Abstract
Pseudomonas aeruginosa lung infection is a major cause of morbidity and mortality worldwide. P. aeruginosa flagellin, the main structural protein of the flagellar filament, is a virulence factor with proinflammatory activity on respiratory epithelial cells. P. aeruginosa bacteria express one of two isoforms of flagellin (type a or b) that differ in their primary amino acid sequences as well as in posttranslational glycosylation. In this study, the distribution of type a and b flagellins among 3 P. aeruginosa laboratory strains and 14 clinical isolates (1 ulcerative keratitis, 3 cystic fibrosis, and 10 acute pneumonia isolates) was determined, and their abilities to stimulate interleukin-8 (IL-8) production by human airway epithelial cells was compared. By comparison with the PAK (type a) and PAO1 (type b) prototype laboratory strains, 10/14 (71.4%) of clinical isolates expressed type a and 4/14 (28.6%) expressed type b flagellins. Among four cell lines surveyed, BEAS-2B cells were found to give the greatest difference between constitutive and flagellin-stimulated IL-8 production. All 17 flagellins stimulated IL-8 production by BEAS-2B cells (range, 700 to 4,000 pg/ml). However, no discernible differences in IL-8 production were evident when comparing type a versus type b flagellins or flagellins from laboratory versus clinical strains or among the clinical strains.
Pseudomonas aeruginosa is a Gram-negative, aerobic, rod-shaped bacterium with a unipolar flagellum. P. aeruginosa is a clinically important opportunistic human pathogen, and its respiratory tract infections are a leading cause of morbidity and mortality in patients with cystic fibrosis, ventilator-associated pneumonia, and compromised immune systems (6). Hospital-acquired pneumonia constitutes the second leading type of nosocomial infection, and P. aeruginosa is the most commonly isolated bacterium from these cases (36). P. aeruginosa lung colonization in cystic fibrosis patients induces a neutrophil-dominated airway inflammatory response that, if untreated, ultimately leads to lung failure and death (41). P. aeruginosa also causes severe eye and urinary tract infections in immunocompromised patients, particularly those with HIV, and in individuals with severe burn wounds (42). Despite antibiotic treatment, mortality rates as high as 40% may occur in acute infections, and multidrug-resistant isolates are increasingly reported (11).
Respiratory epithelial cells play a crucial role in the inflammatory response during P. aeruginosa infection (33). Airway epithelial cells produce cytokines and chemokines that initiate and amplify host innate and adaptive immune responses following bacterial colonization. For example, epithelial cells exposed to P. aeruginosa produce interleukin-8 (IL-8), the major chemokine associated with neutrophil extravasation from the vasculature into the lumen of the airways (17). IL-8 and neutrophils are present in increased amounts in the lungs of patients with P. aeruginosa infections (8). A diverse array of P. aeruginosa gene products stimulate IL-8 production by respiratory epithelial cells, including flagellin and pilin, the primary structural proteins of bacterial flagella and pili respectively (9).
In addition to its ability to stimulate a proinflammatory host response, P. aeruginosa flagellin also constitutes a bacterial virulence factor. Multiple studies have demonstrated a role for P. aeruginosa flagella in the pathogenesis of experimental and clinical diseases (16, 22, 25). Using a burned-mouse model, nonflagellated P. aeruginosa strains expressing a mutant flagellin gene showed a significant decrease in virulence that was restored when flagellin expression was reinstated (29). Pulmonary infection of mice with P. aeruginosa devoid of flagella also resulted in reduced airway colonization and decreased mortality compared with those in mice infected with flagellated bacteria (12). Because flagella are one of the most immunostimulatory products of P. aeruginosa, it should be possible to modulate airway inflammation and reduce mortality using flagellin-based therapeutics without predisposing the host to invasive bacterial infection. However, to develop such therapies, the immunostimulatory bacterial component, as well as the epithelial cell responses that are activated, requires thorough characterization.
Flagellins isolated from laboratory reference strains of P. aeruginosa have been classified as type a or b based upon molecular mass and reactivity with specific antisera (28). The type a flagellins have more variable molecular masses (45 to 52 kDa), whereas the type b proteins show an invariant size of about 53 kDa (1, 4). The discrepancy in sizes between type a and b flagellins results from differences in their primary amino acid sequences as well as in posttranslational glycosylation (4, 39, 43-45). The P. aeruginosa flagellar typing system was developed based upon the analysis of defined laboratory strains, and to our knowledge, clinical isolates, particularly from acute bacterial pneumonia patients, have not been extensively characterized in this manner. Therefore, the present study was undertaken to assess the distribution of type a and b flagellins among a panel of P. aeruginosa clinical isolates and to compare the abilities of the two protein isoforms to stimulate a proinflammatory response by respiratory epithelial cells.
MATERIALS AND METHODS
Cells.
16HBE14o− is a simian virus 40 (SV40) T-antigen-transformed human bronchial epithelial cell line (7) provided by Dieter Gruenert (California Pacific Medical Center Research Institute, San Francisco, CA). BEAS-2B is an SV40 T-antigen-transformed human bronchial cell line (34) provided by Sekhar Reddy (Johns Hopkins University, Baltimore, MD). A549, an alveolar type II cell line derived from a lung adenocarcinoma (14), and NCI-H292, a human mucoepidermoid pulmonary carcinoma (2), were from the American Type Culture Collection (Manassas, VA). Human embryonic kidney (HEK) 293T cells were provided by Stephanie Vogel (University of Maryland, Baltimore, MD). All cells were cultured at 37°C in 5% CO2 with Dulbecco's modified Eagle's medium containing 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin (Invitrogen, Carlsbad, CA).
Bacteria.
