Abstract
To develop a vaccine to block the transmission of vivax malaria, the gene encoding the ookinete surface protein Pvs25 was cloned from a Korean malaria patient. The Pvs25 gene was 660 bp long, encoding 219 amino acids. It was subcloned into the expression vector pQE30 and expressed in Escherichia coli. The expressed recombinant protein, named rPvs25, showed a molecular mass of approximately 25 kDa by SDS-PAGE analysis. An anti-rPvs25 monoclonal antibody produced in BALB/c mice was able to inhibit sporozoite development in the mosquito Anopheles sinensis, which is known as the malaria transmission vector in the Republic of Korea. In addition, rPvs25 produced a relatively high antibody titer in BALB/c mice that lasted for more than 6 months. Based on these results, we suggest that recombinant Pvs25 could be a useful antigen in the development of a vaccine to prevent local malaria transmission in the Republic of Korea.
Although Plasmodium vivax has presumably been prevalent in the Republic of Korea for several centuries, the incidence of vivax malaria has rapidly decreased as a result of a national malaria eradication program conducted by the Korean government in collaboration with the World Health Organization (WHO) (12, 13, 24). After a report of two malaria patients in 1985 (18), there were no further reported cases until 1993. Between 1993 and 2000, the number of malaria cases rapidly increased. Thus, starting in 2000, the Korean government made efforts to reduce the incidence of malaria, resulting in a decrease in reported malaria cases over the course of several years. Nevertheless, it is unlikely that malaria has been eradicated thoroughly from the Republic of Korea; treatment failure is reported for approximately 3 to 4% of cases every year (Korean Centers for Disease Control and Prevention, unpublished data), and there is a steady influx of travelers and workers from countries where malaria is prevalent. Malaria is caused by protozoan parasites of the genus Plasmodium, primarily Plasmodium falciparum and P. vivax, and is transmitted in nearly 100 countries, where approximately 2.0 billion people are exposed to infection. The WHO estimates that the global burden of malaria is approximately 500 million clinical cases per year, and recent estimates suggest that 70 to 80 million of those cases are due to P. vivax (24). Unfortunately, most trials for developing a malaria vaccine have not been successful because of the complicated life cycle of the malaria parasite. To overcome this problem, several researchers began to develop transmission-blocking vaccines (TBVs), which are designed to induce an immune response in the human host, inhibiting the formation of ookinetes or oocysts in the mosquito vector and thus preventing the spread of the parasites between humans. For example, in P. falciparum, monoclonal antibodies against the Pfs230 and Pfs48/45 proteins, which are expressed in the gametocytes/gametes, can block fertilization. In addition, monoclonal antibodies against the P25 and P21/28 surface protein family members, which are expressed in zygotes and ookinetes, can inhibit postfertilization events (2, 10). Proteins in the P25 and P21/28 families are distinctive, having four evolutionarily conserved tandem epidermal growth factor (EGF)-like domains attached to the parasite surface by a glycosylphosphatidylinositol (GPI) anchor. Pfs25 and Pfs28 of P. falciparum and Pfs28 of rodent malaria parasites have been cloned and are well characterized (5, 6, 20). In Plasmodium yoelii, antibodies against the P25 homolog Pys25 can inhibit zygote formation (22). In Plasmodium gallinacerum, polyclonal antibodies against Pgs28, the homolog of P21/28, inhibit the development of zygotes into ookinetes in vitro and the development of ookinetes into oocysts (4). In P. vivax, antibodies against Pvs25 and Pvs28, which were cloned from the SalI strain, have the ability to block parasite formation in infected mosquitoes (8). However, Pvs25 contains an antigenic polymorphism that causes problems with a SalI strain-based vaccine. Therefore, in this study, we report the cloning of the ookinete surface protein Pvs25 from a Korean isolate that could be a suitable antigen for the development of a transmission-blocking vaccine for use in South Korea. It would be time-consuming and laborious to immunize the more than 10 million individuals in areas of malaria endemicity in South Korea with precise immunization schedules. One way to circumvent the logistical difficulties would be to develop an edible malaria vaccine, so we also addressed whether immune responses could be evoked in vivo by the oral administration of the recombinant Pvs25 (rPvs25) protein.
