Abstract
The ABO blood group system has been implicated in susceptibility to cholera or in explaining variability in the immune response to a cholera vaccine. O blood group individuals were found to be more susceptible to cholera and elicited lower vibriocidal antibody response to cholera toxin B subunit-killed oral vaccine. Based on the observations that O blood group individuals were more susceptible to cholera and that high mortality was associated with cholera, an evolutionary explanation was provided for the extremely low prevalence of the O blood group in the Gangetic Delta (West Bengal, India, and Bangladesh). However, conflicting results were reported from a later study conducted in Indonesia using a live attenuated oral cholera vaccine; O blood group individuals showed a higher vibriocidal antibody response. In a study conducted in a region of India where cholera is endemic (Kolkata, West Bengal) that comprised 992 individuals vaccinated by a killed whole-cell oral cholera vaccine, we found no statistically significant difference between O and non-O individuals either in the frequency distributions of the fold increase or in the postvaccination increase in geometric mean titer compared to the baseline. Further, in contrast to the earlier observation that the O allele frequency is extremely low in the Gangetic Delta, we have noted that the O allele frequency exceeds 0.5 in the vast majority of ethnic groups of this region. In addition, we have found large differences in response to the vaccine among residents of an area where cholera is not endemic compared to an area where cholera is endemic to The percentages of vaccinees who seroconverted in an area where cholera is not endemic (Son La province of Vietnam) was >90% compared to ∼50% in Kolkata, India, an area where cholera is endemic.
Cholera continues to be endemic in many countries, including India (12). It also accounts for a significant fraction of mortality in developing countries (7). The Gram-negative bacterium, Vibrio cholerae, has two major serogroups—O1 and O139—that are responsible for most cholera cases. These serogroups are defined by characteristics of the O side chain of its polysaccharide. The O1 serogroup is subclassified into two biotypes—classical and El Tor—and two major serotypes—Inaba and Ogawa.
Among other control measures, the World Health Organization (WHO) advocates vaccination for cholera (11). Vietnam is the only country in the world with large areas where cholera is endemic and where a vaccine for cholera has been widely used (1) for a long time. The bivalent oral cholera vaccine that has been used there since 1997 was recently reformulated for international use, in accord with WHO requirements (1). In a clinical trial of this vaccine (1) conducted in the Son La province, an area in Vietnam where cholera is not endemic, it has been shown that the vaccine is safe, well tolerated, and immunogenic. Ninety percent of the vaccine recipients developed ≥4-fold increases in vibriocidal antibodies to V. cholerae O1. Among vaccinees, there was a 27-fold rise in the geometric mean titers. There was, of course, considerable variation in postvaccination titers of vibriocidal antibodies and fold increases among vaccinees. One contributing factor to this postvaccination variation was the prevaccination titer, since it was found (1) that all vaccinees with a baseline titer ≤80 seroconverted, while only 12% of those with baseline titer ≥160 seroconverted. In order to investigate whether the genomic backgrounds of vaccinees also contributed to variation in immunological response to the vaccine, we undertook a large study (n = 1,000) in an area of Kolkata, India, where cholera is endemic. One of the genetic factors reported earlier in the literature (3, 5, 6, 8) is that persons belonging to the O blood group are more susceptible to cholera than persons belonging to a non-O blood group. Past studies on the relationship of the blood group distribution to the efficacy of a cholera vaccine have yielded equivocal results. In a trial of a cholera toxin B subunit-killed oral vaccine in Bangladesh, O blood group vaccinees showed lower protective efficacy (3). However, in a study in Indonesia, O blood group individuals elicited higher vibriocidal antibody responses to a live attenuated oral cholera vaccine, CVD 103-HgR (8). In view of these conflicting reports and the fact that O blood group individuals are more susceptible to cholera, we sought to test whether O blood group vaccinees in Kolkata (an area where cholera is endemic), India, elicit a lower immunological response, as assessed by the serum vibriocidal antibody assay, to a two-dose whole-cell killed oral cholera vaccine. We report our findings here. We also report significant differences in the nature and extent of the immunological response to the vaccine in India compared to those found in Vietnam (1).