P. aeruginosa laboratory strains PAK, PAK/ΔfliC, PAO1, and Nottingham were provided by Alice Prince (Columbia University, New York, NY). PAK/ΔfliC (12) is a nonmotile isogenic mutant of PAK in which the fliC gene encoding flagellin was replaced with a homologous gene interrupted by a gentamicin resistance cassette (38). P. aeruginosa clinical isolates from cystic fibrosis (CF) patients (PA149, PA383, and CF3) and an ulcerative keratitis patient (PA6294) were provided by Gerald Pier (Harvard University, Boston, MA) (40). Ten P. aeruginosa isolates (PA50241, PA50255, PA50296, PA50312, PA50327, PA50476, PA50542, PA50554, PA50403, and PA50482) from acute pneumonia patients at the University of Maryland Medical Center in Baltimore, MD, were provided by Richard Venezia. Bacteria were collected on throat swabs and eluted into 1.0 ml of Trypticase soy broth (Becton Dickenson, Franklin Lakes, NJ), and 0.1-ml aliquots were streaked for isolation on MacConkey agar plates (Becton Dickenson). Isolated colonies were cultured in Luria-Bertani (LB) broth, confirmed as P. aeruginosa by Gram staining and growth on Cetrimide agar (Becton Dickenson), and stored at −80°C in 50% glycerol in LB broth.
Flagellin purification.
Flagellin was purified as described previously (47). Briefly, overnight liquid cultures in mineral salts medium (7.0 mg/ml K2HPO4, 3.0 mg/ml KH2PO4, 1.0 mg/ml [NH4]2SO4, 50 μg/ml MgSO4·7H2O, 2.5 μg/ml FeCl3·H2O, 7.4 μg/ml l-methionine, 4.0 mg/ml sodium succinate, pH 7.0) were centrifuged at 5,000 × g for 15 min at 4°C, the bacterial pellet was resuspended in 20 mM Tris-HCl, pH 8.0, and flagella were sheared in a commercial blender (Black and Decker, Towson, MD) for 90 s at room temperature. The suspension was centrifuged at 16,000 × g for 30 min at 4°C to remove bacteria, and saturated [NH4]2SO4 was added to the supernatant to a final concentration of 20% (vol/vol). The precipitate was collected by centrifugation at 16,000 × g for 30 min at 4°C, resuspended in 20 mM Tris-HCl, pH 8.0, and extensively dialyzed in resuspension buffer. Protein concentrations were determined by the method of Bradford (3) with crystalline bovine serum albumin (BSA) as the standard (Bio-Rad, Richmond, CA). As a negative control, a mock flagellin preparation was prepared in an identical manner using an extract of nonmotile PAK/ΔfliC bacteria.
Endotoxin removal.
Endotoxins were removed from flagellar preparations using polymyxin B-agarose (Sigma, St. Louis, MO). Flagellin proteins (10 μg) were resuspended in phosphate-buffered saline (PBS), 100 mg of polymyxin B-agarose was added, and the samples were incubated for 1 h at 4°C with constant agitation. The suspensions were centrifuged at 18,000 × g for 5 min, and the supernatant was aspirated and saved. The Limulus amebocyte lysate assay (Sigma) was used according to the manufacturer's directions to detect the presence of any remaining endotoxins in the samples. No endotoxin was detected above the lower limit of the assay (<0.06 endotoxin unit/ml).
SDS-PAGE and immunoblot analysis of purified flagellin.
Proteins were resolved on 10% SDS-acrylamide gels according to the method of Laemmli (21) and either stained with 0.25% Coomassie blue or transblotted to polyvinylidene difluoride (PVDF) membrane as described previously (23). The membrane was blocked with 5% nonfat dry milk in 10 mM Tris-HCl, pH 7.4, containing 1.5% Tween 20, and reacted with rabbit antisera to PAK flagellin or pilin (provided by Daniel J. Wozniak, Wake Forest University, Winston-Salem, NC), diluted 1/3,000 in blocking buffer, or with monoclonal antibody to PAK endotoxin (provided by Joseph S. Lam, University of Guelph, Guelph, Ontario, Canada) diluted 1/20 in blocking buffer. After washing the membrane, bound antibodies were detected with horseradish peroxidase-conjugated goat anti-rabbit IgG or goat anti-mouse IgG antibodies (KPL, Gaithersburg, MD) diluted 1/10,000 in blocking buffer and enhanced chemiluminescence substrate (GE Healthcare, Piscataway, NJ).
TLR5 immunoblotting.
Confluent cultures of human airway epithelial cells were washed with PBS and lysed at 4°C with PBS containing 0.1% SDS, 0.5% sodium deoxycholate, 1.0% Triton X-100, 5.0% glycerol, and 1.0% protease inhibitor cocktail (Sigma). Equal protein aliquots (20 μg) were resolved on 10% SDS-acrylamide gels, transferred to PVDF membrane, reacted with 1.0 μg/ml of Toll-like receptor 5 (TLR5) antibody (Santa Cruz Biotechnology, Santa Cruz, CA) followed by horseradish peroxidase-conjugated secondary antibody, and developed with enhanced chemiluminescence substrate. To confirm equivalent protein loading and transfer, the blot was stripped with 100 mM 2-mercaptoethanol, 2% SDS, and 62.5 mM Tris-HCl, pH 6.7, reblocked, and probed with β-tubulin antibody. Immunoreactive bands were identified by comigration of prestained protein size markers (Bio-Rad).
IL-8 and TNF-α assays.
Cells in 24-well plates were treated with purified flagellin for 6 h, cell culture media were centrifuged at 18,000 × g for 5 min, and IL-8 and tumor necrosis factor alpha (TNF-α) levels in supernatants were measured by enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN). We chose the 6-h time point based on our previously published study that used 6-h flagellin treatments of human and mouse airway cells and HEK293T cells prior to measurement of IL-8 and TNF-α production (24). As a negative control, the cells were treated with the negative control preparation. In some experiments, flagellin (1.0 μg) was pretreated with trypsin (10 ng; Sigma) for 1 h at 37°C prior to analysis in order to inactivate the cytokine-inducing stimulus and rule out that a non-protein component was responsible for TNF-α production. Excess trypsin was inactivated by addition of soybean trypsin inhibitor (25 ng; Sigma). Supernatants were incubated for 2 h at room temperature in 96-well plates containing capture antibody, and the ELISA plates were blocked with PBS containing 1% BSA and incubated for 2 h at room temperature with biotinylated detection antibody followed by peroxidase-labeled streptavidin and tetramethylbenzidine substrate. Optical density values at 450 nm were measured, and IL-8 and TNF-α levels were determined from standard curves constructed with serial dilutions of purified chemokines. All samples were analyzed in triplicate or quadruplicate, and standard curves were performed on each plate.
