Abstract
Despite the potential for its use as an agent of biowarfare or bioterrorism, no approved vaccine against staphylococcal enterotoxin B (SEB) exists. Nontoxic, mutant forms of SEB have been developed; however, it has been difficult to determine the efficacy of such subunit vaccine candidates due to the lack of superantigen activity of native SEB in rodents and due to the limitations of primate models. Since pigs respond to SEB in a manner similar to that of human subjects, we utilized this relevant animal model to investigate the safety and immunogenicity of a triple mutant of SEB carrying the amino acid changes L45R, Y89A, and Y94A. This recombinant mutant SEB (rmSEB) did not possess superantigen activity in pig lymphocyte cultures. Furthermore, rmSEB was unable to compete with native SEB for binding to pig leukocytes. These in vitro studies suggested that rmSEB could be a safe subunit vaccine. To test this possibility, piglets immunized orally with rmSEB formulations experienced no significant decrease in food consumption and no weight loss during the vaccination regimen. Oral vaccination with 1-mg doses of rmSEB on days 0, 7, 14, and 24 resulted in serum IgG and fecal IgA levels by day 36 that cross-reacted with native SEB. Surprisingly, the inclusion of cholera toxin adjuvant in vaccine formulations containing rmSEB did not result in increased antibody responses compared to formulations using the immunogen alone. Taken together, these studies provide additional evidence for the potential use of nontoxic forms of SEB as vaccines.
Staphylococcus aureus produces several exotoxins that are important determinants of pathogenicity (7). The staphylococcal enterotoxins are among these exotoxins and are produced by S. aureus strains growing in contaminated food, with staphylococcal enterotoxin B (SEB) being the most potent of the exotoxins. SEB mediates its toxicity by linking major histocompatibility complex (MHC) class II molecules with T-cell receptors outside the antigen binding site (24). Several families of T lymphocytes expressing certain V beta T-cell receptors can be stimulated by this toxin, which can include up to 20% of the total T-cell population. The term “superantigen” has been given to SEB and similar toxins which have this ability to bridge MHC class II molecules and T-cell receptors, stimulating a large percentage of T lymphocytes in this unconventional manner (12). One result of this toxin-induced T-lymphocyte activation is the overproduction of certain cytokines which contribute to the clinical symptoms of SEB-induced toxicity and shock (8). S. aureus can produce SEB within the environment, but its production is most problematic following infection or when present in contaminated foodstuffs. Ingestion of the toxin results in symptoms which include anorexia, nausea, vomiting, and diarrhea, which may present with hypotension, tachycardia, and hyperperistalsis (18).
Unfortunately, SEB has several characteristics which make it a candidate for possible use as an agent of biowarfare or bioterrorism. SEB has a very compact, stable protein structure, allowing it to survive the harsh environment of the gastrointestinal tract (24, 31). In addition, its stability to heat and denaturation allowed the weaponization of this toxin for aerosol dispersal in the 1960s (4). Following inhalation of aerosolized SEB, patients experience shortness of breath, chest pain, and some tachycardia (26). If exposure is significant, pulmonary edema, high fever, and a respiratory distress-like syndrome occur. With supportive medical intervention, death following inhalation is not common. However, symptoms and incapacitation can linger for up to 2 weeks following exposure. SEB has been characterized as one of the “two most important toxin threats on the battlefield or in bioterrorism” (17).
At present, there are no approved vaccines for SEB. Early attempts at toxoid-based formalin-inactivated vaccines have been abandoned, since these toxoids were not reproducibly protective (33). This has led to more recent investigations using engineered, nontoxic mutant forms of SEB. These mutant forms of SEB have been designed and tested based on the considerable data defining the structure-function relationships for this toxin (13, 22, 23). Specifically, several studies have focused on the role of particular amino acid residues in SEB that are important in toxinogenic activity (1, 3, 5, 15, 24, 32, 34). Most staphylococcal superantigens have common structures for binding to a subunit of the human MHC class II molecule (34). A hydrophobic binding loop, centered at a leucine residue (e.g., staphylococcal enterotoxin A [SEA] L48, SEB L45, and toxic shock syndrome toxin 1 [TSST-1] L30), is conserved in all superantigens except streptococcal pyrogenic exotoxin C and is essential for the recognition of the class II molecule. A second conserved structure is found in all the superantigens except TSST-1 and consists of a polar pocket that interacts with lysine 39 of the class II molecule (e.g., SEA Y92, Y108, and D70 and SEB Y89, Y115, and E67). These observations on superantigen-receptor complexes have resulted in the generation of mutant proteins that are immunogenic but not toxic. Single mutations of key residues in the polar pocket (e.g., Y89A) or in the hydrophobic binding loop (e.g., L45R) of SEB eliminated binding of the toxin to the MHC class II molecule, with minimal perturbation in SEB structure (34). This altered SEB molecule generated high levels of circulating antibody when injected into mice, and all immunized mice subsequently survived a challenge with wild-type SEB. A triple mutant carrying the L45R, Y89A, and Y94A mutations was subsequently shown to induce immunity in nonhuman primates and to protect them against an aerosol challenge with wild-type SEB (3).
In an effort to develop a needleless vaccine formulation, this triple SEB mutant (L45R, Y89A, Y94A) was used in a piglet model to test oral immunization strategies. Unlike in mouse models (3, 29, 30, 34), native SEB functions as a superantigen in pigs (2, 11, 19, 36), demonstrating the relevance of using this animal species for evaluating vaccination strategies for use in humans. We used the piglet model to address the possibility that an oral vaccine formulation consisting of the triple mutant of SEB and the cholera toxin oral adjuvant would combine to stimulate a mucosal and systemic antibody response against SEB. Surprisingly, oral vaccination was able to stimulate an antibody response against the toxin; however, we observed no adjuvant effect when cholera toxin was included in the vaccine formulations given to the piglets.