MATERIALS AND METHODS
Parasites.
For the artificial blood fed to the Anopheles vector mosquitoes, malaria blood samples were collected from patients infected with P. vivax who had never been abroad. The developmental stage of the parasite in each blood sample was determined by examination of thin blood films. Whole-blood samples were stored in the refrigerator (4°C) or at room temperature until the mosquitoes were ready to feed. When the presence of gametocytes was confirmed, the parasitemia for each sample was calculated. The remaining blood samples were used for genomic DNA preparation. All samples were collected under human use protocols that were reviewed and approved by the Human Ethics Committee of the National Institute of Health of the Republic of Korea.
PCR and cloning.
To amplify the ookinete surface protein gene (Pvs25), a specific primer set for Pvs25 was designed based on the DNA sequences listed in GenBank (accession no. AF083502). The primers contained restriction sites that were used in expression and cloning experiments. Pvs25-F (5′-GGATCCAACTCCTACTACAGCC-3′) contained a BamHI site at the 5′ end, and Pvs25-R (5′-GGTACCTATGACGTACGAAGG-3′) contained a KpnI site at the 5′ end. P. vivax genomic DNA was extracted from the whole blood of a malaria patient by use of a QIAamp blood kit (Qiagen Co., Hilden, Germany). PCR was performed with AccuPower PCR premix (Bioneer Co., Taejeon, South Korea), 50 ng of the purified genomic DNA, and 40 pmol (each) of the reverse and forward primers described above. The total volume was adjusted to 20 μl with distilled water. Cycling conditions were as follows: initial denaturation at 94°C for 5 min, followed by 35 cycles of 94°C for 1 min, 50°C for 1 min, and 72°C for 1 min and a final incubation at 72°C for 5 min. PCR products were confirmed under UV transillumination and were purified with a gel extraction kit (Qiagen). Purified PCR products were ligated into the pCR2.1 cloning vector (Invitrogen Co., Carlsbad, CA) and then transformed into Escherichia coli INVαF′ according to the manufacturer's instructions. Transformants were confirmed by EcoRI digestion.
DNA sequencing and sequence analysis.
The sequence of the Pvs25 gene from the Korean isolate was determined using an ABI Prism BigDye Terminator FS cycle sequencing ready reaction kit (PerkinElmer Co., Boston, MA) according to the manufacturer's protocol. DNA was prepared from E. coli expressing the Pvs25 gene. The M13 reverse (5′-GTCCTTTGTCGATACTG-3′) and M13 forward (−20) (5′-GTAAAACGACGGCCAG-3′) primers were used in the sequencing reaction mix, and nucleotide and deduced amino acid sequences were analyzed using EditSeq and Clustal in the Megalign program, a multiple alignment program in the DNASTAR package (DNASTAR, Madison, WI). The Internet-based BLAST search program of the National Center for Biotechnology Information (NCBI) was used to search protein databases.
Recombinant protein expression and purification.
For expression of the P. vivax ookinete surface protein gene in E. coli, amplified PCR products were digested with BamHI and KpnI, separated in an agarose gel, and purified with a Qiagen gel extraction kit. PCR products were then integrated into the pQE30 expression vector (Qiagen), using the same restriction sites. The resulting plasmid was used to express a Pvs25-His6 fusion protein from E. coli strain M15 (Qiagen). Transformants were confirmed both by restriction enzyme digestion with BamHI and KpnI and by DNA sequence analysis. Recombinant proteins were induced by isopropyl-1-thio-β-d-galactoside (IPTG) and purified by immobilized metal ion affinity chromatography (Qiagen). After each purification step, the proteins were run in 12% SDS-PAGE gels at 30 mA for 35 min and then analyzed.
ELISA.
