.
(B) GAS lesions from Chga+/+ (top) and Chga−/− (bottom) mice were subjected to immunofluorescent staining for CRAMP. Images shown at 10× magnification (left) show region magnified at 40x (right), indicated by white box. Scale bar represents 50 µM.
(C) GAS lesions from Chga+/+ (top) and Chga−/− (bottom) mice were subjected to immunofluorescent staining for mBD3. Same representation as in (B).
(D) GAS lesions immunostained with rabbit IgG.
nAChR Activation by Ach Reduces AMP Gene Expression and Antimicrobial Activity in Epidermal Keratinocytes
To determine whether AMP suppression was attributable, at least in part, to direct cholinergic activation of epidermal cells, isolated cultured normal human keratinocytes were stimulated with Ach in the presence or absence of nAChR antagonists, and changes in the gene expression of cathelicidin and β-defensin was analyzed. Because constitutive expression of AMPs is relatively low in cultured keratinocytes due to the lack of necessary components required for AMP expression seen in normal skin, in vitro keratinocyte cultures must be stimulated with varying exogenous molecules to induce the expression of AMPs. Normal human epidermal keratinocytes (NHEK) were stimulated to increase expression of human cathelicidin (CAMP) by exposure to VD3 and MALP-2, a TLR-2 agonist, whereas MALP-2 alone was used as a positive control for maximal expression of human beta-defensin 2 (hBD-2, DefB2) (Schauber et al., 2006). NHEKs stimulated with Ach demonstrated a statistically significant dose-dependent reduction in the expression of CAMP and DefB2 at concentrations at 0.01– 0.1 nM compared with the positive controls, with the greatest statistical significance occurring at 0.01 nM Ach (data not shown). At .01 nM Ach, the gene expression of CAMP was reduced to 68-fold over untreated NHEKs compared with 292-fold with the positive control of VD3 and MALP-2, while the expression of DefB2 was reduced to 23-fold over untreated NHEKs compared with 52-fold with the positive control of MALP-2 (data not shown). Therefore, we next examined whether this reduction in gene expression could be reversed using Ach nAChR antagonists. The suppression of both CAMP and DefB2 by 0.01nM Ach was rescued by the presence of the endogenous nicotinic antagonist encoded by Chga, catestatin (Cst) (Figure 6A), as well as by the pharmacologic antagonist α-bungarotoxin (Figure 6B). Similar results were observed with another nAChR antagonist, mecamylamine (data not shown). The reduction in CAMP gene expression was accompanied by decreased protein production of hCAP18 protein as detected by immunofluorescent staining with antibody directed against the LL-37 domain (see Figure S1 available online). The specificity of this response to nAChR activation was further confirmed by a dose-dependent suppression of CAMP and DefB2 gene expression in NHEKs observed with nicotine, but not with the muscarinic receptor agonist muscarine, and the β-adrenergic receptor agonist, propranolol (data not shown).
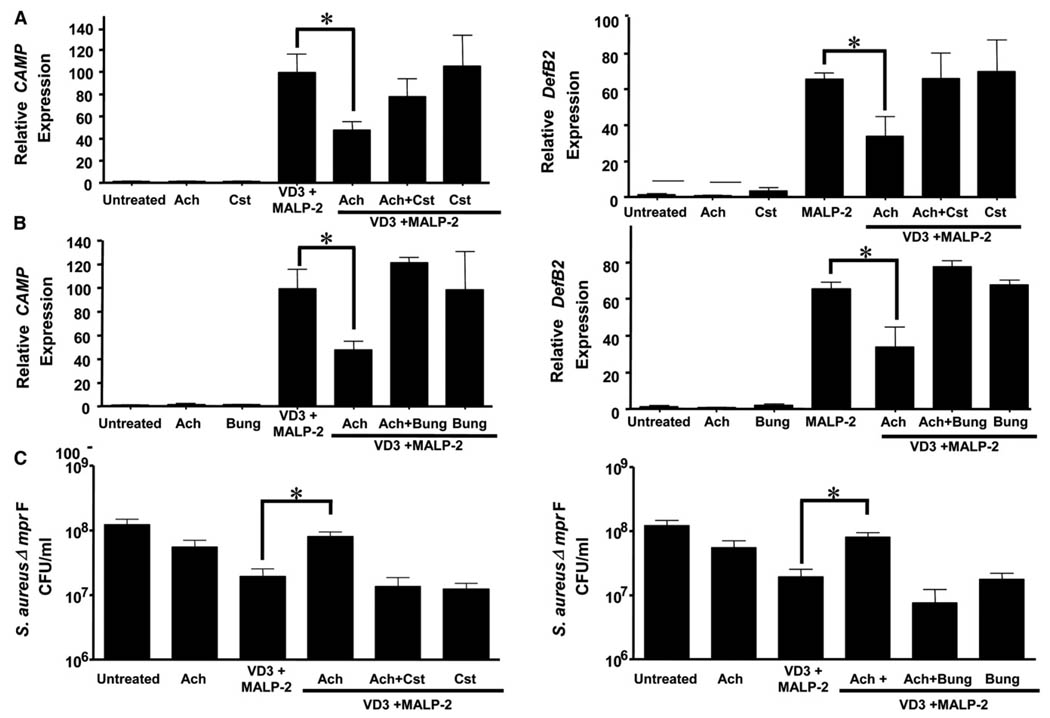
Figure 6. Cholinergic Activation by Ach Reduces AMP Gene Expression and Antimicrobial Activity in Epidermal Keratinocytes.
