The disease caused by the pathogenic fungus Batrachochytrium dendrobatidis (Bd), chytridiomycosis, is one of several factors driving the global decline of amphibian populations (Blaustein and Kiesecker 2002; Lips et al.2006; Muths et al.2003). Bd has been found in amphibians at sites across North America including Minnesota, USA (Ouellet et al. 2005;Woodhams et al. 2008). The prevalence of Bd in wild anuran populations in Minnesota is unknown, and motivated the work described herein.
We investigated the occurrence of Bd at the University of Minnesota’s Itasca Biological Station and Laboratories in Itasca State Park, site of the headwaters of the Mississippi River. Our research objectives were to: 1) verify if Bd is present in the park; 2) determine which anuran species are affected by the fungus; and 3) test if there are differences in infection rate among species.
Methods
We collected frogs in Itasca State park, Clearwater County, Minnesota in June and July 2008. We collected frogs by hand and with nets at night in breeding ponds and during the day near ponds and wetlands. We rinsed nets with 95% ethanol between outings and wore latex gloves in the field and the laboratory to prevent potential transfer of Bd among individuals. A subsample of frogs was toe-clipped and released in the field, but most were vouchered. Toe-clips were collected in individual plastic bags and we extracted genomic DNA immediately upon return to the laboratory. We kept frogs individually in plastic bags from time of capture until they were euthanised in the laboratory. Frogs were humanely euthanised with MS-222 (tricaine methanesulfonate) or topical application of benzocaine (Simmons 2002). MS-222 does not appear to inhibit growth or detection of Bd (Webb et al. 2005) and was the preferred method of euthanasia. We clipped one toe and a portion of adjacent webbing to obtain tissues. We stored tissues in 95% ethanol at 4°C until processing. All vouchers were deposited in the Bell Museum of Natural History, University of Minnesota (JFBM).
We extracted genomic DNA from tissues using the Qiagen extraction kit (Qiagen, Valencia, CA, USA) following the manufacturer’s instructions. We used polymerase chain reaction (PCR) to amplify a 300-bp fragment consisting of part of internal transcribed spacer 1 (ITS1), ribosomal rRNA 5.8S, and part of internal transcribed spacer 2 (ITS2) using, B. dendrobatidis specific primers (Bd1a and Bd2a; Annis et al. 2004). PCR was performed in 12.5 µl reaction volumes under the following conditions: an initial denaturation of 94°C for 5 min; 30 cycles of denaturation (94°C for 45 sec), annealing (50°C for 45 sec) and extension (72°C for 1 min); followed by a final extension of 72°C for 5 min. All PCR reactions contained a negative control. PCR products were run on a 1% agarose gel, stained with ethidium bromide and visualized under ultraviolet light. Presence of a strong, defined band approximately 300 bp was considered a positive result.
We sequenced two positive samples to ensure that we had amplified Bd rather than non-target DNA. We purified PCR products using Exonuclease I and Shrimp Alkaline Phosphatase (Hanke and Wink 1994). Sequencing was performed using Big Dye (perkin Elmer, Boston, MA, USA) terminator cycle sequencing on an ABI 3730×1 at the Advanced Genetic Analysis Center, University of Minnesota. We used BLAST (Altschul et al. 1990) to verify that sequences were Bd.
We tested whether infection rates of Bd were significantly different among sampled species and among sampled families of frogs using a Chi-square contingency test. All statistical analyses were conducted using JMP 7.0 (sAS 2007).
