Abstract
The immune system comprises an innate and an adaptive immune response to combat pathogenic agents. The human enteropathogen Salmonella enterica serovar Typhimurium invades the intestinal mucosa and triggers an early innate proinflammatory host gene response, which results in diarrheal disease. Several host factors, including transcription factors and transcription coregulators, are involved in the acute early response to Salmonella infection. We found in a mouse model of enterocolitis induced by S. Typhimurium that the absence of the nuclear protein poly(ADP-ribose) polymerase 1 (PARP1), a previously described cofactor for NF-κB-mediated proinflammatory gene expression, is associated with a delayed proinflammatory immune response after Salmonella infection. Our data reveal that PARP1 is expressed in the proliferative zone of cecum crypts, where it is required for the efficient expression of proinflammatory genes, many of which are related to interferon signaling. Consequently, animals lacking PARP1 show impaired infiltration of immune cells into the gut, with severely delayed inflammation.
The innate immune system constitutes the first line of host defense during infection. Innate immunity is therefore crucial for the early recognition of invading pathogens and for the subsequent proinflammatory response (26). Recognition of pathogen-associated molecular patterns (PAMPs) is achieved by pattern recognition receptors (PRRs), e.g., Toll-like receptors (TLRs) (3). Activation of PRRs triggers proinflammatory and antimicrobial responses by activating a multitude of intracellular signaling pathways and inducing inflammation-related transcription factors, such as nuclear factor kappa B (NF-κΒ) (2). The protein family of NF-κB comprises an important group of inducible transcription factors involved in proinflammatory gene expression (18). Activation of NF-κB results in the expression and synthesis of a broad range of molecules, including cytokines, chemokines, and adhesion molecules, which together orchestrate the early host response to infection and significantly contribute to inflammation.
The human enteropathogen Salmonella enterica subspecies 1 serovar Typhimurium (S. Typhimurium) invades the intestinal mucosa, causes self-limiting gut infection, and elicits mucosal inflammation and diarrhea. Salmonella infection is a global threat to human health, but the molecular mechanisms underlying Salmonella-induced enteric diseases are not sufficiently understood. A mouse model system with which to study S. Typhimurium enteropathogenesis was described previously (4). In this model system, streptomycin-pretreated mice develop severe colitis after infection with S. Typhimurium, and the disease largely resembles the human infection. The streptomycin-pretreated mouse model has been used as an appropriate model system to study the mechanisms of pathogenesis and the host responses to acute enteric salmonellosis (10, 11, 22, 27, 37).
The protein poly(ADP-ribose) polymerase 1 (PARP1) is an abundant nuclear chromatin-associated enzyme with a variety of cellular functions. PARP1 has been implicated in DNA damage signaling and repair, chromatin remodeling, and transcriptional regulation (16). PARP1 knockout mice are viable and fertile and have a normal life span (40). Interestingly, they are protected from tissue injury in various inflammation-related disease models, ranging from myocardial infarction and streptozotocin-induced diabetes to lipopolysaccharide (LPS)-induced septic shock and arthritis (14, 34, 40). These phenotypes suggest an important role for PARP1 during inflammation. The protective effects observed in PARP1 knockout animals correlate with the reduced expression of proinflammatory cytokines under the control of the inducible transcription factor NF-κΒ. In line with these observations, PARP1 was found to function as a coactivator for NF-κB-dependent gene expression (15).
Here we investigated a possible role of PARP1 in S. Typhimurium-induced colitis. Employing the streptomycin mouse model of enterocolitis, we analyzed the impact of PARP1 on the Salmonella-triggered innate immune response and early development of cecal inflammation. We investigated three different time points after Salmonella infection. The time point of 6 h postinfection (p.i.) was chosen to study the very early response of the host tissue to the invading pathogen. This early response was still independent of infiltrating inflammatory cells and manifested itself mainly in the upregulation of proinflammatory genes. The time point of 8 h p.i. was chosen to study the subsequent infiltration of inflammatory cells into the infected tissue. Finally, 10 h p.i. was chosen as a late time point, when significant and severe gut inflammation was observed. This late time point was also employed to obtain a complete signature of Salmonella-induced genes in the inflamed mouse cecum. Our data reveal that a lack of PARP1, which is normally expressed in the proliferative zone of the cecum, results in a delayed proinflammatory host response. This delayed response involves reduced expression of proinflammatory genes and attenuated inflammation, with diminished infiltration of immune cells. Our findings therefore link, for the first time, PARP1 to Salmonella-induced proinflammatory gene expression, and they suggest an important role for PARP1 in timing the host response to enteric Salmonella infection.