(A) Keratinocytes were stimulated for 24 hr with 1,25 (OH)2 vitamin D3 (VD3) and MALP-2 in the presence or absence of Ach (Ach), catestatin (Cst), or α-bungarotoxin (Bung). Relative mRNA expression of cathelicidin (Camp) (left) and human beta-defensin 2 (DefB2) (right) by qPCR and normalized to GAPDH. *p < 0.05 versus VD3+MALP-2 by one-way ANOVA. p > 0.05 with Cst and Bung.
(B) Keratinocytes were stimulated as above and cell lysates incubated with S. aureus ΔmprF. Surviving bacteria after 12 hr is shown. *p < 0.05 versus VD3+MALP-2 by one-way ANOVA. p > 0.05 with Cst and α-bungarotoxin antagonists. All data are represented as mean ± SEM.
(C) Keratinocytes were stimulated as above, and cell lysates were incubated with S. aureus ΔmprF. Surviving bacteria after 12 hr is shown. *p < 0.05 versus VD3+MALP-2 by one-way ANOVA. p > 0.05 with Cst and α-Bugarotoxin antagonists. All data are represented by mean ± SEM.
To evaluate whether cholinergic activation in NHEKs also inhibits their ability to restrict the growth of bacteria, lysates of NHEKs treated with either Ach in the presence or absence of nAChR antagonists were incubated with S. aureus ΔmprF. Lysates derived from NHEKs stimulated to increase cathelicidin gene expression with VD3 and MALP-2 increased their ability to inhibit bacterial growth compared to untreated cells (Figure 6C). However, the presence of Ach significantly suppressed the capacity of lysates to inhibit bacterial growth, and both Cst and α-bungarotoxin reversed the suppression of antimicrobial activity to levels observed with the positive control.
Suppression of Cathelicidin by Cholinergic Signaling Is a Critical Component for the Susceptibility to Cutaneous Infection
To determine if diminished cathelicidin is responsible in part for the observed susceptibility to infection seen following nAChR activation, cathelicidin-deficient mice (Camp−/−) mice were subjected to PS in the presence or absence of topical α-bungarotoxin, and subsequently challenged with GAS. Vehicle-treated wild-type mice (Figure 7A) exhibited lesions comparable in size to vehicle-treated Camp−/− mice (Figure 7B). α-Bungarotoxin treated wild-type mice (Figure 7C) compared with α-bungarotoxin Camp−/− mice (Figure 7D) also exhibited comparable lesion size. Quantification of the lesion size revealed that only vehicle-treated wild-type mice demonstrated a significant increase in lesion size at both 24 and 96 hr (Figure 7E) and greater bacterial burden in both the skin (Figure 7F) and spleen (Figure 7G) compared with α-bungarotoxin-treated wild-type mice. The lack of significant differences between stressed α-bungarotoxin-treated versus vehicle-treated Camp−/− mice indicates that cathelicidin is a large fraction of AMP activity that is suppressed during either PS or nAChR activation.
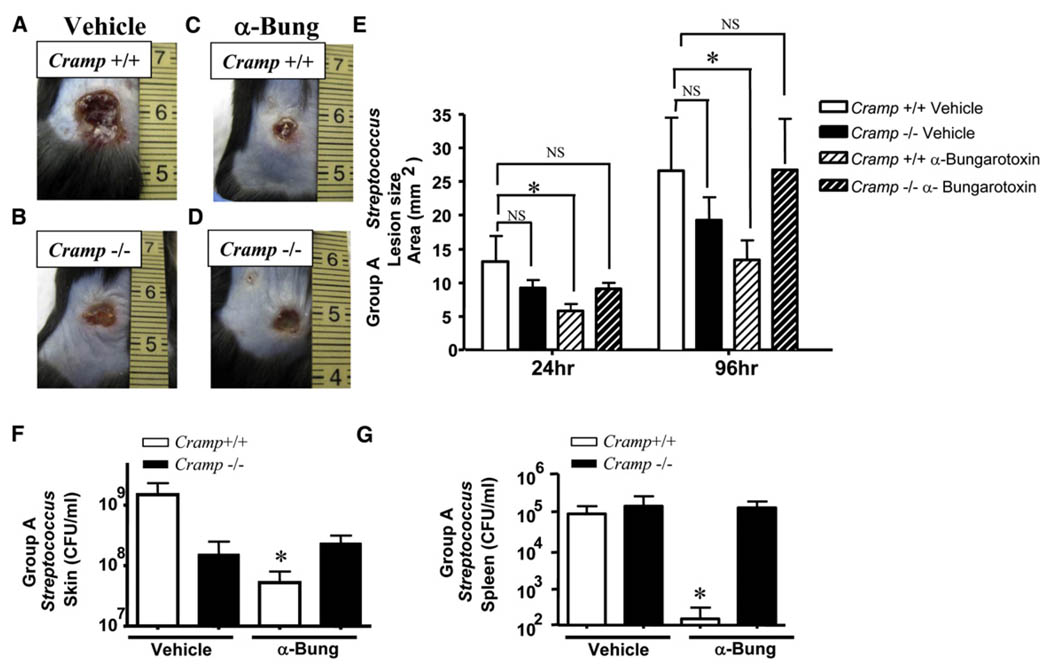
Figure 7. Suppression of Cathelicidin by Cholinergic Signaling Is a Critical Component for the Susceptibility to Cutaneous Infection.