Results
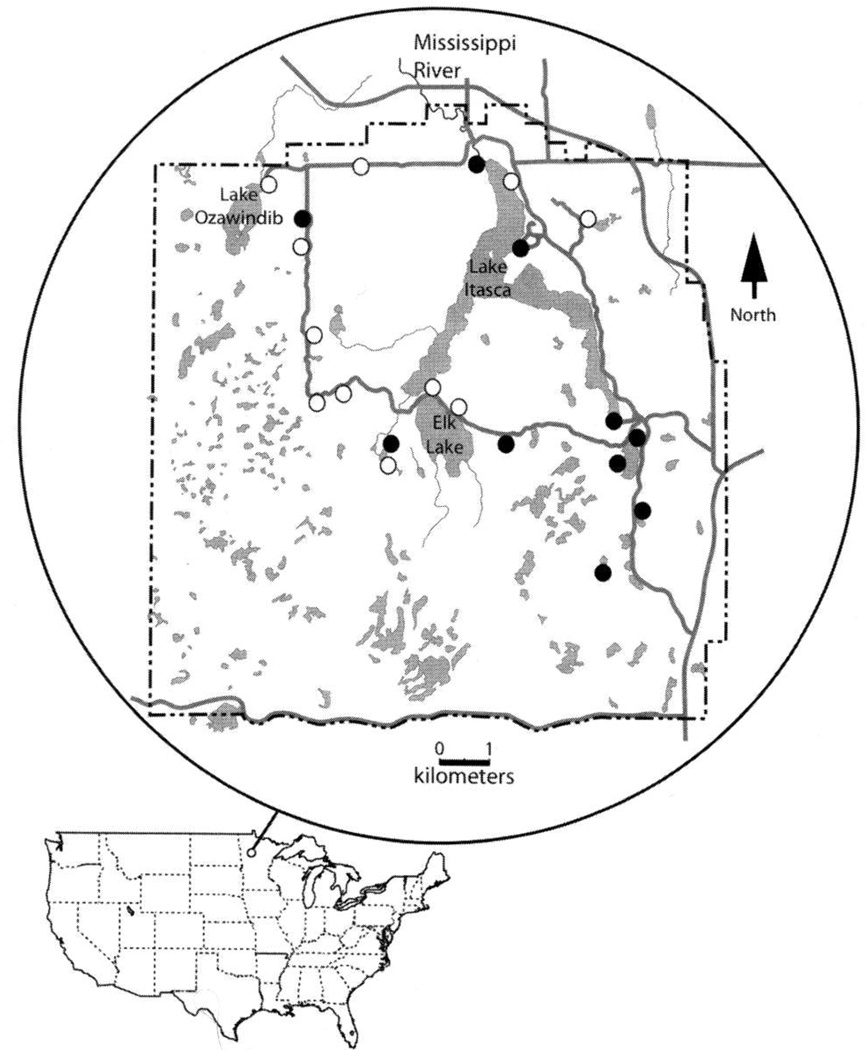
We collected tissues from 147 frogs and toads of three families from around Itasca State Park (Fig. 1), consisting of 133 vouchered specimens and 14 toe-clips of released frogs. Thirty-four of 147 (23%) individuals were Bd-positive. None of our negative controls produced bands on agarose gels. Pseudacris maculata had the highest percentage of positive individuals, 50% (Table 1), although it is difficult to interpret this given the small number of individuals captured. Lithobates pipiens had the second highest infection rate with 33.3% of samples being Bd-positive. Hyla versicolor and Pseudacris crucifer had no positive samples. We collected one dead Lithobates sylvaticus (JFBM 15957) at the Mississippi River headwaters on the North end of Lake Itasca that tested positive for Bd. No other dead animals were found, and no animals were observed with symptoms of illness.
FIG. 1.
Collecting locations (circles) of anurans examined for Batrachochytrium dendrobatitis (Bd) at Itasca State park, Minnesota, USA. Localities with Bd-positive specimens indicated by filled cirlces, localities with no Bd-positive specimens indicated by open circles. Park boundaries shown by a dashed line. Light gray areas are water.
TABLE 1.
Prevalence of Batrachochytrium dendrobatidis in seven frog species collected in Itasca State Park, Minnesota, USA, in 2008.
| Species | Family | No. Bd-Negative Animals |
No. Bd-Positive Animals |
% Positive (95% C.I.) |
|---|---|---|---|---|
| Anaxyrus americanus | Bufonidae | 20 | 1 | 4.8 (0–24.4) |
| Hyla versicolor | Hylidae | 19 | 0 | 0 (0–19.8) |
| Pseudacris crucifer | Hylidae | 2 | 0 | 0 (0–70.1) |
| Pseudacris maculata | Hylidae | 1 | 1 | 50 (9.5–90.6) |
| Lithobates pipiens | Ranidae | 14 | 7 | 33.3(17.1–54.8) |
| Lithobates septentrionalis | Ranidae | 13 | 3 | 18.8( 5.8–43.8) |
| Lithobates sylvaticus | Ranidae | 78 | 22 | 22 (14.9–31.2) |
Rates of Bd infection differed among species (χ2 = 16.505, p < 0.0113) and among families (χ2 = 9.673,P < 0.0079). Frogs in the family Ranidae had a higher infection rate than Bufonidae and Hylidae (Table 1).