MATERIALS AND METHODS
Animals.
Seven-day-old crossbred piglets (n = 24, male and female) were obtained from a commercial swine (Spring Meadow Farms, Springfield, NC) and individually housed in 0.6-m by 1.5-m pens. Piglets were maintained under controlled lighting (a cycle of 15 h of light and 9 h of dark) and temperature. Supplemental radiant heat provided local temperatures up to 35°C. Piglets were assigned to one of three treatment groups to determine the antibody response against SEB and the effects of cholera toxin as an adjuvant. Treatment groups were arranged in a completely randomized design (8 pigs/treatment group). Body weights were recorded on days 0, 1, 2, 3, 7, 8, 9, 10, 14, 15, 16, 17, 25, and 36. Piglets were fed a milk replacer liquid diet (Milk Specialties Company, Carpentersville, IL) each morning, afternoon, and evening for the first 30 days. Feed amounts and leftover (uneaten) feed amounts were recorded at each feeding. After the final vaccination, piglets were switched to a dry pelleted feed (Ralco Nutrition, Marshall, MN) and given access to feed and water ad libitum.
Expression and purification of recombinant mutant SEB (L45R, Y89A, Y94A) in Escherichia coli.
Recombinant mutant SEB (L45R, Y89A, Y94A) (rmSEB) was expressed in E. coli and purified as a C-terminal six-histidine-tagged (6×His) fusion protein. The plasmid harboring the fusion protein was created by PCR amplification of seb with the primers SEB-PCR-F2 (5′-CC TCT AG ATG GAG TCA CAG CCA GAC CCC AAG C-3′) and SEB-PCR-R (5′-AAC TCG AGT CAG TGG TGG TGA TGG TGG TGG CCA CCC TTC TTC TTA GTA GTA AGG TAC ACC TCG-3′) and Pfu Ultra high-fidelity DNA polymerase (Stratagene) using pRG5 plasmid DNA as a template. The amplified PCR product was digested with XbaI and XhoI and subcloned into the vector backbone of pET303/CT-His (Invitrogen, Carlsbad, CA). The plasmid pET303/R-SEB harboring rmSEB-6×His was used to transform E. coli (BL21 Star strain) for the heterologous protein expression. rmSEB was purified from the cleared bacterial cell lysate using HIS-Select nickel affinity gel (Sigma-Aldrich, St. Louis, MO) according to the manufacturer's protocol. Quantification of purified rmSEB was performed using several methods, including a Bradford assay (Pierce, Rockford, IL) and by comparison of the amount of rmSEB with standard amounts of proteins using Western blot analyses and an SEB capture enzyme-linked immunosorbent assay (ELISA).
For the capture ELISA, plates were coated overnight with a monoclonal anti-SEB antibody (clone S222; Abcam, Cambridge, MA). After the plates were blocked with phosphate-buffered saline (PBS)-1% bovine serum albumin (BSA), dilutions of rmSEB or known amounts of native SEB (Sigma-Aldrich) were added to the wells. Bound material was detected using a horseradish peroxidase-conjugated polyclonal anti-SEB antibody (Abcam). After being washed, the plates were incubated with tetramethylbenzidine (TMB) substrate (BioFX, Owings Mills, MD) at room temperature. The enzymatic reactions were stopped by the addition of 1 M sulfuric acid, and absorbances were read at 405 nm. The quantity of rmSEB present in each dilution was determined by extrapolation from standard curves.
Immunization of piglets.
Groups of 7-day-old piglets (n = 8) were orally immunized (day 0) and then boosted 7, 14, and 24 days later with formulations of 1 mg of rmSEB with or without 100 μg of the cholera toxin oral adjuvant. The immunogen, with or without adjuvant, was formulated in soy milk to facilitate oral delivery to the piglets. Control piglets received only soy milk as a vehicle control. Piglets were bled on days 0, 7, 14, 24, and 36 postimmunization. On day 36, piglets were euthanized and fecal material was collected for ELISA analyses.
ELISA to detect anti-SEB antibodies.
To determine the anti-SEB titers in immunized animal serum and fecal samples, microtiter plates were coated with 200 ng/well of native SEB (Sigma-Aldrich) in 100 μl of carbonate buffer overnight at 4°C. Wells were then blocked with 1% BSA in PBS. After the wells were washed, serial dilutions of sera or fecal extracts were incubated in the wells for 2 h at room temperature. After unbound material was washed off, a horseradish peroxidase-conjugated goat anti-swine IgG (Southern Biotech, Birmingham, AL) was added for 2 h. After being washed, plates were incubated with TMB substrate (BioFX) at room temperature. The enzymatic reactions were stopped by the addition of 1 M sulfuric acid, and absorbances were read at 405 nm. Endpoint titers were defined as the last serum dilution with an absorbance double that of sera from animals which received the vehicle only.
Fecal extracts were diluted in PBS (7 μl/mg fecal pellet) supplemented with protease inhibitors (1 mM phenylmethylsulfonyl fluoride [PMSF], 1 mM iodoacetic acid [IAA], 1 mM pepstatin A, and 5 mM EDTA). Following homogenization, particulate matter was removed by passage over nylon wool columns, followed by centrifugation (13,000 × g). The soluble fraction was lyophilized and reconstituted for determination of total IgA using a capture ELISA. Microtiter plates were coated with monoclonal anti-swine IgA antibody (U.S. Biological, Swampscott, MA), and dilutions of fecal samples were added. Total bound IgA was detected using a horseradish peroxidase-conjugated polyclonal anti-swine IgA antibody (U.S. Biological). Total IgA levels present in each fecal sample were then determined by comparison to a standard curve. For the determination of anti-SEB fecal IgA levels, ELISAs were performed as described above, except that 1 μg of total IgA from each fecal sample was incubated on SEB-coated plates and a horseradish peroxidase-conjugated polyclonal anti-swine IgA antibody (U.S. Biological) was used to determine reactivity with native SEB.