MATERIALS AND METHODS
Study participants.
Institutional ethical approvals were obtained from all collaborating institutions before initiation of the present study. Individuals (n = 1,000), unrelated at least to the first-cousin level based on family history report, aged 14 years or older, inhabiting a socioeconomically depressed locality of Kolkata, India, were recruited into the present study with written informed consent. Cholera is endemic in this locality. The residents belonged to two maritally isolated, religious groups, Muslim and Hindu. The Muslims of this locality are mostly religious converts, during the last 100 years, to Islam from Hinduism. The individuals recruited into the present study, for which a stratified random sampling scheme was used, are representative of the entire locality. Based on self-report, individuals who had ever been diagnosed with cholera or who had experienced diarrhea or vomiting during the week preceding recruitment were excluded. Pregnant or lactating women were also excluded.
Vaccination and collection of blood samples.
A two-dose vaccine, orally administered 14 days apart, was used. Each dose of the vaccine contained 600 enzyme-linked immunosorbent assay units (EU) of lipopolysaccharide (LPS) of formalin-killed V. cholerae Inaba, El Tor biotype (strain Phil 6973); 300 EU of LPS of heat-killed V. cholerae Ogawa classical biotype (Cairo 50); 300 EU of LPS of formalin-killed V. cholerae Ogawa classical biotype (Cairo 50); 300 EU of LPS of heat-killed V. cholerae Inaba, classical biotype (Cairo 48); and 600 EU of LPS of formalin-killed V. cholerae O139 (4260B). Approval was obtained from the Drug Controller General, Government of India, for the use of this vaccine in the present study. The vaccine doses were procured from Shantha Biotechnics, Hyderabad, India. The vaccine was stored at 4 to 8°C before administration, including during transport from the base laboratory to the vaccination field-site.
From each study participant, a blood sample was collected immediately prior to vaccination (day 0 sample) and 28-days postvaccination (day 28 sample) and used for the vibriocidal assay and determination of the blood group.
Vibriocidal assay.
Vibriocidal assay was performed with the V. cholerae O1 Inaba (OS-418), Ogawa (MAK757) and uncapsulated O139 (MO-10T4) strains using sera collected during pre- and post-vaccine trials according to published methods (2). Commercially available guinea pig serum (Rockland, Gilbertsville, PA) was used as a source of complement. The sera (100 μl) were added to 100 μl of phosphate-buffered saline in the first well to give a 2-fold dilution, and the subsequent dilutions were made reciprocally up to 4,800. A ≥4-fold increase in titer between the day 0 and day 28 serum samples was used to identify seroconversion. Reference rabbit antisera against V. cholerae O1 Inaba, Ogawa, and O139 were included in each set of assay as controls. In addition, in every batch of the assay, serum obtained from a healthy volunteer and a high-titer antiserum obtained from one of the volunteers in the present study were included as negative and positive controls, respectively.
Blood grouping.
For each study participant, the blood group with respect to the ABO system was determined. For each participant, either a day 0 or a day 28 blood sample was used for blood group determination. A, B, and AB antisera were procured from CSL Biotherapies, Melbourne, Australia. Blood grouping was carried out on microplates according to standard protocols.
Statistical analysis.
Rank correlation between titers to different serotypes was estimated by using Spearman's ρ statistic. The effect of covariates on titers was estimated using regression analysis. Comparison of the rates of seroconversion was carried out using a binomial test of proportions. A test of significant differences in frequencies of the blood groups between groups was performed using the contingency χ2 statistic.
RESULTS
Demographic characteristics of vaccine recipients.
After all missing or ambiguous data were eliminated, the data on a total of 992 study participants (536 [54%] male and 456 [46%] female; 406 [41%] Muslim and 586 [59%] Hindu) were included in our analysis. The demographic characteristics of the study participants are provided in Table 1.
TABLE 1.