We confirmed that DNA fragments amplified using PCR were Bd by sequencing two of our positive PCR products. Both sequenced samples, one from Lithobates sylvaticus (JFBM 15884, Genbank accession number FJ229469) and the other from Lithobates pipiens (JFBM 15918, Genbank accession number FJ229470) had an identical sequence. We compared our sequences to Bd sequences on Genbank using BLAST, confirming our samples as Bd. Our samples had 100% identity with the following Bd sequences from Genbank: EU779867, EU779864, EU779863, EU779862, EU779860, EU779859. Our samples had a 98% identity with Bd sequences AY997031 and EU779866.
Discussion
Rates of Bd infection in Itasca State Park varied among species and families. This variance is similar to observations from other North American sites for the same species (Longcore et aI.2007: Ouellet et al. 2005). North American hylid frogs, for example, typically have had low Bd infection rates (Longcore et al. 2007; Ouellet et al. 2005; Pearl et al. 2007) and we found no evidence of Bd in the hylid species Hyla versicolor and Pseudacris crucifer. Pseudacris maculata and Acris blanchardi appear to be exceptions to this rule (Ouellet et al. 2005; Steiner and Lehtinen 2008) and we found one infected P.maculata in the Park. We found the highest rates of infection in the three ranid species examined, L. sylvaticus, L. pipiens and L. septentrionalis. Several hypotheses have been proposed to explain the species-specific variance in Bd infection rates. Because Bd can persist in aquatic environments (Johnson and Speare 2003), species that spend more time in the water are thought to be at greater risk of infection than species spending less time in the water (Hero et al. 2005; Lips et al. 2003). Bd is prevalent in species breeding in permanent wetlands and streams (Kriger and Hero 2007) and in species that overwinter in aquatic environments (Longcore et al. 2007). There is also, potentially, a phylogenetic component to Bd (Corey and Waite 2008) with some amphibian lineages showing greater susceptibility to chytridiomycosis than others.
The presence of Bd in multiple frog species in Itasca State Park highlights the pathogen’s pervasiveness in North America. North American amphibian populations have been exposed to Bd since the early 1960s and have been found across the continent (Ouellet et al. 2005). Presence of Bd does not always lead to mortality or population declines (Retallick and Miera 2007) but, given its potential as a pathogen, the presence of the fungus is cause for further monitoring of infected populations.
Acknowledgments
We thank Dede Olson and two anonymous reviewers for helpful comments on the manuscript and Andrew Simons and Ken Kozak for access to lab space. Special thanks to the students of the Global Change and Ecology Program for help in the field. Funding for this research was provided by NSF REU (DBI 0648931) “Field Studies in Global Change at the Headwaters of the Mississippi” to Jim and Sehoya Cotner and the Life Sciences Summer Undergraduate Research Programs (LSSURP), University of Minnesota. TG was partially supported by Grant Number T32DE007288 from the National Institute of Dental & Craniofacial Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Dental & Craniofacial Research or the National Institutes of Health
Contributor Information
Edmarie Martinez Rodriguez, Department of Biology, University of Puerto Rico at Cayey, Avenida Antonio R. Barceló, Cayey, Puerto Rico 00736.
Tony Gamble, Department of Genetics, Cell Biology & Development, University of Minnesota, 321 Church St NE, Minneapolis, Minnesota 55455, USA.
M. Vincent Hirt, Department of Ecology, Evolution and Behavior, University of Minnesota, 1987 Upper Buford Circle, St. Paul, Minnesota 55108, USA.
Sehoya Cotner, College of Biological Sciences, University of Minnesota, 420 Washington Avenue SE, Minneapolis, Minnesota 55455, USA.