MATERIALS AND METHODS
Bacteria.
S. Typhimurium strains were isogenic derivatives of the naturally streptomycin-resistant wild-type strain SL1344 (19). The avirulent strain S. Typhimurium SL1344 ΔinvG was also used (24). Strains were grown at 37°C in LB (0.3 M NaCl) overnight and subcultivated for 4 h as described before (9).
Mice.
Wild-type and isogenic PARP1 knockout mice (40) were bred in a C57BL/6J background. Regular backcrossings were performed to maintain isogenicity. Animals were genotyped by PCR (primer sequences for wild type, 5′-GTTGTGAACGACCTTCTGGG-3′ and 5′-CCTTCCAGAAGCAGGAGAAG-3′; and primer sequences for PARP1 knockout mice, 5′-GTTGTGAACGACCTTCTGGG-3′ and 5′-GCTTCAGTGACAACGTCGAG-3′). All mice were bred and kept in a specific-pathogen-free area. Streptomycin-pretreated mice (20 mg/animal) were infected by gavage (5 × 107 CFU) as published previously (4, 11). Bacterial colonization was determined by plating (11, 27). The minimum detectable values were 10 CFU/sample (between 25 and 150 mg) for intestinal content, 20 CFU/organ in the spleen, and 10 CFU/organ in the mesenteric lymph nodes (mLN). All animal experiments were approved and performed as legally required (Kantonales Veterinäramt Zürich license 201/2007).
Histology.
Hematoxylin-and-eosin (HE)-stained cecum cryosections were scored as described previously, by evaluating submucosal edema, polymorphonuclear leukocyte (PMN) infiltration, goblet cells, and epithelial damage, yielding a total score of 0 to 13 points (4, 27).
Immunofluorescence microscopy.
Immunofluorescence microscopy was performed as described previously (12). Cryosections were stained with rabbit anti-PARP1 antibody H-250 (Santa Cruz), mouse anti-PAR antibody 10H (kindly provided by Alexander Bürkle), fluorescein isothiocyanate (FITC)-conjugated or Cy3-conjugated secondary antibodies (Covance), and DAPI (4′,6-diamidino-2-phenylindole). Images were taken using an Olympus Mx51 (1.3 numerical aperture [NA]) fluorescence microscope.
Western blotting.
Cecum tissues were washed in ice-cold phosphate-buffered saline (PBS), shock frozen in liquid nitrogen, mechanically pulverized, and lysed in 50 mM Tris-HCl, pH 7.5, 400 mM NaCl, 25 mM NaF, and 1% (vol/vol) Triton X-100. The lysate was used for standard Western blot procedures, using the anti-PARP1 antibody H-250 (Santa Cruz) or anti-tubulin (Sigma).
RNA isolation.
The intestinal tract was excised and the cecum was isolated. The cecum was then cut into four slices. Slices 1 and 3 were washed in cold PBS to remove the cecum content and, afterwards, were frozen in liquid nitrogen in 300 μl RLT buffer (RNeasy Mini kit; Qiagen) with 1% β-mercaptoethanol and stored at −80°C until RNA extraction. Frozen tissue was homogenized in RLT buffer for 3 min at 25 Hz, using a tissue lyser (Qiagen). RNA was processed using an RNeasy Mini kit (Qiagen) and an RNase-free DNase set (Qiagen) according to the manufacturer's instructions. Slices 2 and 4 were cryo-embedded for HE staining.
Quantitative RT-PCR.