NF-κB reporter assay.
HEK293T cells at ∼80% confluence in 24-well plates were cotransfected with 0.8 μg/well of a TLR5 expression plasmid (Addgene, Cambridge, MA), or the pcDNA empty vector (Invitrogen), plus 0.8 μg/well of the pGL4.32 plasmid containing NF-κB response elements of the endothelial-leukocyte adhesion molecule 1 (ELAM-1) gene promoter linked to a firefly luciferase reporter gene and 40 ng/well of the pRL-SV40 plasmid encoding Renilla luciferase as an internal control (Promega, Madison, WI). The cells were incubated for 24 h, either untreated or treated with purified flagellin or the mock negative control, and luciferase activity was measured in cell lysates using the dual-luciferase assay system according to the manufacturer's instructions (Promega).
Statistical analysis.
Replicates of three or four samples were used for each group. Mean ± standard deviation (SD) values were calculated, and differences between groups were determined using the Students t test and considered significant at P < 0.05. All experiments were repeated at least two times.
RESULTS
P. aeruginosa flagellin stimulates IL-8 production by human airway epithelial cells.
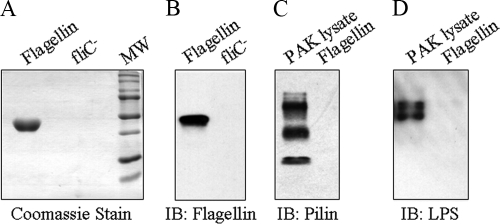
Initially, we compared four human airway epithelial cell lines for flagellin-stimulated IL-8 production. Flagellin purified from the PAK laboratory strain migrated as a single band on SDS-acrylamide gels that was immunoreactive with a flagellin polyclonal antiserum and devoid of detectable immunoreactivities with antisera against P. aeruginosa pilin and endotoxin (Fig. 1). A mock preparation purified in an identical manner from the PAK/ΔfliC isogenic mutant lacking a functional fliC gene was free of detectable protein and nonreactive with the flagellin antiserum. Treatment of human airway epithelial cell lines with the mock and purified flagellin preparations revealed a diverse pattern of proinflammatory responses, as assessed by IL-8 production (Fig. 2 A). 16HBE14o− cells produced relatively high IL-8 levels in response to treatment with 10 ng/ml of flagellin or an identical amount of the negative control preparation. Flagellin-treated BEAS-2B and A549 cells produced intermediate levels of IL-8, while NCI-H292 cells produced the lowest chemokine levels, all 3 of which were significantly greater than those of negative control-treated cells. The greatest difference between IL-8 production by flagellin and the control was manifested by BEAS-2B cells (10.1-fold). The heterogeneous IL-8 responses could not be explained by disparate expression of TLR5 protein, the cellular receptor for bacterial flagellin (Fig. 2B). Whole-cell lysates of all four cell lines contained TLR5 protein as an ∼100-kDa doublet, which is a result of differential glycosylation (35), although it is possible that different levels of TLR5 expression on the cell surface may have accounted for the varied IL-8 responses. Using a range of flagellin concentrations, a dose response in IL-8 (0 to 30 ng/ml flagellin) and TNF-α (0 to 50 ng/ml flagellin) production by BEAS-2B cells was observed (Fig. 3).
FIG. 1.
Purification of PAK flagellin. PAK flagellin (5.0 μg) and an equal volume of a negative control preparation from PAK/ΔfliC bacteria were analyzed by SDS-PAGE and stained with Coomassie blue (A) or immunoblotted with antiflagellin antibody (B). To verify protein purity, samples of purified flagellin or a PAK whole bacterial cell lysate were immunoblotted with antibodies against PAK pilin (C) or endotoxin (lipopolysacharide [LPS]) (D). MW, prestained molecular weight marker proteins; IB, immunoblot.
FIG. 2.
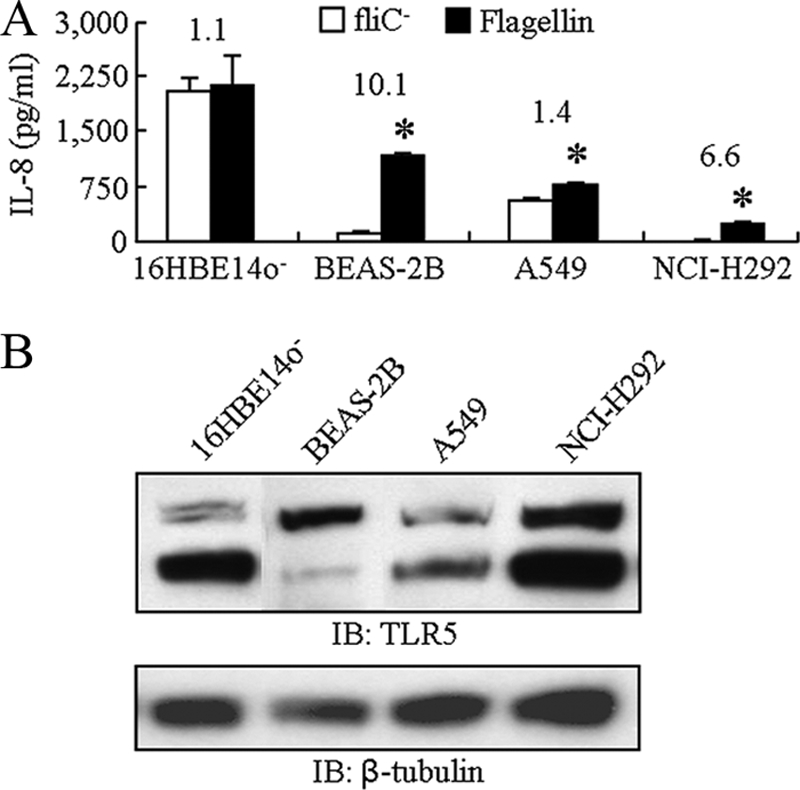
Flagellin stimulates IL-8 production by human airway epithelial cells. (A) Cells were treated for 6 h with 10 ng/ml of PAK flagellin or an equal volume of the negative control, and IL-8 levels in culture supernatants were measured by ELISA. The numbers above each pair of bars illustrate the fold increase in IL-8 levels with flagellin versus the negative control. (B) Immunoblot analysis of TLR5 protein expression by airway epithelial cells (upper panel). The blot in the lower panel was stripped and reprobed with β-tubulin antibody.