(A) Stress increases susceptibility to GAS infection in wild-type mice. Wild-type mice were subjected to PS and simultaneously treated topically with vehicle for 3 days and then injected with GAS. A representative photograph of day 4 lesions is shown.
(B) Stress does not increase susceptibility to GAS infection in Cramp−/− mice. Mice were subjected to PS and simultaneously treated topically with vehicle for 3 days and then injected with GAS. Lesions are represented as in (A).
(C) Wild-type mice are rescued from susceptibility GAS infection by α-bungarotoxin treatment during stress. Mice were subjected to PS and simultaneously treated topically with α-bungarotoxin (α-Bung) for 3 days and then injected with GAS. Lesions are represented as in (A).
(D) Cramp−/− mice are not rescued from susceptibility GAS infection by α-bungarotoxin treatment during stress. Mice were subjected to PS and simultaneously treated topically with α-bungarotoxin (α-Bung) for 3 days and then injected with GAS. Lesions are represented as in (A).
(E) Lesion size (in mm2) was calculated in each group for day 1 and day 4 lesions. *p < 0.05 by unpaired t test.
(F) Day 4 lesions were excised from stressed Cramp+/+ and Cram−/− vehicle or α-bungarotoxin-treated mice for quantitation of bacteria. The CFU/ml GAS in day 4 lesions is shown. *p < 0.05 by unpaired t test.
(G) Spleens were excised from stressed Cramp+/+ and Cramp−/− vehicle or α-bungarotoxin-treated mice 4 days following injection for quantitation of bacteria. The CFU/ml GAS in spleen is shown. *p < 0.05 by unpaired t test. All data are represented as mean ± SEM.
DISCUSSION
At the interface with the external world, resident epithelia and bone-marrow-derived cells employ multilayered immune strategies to defend against infection. In the present study, we establish that nAChR activation suppresses innate antimicrobial defense of the skin. The decrease in early host responses to pathogen invasion results in enhanced infection in mouse models of invasive MRSA and GAS infection. Furthermore, these findings are consistent with clinical observations in humans that have increased incidence of infection as a consequence of nicotine exposure or prolonged stress, two common events that enhance nAChR activation. These observations provide insight into an element previously unknown to influence the susceptibility to infection, and present immediate opportunities to improve disease resistance in significant human populations exposed to stress.
The direct effects of nAChR stimulation were assessed by using topical nicotine specific to nAChRs. The diminished capacity of nicotine-treated extracts to impart AMP activity against the AMP-susceptible mutant, S. aureus ΔmprF, compared with MRSA, indicates that the nAChR activation promotes a selective reduction of antimicrobial peptide activity, as opposed to other antimicrobial molecules in the skin. The direct effect of nAChR activation on AMP activity was further confirmed by using Chga−/−mice having unopposed nAChR activation, because these extracts also exhibited a greater diminished capacity against S. aureus ΔmprF compared with MRSA when evaluated against wild-type mice. The physiologic relevance of this was confirmed using two models of infection with MRSA and GAS, which represent two important pathogens that account for most acute skin infections. However, there is a dramatic difference between the modest changes in antimicrobial activity against MRSA in our radial diffusion assays, compared with S. aureus ΔmprF, and the very substantial and impressive change in lesion size. This indicates that the diminished immune response following nAChR activation is quite complex and likely involves the suppression of immunostimulatory functions normally induced by the increase in or activation of AMPs following infection. The increase in the number of surviving bacteria may be due to several defects, such as recruitment of phagocytes and/or oxidative killing capacity, which may be a direct or indirect consequence of global AMP suppression during stress.
These results substantiate the detrimental effects that increased nAChR activation caused by stress and/or smoking have on the host response to infection. Interestingly, most epithelia, including the gut and lung similarly respond to nAChR activation through immunosuppression of immune cells (Giebelen et al., 2009; Pullan et al., 1994). This dampened response to infection may increase dissemination of bacteria from initial sites of infection to distal organs or tissues. Catecholamines have been shown to increase the expression of virulence factors to promote survival of pathogens (Freestone et al., 2008). Thus, the effect of the stress response may be twofold to suppress host response to infection, while enhancing the microenvironment for survival and proliferation of bacteria. It is attractive to speculate that some microorganisms might utilize this system to enhance their pathogenic potential, a concept that should be investigated in the future.
The focus of this investigation was on the epithelial defense against bacterial infection, in particular keratinocytes of the epidermis. Normal, uninfected skin was used for topical nicotine and antagonist treatment, and skin extracts were evaluated from Chga−/− mice to allow for the extraction of acid-soluble AMPs and selective exclusion of infiltrating immune cells and reactive oxygen species. This approach enabled analysis of direct effects of nAChR stimulation or antagonism, and is justified because keratinocytes comprise ~95% of the cells within the epidermis and have previously been shown to be an essential cell type for production of AMPs in the skin (Braff et al., 2005a, 2005b). Nonetheless, the topical administration of nicotine may expose not only epidermal keratinocytes, but also other resident epidermal cells including dendritic cells and mast cells that participate in the response to infection. The results from quantitative polymerase chain reaction (qPCR) and immunostaining of bacterial lesions further confirmed that the reduction of selected AMP expression was primarily observed in the epidermis. The proportion of nAChR signaling from other cell types relative to the keratinocyte remains unknown in response to infection and requires further scrutiny. However, activation of a cholinergic anti-inflammatory pathway has been previously shown to be mediated by vagal nerve stimulation and α7 cholinergic receptors and can decrease inflammatory responses (Tracey, 2007). Therefore, it is likely that nAChR activation can enhance susceptibility to infection by action on several cell types.