Demographic characteristics of the vaccine recipients
| Age group (yr) | Gender | No. of recipients (%) |
Total | |
|---|---|---|---|---|
| Muslim | Hindu | |||
| 14-18 | Male | 10 (48) | 11 (52) | 21 |
| Female | 8 (62) | 5 (38) | 13 | |
| Total | 18 (53) | 16 (47) | 34 | |
| 18-30 | Male | 126 (55) | 105 (45) | 231 |
| Female | 52 (27) | 140 (73) | 192 | |
| Total | 178 (42) | 245 (58) | 423 | |
| 31-40 | Male | 61 (43) | 80 (57) | 141 |
| Female | 45 (39) | 69 (61) | 114 | |
| Total | 106 (42) | 149 (58) | 255 | |
| >40 | Male | 59 (41) | 84 (59) | 143 |
| Female | 45 (33) | 92 (67) | 137 | |
| Total | 104 (37) | 176 (63) | 280 | |
Pre- and postvaccination titer levels, effects of covariates, and seroconversion rates.
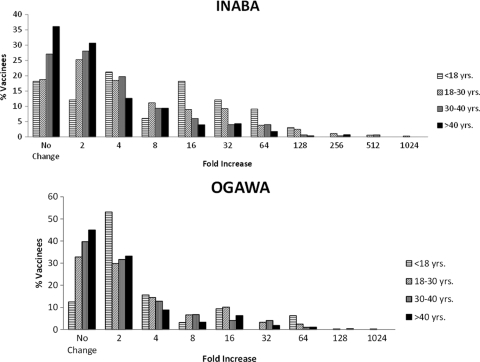
Considerable variation was observed in pre- and postvaccination titers to Inaba and Ogawa but not to O139 (see Fig. S1 in the supplemental material). Absolute titers to O139 were also very low for the vast majority of individuals (98% for prevaccination and 95% for postvaccination). The correlation between prevaccination titers to Inaba and Ogawa was high (Spearman's ρ = 0.544) and statistically significant (P < 0.0006). The fold increases of post- to prevaccination titers to Inaba and Ogawa were highly variable across vaccinees. Although the effects of covariates (age, religion, and gender) were variably significant on pre- and postvaccination titers, only the effect of age, but not religion or gender, on variation in fold increase was significant with respect to both Inaba and Ogawa (Table 2). The fold increases to in response to both Inaba and Ogawa were significantly higher if the vaccine was administered at a younger age (Fig. 1; Spearman's ρ values were −0.237 and −0.168 for Inaba and Ogawa, respectively; the P values for both of these correlations were <0.0006). This effect was primarily due to significantly lower prevaccination titers among younger individuals for both Inaba and Ogawa (both P values were <0.0006). The correlation (Spearman's ρ = 0.434) between the fold increase for Inaba and Ogawa was statistically significant (P < 0.0006). The percentages of vaccinees who seroconverted (postvaccination fold increase of ≥4) with respect to Inaba and Ogawa were, respectively, 46.47 and 30.23. Seroconversion rates monotonically and significantly (P < 0.0006) decreased with age for Inaba and nearly so for Ogawa (P = 0.001) as well (Table 3). The seroconversion rate for Inaba was >2-fold in individuals younger than 18 years of age (70%) compared to those older than 40 years (33%), and for Ogawa was the rate was more than 1.5-fold (34% versus 22%).
TABLE 2.
Effects of religion, gender, and age on antibody titers, pre- and postvaccination, to V. cholerae strains Inaba, Ogawa, and O139
| Strain | Assay day or fold increasea | Effect (P) on antibody titerb |
||
|---|---|---|---|---|
| Religion | Gender | Age | ||
| Inaba | Day 0 | 0.2 | 0.02 | 0.009 |
| Day 28 | 0.1 | <0.0006 | 0.4 | |
| Fold increase | 0.1 | 0.6 | 0.004 | |
| Ogawa | Day 0 | 0.1 | 0.001 | 0.9 |
| Day 28 | 0.03 | <0.0006 | 0.1 | |
| Fold increase | 0.3 | 0.7 | 0.04 | |
| O139 | Day 0 | 1 | 0.2 | 0.2 |
| Day 28 | 0.7 | 0.5 | 0.3 | |
| Fold increase | 0.8 | 0.7 | 0.9 | |
Day 0, prevaccination; day 28, postvaccination.