Polystyrene 96-well plates were coated with 1 μg per well of purified recombinant antigen diluted in phosphate-buffered saline (PBS), pH 7.4. The plates were incubated at 4°C for 18 h, washed three times with PBS containing 0.05% Tween 20 (PBST), and blocked with 200 μl of blocking solution (3% bovine serum albumin [BSA] in PBST) per well for 2 h at 37°C. After three additional washes with PBST, 100 μl of diluted mouse serum (1:100 [vol/vol]) was added to each well, followed by 2 h of incubation at 37°C. Following another series of washes as described above, the samples were incubated at 37°C for 2 h with 100 μl of peroxidase-conjugated anti-mouse IgG (Sigma Co., St. Louis, MO) diluted in the blocking solution (1:1,000 [vol/vol]). The plates were then washed three times with PBST before the addition of 100 μl of substrate solution, which was prepared immediately before use by dissolving 30 mg of o-phenylenediamine (Sigma) per 60 ml in peroxidase solution B (H2O2 in 0.1 M phosphate-citric acid buffer, pH 5.0), to the wells. The plates were incubated for 30 min in darkness, and the reaction was then terminated with 50 μl of 4 N H2SO4. Optical densities were measured at 405 nm with an enzyme-linked immunosorbent assay (ELISA) reader.
Production of MAbs.
Monoclonal antibodies (MAbs) specific to rPvs25 were produced using myeloma cells. Briefly, BALB/c mice were immunized with rPvs25, and the antibody titer of each mouse was measured. The spleens were removed from immunized mice, and the spleen cells were separated. Spleen cells were then cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) after being washed three times with serum-free DMEM. Sp2/O myeloma cells, at a concentration of no more than 105/ml, were cultured in 15% FBS-DMEM. After 7 days of cell fusion, 8-azaguanine was added to the myeloma cell culture medium. Spleen cells and myeloma cells were mixed at a 5:1 ratio. Fused cells were selected using hypoxanthine aminopterin thymidine (HAT) medium, and their antibody secretion abilities were measured by ELISA and Western blot analysis. To produce ascites, selected cell clones were injected into the peritoneal cavity of BALB/c mice. Ascites fluid was recovered 1 week later by use of an 18-gauge needle. Pvs25 MAb was then purified using protein G Sepharose 4 Fast Flow chromatography (Pharmacia, Uppsala, Sweden).
Western blot analysis.
Recombinant proteins were run in 12% SDS-PAGE gels and then transferred to nitrocellulose membranes. Membranes were blocked to prevent nonspecific binding by use of a blocking buffer (0.01 M PBS, 5% skim milk, 0.05% Tween 20, pH 7.4) for 12 h at 4°C and then were washed three times with washing buffer (0.01 M PBS, 0.15% Tween 20, pH 7.4) for 10 min each time. Membranes were incubated for 2 h with Pvs25 MAb or human sera (1:100 [vol/vol]), washed as described above, and incubated with peroxidase-conjugated anti-mouse or anti-human IgG secondary antibodies (1:1,000 [vol/vol]; Sigma) for 1 h at room temperature. For color development, a mixture of 0.2% 3,3-diaminobenzidine and 0.02% H2O2 was applied.
Assay of inhibition of sporozoite development in mosquito salivary glands.
Female Anopheles sinensis mosquitoes were fed with a glass feeder that maintained a temperature of 37°C. Blood samples were collected aseptically by use of a syringe from patients infected with P. vivax, and the presence of gametocytes was confirmed by microscopic examination. Infected erythrocytes were washed three times with McCoy5A medium (BioWhittaker, Inc., Walkersville, MD) to remove the lymphocytes and serum and then were suspended in McCoy5A medium containing 20% AB-type human serum (Atlanta Biological, Lawrenceville, GA). All mosquitoes were starved for 24 h before artificial blood feeding. To accommodate egg laying, the blood-fed mosquitoes were transferred to paper cups filled with 10 mm of water and equipped with a wire net in the bottom to prevent the mosquitoes from sinking. Female mosquitoes were allowed to engorge on blood, with or without 200 μg/ml Pvs25 MAb, through a Parafilm-covered glass feeder. After feeding was completed, unengorged mosquitoes were discarded. Fed mosquitoes were maintained at 22 ± 2°C with 75% ± 12% relative humidity. Mosquitoes were provided with cotton wool pads soaked in 10% sucrose and were housed with an equatorial photo period (16). Sixteen days after blood feeding, salivary glands were removed and dissected, and the sporozoites were observed under a microscope. Salivary glands were transferred into Eppendorf tubes for further analysis by ELISA. Each tube was filled with 200 μl of blocking buffer (1% bovine serum albumin, 0.5% casein, 0.01% thimerosal, 0.5% NP-40, and 0.002% phenol red dissolved in 0.01 M PBS, pH 7.4), and the contents were homogenized using a pestle (Eppendorf Co., Hamburg, Germany). A two-site homologous sandwich ELISA with circumsporozoite protein (CSP) monoclonal antibody was performed to confirm sporozoite development in mosquitoes, as described by Lee et al. (11).