LITERATURE CITED
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J. Mol. Biol. 1990;215:4O3–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Annis SL, Dastoor FP, Ziel H, Daszak P, Longcore JE. A DNA-based assay identifies Batrachochytrium dendrobatidis in amphibians. J. Wildl. Dis. 2004;40:420–428. doi: 10.7589/0090-3558-40.3.420. [DOI] [PubMed] [Google Scholar]
- Blaustein AR, Kiesecker JM. Complexity in conservation: lessons from the global decline of amphibian populations. Ecol. Lett. 2002;5:597–608. [Google Scholar]
- Corey SJ, Waite TA. Phylogenetic autocorrelation of extinction threat in globally imperilled amphibians. Divers. Distrib. 2008;14:614–629. [Google Scholar]
- Hanke M, Wink M. Direct DNA-Sequencing of Pcr-Amplified Vector Inserts Following Enzymatic Degradation of Primer and Dntps. BioTechniques. 1994;17:858–860. [PubMed] [Google Scholar]
- Hero JM, Williams SE, Magnusson WE. Ecological traits of declining amphibians in upland areas of eastern Australia. J. Zool. 2005;267:221–232. [Google Scholar]
- Johnson ML, Speare R. Survival of Batrachochytrium dendrobatidis in water: Quarantine and disease control implications. Emerg. Infect. Dis. 2003;9:922–925. doi: 10.3201/eid0908.030145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriger KM, Hero JM. The chytrid fungus Batrachochytrium dendrobatidis is non-randomly distributed across amphibian breeding habitats. Divers. Distrib. 2007;13:78l–788. [Google Scholar]
- Lips KR, Brem F, Brenes R, Reeve JD, Alford RA, Voyles J, Carey C, Livo L, Pessier AP, Collins Jp. Emerging infectious disease and the loss of biodiversity in a Neotropical amphibian community. Proc. Natl. Acad. Sci. U. S. A. 2006;103:3165–3170. doi: 10.1073/pnas.0506889103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lips KR, Reeve JD, Witters LR. Ecological traits predicting amphibian population declines in Central America. Conserv. Biol. 2003;17:1078–1088. [Google Scholar]
- Longcore JR, Longcore JE, Pessier AP, Halteman WA. Chytridiomycosis widespread in anurans of northeastem United States. J. Wildl. Manage. 2007;71:435–444. [Google Scholar]
- Muths E, Corn PS, Pessier AP, Green DE. Evidence for disease-related amphibian decline in Colorado. Biol. Conserv. 2003;110:357–365. [Google Scholar]
- Ouellet M, Mikaelian L, Pauli BD, Rodrigue J, Green DM. Historical evidence of widespread chytrid infection in North American amphibian populations. Conserv. Biol. 2005;19:1431–1440. [Google Scholar]
- Pearl CA, Bull EL, Green DE, Bowerman J, Adams MJ, Hyatt A, Wente WH. Occurrence of the amphibian pathogen Batrachochytrium dendrobotidis in the Pacific Northwest. J. Herpetol. 2007;4l:145–149. [Google Scholar]
- Retallick RWR, Miera V. Strain differences in the amphibian chytrid Batrachochytrium dendrobatidis and non-permanent, sub-lethal effects of infection. Dis. Aquat. Org. 2007;75:201–207. doi: 10.3354/dao075201. [DOI] [PubMed] [Google Scholar]
- SAS. JMP. Cary, North Carolina, USA: SAS Institute Inc; 2007. [Google Scholar]
- Simmons JE. Herpetological Collecting and Collections Management. Revised Edition. Shoreview, MN: SSAR; 2002. [Google Scholar]
- Steiner SL, Lehtinen RM. Occurrence of the amohibian pathogen Batrachochytrium dendrobatidis in Blanchard’s cricket frog(Acris creptians blanchardi) in the U.S. Midwest. Herpetol. Rev. 2008;39:193–196. [Google Scholar]
- Webb R, Berger L, Mendez D, Speare R. MS-222 (tricaine methane sulfonate) does not kill the amphibian chytrid fungus Batrachochytrium dendrobatidis. Dis. Aquat. Org. 2OO5;68:89–90. doi: 10.3354/dao068089. [DOI] [PubMed] [Google Scholar]
- Woodhams DC, Hyatt AD, Boyle DG, Rollins-Smith LA. The northem Leopard frog Rana pipiens is a widespread reservoir species harboring Batrachochytrium dendrobatidis in North America. Herpetol. Rev. 2008;39:66–68. [Google Scholar]