Total RNA isolated from cecum was reverse transcribed using a high-capacity cDNA reverse transcription (RT) kit (Applied Biosystems) and was used for quantitative real-time RT-PCR. Reactions were performed with a Sensimix Plus Sybr kit (Quantace) and gene-specific primer pairs in a RotorGene3000 machine (Corbett Life Science). Rps12 was used to normalize for differences in cDNA input. RNAs from ≥5 individual mice per condition were pooled. All reactions were performed in triplicate. Primer pairs (5′ to 3′) were as follows: for mouse Rps12 (mRps12), GAAGCTGCCAAAGCCTTAGA and AACTGCAACCAACCACCTTC; for mouse glyceraldehyde-3-phosphate dehydrogenase (mGAPDH), GCTACACTGAGGACCAGGTTG and GCCCCTCCTGTTATTATGGGGG; for mPARP1, GCAGTCACCCATGTTCGATGG and GCTTCTCTGGATCCACCATC; for mCxcl9, GGAGTTCGAGGAACCCTAG and CTTCTTCACATTTGCCGAGTCC; for mGbp2, CTTGAAGATGTTGAGAAGGGTGACAACC and GATCAGTTAGCTCCGTCACATAGTGC; for mCxcl10, GCACGAACTTAACCACCATCTTCC and CTACCCATTGATACATACTTGATGACAC; for mIigp1, GATAGTAGTGTGCTCAATGTTGC and GGTATATTGGGGTGTTTGTATGG; for mTgtp, CTCAGGGAGATCCAACTGTCCATCC and CTGTATGGTAGAAGCTCAGCAGTGG; for mIgtp, GCTTTGTAAGGCTTCTGAGCAGG and CTGATGAGGCGCTTGAGATAATTTGC; for mCd274, CTCCTCGCCTGCAGATAGTTCC and CTTCCTTTTCCCAGTACACCACTAACG; and for mMpa2l, CTTGGAGAAGCCTACTTCGTCTCT and AAATCTGCCAGCAGACCCTAACCT.
Whole-mouse-genome arrays.
Agilent 4x44K whole-mouse-genome arrays (Agilent Technologies) were used for the analysis of host gene expression profiles. The quantified data were subsequently loaded into GeneSpring GX 10 for further analysis. Data from individual mice were grouped according to condition. For the 10-h time point, data from two S. Typhimurium SL1344 ΔinvG-infected and three wild-type S. Typhimurium-infected wild-type mice were compared. For the 6-h time point, data from three S. Typhimurium-infected wild-type and three S. Typhimurium-infected knockout animals were compared. P values were obtained by an unpaired t test, using GeneSpring GX 10. Gene ontology (GO) and pathway analyses were also performed with GeneSpring GX 10.
Statistical analysis.
Pathoscores were analyzed by the Mann-Whitney U test, and gene expression data were analyzed by an unpaired t test. P values of <0.05 were regarded as statistically significant.
Microarray data accession number.
The microarray gene expression data are available under Gene Expression Omnibus accession no. GSE19174 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=fzaxzcmcgagwazm&acc=GSE19174).
RESULTS
Salmonella serovar Typhimurium induces colitis and a proinflammatory gene expression response in streptomycin-pretreated mice.
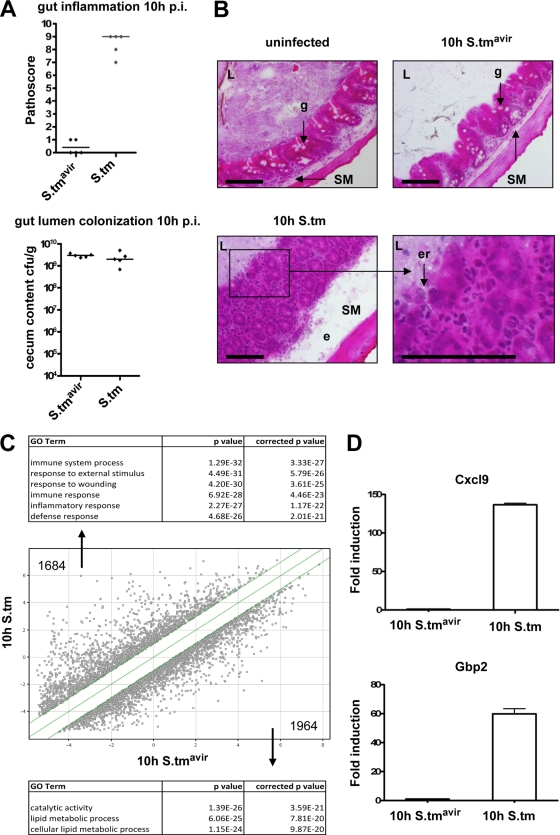
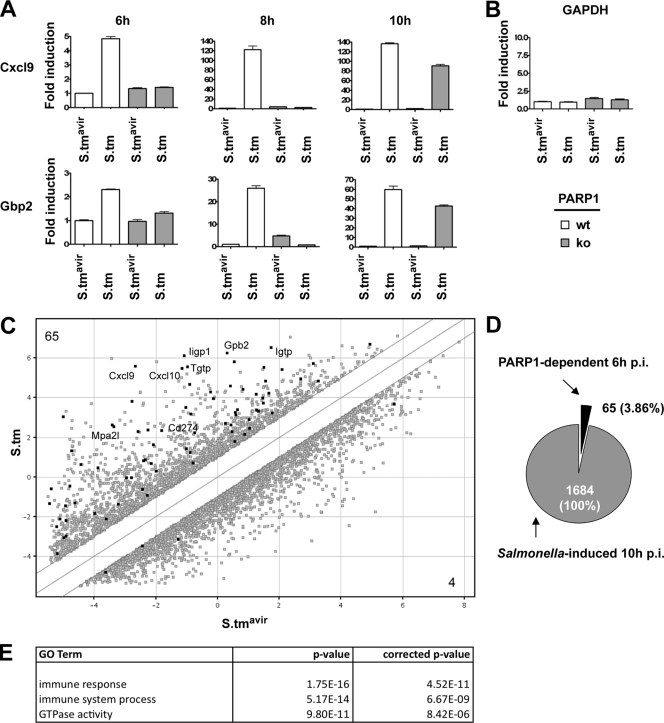