Oral and parenteral immunization.
rPvs25 was dialyzed in 0.01 M PBS and then adjusted to a final concentration of 200 μg in 250 μl of PBS, with or without 10 μg of cholera toxin B subunit (CTB) (1, 19). BALB/c mice were deprived of food and water for 24 h and then orally immunized with 250 μl of PBS containing the proper amounts of antigens. Serum was collected from the eye before the first immunization, as a control, and once each month thereafter to measure the level of antibodies against rPvs25. Three immunizations were given, one each at weeks 0, 1, and 3. Parenteral immunization was also performed, using rPvs25 doses similar to those used for the oral immunization, with the exception of the incomplete adjuvant (23).
Antibody isotyping.
To analyze the isotypes of the monoclonal antibody and the sera from immunized mice, a Beadlyte mouse immunoglobulin isotyping kit (Upstate Co., Temecula, CA) was used. Purified MAbs were diluted with Beadlyte mouse isotyping serum diluent (Upstate) at 1:25,000 (vol/vol), and the amount of each antibody (IgG1, IgG2a, IgG2b, IgG3, IgM, IgE, and IgA) was determined according to the manufacturer's instructions. Mean fluorescence intensity (MFI) was measured with a Luminex 100 instrument (Upstate) (9). To assess the sera of immunized mice, the levels of IgG1, IgG2a, IgG2b, IgG3, and IgA antibodies were determined by ELISA, and the absorbance was measured at 490 nm.
Nucleotide sequence accession number.
The sequence of Pvs25 from the Korean isolate was deposited in GenBank under accession no. GU256271.1.
RESULTS
Amino acid sequence variation in Pvs25.
To determine the complete gene sequence encoding the ookinete surface protein Pvs25, an approximately 600-bp genomic DNA fragment from a Korean isolate was amplified by PCR, using a Pvs25-specific primer set. The PCR products were ligated into a pCR2.1 cloning vector (3.9 kb) and transformed into E. coli INVαF′. The plasmid containing the PCR product (pPvs25) was used for DNA sequence analysis. The cloned Pvs25 gene was 660 bp long and encoded a 219-amino-acid protein with a signal sequence at the N terminus, four tandem EGF-like domains, and a C-terminal putative GPI attachment sequence (GenBank accession no. GU256271.1) (8). Three amino acid polymorphisms were identified between the Korean strain and the SalI strain (GenBank accession no. AF083502). Amino acid (aa) 97 (E in the SalI strain and Q in the Korean strain) and aa 130 (I→T) differed between the two strains, but aa 131 (Q) was the same in both. The amino acid sequence of the Korean isolate was similar to those of Thai isolates (17). In contrast, the amino acid sequence was totally different from those of P. falciparum (GenBank accession no. X07802) and Plasmodium ovale (GenBank accession no. AB051631) (data not shown).
Recombinant protein expression and monoclonal antibody production.
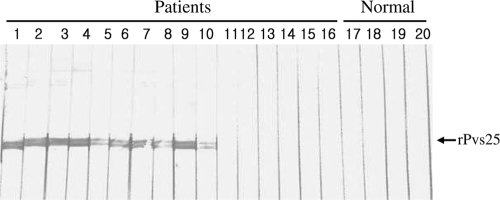
rPvs25 was expressed in E. coli strain M15. Protein expression was induced with 0.5 M IPTG, and recombinant protein was purified by Ni-nitrilotriacetic acid chromatography. The protein had a molecular mass of 25 kDa, as determined by SDS-PAGE analysis (Fig. 1 A). A monoclonal antibody (Pvs25 MAb) specific to rPvs25 was produced using Sp2/O myeloma cells and BALB/c mice. The Pvs25 MAb was purified from the ascites fluid of immunized mice by protein G Sepharose 4 Fast Flow chromatography. A specific immune response between rPvs25 and the Pvs25 MAb was confirmed by Western blot analysis (Fig. 1B). The monoclonal antibody consisted mainly of IgG2b (Fig. 1C). Additionally, sera of 10 of 16 P. vivax-infected patients were found to react with the purified recombinant antigen (Fig. 2).