That is, the antibody titer to the strains listed in column 1.
FIG. 1.
Frequency distributions in the fold increase of postvaccination to prevaccination titer levels to Inaba and Ogawa for different age groups of the vaccinees.
TABLE 3.
Seroconversion rates with respect to strains Inaba and Ogawa by age group
| Age group (yr) | No. of subjects examined (% seroconverted) |
|
|---|---|---|
| Inaba | Ogawa | |
| <18 | 33 (70) | 32 (34) |
| 18-30 | 417 (55) | 414 (36) |
| 31-40 | 250 (44) | 249 (28) |
| >40 | 277 (33) | 271 (22) |
| Total | 977 (46) | 966 (30) |
Comparison to the characteristics in a population where cholera is not endemic.
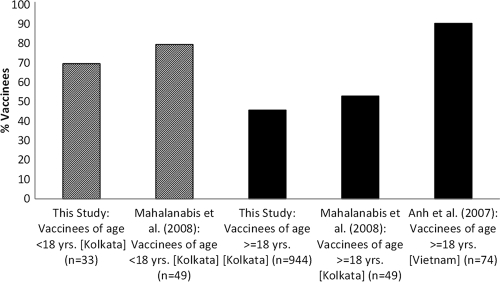
We have compared our data from an area where cholera is endemic to that of a study (9) with a very small sample size conducted in the same area (Kolkata, India) and a study (1) conducted in Son La province of Vietnam, where cholera is not endemic. The study in Vietnam (1) was restricted to adults in the age group of 18 to 40 years. Considerable variation was observed in the frequency distributions of prevaccination titers to Inaba (see Fig. S2 in the supplemental material). Prevaccination titer to Inaba was generally lower (but not statistically significant [P = 0.20]), and the postvaccination titer was significantly (P < 0.05) higher (see Fig. S3 in the supplemental material) in an area of nonendemicity (Vietnam) than in an area of endemicity (Kolkata). The rates of seroconversion observed in these studies are presented in Fig. 2. The rates of seroconversion observed in the two studies conducted in Kolkata were not statistically significant either among vaccinees younger than 18 years (P = 0.432) or among those who were 18 years or older (P = 0.378). However, the percentage of seroconverted adult vaccinees (i.e., ≥18 years) in Vietnam (90.2%) was significantly (P < 0.00001) higher than in Kolkata (∼50%). In other words, the rate of seroconversion among adults was 1.8-fold higher in the area of nonendemicity than in the area of endemicity.
FIG. 2.
Percentages of vaccinees, by age group, who seroconverted in three separate studies that used the same vaccine against cholera.
Relationship of postvaccination titer with blood group.
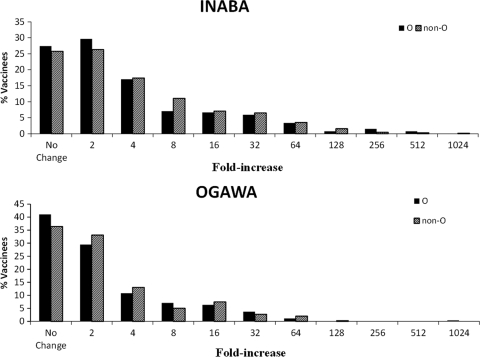
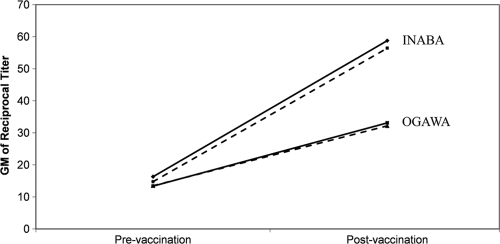
The frequencies of A, B, AB, and O blood groups among the vaccinees are presented in Table 4. There was no statistically significant difference in the frequencies of the various blood groups between the two religious groups (P = 0.822) or between genders within the two religious groups (P = 0.426 for Muslim and P = 0.665 for Hindu). Therefore, the data on all vaccinees were pooled. Overall, 27.7% of vaccinees were of O blood group. Figure 3 presents the frequency distributions of postvaccination fold increase in response to Inaba and Ogawa by the blood group (O and non-O) of the vaccinees. The frequency distributions of the fold increase were not significantly different between O and non-O blood group vaccinees, either for Inaba (P = 0.464) or for Ogawa (P = 0.311). The postvaccination increase in geometric mean titer compared to the baseline were also similar for O blood group and non-O blood group vaccinees for both the Inaba and Ogawa strains (Fig. 4).