Topical application of α-bungarotoxin rescued Chga−/− mice from suppression of AMP activity, and wild-type mice from susceptibility to GAS infection. The efficiency with which α-bungarotoxin restored AMP levels in skin and the response to infection to that of controls corroborate our results and establish that the effects of stress on AMP suppression and susceptibility to infection are, at least in part, through nAChR signaling. This becomes relevant in a setting of sustained PS, which appears to increase susceptibility to invasive infection.
Because α-bungarotoxin is a α7 nAChR antagonist, this further implicates the α7 subtype in cutaneous immunosuppression, which now includes epithelial AMPs. nAChR subunit composition determines both activation and desensitization in response to specific agonists. Work is currently ongoing to determine which specific receptor subtypes and signaling pathways are responsible for suppression of AMPs. Interestingly, GCs and/or steroids have been shown to influence acetylcholine (Ach) content in tissue (Reinheimer et al., 1998), and modulate nAChR activity and sensitization (Valera et al., 1992). This may account for the partial rescue of AMP suppression in stressed mice by RU-486, a GC/progesterone receptor antagonist (Aberg et al., 2007). This convergent mechanism for the suppression of AMPs during stress is depicted in Figure S2. Stress increases both GC and Ach that activates their respective receptors mAChR, nAChR, and GC receptor. GCs can further enhance nAChR activation to augment the suppression of AMP and lipid production. Blocking nAChR and GC receptor activation using specific antagonists may counteract these effects to restore normal production of AMPs and lipids to the epithelial surface.
The in vitro stimulation of keratinocytes with Ach and nAChR antagonists confirms our in vivo results and indicates a direct effect of nAChR activation on AMP expression and activity. Previously published results from other laboratories showed that nicotine was much more efficient than Ach in inhibiting the production of proinflammatory cytokines in macrophages, and virtually no effect was seen with muscarine (Wang et al., 2003). Our in vitro stimulation with the nAChR agonists Ach and nicotine, the adrenergic agonist propranolol, and the muscarinic agonist muscarine parallels these previous results, as only nicotine and Ach were able to suppress gene and protein expression of AMPs. Furthermore, the nAChRs Cst, α-bungarotoxin, and mecamylamine were able to reverse the suppression of AMP gene expression and activity by Ach, with bungarotoxin being the most effective. Mecamylamine, an α3β4 nAChR antagonist, was slightly less effective than α-bungarotoxin, an α7 Ach receptor antagonist, in restoring AMP gene expression, implicating the α7 nAChR as a key subtype involved in AMP suppression. Although the specificity of Cst for nAChRs in the skin is not currently known, it appears that it may have a different nAChR subtype specificity compared with α-bungarotoxin, because its ability to restore AMP expression of both cathelicidin and β-defensin 2 was somewhat less effective. However, the ability of Cst to restore both AMP expression and activity to levels seen with the control suggests a direct role of Cst in maintaining AMP expression under normal homeostasis. We previously determined that Cst expression increases following barrier disruption and infection (Radek et al., 2008), and Ach levels increase in skin following UV exposure and tactile stimulation (Schlereth et al., 2007), which simulates injury. Thus, Cst may function as an endogenous nAChR antagonist in the skin to balance the effects of Ach under normal or acute stressed conditions. Under prolonged periods of stress, the ability of Cst to act as a nAChR antagonist may be limited by the ability of keratinocytes to synthesize or secrete Cst. This would skew the balance toward increased nAChR activation, suppression of AMPs, and ultimately increased infection.
The observation that stress did not exacerbate the susceptibility to infection in either Chga−/− or Camp−/− mice provides two key pieces of information that are critical toward elucidating the mechanism behind AMP suppression during stress. First, GCs, catecholamines, and Ach are all secreted from various tissues during the stress response. If the effects of either GCs or catecholamines were the primary cause for susceptibility to infection during stress, an increase in susceptibility to infection characterized by larger lesions and a greater bacterial burden would be expected. This supports the notion that perhaps GCs and catecholamines may be modifying the nAChR to enhance the effect of Ach during stress. In parallel, the finding that stress did not augment the susceptibility to infection in Camp−/− mice implicates cathelicidin as a key AMP involved following cholinergic activation of nAChRs. If other AMPs, such as β-defensins, were contributing more to the response to stress, larger lesions and greater bacterial burden would be expected as described for stressed Chga−/− mice. This does not exclude the involvement of other AMPs to the susceptibility to infection during stress, but it does suggest that the global effects of nAChR signaling on AMP suppression include cathelicidin. The contribution of cathelicidin to innate immune function of the skin during infection is complex and integrates both its antimicrobial and immunomodulatory functions that may influence the activity of numerous peptides produced by the epidermis, including β-defensins, Psoriasin, or RNase 7, as well as the antimicrobial and proinflammatory capacity of infiltrating immune cells. This may explain the discrepancy between our in vitro and in vivo results. The global suppression of antimicrobial activity seen with the radial diffusion assays may exacerbate the immunosuppression by nAChR activation by blocking necessary stimulation of later immune responses that are AMP-dependent. Because cathelicidin is also immunomodulatory, this does not rule out the fact that other immune responses are indirectly dampened by nAChR activation. Cathelicidin may be a key regulatory peptide for normal AMP function that directs both microbicidal and immunostimulatory functions of additional AMPs in the skin, and its activity and/or expression may be a direct target of nAChR activation. Furthermore, cathelicidin is also involved in epidermal permeability homeostasis, because Cramp−/− mice exhibit defects in membrane permeability and lipid content, demonstrating the interdependence between AMPs and barrier function (Aberg et al., 2008). This emphasizes the importance of AMP regulation in host defense, as well as a natural structural component of the epithelial barrier. Thus, enhanced cholinergic signaling may likely disrupt permeability homeostasis in parallel, further contributing to the increase in bacterial susceptibility.