Induction of gamma interferon production by native SEB and rmSEB.
To assess the ability of native SEB and rmSEB to function as a superantigen, mononuclear leukocytes were isolated from normal pig spleen tissue. Following aseptic removal of spleens, single-cell suspensions were made by pressing tissue through 30-gauge wire mesh screens, followed by passage over nylon wool to remove cellular debris. Cells were then pelleted and mononuclear leukocytes isolated by centrifugation on Histopaque 1077 density medium (Sigma-Aldrich). After being washed, cells were counted and plated at 500,000 per well (Corning, Corning, NY) and then incubated in RPMI 1640 (Mediatech, Manassas, VA) supplemented with 10% fetal calf serum (Atlanta Biologicals, Atlanta, GA).
Various concentrations of native SEB (0.01 to 10 μg/ml; Sigma-Aldrich) and rmSEB (0.1 to 100 μg/ml) were added to wells in triplicate, as indicated. Culture supernatants were harvested 40 h later, and porcine gamma interferon production was quantified using an ELISA (R&D Systems, Minneapolis, MN) and the instructions supplied by the manufacturer.
Statistical analysis.
For analysis of growth performance, data were analyzed using the MIXED procedure of the Statistical Analysis System (SAS 9.1; Cary, NC). Significance was declared when P values were <0.05. Data are presented as least-square means with pooled standard errors of the mean.
For analysis of immune responses in mice, one-way analysis of variance was performed, followed by the post hoc Tukey-Kramer test using Graph Pad Prism 4.0 software (Innotech, Schonaich, Germany). Statistical significance was declared when P values were <0.05, and data are presented as means ± standard errors.
RESULTS
Characterization and quantification of rmSEB.
To test the safety and immunogenicity of recombinant mutant SEB (L45R, Y89A, Y94A), this form of the toxin was purified and quantified prior to its use. Following affinity chromatography, a single band migrating at approximately 28 kDa on a Coomassie blue-stained SDS-PAGE gel was observed (Fig. 1 A). Western blot analyses demonstrate that this protein band is immunoreactive with anti-SEB antibodies (Fig. 1B). Bradford protein assays (data not shown), coelectrophoresis of known quantities of standard proteins on a Coomassie blue-stained SDS-PAGE gel (Fig. 1C), and a capture ELISA specific for native SEB (data not shown) were used to quantify purified rmSEB prior to its use in vitro and in vivo.
FIG. 1.
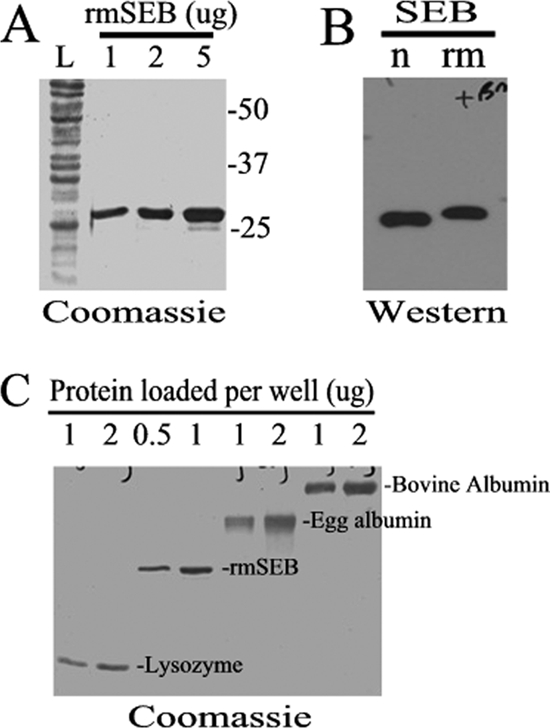
Characterization and quantification of rmSEB. Recombinant mutant SEB (rmSEB) was expressed in E. coli and purified as a C-terminal six-histidine-tagged fusion protein. (A) A Coomassie blue-stained SDS-PAGE gel of 1, 2, and 5 μg of purified rmSEB showed a single band migrating at approximately 28 kDa compared to the total E. coli protein lysate (lane L). Migration of protein standards are shown to the right of the gel. (B) Western blot analyses demonstrated that rmSEB (rm) could be recognized by the same antibodies used to detect native SEB (n). Note that rmSEB migrates at a slightly higher molecular weight due to the amino acid additions made to this recombinant protein. (C) Known quantities of standard proteins (bovine albumin, egg albumin, and lysozyme) were coelectrophoresed on a Coomassie blue-stained SDS-PAGE gel to quantify the amounts of rmSEB used in these studies.
rmSEB lacks superantigen activity for pig leukocytes.
Safety of human vaccine formulations is a paramount concern (38), and nonclinical evaluations of vaccines using relevant animal models is required prior to their use. For mutant forms of SEB, the piglet provides an excellent model to assess the safety of nontoxic mutants such as rmSEB (2, 36). Using cultured pig splenic leukocytes, we questioned whether rmSEB showed any detectable superantigen activity. Figure 2 shows that pig leukocytes are quite responsive to native SEB, with as little as 1 μg/ml of the toxin stimulating a robust gamma interferon response. In fact, concentrations of rmSEB as high as 100 μg/ml showed no detectable gamma interferon secretion (Fig. 2) despite the fact that such a high concentration of native SEB was nearly 100% toxic to pig leukocytes (data not shown). These in vitro studies suggested that rmSEB would be nontoxic to piglets when used in subunit vaccine formulations.