FIG. 3.
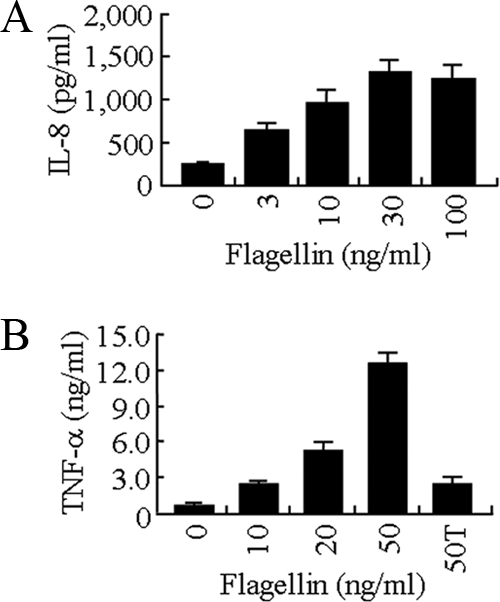
Dose-response analysis of flagellin-stimulated IL-8 and TNF-α production. BEAS-2B cells were treated for 6 h with the indicated concentrations of PAK flagellin, and IL-8 (A) and TNF-α (B) levels in culture supernatants were measured by ELISA. Each bar represents the mean ± SD (n = 4). 50T in panel B refers to 50 ng/ml of flagellin that was pretreated with trypsin prior to analysis.
P. aeruginosa flagellin stimulates NF-κB and IL-8 production by TLR5-transfected HEK293T cells.
Flagellin binding to TLR5 activates the NF-κB transcription factor, leading to induction of IL-8 and TNF-α by epithelial cells (13). To directly demonstrate that TLR5 was responsible for flagellin-stimulated IL-8 production, TLR5 was overexpressed in HEK293T cells in conjunction with an NF-κB-luciferase gene reporter plasmid. HEK293T cells were shown previously to be minimally responsive to non-pseudomonad flagellins, while TLR5-transfected HEK293T cells exhibited robust IL-8 production following flagellin stimulation (15). HEK293T cells transfected with a TLR5 expression plasmid, but not the pcDNA empty vector, had significantly greater NF-κB activation, as measured by increased luciferase activity, following treatment with 1, 10, or 100 ng/ml of flagellin, compared with cells treated with the negative control (Fig. 4 A). Treatment of TLR5-HEK293T cells with 10 ng/ml of flagellin also was correlated with increased IL-8 levels in culture supernatants compared with negative control-treated cells or compared with flagellin-treated pcDNA-transfected cells (Fig. 4B). Thus, P. aeruginosa flagellin stimulated NF-κB and IL-8 production through TLR5.
FIG. 4.
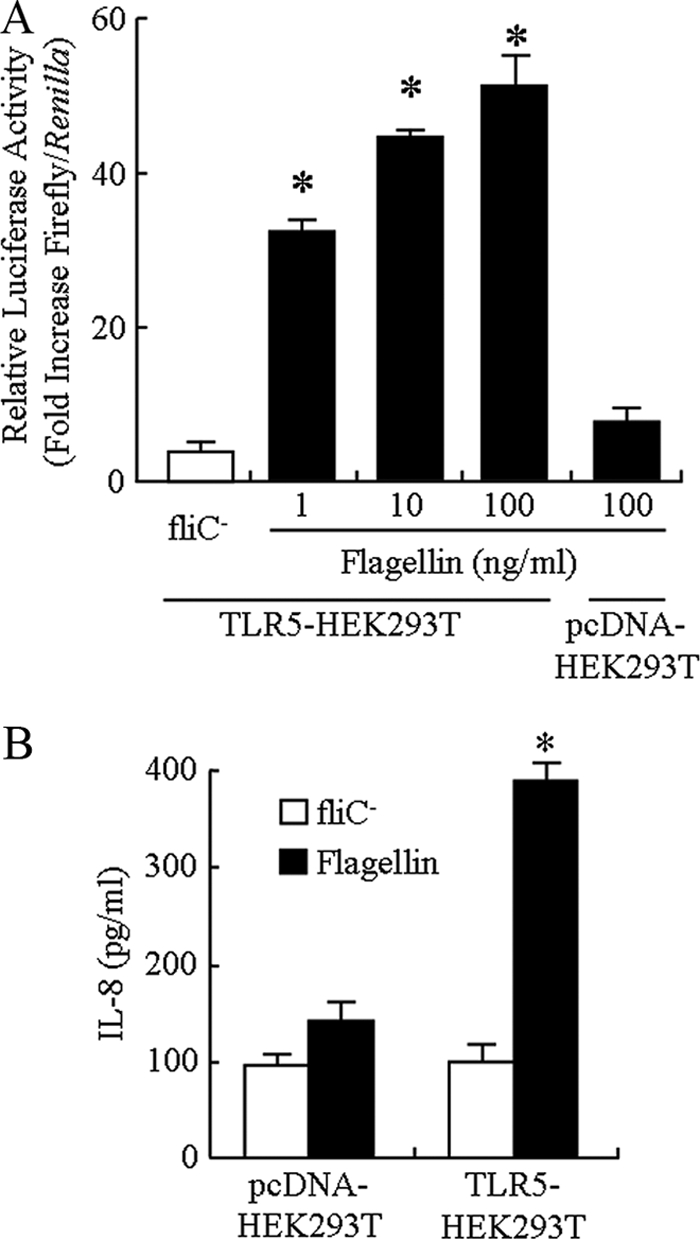
Flagellin stimulates NF-κB and IL-8 production by TLR5-transfected HEK293T cells. (A) HEK293T cells were cotransfected with a TLR5 expression plasmid or pcDNA empty vector plus the pGL4.32 plasmid containing NF-κB response elements linked to a firefly luciferase reporter gene and the pRL-SV40 plasmid encoding Renilla luciferase, the cells were incubated for 24 h and treated for 6 h with the indicated concentrations of PAK flagellin or the negative control, and relative luciferase activity in cell lysates was determined. (B) HEK293T cells were transfected with the TLR5 plasmid or pcDNA vector, incubated for 24 h, and treated for 6 h with 10 ng/ml of flagellin, and IL-8 levels in culture supernatants were measured by ELISA. Each bar represents the mean ± SD (n = 3). *, significantly increased IL-8 levels comparing flagellin- and negative control-treated cells (P < 0.05).