Collectively, our study identifies two unique pathways of the epithelial response to infection. First, the AMP response is regulated at the molecular level by Ach via nAChRs and is dependent upon the presence of the nAChR antagonist, Cst. Second, the suppression of AMP response to infection during stress is mediated primarily by cathelicidin following increased nAChR stimulation. Further insight into how bacteria utilize the host nAChR pathway to augment their invasive capacity and virulence will be critical to our understanding of the pathogen-host interactions at the epithelial interface.
EXPERIMENTAL PROCEDURES
Animals
CHGA wild-type (Chga+/+) and knockout mice (Chga−/−) were generated as described previously (Mahapatra et al., 2005) and bred internally. Cramp−/− mice were bred internally. C57Bl/6 mice (8–10-week-old males) were purchased from Jackson Laboratories. All mice were housed in the VA San Diego VMU. All animal experiments were approved by the VA San Diego IACUC.
HPLC Purification of Skin Extracts
Mice were sacrificed and dorsal skin extracted overnight in 1 N acetic acid at 4°C. Samples were homogenized, and supernatant was lyophilized and dissolved in water. HPLC peptide separation was performed on a C18 column (12 µm, ST 4.6/250). Equilibration was done in 0.1% trifluoroacetic acid. Peptides were eluted with an acetonitrile gradient (10%–100%). Fractions were lyophilized and dissolved in 10 µl water.
Radial Diffusion Assay
HPLC fractions were evaluated using the radial diffusion assay as described elsewhere (Lehrer et al., 1991). Antimicrobial activity was analyzed against S. aureus ΔmprF (Peschel et al., 2001) and S. aureus Sanger 252 (MRSA). Approximately 5 × 106 cells/ml of bacteria were used. Water was the negative control. Synthetic CRAMP (32 µM) was the positive control. The zone of inhibition (Area) was quantified using Image J Software.
Topical Nicotine Application
Three-day-old C57Bl/6 neonatal mice had either vehicle (1:1000 ethanol:- water) or 1 nM nicotine (Sigma) topically applied to skin twice daily for 3 days. Whole mouse skin was excised and subsequently purified by HPLC for radial diffusion assays (n = 4 per group, repeated twice).
Topical α-Bungarotoxin Application
Vehicle (water) or 100 nM α-bungarotoxin (Sigma) was topically applied to mouse skin twice daily for 3 days. For some experiments, whole mouse skin was excised and subsequently purified by HPLC for radial diffusion assays as described above. For some experiments, mice were also subjected to PS and injected with bacteria as indicated below (n = 6 per group, repeated twice).
Psychological Stress Model
Mice were subjected to psychological insomnia stress as previously described (Aberg et al., 2007). Some mice also had topical α-bungarotoxin applied daily as indicated above during stress. Mice were subsequently injected with bacteria as described below.
GAS and Staphylococcus aureus Infection
GAS was grown in Todd Hewitt broth (THB) and Staphylococcus aureus was grown in tryptic soy broth (TSB). Infection was performed as previously described (Nizet et al., 2001). For GAS, hair was plucked and skin injected with 100 µl of a mid-logarithmic growth phase of bacteria and sterile Cytodex beads. On day 4 postinjection, lesions were excised and homogenized in sterile PBS with 1 mm zirconia beads in a mini bead-beater (BioSpec Products). Supernatants were diluted and plated on THA or TSA to quantify the colony-forming units per milliliter (CFU/ml).
Immunohistochemistry on Mouse Skin
Immunohistochemistry was performed on 24 hr GAS lesions and immunostained for CRAMP or mBD3 as previously described (Dorschner et al., 2001) (Aberg et al., 2007). Cells were counterstained with DAPI. Fluorescent images were captured on an Olympus BX51 microscope equipped with a DC71 camera using 10× or 40× objectives (n = 6 per group, repeated twice).
Cell Culture and Stimulation
Normal human keratinocytes were cultured in EpiLife medium (Cascade Biologics) supplemented with 0.06 mM calcium, EDGS, and penicillin/streptomycin. For antimicrobial lysate assays, cells were grown in the same media without antibiotics for 2 passages prior to stimulation. For RNA and antimicrobial assays, cells were grown to confluency and differentiated in high calcium (1.2 mM) for 3 days prior to stimulation to allow for more uniform expression of nAChRs. Cells were stimulated for 24 hr separately or in combination with the following reagents: 1nM 1,25 (OH)2 Vitamin D3 (Sigma), 100 ng/ml MALP-2 (Alexis), 0.01 nM Ach (Sigma), 0.001 nM to 1 µM nicotine (Sigma), 10n M Cst (Imgenex), 100 nM α-bungarotoxin (Sigma), 5 µM mecamylamine (Sigma).