FIG. 2.
rmSEB lacks superantigen activity for pig leukocytes. Pig splenic leukocytes were isolated and cultured in the presence of the indicated concentrations of native SEB (nSEB) or rmSEB. After 40 h of culture, supernatants were taken, and porcine gamma interferon (IFN gamma) secretion was determined using an ELISA. It should be noted that no results are shown for 100 μg/ml of native SEB, as such a high concentration of toxin was nearly 100% toxic to pig leukocytes under these culture conditions. Results are presented as mean values (±standard errors of the mean [SEM]) for triplicate determinations. Levels of gamma interferon that were below the 50-ng/ml detection limit for this ELISA were designated nondetectable (ND). This study was performed twice, with similar results.
rmSEB is not a competitive inhibitor of native SEB binding to MHC class II molecules.
Native SEB can induce the secretion of cytokines, like gamma interferon, by virtue of its ability to bridge MHC class II molecules and T-cell receptors, stimulating a large percentage of T lymphocytes (12). The mutations L45R, Y89A, and Y94A targeted residues necessary for binding to MHC class II molecules (3), in theory making rmSEB incapable of binding to these molecules. To test this possibility, excess amounts of rmSEB were added to pig splenic leukocyte cultures containing 1 μg/ml of native SEB, and toxin-induced gamma interferon production was assessed. No significant reduction in cytokine secretion was observed, even when a 100-fold excess of rmSEB (i.e., 100 μg/ml) was present in the native SEB-stimulated cultures (Fig. 3). These results strongly suggest that rmSEB could not function as a competitive inhibitor of native SEB binding to MHC class II molecules.
FIG. 3.
rmSEB is not a competitive inhibitor of native SEB for pig leukocytes. Pig splenic leukocytes were isolated and cocultured in the presence of 1.0 μg/ml of native SEB (nSEB) with the indicated concentrations of rmSEB. After 40 h of culture, supernatants were taken, and porcine gamma interferon (IFN gamma) secretion was determined using an ELISA. Results are presented as mean values (±SEM) for triplicate determinations.
Administration of oral vaccine formulations had no significant effect on piglet weight or food consumption.
The in vitro results from Fig. 1 and 2 suggested that rmSEB would be nontoxic to pigs even when given orally in formulations containing relatively large amounts of this subunit vaccine (e.g., 1 mg). To demonstrate the safety of such vaccine formulations, groups of piglets received an immunization regimen and food consumption and body weight were monitored during the course of treatment. As shown in Table 1, there was no significant difference in feed intake or weight gain between the three groups of piglets throughout the immunization regimen. Since weight loss over time can be a sensitive measure of adverse treatments, these results indicate the in vivo safety of using rmSEB, with or without cholera toxin adjuvant, as an oral vaccine.
TABLE 1.
Administration of oral vaccine formulations had no significant effect on feed intake or piglet weight gaina
| Piglet group | Feed intake (g/day) for days: |
Wt gain (g/day)b for days: |
Gain feed ratio for days: |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0-7 | 7-14 | 14-24 | 24-36 | 0-36 | 0-7 | 7-14 | 14-24 | 24-36 | 0-36 | 0-7 | 7-14 | 14-24 | 24-36 | 0-36 | |
| Vehicle treated | 208 | 276 | 349 | 304 | 320 | 228 | 364 | 453 | 457 | 372 | 1.21 | 1.21 | 1.19 | 1.17 | 0.95 |
| rmSEB immunized | 202 | 274 | 349 | 322 | 321 | 221 | 371 | 451 | 503 | 377 | 1.1 | 1.23 | 1.18 | 1.26 | 0.96 |
| rmSEB + CT immunized | 187 | 268 | 348 | 301 | 315 | 215 | 344 | 459 | 472 | 365 | 1.17 | 1.14 | 1.2 | 1.25 | 0.93 |
| All (pooled SEM) | 13.72 | 8.37 | 1.14 | 22.94 | 4.07 | 34.64 | 15.58 | 13.18 | 55.48 | 18.94 | 0.13 | 0.05 | 0.03 | 0.14 | 0.05 |
Values are expressed as least-square means of results for 8 animals per group. CT, cholera toxin.
Initial body weights were used as covariates. Initial mean body weights for each of the groups of immunized animals were as follows: vehicle, 2,773 g; rmSEB, 2,731 g; rmSEB + CT, 2,552 g.
Oral administration of rmSEB results in an antibody response in a piglet model.
While many subunit vaccine candidates cannot survive the harsh environment of the gastrointestinal tract long enough to stimulate immune responses, SEB has a very compact, stable protein structure (24, 31). We questioned whether an oral formulation containing rmSEB might be capable of transversing the gut to stimulate an antibody response in piglets. For these studies, groups of piglets were orally administered a soy formulation or a soy formulation containing rmSEB on day 0, followed by boosts on days 7, 14, and 24. Blood was taken from each animal at the indicated times, and the presence of antibodies against native SEB was detected by ELISA. Figure 4 A shows that on day 36 postimmunization, significant levels of IgG antibody against native SEB were present in sera of piglets exposed to this antigen compared to that in sera of piglets that received only the soy formulation.
FIG. 4.