FIG. 1.
Expression and purification of rPvs25 from E. coli strain M15 and monoclonal antibody production. (A) Expression of the cloned Pvs25 gene was induced by adding IPTG to a final concentration of 0.5 mM, and protein expression was analyzed by SDS-PAGE followed by Coomassie blue staining. Lanes: M, molecular size marker; 1, uninduced E. coli; 2, IPTG-induced E. coli; 3, purified sexual-stage surface protein. (B) Antibody reactivity with the recombinant protein, as confirmed by Western blot analysis. Lane 1, rPvs25 detected with the Pvs25 MAb. (C) Isotype analysis of the eluate of protein G affinity chromatography purification of ascites fluid from an rPvs25-immunized mouse. OD, optical density.
FIG. 2.
Western blot analysis of the ability of sera from human subjects infected with P. vivax to react with rPvs25. Lanes 1 to 16, sera of humans infected with P. vivax; lanes 17 to 20, sera of healthy humans.
Pvs25 MAb-blocked sporozoite development in Anopheles sinensis.
In the control group (n = 100), in which mosquitoes were fed by a glass apparatus with patient blood only, 34 A. sinensis mosquitoes tested positive for sporozoite development both by microscopic examination and by ELISA. In contrast, none of the mosquitoes in the Pvs25 MAb-treated group (n = 80) developed sporozoites in their salivary glands.
IgG persistence after parenteral immunization.
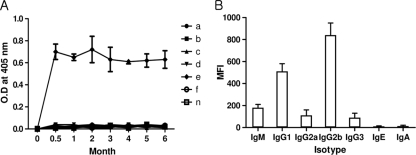
To investigate the longevity of IgG levels in immunized mice, sera were collected from each group 2 weeks after the final immunization, and their IgG levels were tested against rPvs25 by use of ELISA. The IgG levels of the mice that were immunized parenterally with rPvs25 were higher than those of mice immunized by any of the other antigen delivery methods. In addition, the levels remained high for 6 months. In contrast, no antibodies were produced in mice receiving oral immunizations (Fig. 3 A). The IgG2b isotype was dominant in mice immunized with rPvs25 (Fig. 3B).
FIG. 3.
(A) Antibody longevity in mice immunized with rPvs25 through different immunization routes. Lines: a, oral immunization with rPvs25; b, oral immunization with rPvs25 and 10 μg CTB; c, oral immunization with PBS; d, oral immunization with PBS and 10 μg CTB; e, parenteral immunization with rPvs25; f, parenteral immunization with PBS; n, untreated control. (B) Analysis of the isotypes of sera from parenterally rPvs25-immunized mice after 6 months (e group from panel A).