Purification of flagellins from P. aeruginosa clinical isolates and IL-8 production.
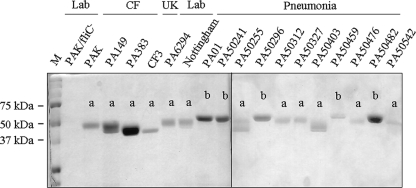
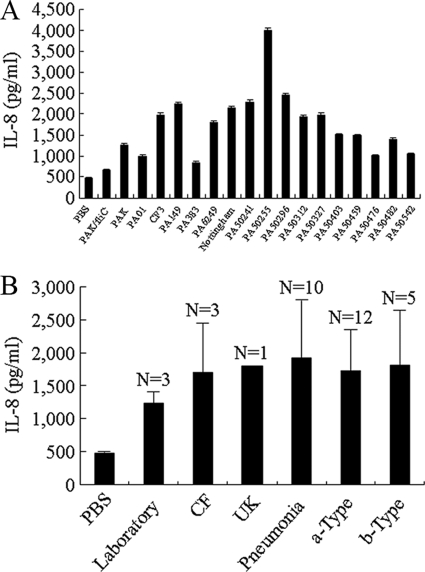
Flagellins isolated from laboratory reference strains of P. aeruginosa have been classified as type a (e.g., the PAK strain) or type b (e.g., the PAO1 strain) based upon their molecular mass and reactivity with type-specific antisera (28). However, no published studies have compared the distributions of type a and b flagellins among P. aeruginosa clinical isolates. Therefore, we purified flagellin proteins from a panel of bacterial strains isolated from ulcerative keratitis (n = 1), cystic fibrosis (n = 3), or acute pneumonia (n = 10) patients and classified them as type a or b based upon their mobilities on SDS-acrylamide gels (Fig. 5) and reactivities with flagellin typing antiserum (Table 1). We first confirmed that all isolates were motile on 0.3% LB agar (data not shown). Next, the purified flagellins were used to stimulate IL-8 production by BEAS-2B cells. As shown in Fig. 6 A, a broad distribution in IL-8 levels in cell culture supernatants was observed, ranging from 700 to 4,000 ng/ml. However, no significant differences in IL-8 levels were observed when grouped according to clinical status or flagellin type (Fig. 6B). In summary, the distribution of type a and b flagellins had no specified pattern among the P. aeruginosa clinical strains, and no differences in IL-8 production were seen between the type a and b flagellins.
FIG. 5.
SDS-PAGE of flagellins from P. aeruginosa laboratory and clinical strains. Purified flagellins were resolved by SDS-PAGE and stained with Coomassie blue. The migration of prestained molecular mass marker proteins (M) is indicated on the left. The assignment as type a or b flagellins based upon molecular mass and reaction with typing antiserum is indicated above each flagellin band. Lab, laboratory strains; CF, cystic fibrosis isolates; UK, ulcerative keratitis isolate.
TABLE 1.
Pseudomonas aeruginosa flagellin purification and type classification
| PA isolate | Source | Flagellin yield (μg/liter)a | Flagellin mol mass (kDa)b | Flagellin typec |
|---|---|---|---|---|
| PAK/ΔfliC | Laboratory | NAd | NA | NA |
| PAK | Laboratory | 65.6 | 47.0 | a |
| PAO1 | Laboratory | 50.5 | 54.0 | b |
| Nottingham | Laboratory | 76.8 | 52.0 | a |
| PA149 | Cystic fibrosis | 42.7 | 46.0 | a |
| PA383 | Cystic fibrosis | 96.8 | 46.0 | a |
| CF3 | Cystic fibrosis | 40.6 | 50.0 | a |
| PA6294 | Ulcerative keratitis | 22.5 | 52.0 | a |
| PA50241 | Pneumonia | 61.9 | 54.0 | b |
| PA50255 | Pneumonia | 48.4 | 50.0 | a |
| PA50296 | Pneumonia | 31.1 | 54.0 | b |
| PA50312 | Pneumonia | 29.6 | 52.0 | a |
| PA50327 | Pneumonia | 51.3 | 52.0 | a |
| PA50403 | Pneumonia | 24.2 | 52.0 | a |
| PA50459 | Pneumonia | 36.4 | 54.0 | b |
| PA50476 | Pneumonia | 28.8 | 52.0 | a |
| PA50482 | Pneumonia | 21.8 | 54.0 | b |
| PA50542 | Pneumonia | 49.7 | 50.0 | a |
Micrograms of purified flagellin per liter of bacterial culture.
Flagellin molecular mass determined by SDS-PAGE with reference to prestained protein markers: type a flagellin, 54.0 kDa; type b flagellin, 45.0 to 52.0 kDa.
Flagellin type based upon molecular mass and reaction with flagellin typing antiserum.