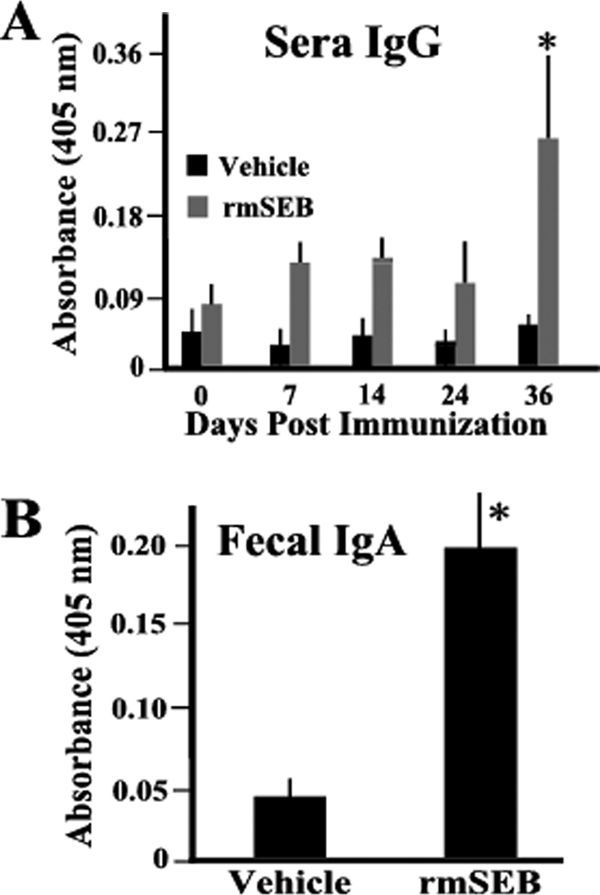
Oral administration of rmSEB results in significant antibody responses. Groups of 7-day-old piglets (n = 8) were orally immunized (day 0) and then boosted 7, 14, and 24 days later with formulations of 1 mg of rmSEB in soy milk (rmSEB) or with soy milk alone (vehicle). Each animal was bled at days 0, 7, 14, 24, and 36 postimmunization to obtain sera. Fecal samples were also collected at the time of euthanasia (day 36) for ELISA to determine serum IgG (A) or fecal IgA (B) anti-SEB reactivity. Results are presented as mean absorbance values (±SEM) using a serum dilution of 1:200 or a total fecal IgA level of 1 μg/ml. Asterisks indicate a statistically significant difference (P < 0.05) compared to all other determinations.
In addition, we questioned whether a mucosal IgA response could be detected in these piglets by day 36 postimmunization. Fecal samples were collected from piglets, extracts made, and the levels of mucosal IgA specific for native SEB determined. Consistent with the results obtained for serum antibody, significant IgA titers were observed in fecal extracts obtained at day 36 postimmunization from piglets who received the soy formulation containing rmSEB compared to those from piglets who received only the soy formulation (Fig. 4B). It should be noted that no significant fecal IgA responses were detected prior to day 36 (data not shown).
Oral administration of rmSEB plus cholera toxin adjuvant does not augment the antibody response.
The results shown in Fig. 4 were quite surprising, since most oral subunit vaccines require formulation with an adjuvant to induce any detectable antibody responses (14). While the anti-SEB antibody responses in piglets exposed to rmSEB alone were significant (Fig. 4), we sought to boost the responses even further by including an adjuvant in the oral vaccine formulations. For these studies, groups of piglets were immunized with rmSEB alone or with rmSEB plus the cholera toxin oral adjuvant (14). On day 36 following the immunization regimen, serum was obtained from each piglet, and antibody titers against the adjuvant and the toxin were determined using ELISAs. As expected (10), piglets that received adjuvant formulated with immunogen produced high titers of serum IgG antibodies against cholera toxin (Fig. 5 A), demonstrating that oral administration efficiently delivered this adjuvant to the gut. Surprisingly, there was no significant difference in the anti-SEB antibody titers between piglets that received rmSEB alone and animals that received rmSEB formulated with adjuvant (Fig. 5B). Taken together, these studies clearly demonstrate that cholera toxin does not function as an adjuvant when given with rmSEB with this immunization regimen and with piglets of this age.
FIG. 5.
Oral administration of rmSEB plus cholera toxin adjuvant does not augment the antibody response. Groups of 7-day-old piglets (n = 8) were orally immunized (day 0) and then boosted 7, 14, and 24 days later with formulations of 1 mg of rmSEB in soy milk (rmSEB) with (+) or without (−) 100 μg of cholera toxin (CT). On day 36 postimmunization, piglets were euthanized and sera collected for ELISA to determine serum IgG anti-CT (A) or serum IgG anti-SEB (B) titers. Endpoint titers were defined as the last serum dilution with an absorbance double that of sera from animals which received the vehicle only. Results are presented as mean titers (±SEM). Asterisks indicate a statistically significant difference (P < 0.05) compared to the result for animals receiving the vehicle only.
DISCUSSION
SEB is currently classified as a category B agent of biowarfare or bioterrorism. In fact, it is one of the few agents which has actually been weaponized (4) and whose effects on human subjects have been documented (35). While debilitating, exposure to the toxin is usually not fatal. However, the nature of the symptoms and the ability to incapacitate victims for days to weeks following exposure likely heighten the attractiveness of this toxin for weaponization.
No approved vaccine currently exists for SEB, and the lack of such a vaccine likely stems from at least two facts. First, widespread immunization of the population against this toxin has not been justified, especially in westernized societies where the incidence of SEB-induced hospitalizations and deaths is circumscribed (20). Second, the threat of large-scale bioterrorism has only recently received renewed attention (28), and achieving prevention and protection against such attacks will require time and effort. Therefore, the availability of an efficacious vaccine for use by the military or other at-risk populations could represent a significant deterrent for those considering the use of SEB to induce injury.
