Abstract
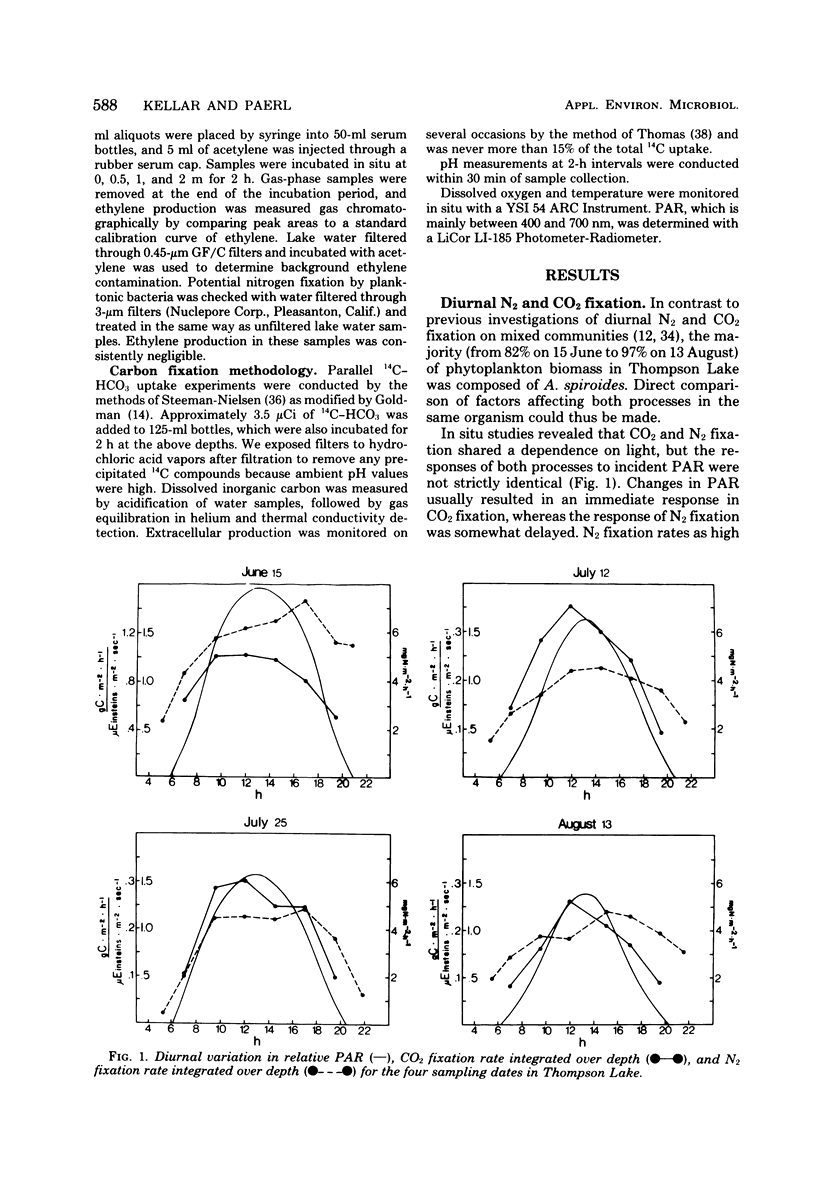
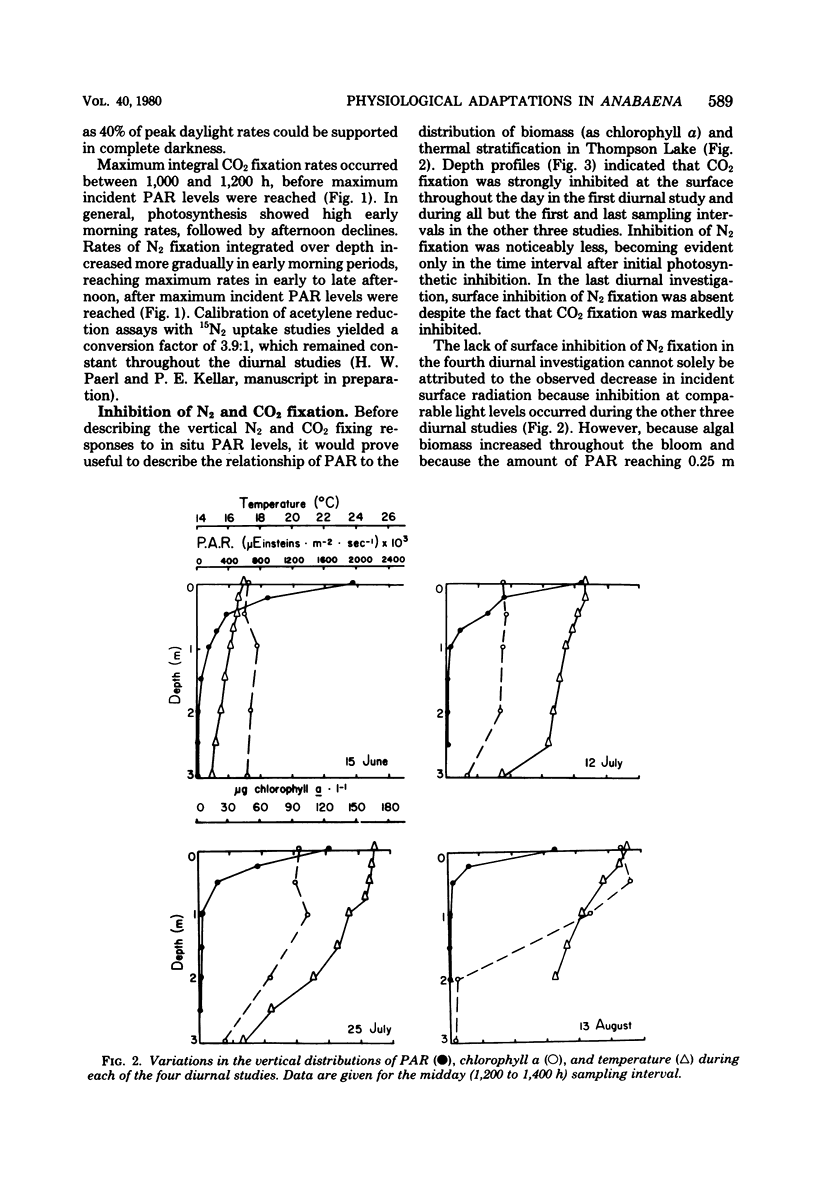
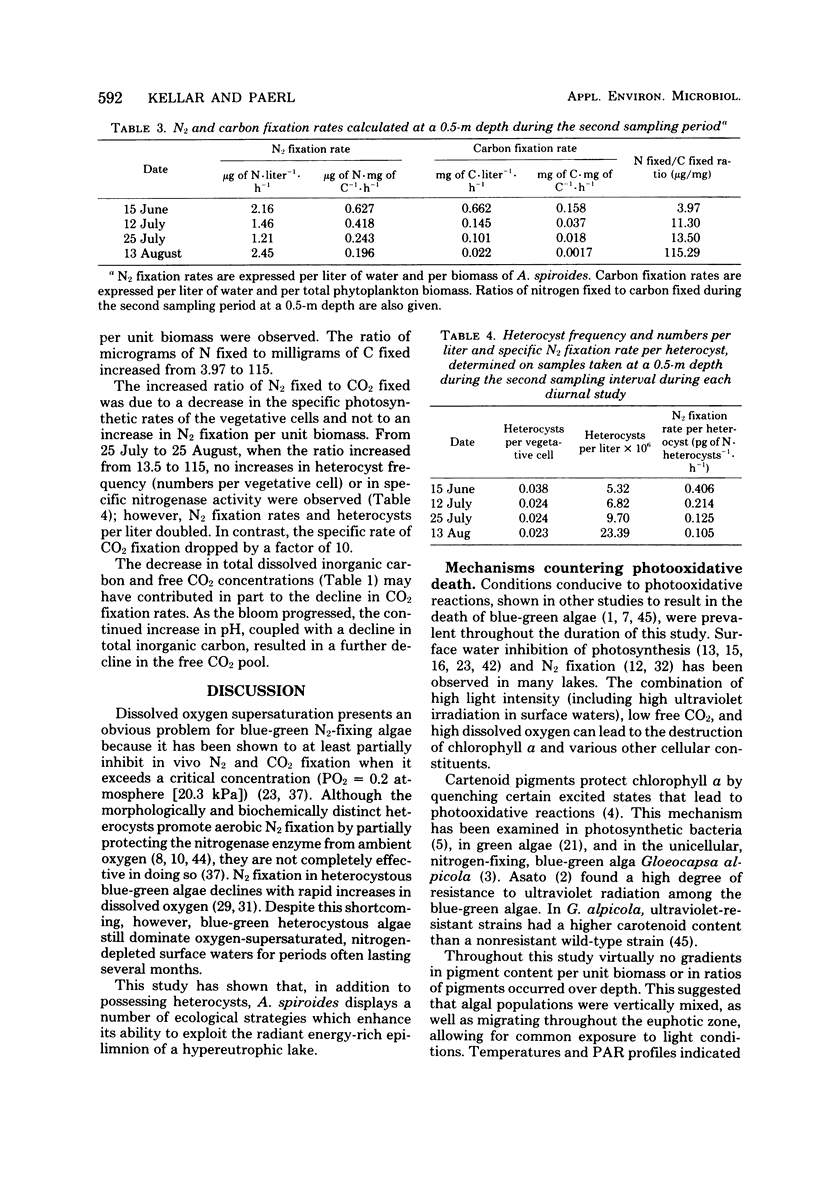
Anabaena spiroides has the ability to maintain intense biomass production for extensive periods in the epilimnion of a small eutrophic lake characterized by conditions shown to cause photooxidative death in a number of other phytoplankton. By the enhancement of carotenoid synthesis chlorophyll a was protected from photooxidation and prevented from catalyzing other photooxidative reactions within the cells. By temporally separating CO2 and N2 fixation, maximum utilization of photosynthetically active radiation was achieved. Because CO2 fixation was more sensitive than N2 fixation to a high oxygen concentration, the former was maximized during morning hours, before the afternoon buildup of dissolved oxygen. The diurnal partitioning of carbon and N2 fixation has two additional advantages; possible competition for reductant-generating compounds is minimized, and adequate endogenous pools of carbon skeletons are assured to accept newly fixed ammonia. Hence, Anabaena, far from undergoing photooxidative death, appears to utilize a physiological strategy which allows optimization of radiant energy use for reductive processes and dominance of surface waters and shading of deeper phytoplankton during summer blooms.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeliovich A., Shilo M. Photooxidative death in blue-green algae. J Bacteriol. 1972 Sep;111(3):682–689. doi: 10.1128/jb.111.3.682-689.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asato Y. Isolation and characterization of ultraviolet light-sensitive mutants of the blue-green alga Anacystis nidulans. J Bacteriol. 