TABLE 4.
Prevalence of O, A, B, and AB blood groups among vaccinees by religious group and gender
| Religion | Gender | No. (%) of vaccinees of blood group: |
Total | |||
|---|---|---|---|---|---|---|
| O | A | B | AB | |||
| Muslim | Male | 72 (28) | 60 (23) | 99 (39) | 25 (10) | 256 |
| Female | 35 (23) | 35 (23) | 58 (39) | 22 (15) | 150 | |
| Total | 107 (26) | 95 (23) | 157 (39) | 47 (12) | 406 | |
| Hindu | Male | 81 (29) | 68 (24) | 106 (38) | 25 (9) | 280 |
| Female | 87 (28) | 72 (23) | 110 (36) | 37 (12) | 306 | |
| Total | 168 (29) | 140 (24) | 216 (37) | 62 (10) | 586 | |
FIG. 3.
Frequency distributions in the fold increase in response to the Inaba and Ogawa strains among individuals of the O and non-O blood groups.
FIG. 4.
Postvaccination increase in geometric mean titer compared to prevaccination value for O blood group (solid line) and non-O blood group (broken line) vaccinees for both Inaba and Ogawa.
DISCUSSION
The new two-dose whole-cell killed oral vaccine for cholera exhibited large differences in immune response. Prevaccination titers to the O1 serotypes Inaba and Ogawa were highly correlated, but postvaccination titers showed considerable differences. The fold increase in response to both of these serotypes was greater if the vaccine was administered at a younger age. The immunological response to O139 was minimal. Until 1992, V. cholerae serogroup O1 was the only serogroup responsible for large epidemics and pandemics of cholera. In 1992, a new serotype—O139—emerged in southeast Asia that caused a large cholera outbreak in India and neighboring countries. Serotype O139 is no longer prevalent in India. Unlike O1, O139 possesses a capsular polysaccharide that, in general, is a T-independent antigen giving rise to poor immune response and lacking immunological memory. This is the most likely reason why we have observed low response to O139.
Large differences were found in response if the vaccinees were drawn from an area of nonendemicity compared to vaccinees from an area of endemicity. The percentages of vaccinees who seroconverted to Inaba in an area of nonendemicity (Son La province of Vietnam) was >90% (1) compared to ∼50% in the Kolkata, India (both in a study of 101 vaccinees [9] and in the present study, with a sample size of 992), area of endemicity. It is interesting that the significantly lower rate of seroconversion among residents of Kolkata compared to Son La was not due to higher prevaccination titer levels among the Kolkata residents; in fact, there was no statistically significant difference in the prevaccination titer levels of residents of these two regions. It is also intriguing that differences in age distribution of vaccinees also do not provide an adequate distribution of the observed differences in the rate of seroconversion between the two regions. In the Son La study (1), all vaccinees were older than 18 years of age, whereas in our (Kolkata) study a small number of (33 of 977) of vaccinees were younger than 18 years of age. Among vaccinees in the age range from 18 to 40 years (which was the age range of vaccinees included in the Son La study), the observed seroconversion rate in Son La was 90% compared to only ca. 51% observed in the present study in Kolkata. It is possible that the observed differences in immunological response between the two regions may be due largely to differences in ethnicity.