DISCUSSION
This study shows that a monoclonal antibody against Pvs25 can efficiently block parasite development in the A. sinensis mosquito, which is a malarial vector in the Republic of Korea. Pvs25 is not expressed by P. vivax during its life cycle in humans; therefore, it is not subject to immune selection pressure in the human host. As a consequence, its sequence diversity is low, which simplifies vaccine development (17, 21). The Pvs25 gene is highly conserved between many isolates, but three amino acid substitutions have been identified. One amino acid substitution (E/Q97) is located in the second EGF-like domain, whereas the other two (I/T130 and Q131) are located in the third EGF-like domain (21, 25). The Korean isolate from the present study differs from the SalI strain at aa 97 (Q in the Korean isolate and E in the SalI strain) and aa 130 (T→I), but the two strains are identical at aa 131 (Q). Interestingly, the North Korean strain (AY639971) has a unique combination of polymorphisms at these amino acid positions, with an E at aa 97, a T at aa 130, and a Q at aa 131. The polymorphic differences in the Pvs25 gene between the North and South Korean strains should be studied further prior to the application of a TBV in the Republic of Korea. Despite the presence of polymorphisms in Pvs25, a SalI-based vaccine against the polymorphic target antigen has been found to be effective against field isolates, likely because most of these amino acid substitutions are conservative and, therefore, the tertiary structure and epitopes of the transmission-blocking antibodies are preserved. The low substitution frequencies and bias toward conservative substitutions in Pvs25, combined with the absence of selection due to immune pressure, suggest that antigenic diversity will not limit the efficacy of Pvs25-based TBVs. Therefore, SalI-based vaccines should not be affected by Pvs25 polymorphisms in natural isolates of P. vivax and, consequently, should have wide-range applicability (17). Given these theories, a Pvs25-based vaccine developed from the South Korean isolate will likely be effective against the North Korean strain because A. sinensis is also the malarial vector in North Korea.
Although the blocking effect of antisera produced against Pvs25 (Saccharomyces cerevisiae-produced recombinant protein) on oocyst development in Anopheles dirus A has been well studied (8, 17), the transmission-blocking effect of Pvs25 on sporozoite development in A. sinensis has not yet been investigated. Therefore, the efficacy of Pvs25 as the basis for a vaccine to block malaria transmission in South Korea was investigated in this study. rPvs25 was produced in E. coli strain M15. The rPvs25 protein is larger than the Pvs25 protein produced in yeast because it contains the full Pvs25 sequence, including the signal sequence. A monoclonal antibody specific to the recombinant protein was then produced, using Sp2/O myeloma cells and BALB/c mice. A specific immune response between the rPvs25 antigen and the monoclonal antibody (Pvs25 MAb), which consisted mainly of IgG2b (Fig. 1C), was confirmed by Western blot analysis (Fig. 1B). It is possible that hybridoma supernatants would provide clearer results about immunoglobulin isotype than did mouse sera. To test the biological function of the Pvs25 MAb in sporozoite development, A. sinensis mosquitoes were fed patient blood containing gametocytes and the monoclonal antibody. After 16 days, dissected salivary glands were examined for the presence of sporozoites under a microscope, and the results were also determined by an ELISA with a CSP antibody. In the control group (n = 100), comprising A. sinensis mosquitoes fed with patient blood only, 34 A. sinensis mosquitoes showed sporozoite development in both tests. In contrast, none of the mosquitoes in the experimental group (n = 80), treated with the Pvs25 MAb, developed sporozoites in the salivary glands. The low level of infection in the control group prevents us from concluding that the Pvs25 MAb can effectively block sporozoite development in A. sinensis. The low parasitemias in Korean malaria patients make the acquisition of more accurate data very difficult. Future work should aim to increase the numbers of gametocytes in blood samples to overcome this experimental limitation; however, this process will be difficult due to the lack of available P. vivax cultivation methods. In addition, we will modify the transmission blocking assay based on the present study; for example, an isotype control MAb must be used in the control group to exclude a nonspecific reduction of sporozoites, and we will count the number of oocysts in the midguts of parasite-infected mosquitoes in future experiments because it is a direct marker of blocked transmission.
The longevity of antisera produced in response to rPvs25 in BALB/c mice was investigated to evaluate the potential of rPvs25 as a vaccine. The protein was administrated to BALB/c mice both orally and parenterally. Orally immunized mice did not produce antibodies against rPvs25 (Fig. 3A, line b), while parenterally immunized mice did (Fig. 3A, line f). Antibodies persisted at high levels in the parenterally immunized mice for at least 6 months and consisted primarily of IgG2b (Fig. 3B), as shown in the test of monoclonal antibody production (Fig. 1C). During this experiment, we checked only IgG levels in immunized mice because we obtained small amounts of serum from each mouse; however, in further studies, we will check both IgG levels and the transmission-blocking effect at each time point.