NA, not applicable.
FIG. 6.
IL-8 production by flagellins from P. aeruginosa laboratory and clinical strains. (A) BEAS-2B cells were treated for 6 h with 100 ng/ml of the indicated flagellins, and IL-8 levels in culture supernatants were measured by ELISA. (B) IL-8 levels grouped according to clinical status or flagellin type. Each bar represents the mean ± SD (n = 3).
DISCUSSION
The results of this study are summarized as follows. (i) 16HBE14o− cells displayed a high level of constitutive IL-8 production that was not further increased by treatment with flagellin, while BEAS-2B, A549, and NCI-H292 cells had minimal constitutive chemokine production that was additionally enhanced following flagellin treatment. (ii) All four airway epithelial cell lines expressed TLR5 protein. (iii) Flagellin stimulated NF-κB and IL-8 production through TLR5. (iv) The majority of P. aeruginosa clinical isolates (10/14) expressed type a flagellin. (v) While differences in the ability of flagellins from bacterial strains to stimulate IL-8 production were observed (700 to 4,000 pg/ml), a correlation between expression of type a or b proteins and IL-8 levels could not be made. These results lend credence to previous studies that revealed no relationship between the source of P. aeruginosa isolates (clinical versus environmental) and the types of flagellins expressed (30) as well as the prior report that 76% of ulcerative keratitis isolates expressed type a fliC genes versus 24% for type b (46). Of note, all isolates (although limited in number) from CF cases were type a, but whether this relationship will remain with a larger number of CF clinical samples remains to be established. Furthermore, while airway cell lines were exclusively used in this study, we plan to use human primary tracheobronchial epithelial cells in future experiments to compare with the results from airway cell lines.
A short segment of 10 amino acids (LQRIRDLALQ) in the NH2-terminal region of P. aeruginosa flagellin has been shown to be important for binding to TLR5 and downstream NF-κB activation and proinflammatory cytokine/chemokine production (18, 45). A homologous region is present in the flagellins of most Gram-negative bacteria that have been examined (Salmonella enterica, Escherichia coli, Vibrio cholerae, and Listeria monocytogenes) (15, 18). Therefore, it is unlikely that sequence differences in the TLR5-binding region of the conserved NH2-terminal domain of P. aeruginosa flagellin account for variation in IL-8 production from the different bacterial strains. More plausible, however, is a potential role for alteration in posttranslational modifications, particularly O-linked glycosylation (1, 4) and/or tyrosine phosphorylation (19, 20). For example, IL-8 release from A549 cells stimulated with nonglycosylated flagellins was significantly reduced compared with that in wild-type flagellar preparations, indicating a role for glycosylation in the proinflammatory action of P. aeruginosa flagellin (44). Mapping of these glycosylation sites has revealed not only strain-specific variation in the sites of glycosylation (Thr-189, Ser-191, Ser-195, and Ser-260) but also heterogeneity in the particular glycan moieties attached to these sites (39, 45). Differences in flagellin glycosylation can be explained, in part, through strain-dependent genetic divergence of the respective flagellar glycosylation islands in the P. aeruginosa genome.
Given that the P. aeruginosa flagellum contributes to bacterial virulence and stimulates host innate immunity through a TLR5 → NF-κB → IL-8 pathway, recent interest has been generated in the development of a flagellar vaccine and vaccination efficacy has been demonstrated in several animal models of active and passive immunization. For example, Holder et al. (16) observed that immunization with flagella protected against subsequent P. aeruginosa challenge infection in the burned-mouse model. Saha et al. (37) developed a DNA vaccine encoding a mutant flagellin protein that was highly immunogenic, but its ability to interact with TLR5 was reduced by more than 100-fold. DNA vaccination of mice induced flagellum-reactive antibodies and provided protection against lethal P. aeruginosa infection in the lungs without blocking TLR5 activation. In mouse and rat models of P. aeruginosa-induced pneumonia, administration of human monoclonal antibodies to flagella provided protection against infection and decreased bacterially induced lung injury (22, 32). Active vaccination of mice with intact flagella induced protection against infection by PAK and PA01, although it was much less effective against P. aeruginosa clinical isolates (5). In contrast, immunization with flagellin monomers was suggested to induce antibodies capable of neutralizing innate immunity due to blockade of TLR5 activation. In human studies, P. aeruginosa lung infections were reduced in patients passively immunized with chicken IgY antiflagellin antibodies (31). Additionally, a double-blind randomized placebo-controlled phase III study of a P. aeruginosa flagellar vaccine in CF patients has been published, albeit these patients nonetheless became colonized with P. aeruginosa in spite of being vaccinated (10). Part of the problem in the latter study relates to the ability of the bacteria to downregulate flagellin biosynthesis in colonized CF patients who harbor biofilms of P. aeruginosa in the lungs (26, 27). In summary, while promising experimental results have been obtained following flagellar vaccination in animal models of P. aeruginosa infection, the limited human clinical trails that have been performed are less encouraging. Future studies to identify optimal flagellar/flagellin vaccine candidates that elicit broadly protective immunity against P. aeruginosa infection may be applicable to patients with acute infections as well as to high-risk patients before the onset of chronic infection.
Acknowledgments
This work was supported by Public Health Service grants AI072291 and AI083463 from the National Institute of Allergy and Infectious Diseases.
Footnotes
Published ahead of print on 30 June 2010.