Fortunately, much is known regarding the structure-function relationships mediating the pathology of this protein (15, 22, 24, 31). The ability of SEB to bridge MHC class II molecules with the beta chain of T-cell receptors results in immune activation, cytokine secretion, and toxin-induced illness. By altering key amino acid residues which allow SEB to bind these molecules, no bridging can occur, and the toxic activity can be eliminated. Previous work demonstrated that the SEB mutant carrying the L45R, Y89A, and Y94A mutations could not bind or stimulate human leukocytes to produce cytokines (3). Like human cells, pig leukocytes readily respond to native SEB (Fig. 2), and our results extend the previous work demonstrating a lack of binding (Fig. 3) and a lack of toxicity (Fig. 2) for this SEB mutant using this animal species. Furthermore, during the course of immunization, no significant alteration in food intake or weight gain was noted in groups of control versus immunized piglets (Table 1), demonstrating the safety of using oral rmSEB in vivo. In fact, a strong case has been made for the use of the piglet model in assessing and understanding the pathology and toxicity following native SEB challenge (2, 36). The piglet model is superior to mouse models, which require lipopolysaccharide to potentiate SEB toxicity (3), and to the DR3 transgenic mouse model (6) in mirroring the biphasic clinical response and overall pathology observed in humans (2, 36). The advantages of using the piglet model rather than the rhesus macaque model (3) are that the high expense, complexity of experimental manipulations, and some biosafety concerns about using these primates are overcome (36). Therefore, the piglet model seemed ideal for use in studies to determine whether oral administration of a formulation containing rmSEB could stimulate an immune response following an immunization regimen. Future studies are aimed at using this model to demonstrate the efficacy of vaccination formulations and regimens by challenging immunized pigs with native toxin following therapy.
Previous studies have demonstrated that the SEB mutant carrying the L45R, Y89A, and Y94A mutations was antigenically related to native SEB and that immunization of mice and rhesus macaques resulted in the production of antibodies (3, 34). In the present study, we extend the observation that rmSEB can function as an immunogen and produce antibodies in swine that cross-react with native SEB (Fig. 4 and 5). In addition, we made the surprising observation that an oral formulation containing rmSEB could induce a systemic IgG (Fig. 4A and 5) and a mucosal IgA (Fig. 4B) antibody response following an immunization regimen.
Oral immunization strategies have some significant advantages compared to conventional vaccinations; however, there are some hurdles which remain when trying to develop efficacious mucosal vaccines (21, 27). Most pathogens and toxins enter the host via mucosal surfaces, and it is logical to suggest that existing immunity at such surfaces could prevent or limit entry. Unfortunately, parenteral immunization regimens do not routinely stimulate high levels of mucosal IgA antibodies or cellular immunity at mucosal surfaces. Efficacious oral vaccinations not only stimulate mucosal immunity but also often result in the induction of peripheral IgG and cellular responses. In this manner, mucosal immunizations can have the advantage of providing local, as well as systemic, protection against a particular pathogen or toxin. Injection-based vaccines must contend with the problems and limitations associated with the use of needles for delivery (27). The concept of needleless, oral administration is an attractive one that would reduce the need for medically trained personnel, eliminate disease transmission by contaminated needles, and provide the safest method of delivery. Despite these advantages, the development of efficacious oral immunization strategies has been slowed by the recognition that subunit protein antigens can be degraded in the gut, that these proteins are poorly immunogenic, and that new mucosal adjuvants must be developed to augment the response.
One class of oral adjuvants which may have the most promise for use in efficacious oral vaccine formulations is the alpha-beta bacterial toxins, including cholera toxin (9, 39). While there remains some controversy about how these bacterial proteins induce their effects, a variety of laboratories have demonstrated adjuvanticity of these toxins and their mutants in rodent models of immunization. Surprisingly, the ability of cholera toxin to function as an oral adjuvant in swine appears to depend upon the antigen which is being coadministered. Cholera toxin demonstrated adjuvant activity when coadministered with the beta chain of cholera toxin but not when it was coadministered with keyhole limpet hemocyanin (10). In another study using swine, coadministration of cholera toxin was shown to improve the response against a fimbrial protein of E. coli following oral immunization (37). In the present study, however, no adjuvant effect was observed when cholera toxin was added to oral vaccine formulations containing rmSEB (Fig. 5B). Despite the presence of substantial anti-cholera toxin antibody levels in piglets coadministered this bacterial protein (Fig. 5A), this exposure was not sufficient to augment the rmSEB response (Fig. 5B). Therefore, the inability of orally administered cholera toxin to augment immune responses when coadministered with rmSEB (Fig. 5B) or keyhole limpet hemocyanin (10) suggests that the activity of this potential oral adjuvant in swine must be empirically determined for each immunogen.
The inconsistency of adjuvant activity for coadministered cholera toxin illustrates some hurdles which still need to be overcome when constructing efficacious oral vaccine formulations (21, 27). In addition to discovering new oral adjuvants for human use, it will also be important to consider additives which might facilitate the delivery of immunogens safely through the gastrointestinal tract to mucosal antigen-presenting cells. Many subunit protein vaccines can be destroyed or altered following oral administration, often making them very poor immunogens by themselves. Our results demonstrated that once administered orally, rmSEB remained sufficiently intact to stimulate a systemic (Fig. 4A) and mucosal (Fig. 4B) antibody response. To facilitate oral delivery of rmSEB to the piglets, this immunogen was formulated with soy milk. Whether such a formulation contributed to the stability of rmSEB as it traversed the gastrointestinal tract will need further study. Soy milk formulations have been shown to have inherent buffering capacity (16, 25), which might aid in protein stability in the acidic environment of the gut. Furthermore, excess soy proteins in a milk formulation may facilitate the passage of proteins through the gut by competitively limiting protease activity. Whether the inherent acid-neutralizing ability of soy milk, the presence of excess soy protein inhibiting proteases, or some other property of such soy formulations contributes to safe passage through the gut is not presently clear. However, these studies clearly demonstrate that soy formulations containing rmSEB can induce an immune response against this immunogen when given orally.