Keratinocyte Lysate Antimicrobial Assay
Protein was extracted with 1 N acetic acid. Samples were sonicated, lyophilized and dissolved in 100 µl sterile water. Protein concentration was normalized between samples. S. aureus ΔmprF was grown in TSB overnight and then subcultured in 30% TSB, 25 mm NaHCO3, and 1 mm NaHPO4 until log phase was reached. 103 bacteria was incubated with lysates from stimulated keratinocytes (7 µg total protein) at 37°C in 30% TSB, 25 mm NaHCO3, 1 mm NaHPO4. Bacterial growth over time was determined by optical density at 600 nm. The number of surviving bacteria was determined after 4, 8, and 12 hr.
qPCR for Skin and Keratinocytes
RNA from mouse skin and keratinocytes was extracted as described elsewhere (Schauber et al., 2006). Assays were performed in triplicate and repeated at least three times. All assays using mouse skin RNA used at least five mice per group and were repeated twice.
Statistical Analysis
Data were analyzed using GraphPad Prism, v4 (GraphPad Software). The means and standard error of the mean (SEM) were calculated for each data set. Data were analyzed by an unpaired t test or analysis of variance (ANOVA) with post-tests when appropriate, and p values < 0.05 were considered significant.
ACKNOWLEDGMENTS
Supported by VA and NIH grants NIH/NIAID HHSN26620040029C; ADB contracts no. N01-AI-40029AI48176, AI052453, and AR45676 NIH F32-AR054220-01A2 (KAR-UCSD); and NIH-1P30AA019373-01 (Kovacs, PI-LUMC). We acknowledge Steven Droho and Keith Welker for technical expertise (LUMC), and Dr. Sergei Grando from UC Irvine for valuable technical advice and expertise. K.A.R. dedicates this manuscript to her mother, Rita M. Radek (1940–2009), in remembrance of her endless love, sacrifices, and encouragement.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes two figures and can be found with this article online at http://doi:10.1016/j.chom.2010.03.009.
The authors have no conflict of interest.
REFERENCES
- Aberg KM, Man MQ, Gallo RL, Ganz T, Crumrine D, Brown BE, Choi EH, Kim DK, Schroder JM, Feingold KR, et al. Co-regulation and interdependence of the mammalian epidermal permeability and antimicrobial barriers. J. Invest. Dermatol. 2008;128:917–925. doi: 10.1038/sj.jid.5701099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aberg KM, Radek KA, Choi EH, Kim DK, Demerjian M, Hupe M, Kerbleski J, Gallo RL, Ganz T, Mauro T, et al. Psychological stress downregulates epidermal antimicrobial peptide expression and increases severity of cutaneous infections in mice. J. Clin. Invest. 2007;117:3339–3349. doi: 10.1172/JCI31726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcraft KA, Bonneau RH. Psychological stress exacerbates primary vaginal herpes simplex virus type 1 (HSV-1) infection by impairing both innate and adaptive immune responses. Brain Behav Immun. 2008;22:1231–1240. doi: 10.1016/j.bbi.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson BJ, Hadley ME. In vitro characterization of adrenergic receptors controlling skin gland secretion in two anurans Rana pipiens and Xenopus laevis. Comp. Biochem. Physiol. 1969;30:857–864. doi: 10.1016/0010-406x(69)90040-1. [DOI] [PubMed] [Google Scholar]
- Bernik TR, Friedman SG, Ochani M, DiRaimo R, Susarla S, Czura CJ, Tracey KJ. Cholinergic antiinflammatory pathway inhibition of tumor necrosis factor during ischemia reperfusion. J. Vasc. Surg. 2002;36:1231–1236. doi: 10.1067/mva.2002.129643. [DOI] [PubMed] [Google Scholar]
- Braff MH, Di Nardo A, Gallo RL. Keratinocytes store the antimicrobial peptide cathelicidin in lamellar bodies. J. Invest. Dermatol. 2005a;124:394–400. doi: 10.1111/j.0022-202X.2004.23443.x. [DOI] [PubMed] [Google Scholar]
- Braff MH, Zaiou M, Fierer J, Nizet V, Gallo RL. Keratinocyte production of cathelicidin provides direct activity against bacterial skin pathogens. Infect. Immun. 2005b;73:6771–6781. doi: 10.1128/IAI.73.10.6771-6781.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogden KA, Guthmiller JM, Salzet M, Zasloff M. The nervous system and innate immunity: the neuropeptide connection. Nat. Immunol. 2005;6:558–564. doi: 10.1038/ni1209. [DOI] [PubMed] [Google Scholar]
- Davidson DJ, Currie AJ, Reid GS, Bowdish DM, MacDonald KL, Ma RC, Hancock RE, Speert DP. The cationic antimicrobial peptide LL-37 modulates dendritic cell differentiation and dendritic cellinduced T cell polarization. J. Immunol. 2004;172:1146–1156. doi: 10.4049/jimmunol.172.2.1146. [DOI] [PubMed] [Google Scholar]
- De Rosa MJ, Dionisio L, Agriello E, Bouzat C, Esandi Mdel C. Alpha 7 nicotinic acetylcholine receptor modulates lymphocyte activation. Life Sci. 2009;85:444–449. doi: 10.1016/j.lfs.2009.07.010. [DOI] [PubMed] [Google Scholar]