REFERENCES
- 1.Arora, S. K., M. C. Wolfgang, S. Lory, and R. Ramphal. 2004. Sequence polymorphism in the glycosylation island and flagellins of Pseudomonas aeruginosa. J. Bacteriol. 186:2115-2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banks-Schlegel, S. P., A. F. Gazdar, and C. C. Harris. 1985. Intermediate filament and cross-linked envelope expression in human lung tumor cell lines. Cancer Res. 45:1187-1197. [PubMed] [Google Scholar]
- 3.Bradford, M. M. 1976. A rapid sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 4.Brimer, C. D., and T. C. Montie. 1998. Cloning and comparison of fliC genes and identification of glycosylation in the flagellin of Pseudomonas aeruginosa a-type strains. J. Bacteriol. 180:3209-3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campodónico, V. L., N. J. Llosa, M. Grout, G. Döring, T. Maira-Litrán, and G. B. Pier. 2010. Evaluation of flagella and flagellin of Pseudomonas aeruginosa as vaccines. Infect. Immun. 78:746-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carmeli, Y. 2008. Strategies for managing today's infections. Clin. Microbiol. Infect. 14:22-31. [DOI] [PubMed] [Google Scholar]
- 7.Cozens, A. L., M. J. Yezzi, M. Yamaya, J. A. Wagner, D. Steiger, S. S. Garber, L. Chin, E. M. Simon, G. R. Cutting, P. Gardner, D. S. Friend, C. B. Basbaum, and D. C. Gruenert. 1992. A human transformed epithelial cell line that retains tight junctions. In Vitro Cell. Dev. Biol. 28:735-744. [DOI] [PubMed] [Google Scholar]
- 8.Dean, T. P., Y. Daij, J. K. Shute, M. K. Church, and J. O. Warner. 1993. Interleukin-8 concentrations are elevated in bronchoalveolar lavage, sputum, and sera of children with cystic fibrosis. Pediatr. Res. 34:159-161. [DOI] [PubMed] [Google Scholar]
- 9.DiMango, E., H. J. Zar, R. Bryan, and A. Prince. 1995. Diverse Pseudomonas aeruginosa gene products stimulate respiratory epithelial cells to produce interleukin-8. J. Clin. Invest. 96:2204-2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Döring, G., C. Meisner, and M. Stern. 2007. A double-blind randomized placebo-controlled phase III study of a Pseudomonas aeruginosa flagella vaccine in cystic fibrosis patients. Proc. Natl. Acad. Sci. U. S. A. 104:11020-11025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engel, J., and P. Balachandran. 2009. Role of Pseudomonas aeruginosa type III effectors in disease. Curr. Opin. Microbiol. 12:61-66. [DOI] [PubMed] [Google Scholar]
- 12.Feldman, M., R. Bryan, S. Rajan, L. Scheffler, S. Brunnert, H. Tang, and A. Prince. 1998. Role of flagella in the pathogenesis of Pseudomonas aeruginosa pulmonary infection. Infect. Immun. 66:43-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gewirtz, A. T., T. A. Navas, S. Lyons, P. J. Godowski, and J. L. Madara. 2001. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J. Immunol. 167:1882-1885. [DOI] [PubMed] [Google Scholar]
- 14.Giard, D. J., S. A. Aaronson, G. J. Todaro, P. Arnstein, J. H. Kersey, H. Dosik, and W. P. Parks. 1973. In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J. Natl. Cancer Inst. 51:1417-1423. [DOI] [PubMed] [Google Scholar]
- 15.Harrison, L. M., P. Rallabhandi, J. Michalski, X. Zhou, S. R. Steyert, S. N. Vogel, and J. B. Kaper. 2008. Vibrio cholerae flagellins induce Toll-like receptor 5-mediated interleukin-8 production through mitogen-activated protein kinase and NF-κB activation. Infect. Immun. 76:5524-5534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holder, I. A., R. Wheeler, and T. C. Montie. 1982. Flagellar preparations from Pseudomonas aeruginosa: animal protection studies. Infect. Immun. 35:276-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huber, A. R., S. J. Kunkel, R. F. Todd, and S. J. Weiss. 1991. Regulation of transendothelial neutrophil migration by endogenous interleukin-8. Science 254:99-102. [DOI] [PubMed] [Google Scholar]
- 18.Jacchieri, S. G., R. Torquato, and R. R. Brentani. 2003. Structural study of binding of flagellin by Toll-like receptor 5. J. Bacteriol. 185:4243-4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelly-Wintenberg, K., T. Anderson, and T. C. Montie. 1990. Phosphorylated tyrosine in the flagellum filament protein of Pseudomonas aeruginosa. J. Bacteriol. 172:5135-5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelly-Wintenberg, K., S. L. South, and T. C. Montie. 1993. Tyrosine phosphate in a- and b-type flagellins of Pseudomonas aeruginosa. J. Bacteriol. 175:2458-2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laemmli, U. K. 1970. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 22.Landsperger, W. J., K. D. Kelly-Wintenberg, T. C. Montie, L. S. Knight, M. B. Hansen, C. C. Huntenburg, and M. J. Schneidkraut. 1994. Inhibition of bacterial motility with human antiflagellar monoclonal antibodies attenuates Pseudomonas aeruginosa-induced pneumonia in the immunocompetent rat. Infect. Immun. 62:4825-4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lillehoj, E. P. 1994. Protein immunoblotting, p. 273-289. In V. S. Malik and E. P. Lillehoj (ed.), Antibody techniques. Academic Press, San Diego, CA.