1972 Jun;110(3):1058–1064. doi: 10.1128/jb.110.3.1058-1064.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley C. E., Houghton J. A. A study of the effects of near UV radiation on the pigmentation of the blue-green alga Gloeocapsa alpicola. Arch Microbiol. 1976 Feb;107(1):93–97. doi: 10.1007/BF00427873. [DOI] [PubMed] [Google Scholar]
- COHEN-BAZIRE G., STANIER R. Y. Specific inhibition of carotenoid synthesis in a photosynthetic bacterium and its physiological consequences. Nature. 1958 Jan 24;181(4604):250–252. doi: 10.1038/181250a0. [DOI] [PubMed] [Google Scholar]
- Eloff J. N., Steinitz Y., Shilo M. Photooxidation of cyanobacteria in natural conditions. Appl Environ Microbiol. 1976 Jan;31(1):119–126. doi: 10.1128/aem.31.1.119-126.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay P., Stewart W. D., Walsby A. E., Fogg G. E. Is the heterocyst the site of nitrogen fixation in blue-green algae? Nature. 1968 Nov 23;220(5169):810–812. doi: 10.1038/220810b0. [DOI] [PubMed] [Google Scholar]
- Flett R. J., Hamilton R. D., Campbell N. E. Aquatic acetylene-reduction techniques: solutions to several problems. Can J Microbiol. 1976 Jan;22(1):43–51. doi: 10.1139/m76-006. [DOI] [PubMed] [Google Scholar]
- Horne A. J., Viner A. B. Nitrogen fixation and its significance in tropical Lake George, Uganda. Nature. 1971 Aug 6;232(5310):417–418. doi: 10.1038/232417a0. [DOI] [PubMed] [Google Scholar]
- Lex M., Silvester W. B., Stewart W. D. Photorespiration and nitrogenase activity in the blue-green alga, Anabaena cylindrica. Proc R Soc Lond B Biol Sci. 1972 Jan 18;180(1058):87–102. doi: 10.1098/rspb.1972.0007. [DOI] [PubMed] [Google Scholar]