One of the genetic factors implicated in susceptibility to cholera or in explaining variability in immune response to a cholera vaccine is the ABO blood group system. Blood group O individuals were found to be more susceptible (5). Interestingly, in a prospective study of a cohort of household contacts of patients with cholera in Bangladesh (6), it was found that persons with blood group O were less likely than those with other blood groups to become infected with V. cholera O1. A trial of a cholera toxin B subunit-killed oral vaccine in Bangladesh showed a lower protective efficacy in blood group O individuals (3), but a live oral attenuated cholera vaccine, CVD 103-HgR, resulted in higher vibriocidal antibody responses in blood group O individuals in Indonesia (8). We have found no significant difference between O and non-O individuals either in frequency distributions of the fold increase or in the postvaccination increase in geometric mean titer compared to baseline values. The susceptibility of O blood group individuals to cholera was postulated (5) to be the reason, via pressures of natural selection, for the “extremely low prevalence of O blood group genes … among people living in the Gangetic Delta,” i.e., the area around Kolkata, including the states of West Bengal (India) and Bangladesh. However, from an extensive analysis of ABO blood group profiles of a large number of ethnic groups of India, including 75 data sets from ethnic groups of West Bengal, India, and Bangladesh (10), the frequency of O blood group individuals varied between 15 and 64%, and the O allele was the major allele in most ethnic groups, with frequencies exceeding 0.5. Thus, the observation (5) that in the Gangetic Delta there is “extremely low prevalence” of O blood group genes was inaccurate. Even if blood group O individuals are more susceptible to cholera (5) or suffer from increased disease severity if infected (6), our results also show that compared to individuals with non-O blood group, the O blood group individuals do not elicit greater vibriocidal antibody responses to a killed whole-cell cholera vaccine in an area where cholera is endemic.
Although O blood group individuals were found to be more susceptible to develop severe cholera, the biological basis for this phenomenon is not clearly understood. It has been presumed that V. cholerae O1 may adhere better to intestinal mucosa of persons of O blood group. If this hypothesis is correct, then O blood group individuals may be expected to show altered immunogenicity compared to non-O blood group individuals, with implications for vaccination programs for cholera especially in regions of the world where the prevalence of both cholera and the O blood group is high. The present study conducted in such a region failed to find any difference in vibriocidal antibody response between O and non-O individuals, indicating that inactivated oral vaccines may not necessarily provide less protection to O blood-group individuals, as was found in a previous study (3). The reasons for conflicting findings for the susceptibility to cholera and the antibody responses to vaccines for cholera in relation to O blood group may, in part, be due to other factors, such as helminth infection and the use of antihelminth drugs. Indeed, it has been found (4) that among vaccinees who were recipients of albendazole (an antigeohelminth drug), postvaccination titers were significantly greater in non-O blood group individuals than in those belonging to the O blood group.
Supplementary Material
Acknowledgments
Financial support for this study was provided by U.S. National Institute of Allergy and Infectious Diseases, National Institutes of Health, contract HHSN200400067C.
We thank all members of The Chatterjee Group-Indian Statistical Institute Centre for Population Genomics for infrastructural and logistical support. In particular, we are grateful to Biplab Dey and Sonia Poddar for ABO blood group typing. The use of this vaccine in this study was approved by the Drug Controller General of India, and the entire study was approved by the Health Ministry Monitoring Committee, Government of India. We are grateful to them for these approvals. We are also grateful to Herman Staats (Duke University) and Carol Whisnant (RTI International) for valuable comments on an earlier version of the manuscript.
Footnotes
Published ahead of print on 16 June 2010.
Supplemental material for this article may be found at http://cvi.asm.org/.