- 24.Lu, W., A. Hisatsune, K. Kato, T. Koga, E. P. Lillehoj, W. Chen, A. S. Cross, S. J. Gendler, A. T. Gewirtz, and K. C. Kim. 2006. Enhanced pulmonary clearance of Pseudomonas aeruginosa by Muc1 knockout mice. J. Immunol. 176:3890-3894. [DOI] [PubMed] [Google Scholar]
- 25.Luzar, M. A., M. J. Thomassen, and T. C. Montie. 1985. Flagella and motility alterations in Pseudomonas aeruginosa strains from patients with cystic fibrosis: relationship to patient clinical condition. Infect. Immun. 50:577-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahenthiralingam, E., M. E. Campbell, and D. P. Speert. 1994. Nonmotility and phagocytic resistance of Pseudomonas aeruginosa isolates from chronically colonized patients with cystic fibrosis. Infect. Immun. 62:596-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahenthiralingam, E., and D. P. Speert. 1995. Nonopsonic phagocytosis of Pseudomonas aeruginosa by macrophages and polymorphonuclear leukocytes requires the presence of the bacterial flagellum. Infect. Immun. 63:4519-4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montie, T. C., R. C. Craven, and I. A. Holder. 1982. Flagellar preparations from Pseudomonas aeruginosa: isolation and characterization. Infect. Immun. 35:281-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montie, T. C., D. Doyle-Huntzinger, R. C. Craven, and I. A. Holder. 1982. Loss of virulence associated with the absence of flagellum in an isogenic mutant of Pseudomonas aeruginosa in the burned-mouse model. Infect. Immun. 38:1296-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morgan, J. A., N. F. Bellingham, C. Winstanley, M. A. Ousley, C. A. Hart, and J. R. Saunders. 1999. Comparison of flagellin genes from clinical and environmental Pseudomonas aeruginosa isolates. Appl. Environ. Microbiol. 65:1175-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nilsson, E., A. Amini, B. Wretlind, and A. Larsson. 2007. Pseudomonas aeruginosa infections are prevented in cystic fibrosis patients by avian antibodies binding to Pseudomonas aeruginosa flagellins. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 856:75-80. [DOI] [PubMed] [Google Scholar]
- 32.Oishi, K., F. Sonoda, A. Iwagaki, P. Ponglertnapagorn, K. Watanabe, T. Nagatake, A. Siadak, M. Pollack, and K. Matsumoto. 1993. Therapeutic effects of a human antiflagella monoclonal antibody in a neutropenic murine model of Pseudomonas aeruginosa pneumonia. Antimicrob. Agents Chemother. 37:164-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prince, A. 2006. Flagellar activation of epithelial signaling. Am. J. Respir. Cell Mol. Biol. 34:548-551. [DOI] [PubMed] [Google Scholar]
- 34.Reddel, R. R., Y. Ke, and B. I. Gerwin. 1988. Transformation of human bronchial epithelial cells by infection with SV40 or adenovirus SV40 hybrid virus, or transfection via strontium phosphate coprecipitation with a plasmid containing SV40 early region genes. Cancer Res. 8:1904-1909. [PubMed] [Google Scholar]
- 35.Reed, K. A., M. E. Hobert, C. E. Kolenda, K. A. Sands, M. Rathman, M. O'Connor, S. Lyons, A. T. Gewirtz, P. J. Sansonetti, and J. L. Madara. 2002. The Salmonella typhimurium flagellar basal body protein FliE is required for flagellin production and to induce a proinflammatory response in epithelial cells. J. Biol. Chem. 277:13346-13353. [DOI] [PubMed] [Google Scholar]
- 36.Ruimy, R., E. Genauzeau, C. Barnabe, A. Beaulieu, M. Tibayrenc, and A. Andremont. 2001. Genetic diversity of Pseudomonas aeruginosa strains isolated from ventilated patients with nosocomial pneumonia, cancer patients with bacteremia, and environmental water. Infect. Immun. 69:584-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saha, S., F. Takeshita, T. Matsuda, N. Jounai, K. Kobiyama, T. Matsumoto, S. Sasaki, A. Yoshida, K. Q. Xin, D. M. Klinman, S. Uematsu, K. J. Ishii, S. Akira, and K. Okuda. 2007. Blocking of the TLR5 activation domain hampers protective potential of flagellin DNA vaccine. J. Immunol. 179:1147-1154. [DOI] [PubMed] [Google Scholar]
- 38.Saiman, L., and A. Prince. 1993. Pseudomonas aeruginosa pili bind to asialoGM1, which is increased on the surface of cystic fibrosis epithelial cells. J. Clin. Invest. 92:1875-1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schirm, M., S. K. Arora, A. Verma, E. Vinogradov, P. Thibault, R. Ramphal, and S. M. Logan. 2004. Structural and genetic characterization of glycosylation of type a flagellin in Pseudomonas aeruginosa. J. Bacteriol. 186:2523-2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schroeder, T. H., T. Zaidi, and G. B. Pier. 2001. Lack of adherence of clinical isolates of Pseudomonas aeruginosa to asialo-GM1 on epithelial cells. Infect. Immun. 69:719-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith, E. E., D. G. Buckley, Z. Wu, C. Saenphimachak, L. R. Hoffman, D. A. D'Argenio, S. I. Miller, B. W. Ramsey, D. P. Speert, S. M. Moskowitz, J. L. Burns, R. Kaul, and M. V. Olson. 2006. Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc. Natl. Acad. Sci. U. S. A. 103:8487-8492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Starkey, S. D., and J. W. Chandler. 1975. Experimental keratitis due to Pseudomonas aeruginosa: model for evaluation of antimicrobial drugs. Antimicrob. Agents Chemother. 8:350-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Totten, P. A., and S. Lory. 1990. Characterization of the a-type flagellin gene from Pseudomonas aeruginosa PAK. J. Bacteriol. 172:7188-7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verma, A., S. K. Arora, S. K. Kuravi, and R. Ramphal. 2005. Roles of specific amino acids in the N terminus of Pseudomonas aeruginosa flagellin and of flagellin glycosylation in the innate immune response. Infect. Immun. 73:8237-8246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Verma, A., M. Schirm, S. K. Arora, P. Thibault, S. M. Logan, and R. Ramphal. 2006. Glycosylation of b-type flagellin of Pseudomonas aeruginosa: structural and genetic basis. J. Bacteriol. 188:4395-4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Winstanley, C., S. B. Kaye, T. J. Neal, H. J. Chilton, S. Miksch, C. A. Hart, and the Microbiology Ophthalmic Group. 2005. Genotypic and phenotypic characteristics of Pseudomonas aeruginosa isolates associated with ulcerative keratitis. J. Med. Microbiol. 54:519-526. [DOI] [PubMed] [Google Scholar]
- 47.Zhang, J., K. Xu, B. Ambati, and F. S. Yu. 2003. Toll-like receptor 5-mediated corneal epithelial inflammatory responses to Pseudomonas aeruginosa flagellin. Invest. Ophthalmol. Vis. Sci. 44:4247-4254. [DOI] [PubMed] [Google Scholar]