To study the molecular mechanisms of the host response to enteric salmonellosis, we employed a mouse model system for Salmonella-induced colitis (4). We used wild-type S. Typhimurium and an isogenic S. Typhimurium mutant, SB161 (S. Typhimurium SL1344 ΔinvG), which is incapable of actively invading the gut mucosa. Infection of streptomycin-pretreated wild-type C57BL/6J mice with wild-type S. Typhimurium resulted in severe colitis at 10 h p.i., with classical hallmarks of cecal inflammation, including pronounced edema in the submucosa, disruption of the crypt architecture, loss of goblet cells, epithelial erosion, and infiltration of PMNs (Fig. 1A and B). In contrast, infection with the mutant S. Typhimurium SL1344 ΔinvG did not trigger any measurable inflammatory response, although the cecum lumen was heavily colonized. Analysis of the gene transcript profiles for the cecum at 10 h p.i. revealed that 1,684 genes, mainly those involved in the immune response, were upregulated >2-fold in wild-type S. Typhimurium-infected mice compared to those in S. Typhimurium SL1344 ΔinvG-infected control mice (Fig. 1C; see Tables S1 and S3 in the supplemental material). In contrast, 1,964 genes with diverse functions not primarily related to immune system processes were downregulated >2-fold in wild-type S. Typhimurium-infected mice (see Tables S2 and S4 in the supplemental material). The observed changes in gene expression at 10 h p.i. confirmed previous gene expression analyses of S. Typhimurium-infected streptomycin-pretreated mice (8) and reflected, at least in part, the infiltration of immune cells into the cecal mucosa. We further confirmed our microarray results by quantitative RT-PCR for two highly upregulated genes, the neutrophil chemoattractant protein Cxcl9 and the interferon-inducible, antiviral guanylate-binding protein 2 (Gbp2) (Fig. 1D).
FIG. 1.
Salmonella-induced inflammation at 10 h postinfection in wild-type mice. (A) Signs of inflammation and cecum colonization were analyzed in wild-type C57BL/6J mice infected with Salmonella strain SL1344 ΔinvG (S.tmavir) or wild-type S. Typhimurium (S.tm). Cecal pathology was scored, and bacterial colonization was determined as described in Materials and Methods. Five animals were analyzed in each group. Medians are indicated. (B) Representative HE-stained cecum cryosections. L, lumen; g, goblet cell; SM, submucosa; e, edema; er, erosion of the epithelial layer. Bar = 100 μm. (C) Whole-mouse-genome array data for wild-type mice infected with either S. Typhimurium SL1344 ΔinvG or wild-type S. Typhimurium for 10 h. Total numbers of upregulated genes (>2-fold; P < 0.05) and downregulated genes (>2-fold; P < 0.05) are indicated. Highest-scoring GO terms for upregulated genes (top) and downregulated genes (bottom) are listed. (D) Quantitative RT-PCR confirmation for the indicated genes. Values obtained for S. Typhimurium SL1344 ΔinvG-infected mice were set to 1. Reactions were performed in triplicate, and mean values ± standard deviations (SD) are shown.
A pathway analysis of S. Typhimurium-induced genes suggested that NF-κB might be involved in the observed expression profile (see Table S5 in the supplemental material). Furthermore, 25% of the most upregulated genes (with >10-fold induction) contained promoters with NF-κB consensus binding sequences (data not shown).
Absence of PARP1 delays the onset of Salmonella-induced gut inflammation.