REFERENCES
- 1.Anh, D. D., D. G. Canh, A. L. Lopez, V. D. Thiem, P. T. Long, N. H. Son, J. Deen, L. von Seidlein, R. Carbis, S. H. Han, S. H. Shin, S. Attridge, J. Holmgren, and J. Clemens. 2007. Safety and immunogenicity of a reformulated Vietnamese bivalent killed, whole-cell, oral cholera vaccine in adults. Vaccine 25:1149-1155. [DOI] [PubMed] [Google Scholar]
- 2.Benenson, A. S., A. Saad, and W. H. Mosley. 1968. Serological studies in cholera. 2. The vibriocidal antibody response of cholera patients determined by a microtechnique. Bull. World Health Organ. 38:277-285. [PMC free article] [PubMed] [Google Scholar]
- 3.Clemens, J. D., D. A. Sack, J. R. Harris, J. Chakraborty, M. R. Khan, S. Huda, F. Ahmed, J. Gomes, M. R. Rao, and A. M. Svennerholm. 1989. ABO blood groups and cholera: new observations on specificity of risk and modification of vaccine efficacy. J. Infect. Dis. 159:770-773. [DOI] [PubMed] [Google Scholar]
- 4.Cooper, P. J., M. E. Chico, G. Lasonsky, C. Sandoval, I. Espinel, R. Sridhara, M. Aguilar, A. Guevara, R. H. Guderian, M. M. Levine, G. E. Griffin, and T. B. Nutman. 2000. Albendazole treatment of children with ascariasis enhances the vibriocidal antibody response to the live attenuated oral cholera vaccine CVD 103-HgR. J. Infect. Dis. 182:1199-1206. [DOI] [PubMed] [Google Scholar]
- 5.Glass, R. I., J. Holmgren, C. E. Haley, M. R. Khan, A. M. Svennerholm, B. J. Stoll, K. M. B. Hossain, R. E. Black, M. Yunus, and D. Barua. 1985. Predisposition for cholera of individuals with O blood group: possible evolutionary significance. Am. J. Epidemiol. 121:791-796. [DOI] [PubMed] [Google Scholar]
- 6.Harris, J. B., A. I. Khan, R. C. LaRocque, D. J. Dorer, F. Chowdhury, A. S. Faruque, D. A. Sack, E. T. Ryan, F. Qadri, and S. B. Calderwood. 2005. Blood group, immunity, and risk of infection with Vibrio cholerae in an area of endemicity. Infect. Immun. 73:7422-7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Institute of Medicine. 1986. New vaccine development: establishing priorities, vol. II. Diseases of importance in developing countries. National Academy Press, Washington, DC. [PubMed]
- 8.Lagos, R., A. Avendaño, V. Prado, I. Horwitz, S. Wasserman, G. Losonsky, S. Cryz, Jr., J. B. Kaper, and M. M. Levine. 1995. Attenuated live cholera vaccine strain CVD 103-HgR elicits significantly higher serum vibriocidal antibody titers in persons of blood group O. Infect. Immun. 63:707-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahalanabis, D., A. L. Lopez, D. Sur, J. Deen, B. Manna, S. Kanungo, L. von Seidlein, R. Carbis, S. H. Han, S. H. Shin, S. Attridge, R. Rao, J. Holmgren, J. Clemens, and S. K. Bhattacharya. 2008. A randomized, placebo-controlled trial of the bivalent killed, whole-cell, oral cholera vaccine in adults and children in a cholera endemic area in Kolkata, India. PLoS One 3:e2323. doi: 10.1371/journal.pone.0002323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Majumder, P. P., and J. Roy. 1982. Distribution of ABO blood groups on the Indian subcontinent: a cluster-analytic approach. Curr. Anthrop. 23:539-566. [Google Scholar]
- 11.World Health Organization Global Task Force on Cholera Control. 2002. Cholera vaccines: a new public health tool? Report of a WHO meeting, 10 to 11 December 2002. World Health Organization, Geneva, Switzerland.
- 12.World Health Organization. 2007. Cholera 2006. Wkly. Epidemiol. Rec. 82:273-284. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.