Acknowledgments
This work was supported by Public Health Service grant R42 AI72777 from the National Institute of Allergy and Infectious Diseases.
Footnotes
Published ahead of print on 16 June 2010.
REFERENCES
- 1.Baker, M. D., A. C. Papageorgiou, R. W. Titball, J. Miller, S. White, B. Lingard, J. J. Lee, D. Cavanagh, M. A. Kehoe, J. H. Robinson, and K. R. Acharya. 2002. Structural and functional role of threonine 112 in a superantigen Staphylococcus aureus enterotoxin B. J. Biol. Chem. 277:2756-2762. [DOI] [PubMed] [Google Scholar]
- 2.Bi, S., R. Das, E. Zelazowska, S. Mani, R. Neill, G. D. Coleman, D. C. Yang, R. Hammamieh, J. W. Shupp, and M. Jett. 2009. The cellular and molecular immune response of the weanling piglet to staphylococcal enterotoxin B. Exp. Biol. Med. (Maywood) 234:1305-1315. [DOI] [PubMed] [Google Scholar]
- 3.Boles, J. W., M. L. Pitt, R. D. LeClaire, P. H. Gibbs, E. Torres, B. Dyas, R. G. Ulrich, and S. Bavari. 2003. Generation of protective immunity by inactivated recombinant staphylococcal enterotoxin B vaccine in nonhuman primates and identification of correlates of immunity. Clin. Immunol. 108:51-59. [DOI] [PubMed] [Google Scholar]
- 4.Christopher, G. W., T. J. Cieslak, J. A. Pavlin, and E. M. Eitzen, Jr. 1997. Biological warfare. A historical perspective. JAMA 278:412-417. [PubMed] [Google Scholar]
- 5.Coffman, J. D., J. Zhu, J. M. Roach, S. Bavari, R. G. Ulrich, and S. L. Giardina. 2002. Production and purification of a recombinant staphylococcal enterotoxin B vaccine candidate expressed in Escherichia coli. Protein Expr. Purif. 24:302-312. [DOI] [PubMed] [Google Scholar]
- 6.DaSilva, L., B. C. Welcher, R. G. Ulrich, M. J. Aman, C. S. David, and S. Bavari. 2002. Humanlike immune response of human leukocyte antigen-DR3 transgenic mice to staphylococcal enterotoxins: a novel model for superantigen vaccines. J. Infect. Dis. 185:1754-1760. [DOI] [PubMed] [Google Scholar]
- 7.Dinges, M. M., P. M. Orwin, and P. M. Schlievert. 2000. Exotoxins of Staphylococcus aureus. Clin. Microbiol. Rev. 13:16-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drake, C. G., and B. L. Kotzin. 1992. Superantigens: biology, immunology, and potential role in disease. J. Clin. Immunol. 12:149-162. [DOI] [PubMed] [Google Scholar]
- 9.Elson, C. O. 1989. Cholera toxin and its subunits as potential oral adjuvants. Curr. Top. Microbiol. Immunol. 146:29-33. [DOI] [PubMed] [Google Scholar]
- 10.Foss, D. L., and M. P. Murtaugh. 1999. Mucosal immunogenicity and adjuvanticity of cholera toxin in swine. Vaccine 17:788-801. [DOI] [PubMed] [Google Scholar]
- 11.Hammamieh, R., S. Bi, R. Das, R. Neill, and M. Jett. 2004. Modeling of SEB-induced host gene expression to correlate in vitro to in vivo responses. Biosens. Bioelectron. 20:719-727. [DOI] [PubMed] [Google Scholar]
- 12.Herman, A., J. W. Kappler, P. Marrack, and A. M. Pullen. 1991. Superantigens: mechanism of T-cell stimulation and role in immune responses. Annu. Rev. Immunol. 9:745-772. [DOI] [PubMed] [Google Scholar]
- 13.Kotb, M. 1998. Superantigens of gram-positive bacteria: structure-function analyses and their implications for biological activity. Curr. Opin. Microbiol. 1:56-65. [DOI] [PubMed] [Google Scholar]
- 14.Lavelle, E. C., and D. T. O'Hagan. 2006. Delivery systems and adjuvants for oral vaccines. Expert Opin. Drug Deliv. 3:747-762. [DOI] [PubMed] [Google Scholar]
- 15.Li, H., A. Llera, D. Tsuchiya, L. Leder, X. Ysern, P. M. Schlievert, K. Karjalainen, and R. A. Mariuzza. 1998. Three-dimensional structure of the complex between a T cell receptor beta chain and the superantigen staphylococcal enterotoxin B. Immunity 9:807-816. [DOI] [PubMed] [Google Scholar]
- 16.Lutchman, D., S. Pillay, R. Naidoo, N. Shangase, R. Nayak, and A. Rughoobeer. 2006. Evaluation of the buffering capacity of powdered cow's, goat's and soy milk and non-prescription antacids in the treatment of non-ulcer dyspepsia. S. Afr. Med. J. 96:57-61. [PubMed] [Google Scholar]
- 17.Madsen, J. M. 2001. Toxins as weapons of mass destruction. A comparison and contrast with biological-warfare and chemical-warfare agents. Clin. Lab Med. 21:593-605. [PubMed] [Google Scholar]
- 18.Marrack, P., and J. Kappler. 1990. The staphylococcal enterotoxins and their relatives. Science 248:705-711. [DOI] [PubMed] [Google Scholar]
- 19.Mateu de Antonio, E., R. J. Husmann, R. Hansen, J. K. Lunney, D. Strom, S. Martin, and F. A. Zuckermann. 1998. Quantitative detection of porcine interferon-gamma in response to mitogen, superantigen and recall viral antigen. Vet. Immunol. Immunopathol. 61:265-277. [DOI] [PubMed] [Google Scholar]