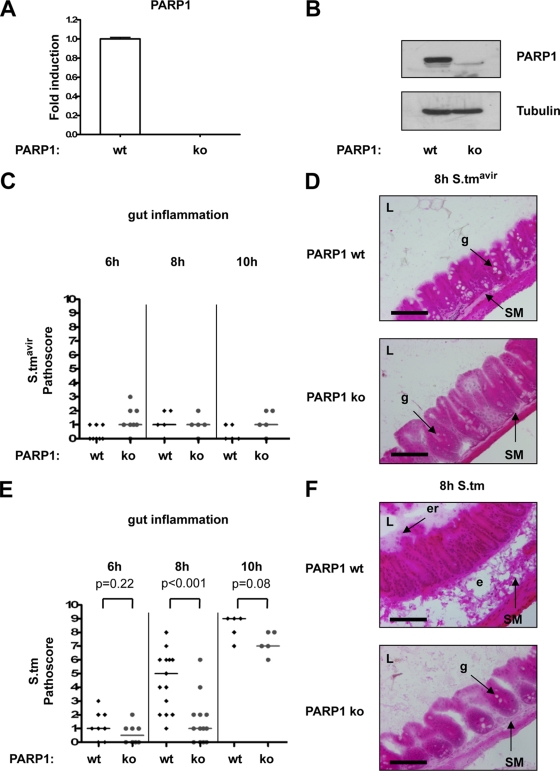
Since PARP1 is a known transcriptional coregulator of NF-κB, and since PARP1 knockout mice are protected from tissue injury in various inflammation-related disease models (1), we investigated the role of PARP1 in S. Typhimurium-induced colitis. As revealed by quantitative RT-PCR and Western blotting, PARP1 was expressed in the cecum in wild-type mice but not in PARP1 knockout mice (Fig. 2A and B). Wild-type and PARP1 knockout mice were pretreated with streptomycin and then infected with either wild-type S. Typhimurium or with the mutant strain S. Typhimurium SL1344 ΔinvG, and Salmonella-induced pathology was analyzed at 6 h to 10 h p.i. As expected, neither wild-type nor PARP1 knockout mice reacted to the S. Typhimurium mutant strain with an inflammatory response (Fig. 2C and D). In contrast, both mouse strains developed gut inflammation in a time-dependent manner when infected with wild-type S. Typhimurium (Fig. 2E). Whereas at 6 h p.i. no animal showed signs of mucosal pathology, at 8 h p.i. only wild-type animals, not PARP1 knockout animals, showed submucosal edema, partly disrupted crypts, beginning erosion of the epithelium, and loss of goblet cells (Fig. 2E and F). At 10 h p.i., signs of inflammation were also observed in PARP1 knockout mice, although they were still less severe than those in wild-type animals (Fig. 2E). These data reveal a significant delay in the host response to Salmonella infection in PARP1 knockout mice compared to that in wild-type control animals.
FIG. 2.
Absence of PARP1 delays Salmonella-induced inflammation. (A) Quantitative RT-PCR analysis of PARP1 mRNA levels in mouse ceca from wild-type (wt) and PARP1 knockout (ko) mice. Wild-type mRNA expression levels were set to 1. Reactions were performed in triplicate, and mean values ± SD are shown. (B) Western blot analysis of PARP1 protein expression in mouse ceca from wild-type and PARP1 knockout mice. (C) Histopathologic analysis of wild-type and PARP1 knockout mice 6 h (n = 8 per group), 8 h (n = 5 per group), and 10 h (n = 5 per group) after S. Typhimurium SL1344 ΔinvG (S.tmavir) infection. Medians are indicated. (D) Representative HE-stained cecum cryosections 8 h after S. Typhimurium SL1344 ΔinvG infection. L, lumen; g, goblet cell; SM, submucosa. Bar = 100 μm. (E) Histopathologic analysis of wild-type (wt) and isogenic PARP1 knockout (ko) mice 6 h (n = 8 per group), 8 h (n = 15 for wt group and n = 12 for ko group), and 10 h (n = 5 per group) after S. Typhimurium infection. Medians are indicated. P values were obtained by the Mann-Whitney U test. (F) Representative HE-stained cecum cryosections 8 h after S. Typhimurium infection. L, lumen; g, goblet cell; SM, submucosa; er, erosion of the epithelial layer. Bar = 100 μm.
Bacterial colonization is similar in wild-type and PARP1 knockout mice.