- Denda M, Tsuchiya T, Elias PM, Feingold KR. Stress alters cutaneous permeability barrier homeostasis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;278:R367–R372. doi: 10.1152/ajpregu.2000.278.2.R367. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS. Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection and immunopathology. Neuroimmunomodulation. 2009;16:300–317. doi: 10.1159/000216188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorschner RA, Pestonjamasp VK, Tamakuwala S, Ohtake T, Rudisill J, Nizet V, Agerberth B, Gudmundsson GH, Gallo RL. Cutaneous injury induces the release of cathelicidin anti-microbial peptides active against group A Streptococcus. J. Invest. Dermatol. 2001;117:91–97. doi: 10.1046/j.1523-1747.2001.01340.x. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve-an integrative interface between two supersystems: the brain and the immune system. Pharmacol. Rev. 2000;52:595–638. [PubMed] [Google Scholar]
- Freestone PP, Sandrini SM, Haigh RD, Lyte M. Microbial endocrinology: how stress influences susceptibility to infection. Trends Microbiol. 2008;16:55–64. doi: 10.1016/j.tim.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Ganz T, Selsted ME, Szklarek D, Harwig SS, Daher K, Bainton DF, Lehrer RI. Defensins. Natural peptide antibiotics of human neutrophils. J. Clin. Invest. 1985;76:1427–1435. doi: 10.1172/JCI112120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giebelen IA, Leendertse M, Florquin S, van der Poll T. Stimulation of acetylcholine receptors impairs host defence during pneumococcal pneumonia. Eur. Respir. J. 2009;33:375–381. doi: 10.1183/09031936.00103408. [DOI] [PubMed] [Google Scholar]
- Grando SA, Kist DA, Qi M, Dahl MV. Human keratinocytes synthesize, secrete, and degrade acetylcholine. J. Invest. Dermatol. 1993;101:32–36. doi: 10.1111/1523-1747.ep12358588. [DOI] [PubMed] [Google Scholar]
- Grando SA, Pittelkow MR, Schallreuter KU. Adrenergic and cholinergic control in the biology of epidermis: physiological and clinical significance. J. Invest. Dermatol. 2006;126:1948–1965. doi: 10.1038/sj.jid.5700151. [DOI] [PubMed] [Google Scholar]
- Harder J, Bartels J, Christophers E, Schroder JM. A peptide antibiotic from human skin. Nature. 1997;387:861. doi: 10.1038/43088. [DOI] [PubMed] [Google Scholar]
- Harder J, Schroder JM. RNase 7, a novel innate immune defense antimicrobial protein of healthy human skin. J. Biol. Chem. 2002;277:46779–46784. doi: 10.1074/jbc.M207587200. [DOI] [PubMed] [Google Scholar]
- Heilborn JD, Nilsson MF, Kratz G, Weber G, Sorensen O, Borregaard N, Stahle-Backdahl M. The cathelicidin anti-microbial peptide LL-37 is involved in re-epithelialization of human skin wounds and is lacking in chronic ulcer epithelium. J. Invest. Dermatol. 2003;120:379–389. doi: 10.1046/j.1523-1747.2003.12069.x. [DOI] [PubMed] [Google Scholar]
- Koczulla R, von Degenfeld G, Kupatt C, Krotz F, Zahler S, Gloe T, Issbrucker K, Unterberger P, Zaiou M, Lebherz C, et al. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J. Clin. Invest. 2003;111:1665–1672. doi: 10.1172/JCI17545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y, Gallo RL. AMPed up immunity: how antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009;30:131–141. doi: 10.1016/j.it.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer RI, Rosenman M, Harwig SS, Jackson R, Eisenhauer P. Ultrasensitive assays for endogenous antimicrobial polypeptides. J. Immunol. Methods. 1991;137:167–173. doi: 10.1016/0022-1759(91)90021-7. [DOI] [PubMed] [Google Scholar]
- Lipsky BA, Weigelt JA, Gupta V, Killian A, Peng MM. Skin, soft tissue, bone, and joint infections in hospitalized patients: epidemiology and microbiological, clinical, and economic outcomes. Infect. Control Hosp. Epidemiol. 2007;28:1290–1298. doi: 10.1086/520743. [DOI] [PubMed] [Google Scholar]
- Mahapatra NR, O'Connor DT, Vaingankar SM, Hikim AP, Mahata M, Ray S, Staite E, Wu H, Gu Y, Dalton N, et al. Hypertension from targeted ablation of chromogranin A can be rescued by the human ortholog. J. Clin. Invest. 2005;115:1942–1952. doi: 10.1172/JCI24354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mookherjee N, Brown KL, Bowdish DM, Doria S, Falsafi R, Hokamp K, Roche FM, Mu R, Doho GH, Pistolic J, et al. Modulation of the TLR-mediated inflammatory response by the endogenous human host defense peptide LL-37. J. Immunol. 2006;176:2455–2464. doi: 10.4049/jimmunol.176.4.2455. [DOI] [PubMed] [Google Scholar]