When the frequency of antibodies against rPvs25 in Korean malaria patients (n = 98) with confirmed vivax malaria infections was tested, the positive rate was only 11.22% (n = 11). This result might be due to the fact that Pvs25 is expressed primarily in the midgut of the mosquito after a blood meal. The purpose of a transmission-blocking vaccine is to block zygote formation in the mosquito midgut by use of an antibody that is produced in the host's bloodstream. In other words, the low antibody frequency in these patients might reflect the active transmission of vivax malaria in South Korea. Given these results, the E. coli-expressed rPvs25 protein could effectively elevate the antibody frequency in the population.
Chemotherapy and vector control have been insufficient to control malaria due to the emergence of both parasite strains resistant to antimalarial drugs and mosquito vectors resistant to insecticides (3, 7, 14, 15). Although TBVs will not prevent the emergence of drug-resistant mutants, these vaccines may prevent their spread. Therefore, the results presented herein are highly encouraging with respect to the applicability of an rPvs25-based vaccine against P. vivax infection in the Republic of Korea.
Acknowledgments
This work was supported by a grant from the Korea Health 21 R&D Project, Ministry of Health and Welfare, Republic of Korea (03-PJ1-PG1-CH01-0001).
Footnotes
Published ahead of print on 16 June 2010.
REFERENCES
- 1.Arakawa, T., D. K. Chong, and W. H. Langridge. 1998. Efficacy of a food plant-based oral cholera toxin B subunit vaccine. Nat. Biotechnol. 16:292-297. [DOI] [PubMed] [Google Scholar]
- 2.Barr, P. J., K. M. Green, H. L. Gibson, I. C. Bathurst, I. A. Quakyi, and D. C. Kaslow. 1991. Recombinant Pfs25 protein of Plasmodium falciparum elicits malaria transmission-blocking immunity in experimental animals. J. Exp. Med. 174:1203-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breman, J. G. 2001. The ears of the hippopotamus: manifestations, determinants, and estimates of the malaria burden. Am. J. Trop. Med. Hyg. 64:1-11. [DOI] [PubMed] [Google Scholar]
- 4.Duffy, P. E., P. Pimenta, and D. C. Kaslow. 1993. Pgs28 belongs to a family of epidermal growth factor-like antigens that are targets of malaria transmission-blocking antibodies. J. Exp. Med. 177:505-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duffy, P. E., P. Pimenta, and D. C. Kaslow. 1997. A novel malaria protein, Pfs28, and Pfs25 are genetically linked and synergistic as falciparum malaria transmission-blocking vaccines. Infect. Immun. 65:1109-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gozar, M. M. G., V. L. Price, and D. C. Kaslow. 1998. Saccharomyces cerevisiae-secreted fusion proteins Pfs25 and Pfs28 elicit potent Plasmodium falciparum transmission-blocking antibodies in mice. Infect. Immun. 60:59-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harinasuta, T., P. Suntharasamai, and C. Viravan. 1965. Chloroquine-resistant falciparum malaria in Thailand. Lancet ii:657-660. [DOI] [PubMed] [Google Scholar]
- 8.Hisaeda, H., A. W. Stowers, T. Tsuboi, W. E. Collins, J. S. Sattabongkot, and N. Suwanabun. 2000. Antibodies to malaria vaccine candidates Pvs25 and Pvs28 completely block the ability of Plasmodium vivax to infect mosquitoes. Infect. Immun. 68:6618-6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kapturczak, M. H., C. Wasserfall, T. Brusko, M. Campbell-Thompson, T. M. Ellis, M. A. Atkinson, and A. Agarwal. 2004. Heme oxygenase-1 modulates early inflammatory responses: evidence from the heme oxygenase-1-deficient mouse. Am. J. Pathol. 165:1045-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaslow, D. C., I. A. Quakyi, C. Syin, M. G. Raum, D. B. Keister, and J. E. Coligan. 1988. A vaccine candidate from the sexual stage of human malaria that contains EGF-like domains. Nature 333:74-76. [DOI] [PubMed] [Google Scholar]
- 11.Lee, H. W., E. H. Shin, S. H. Cho, H. I. Lee, C. L. Kim, W. G. Lee, S. U. Moon, J. S. Lee, W. J. Lee, and T. S. Kim. 2002. Detection of vivax malaria sporozoites naturally infected in anopheline mosquitoes from endemic areas of northern parts of Gyeonggi-do (Province) in Korea. Kor. J. Parasitol. 40:75-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Malaria Eradication Service. 1966. Malaria pre-eradication program in Korea. Progress report 1961-1965, p. 44-70. Ministry of Health and Social Affairs, Seoul, Republic of Korea.