- 20.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neutra, M. R., and P. A. Kozlowski. 2006. Mucosal vaccines: the promise and the challenge. Nat. Rev. Immunol. 6:148-158. [DOI] [PubMed] [Google Scholar]
- 22.Papageorgiou, A. C., and K. R. Acharya. 2000. Microbial superantigens: from structure to function. Trends Microbiol. 8:369-375. [DOI] [PubMed] [Google Scholar]
- 23.Papageorgiou, A. C., and K. R. Acharya. 1997. Superantigens as immunomodulators: recent structural insights. Structure 5:991-996. [DOI] [PubMed] [Google Scholar]
- 24.Papageorgiou, A. C., H. S. Tranter, and K. R. Acharya. 1998. Crystal structure of microbial superantigen staphylococcal enterotoxin B at 1.5 Å resolution: implications for superantigen recognition by MHC class II molecules and T-cell receptors. J. Mol. Biol. 277:61-79. [DOI] [PubMed] [Google Scholar]
- 25.Park, Y. W. 1991. Relative buffering capacity of goat milk, cow milk, soy-based infant formulas and commercial nonprescription antacid drugs. J. Dairy Sci. 74:3326-3333. [DOI] [PubMed] [Google Scholar]
- 26.Rusnak, J. M., M. Kortepeter, R. Ulrich, M. Poli, and E. Boudreau. 2004. Laboratory exposures to staphylococcal enterotoxin B. Emerg. Infect. Dis. 10:1544-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ryan, E. J., L. M. Daly, and K. H. Mills. 2001. Immunomodulators and delivery systems for vaccination by mucosal routes. Trends Biotechnol. 19:293-304. [DOI] [PubMed] [Google Scholar]
- 28.Salerno, R. M., and L. T. Hickok. 2007. Strengthening bioterrorism prevention: global biological materials management. Biosecur. Bioterror. 5:107-116. [DOI] [PubMed] [Google Scholar]
- 29.Stiles, B. G., S. Bavari, T. Krakauer, and R. G. Ulrich. 1993. Toxicity of staphylococcal enterotoxins potentiated by lipopolysaccharide: major histocompatibility complex class II molecule dependency and cytokine release. Infect. Immun. 61:5333-5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stiles, B. G., A. R. Garza, R. G. Ulrich, and J. W. Boles. 2001. Mucosal vaccination with recombinantly attenuated staphylococcal enterotoxin B and protection in a murine model. Infect. Immun. 69:2031-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swaminathan, S., W. Furey, J. Pletcher, and M. Sax. 1992. Crystal structure of staphylococcal enterotoxin B, a superantigen. Nature 359:801-806. [DOI] [PubMed] [Google Scholar]
- 32.Swaminathan, S., W. Furey, J. Pletcher, and M. Sax. 1995. Residues defining V beta specificity in staphylococcal enterotoxins. Nat. Struct. Biol. 2:680-686. [DOI] [PubMed] [Google Scholar]
- 33.Tseng, J., J. L. Komisar, R. N. Trout, R. E. Hunt, J. Y.-J. Chen, A. J. Johnson, L. Pitt, and D. L. Ruble. 1995. Humoral immunity to aerosolized staphylococcal enterotoxin B (SEB), a superantigen, in monkeys vaccinated with SEB toxoid-containing microspheres. Infect. Immun. 63:2880-2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ulrich, R. G., M. A. Olson, and S. Bavari. 1998. Development of engineered vaccines effective against structurally related bacterial superantigens. Vaccine 16:1857-1864. [DOI] [PubMed] [Google Scholar]
- 35.Ulrich, R. G., S. Sidell, and T. J. Taylor. 1997. Staphylococcal enterotoxin B and related pyogenic toxins, p. 621-631. In F. R. Sidell et al. (ed.), Textbook of military medicine. Part I. Warfare, weaponry and the casualty, vol. 3. U.S. Government Printing Office, Washington, DC.
- 36.van Gessel, Y. A., S. Mani, S. Bi, R. Hammamieh, J. W. Shupp, R. Das, G. D. Coleman, and M. Jett. 2004. Functional piglet model for the clinical syndrome and postmortem findings induced by staphylococcal enterotoxin B. Exp. Biol. Med. (Maywood) 229:1061-1071. [DOI] [PubMed] [Google Scholar]
- 37.Verdonck, F., V. Snoeck, B. M. Goddeeris, and E. Cox. 2005. Cholera toxin improves the F4(K88)-specific immune response following oral immunization of pigs with recombinant FaeG. Vet. Immunol. Immunopathol. 103:21-29. [DOI] [PubMed] [Google Scholar]
- 38.World Health Organization. 2005. WHO guidelines on nonclinical evaluation of vaccines, p. 31-62. WHO Technical Report series no. 927. World Health Organization, Geneva, Switzerland.
- 39.Yamamoto, M., J. R. McGhee, Y. Hagiwara, S. Otake, and H. Kiyono. 2001. Genetically manipulated bacterial toxin as a new generation mucosal adjuvant. Scand. J. Immunol. 53:211-217. [DOI] [PubMed] [Google Scholar]