- Mookherjee N, Hancock RE. Cationic host defence peptides: innate immune regulatory peptides as a novel approach for treating infections. Cell. Mol. Life Sci. 2007;64:922–933. doi: 10.1007/s00018-007-6475-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizet V, Ohtake T, Lauth X, Trowbridge J, Rudisill J, Dorschner RA, Pestonjamasp V, Piraino J, Huttner K, Gallo RL. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414:454–457. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- Pavlov VA, Ochani M, Gallowitsch-Puerta M, Ochani K, Huston JM, Czura CJ, Al-Abed Y, Tracey KJ. Central muscarinic cholinergic regulation of the systemic inflammatory response during endotoxemia. Proc. Natl. Acad. Sci. USA. 2006;103:5219–5223. doi: 10.1073/pnas.0600506103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschel A, Jack RW, Otto M, Collins LV, Staubitz P, Nicholson G, Kalbacher H, Nieuwenhuizen WF, Jung G, Tarkowski A, et al. Staphylococcus aureus resistance to human defensins and evasion of neutrophil killing via the novel virulence factor MprF is based on modification of membrane lipids with l-lysine. J. Exp. Med. 2001;193:1067–1076. doi: 10.1084/jem.193.9.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullan RD, Rhodes J, Ganesh S, Mani V, Morris JS, Williams GT, Newcombe RG, Russell MA, Feyerabend C, Thomas GA, et al. Transdermal nicotine for active ulcerative colitis. N. Engl. J. Med. 1994;330:811–815. doi: 10.1056/NEJM199403243301202. [DOI] [PubMed] [Google Scholar]
- Radek K, Gallo R. Antimicrobial peptides: natural effectors of the innate immune system. Semin. Immunopathol. 2007;29:27–43. doi: 10.1007/s00281-007-0064-5. [DOI] [PubMed] [Google Scholar]
- Radek KA, Lopez-Garcia B, Hupe M, Niesman IR, Elias PM, Taupenot L, Mahata SK, O'Connor DT, Gallo RL. The neuroendocrine peptide catestatin is a cutaneous antimicrobial and induced in the skin after injury. J. Invest. Dermatol. 2008;128:1525–1534. doi: 10.1038/sj.jid.5701225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinheimer T, Munch M, Bittinger F, Racke K, Kirkpatrick CJ, Wessler I. Glucocorticoids mediate reduction of epithelial acetylcholine content in the airways of rats and humans. Eur. J. Pharmacol. 1998;349:277–284. doi: 10.1016/s0014-2999(98)00185-x. [DOI] [PubMed] [Google Scholar]
- Schauber J, Dorschner RA, Yamasaki K, Brouha B, Gallo RL. Control of the innate epithelial antimicrobial response is cell-type specific and dependent on relevant microenvironmental stimuli. Immunology. 2006;118:509–519. doi: 10.1111/j.1365-2567.2006.02399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlereth T, Schonefeld S, Birklein F, Kirkpatrick CJ, Wessler I. In vivo release of non-neuronal acetylcholine from human skin by dermal microdialysis: effects of sunlight, UV-A and tactile stimulus. Life Sci. 2007;80:2239–2242. doi: 10.1016/j.lfs.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Scott MG, Davidson DJ, Gold MR, Bowdish D, Hancock RE. The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses. J. Immunol. 2002;169:3883–3891. doi: 10.4049/jimmunol.169.7.3883. [DOI] [PubMed] [Google Scholar]
- Shaykhiev R, Beisswenger C, Kandler K, Senske J, Puchner A, Damm T, Behr J, Bals R. Human endogenous antibiotic LL-37 stimulates airway epithelial cell proliferation and wound closure. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005;289:L842–L848. doi: 10.1152/ajplung.00286.2004. [DOI] [PubMed] [Google Scholar]
- Sorensen LT, Karlsmark T, Gottrup F. Abstinence from smoking reduces incisional wound infection: a randomized controlled trial. Ann. Surg. 2003;238:1–5. doi: 10.1097/01.SLA.0000074980.39700.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani K, Murphy WJ, Chertov O, Salcedo R, Koh CY, Utsunomiya I, Funakoshi S, Asai O, Herrmann SH, Wang JM, et al. Defensins act as potent adjuvants that promote cellular and humoral immune responses in mice to a lymphoma idiotype and carrier antigens. Int. Immunol. 2000;12:691–700. doi: 10.1093/intimm/12.5.691. [DOI] [PubMed] [Google Scholar]
- Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J. Clin. Invest. 2007;117:289–296. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera S, Ballivet M, Bertrand D. Progesterone modulates a neuronal nicotinic acetylcholine receptor. Proc. Natl. Acad. Sci. USA. 1992;89:9949–9953. doi: 10.1073/pnas.89.20.9949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wetering S, Sterk PJ, Rabe KF, Hiemstra PS. Defensins: key players or bystanders in infection, injury, and repair in the lung? J. Allergy Clin. Immunol. 1999;104:1131–1138. doi: 10.1016/s0091-6749(99)70004-7. [DOI] [PubMed] [Google Scholar]
- Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- Weber G, Heilborn JD, Chamorro Jimenez CI, Hammarsjo A, Torma H, Stahle M. Vitamin D induces the antimicrobial protein hCAP18 in human skin. J. Invest. Dermatol. 2005;124:1080–1082. doi: 10.1111/j.0022-202X.2005.23687.x. [DOI] [PubMed] [Google Scholar]
- Wu LR, Zaborina O, Zaborin A, Chang EB, Musch M, Holbrook C, Turner JR, Alverdy JC. Surgical injury and metabolic stress enhance the virulence of the human opportunistic pathogen Pseudomonas aeruginosa. Surg. Infect. (Larchmt) 2005;6:185–195. doi: 10.1089/sur.2005.6.185. [DOI] [PubMed] [Google Scholar]
- Zasloff M. Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc. Natl. Acad. Sci. USA. 1987;84:5449–5453. doi: 10.1073/pnas.84.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]