- 13.Paik, Y. H., H. I. Ree, and J. C. Shim. 1988. Malaria in Korea. Jpn. J. Exp. Med. 58:55-66. [PubMed] [Google Scholar]
- 14.Rieckmann, K. H., D. R. Davis, and C. D. Hutton. 1989. Plasmodium vivax resistance to chloroquine? Lancet ii:1183-1184. [DOI] [PubMed] [Google Scholar]
- 15.Roberts, D. R., and R. G. Andre. 1994. Insecticide resistance issues in vector borne disease control. Am. J. Trop. Med. Hyg. 50:21-34. [DOI] [PubMed] [Google Scholar]
- 16.Rosenberg, R., and J. Rungsiwongse. 1991. The number of sporozoites produced by individual malaria oocysts. Am. J. Trop. Med. Hyg. 45:574-577. [DOI] [PubMed] [Google Scholar]
- 17.Sattabongkot, J., T. Tsuboi, H. Hisaeda, M. Tachibana, N. Suwanabun, T. Rungruang, Y. M. Cao, A. W. Stowers, J. Sirichaisinthop, R. E. Coleman, and M. Torii. 2003. Blocking of transmission to mosquitoes by antibody to Plasmodium vivax malaria vaccine candidates Pvs25 and Pvs28 despite antigenic polymorphism in field isolates. Am. J. Trop. Med. Hyg. 69:536-541. [PubMed] [Google Scholar]
- 18.Soh, J. T., K. T. Lee, K. Y. Im, D. Y. Min, M. H. Ahn, J. J. Kim, and T. S. Yong. 1985. Current status of malaria in Korea. Yonsei Rep. Trop. Med. 16:11-18. [Google Scholar]
- 19.Sun, J. B., J. Holmgren, and C. Czerkinsky. 1994. Cholera toxin B subunit: an efficient transmucosal carrier-delivery system for induction of peripheral immunological tolerance. Proc. Natl. Acad. Sci. U. S. A. 91:10795-10799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor, D., N. Cloonan, V. Mann, Q. Cheng, and A. Saul. 2000. Sequence diversity in rodent malaria of the Pfs28 ookinete surface antigen homologs. Mol. Biol. Parasitol. 110:429-434. [DOI] [PubMed] [Google Scholar]
- 21.Tsuboi, T., D. C. Kaslow, M. M. Gozar, M. Tachibana, Y. M. Cao, and M. Torii. 1998. Sequence polymorphism in two novel Plasmodium vivax ookinete surface proteins, Pvs25 and Pvs28, that are malaria transmission-blocking vaccine candidates. Mol. Med. 4:772-782. [PMC free article] [PubMed] [Google Scholar]
- 22.Tsuboi, T., Y. M. Cao, Y. Hitsumoto, T. Yanagi, and H. Kanbara. 1997. Two antigens of zygotes and ookinetes of Plasmodium yoelii and Plasmodium bergei that are distinct targets of transmission-blocking immunity. Infect. Immun. 65:2260-2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang, L., L. Kedzierski, S. L. Wesselingh, and R. L. Coppel. 2003. Oral immunization with a recombinant malaria protein induces conformational antibodies and protects mice against lethal malaria. Infect. Immun. 71:2356-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization. 1997. World malaria situation in 1994. I. Population at risk. Wkly. Epidemiol. Rec. 72:269-274. [PubMed] [Google Scholar]
- 25.Zakeri, S., S. Razavi, and N. D. Djadid. 2009. Genetic diversity of transmission blocking vaccine candidate (Pvs25 and Pvs28) antigen in Plasmodium vivax clinical isolates from Iran. Acta Trop. 109:176-180. [DOI] [PubMed] [Google Scholar]