- Lex M., Stewart W. D. Algal nitrogenase, reductant pools and photosystem I activity. Biochim Biophys Acta. 1973 Feb 22;292(2):436–443. doi: 10.1016/0005-2728(73)90049-2. [DOI] [PubMed] [Google Scholar]
- Meeks J. C., Wolk C. P., Lockau W., Schilling N., Shaffer P. W., Chien W. S. Pathways of assimilation of [13N]N2 and 13NH4+ by cyanobacteria with and without heterocysts. J Bacteriol. 1978 Apr;134(1):125–130. doi: 10.1128/jb.134.1.125-130.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paerl H. W., Kellar P. E. Nitrogen-fixing anabaena: physiological adaptations instrumental in maintaining surface blooms. Science. 1979 May 11;204(4393):620–622. doi: 10.1126/science.204.4393.620. [DOI] [PubMed] [Google Scholar]
- Peterson R. B., Burris R. H. Conversion of acetylene reduction rates to nitrogen fixation rates in natural populations of blue-green algae. Anal Biochem. 1976 Jun;73(2):404–410. doi: 10.1016/0003-2697(76)90187-1. [DOI] [PubMed] [Google Scholar]
- Peterson R. B., Friberg E. E., Burris R. H. Diurnal variation in n(2) fixation and photosynthesis by aquatic blue-green algae. Plant Physiol. 1977 Jan;59(1):74–80. doi: 10.1104/pp.59.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weare N. M., Benemann J. R. Nitrogen fixation by Anabaena cylindrica. II. Nitrogenase activity during induction and aging of batch cultures. Arch Mikrobiol. 1973 Oct 19;93(2):101–112. [PubMed] [Google Scholar]
- Winkenbach F., Wolk C. P. Activities of enzymes of the oxidative and the reductive pentose phosphate pathways in heterocysts of a blue-green alga. Plant Physiol. 1973 Nov;52(5):480–483. doi: 10.1104/pp.52.5.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt J. T., Silvey J. K. Nitrogen fixation by gloeocapsa. Science. 1969 Aug 29;165(3896):908–909. doi: 10.1126/science.165.3896.908. [DOI] [PubMed] [Google Scholar]