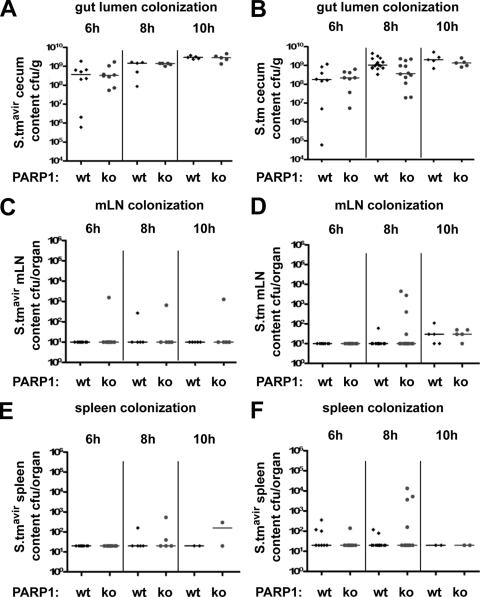
Although S. Typhimurium SL1344 ΔinvG was not able to trigger gut inflammation (Fig. 2C and D), this strain was as capable as wild-type S. Typhimurium of colonizing the gut lumen (Fig. 3A and B). Moreover, mLN and the spleen were also colonized to comparable levels by both Salmonella strains (Fig. 3C to F). While there was a slight trend toward higher colonization levels at 8 h p.i. in mLN and spleens of S. Typhimurium-infected PARP1 knockout animals than in those of wild-type mice, this difference was not statistically significant (P > 0.05; Mann-Whitney U test) (Fig. 3C to F). Thus, the early defect in mucosal immunity observed in PARP1 knockout mice (Fig. 2) did not result in a strong increase in bacterial translocation to the mLN and spleen. The bacterial translocation to these organs might therefore occur in a PARP1-independent manner, either already before an inflammatory response is triggered in the cecum or via the Payer's patches of the small intestine, where Salmonella-induced inflammation typically does not occur.
FIG. 3.
Bacterial colonization is similar in wild-type and PARP1 knockout mice. (A) Cecum colonization of wild-type (wt) and PARP1 knockout (ko) mice after S. Typhimurium SL1344 ΔinvG (S.tmavir) infection. Medians are indicated. (B) Cecum colonization of wild-type and PARP1 knockout mice after wild-type S. Typhimurium infection. Medians are indicated. (C) mLN colonization of wild-type and PARP1 knockout mice after S. Typhimurium SL1344 ΔinvG infection. Medians are indicated. (D) mLN colonization of wild-type and PARP1 knockout mice after wild-type S. Typhimurium infection. Medians are indicated. (E) Spleen colonization of wild-type and PARP1 knockout mice after S. Typhimurium SL1344 ΔinvG infection. Medians are indicated. (F) Spleen colonization of wild-type and PARP1 knockout mice after wild-type S. Typhimurium infection. Medians are indicated.
PARP1 is expressed in the proliferative zone of cecum crypts, and its expression and activity are not markedly affected by S. Typhimurium infection.
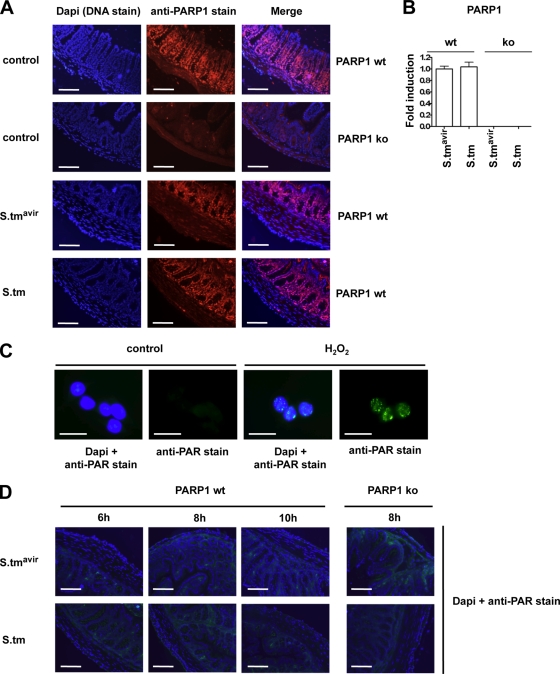
PARP1 is a stably expressed and highly abundant nuclear enzyme. When we investigated PARP1 distribution in the mouse cecum by immunofluorescence microscopy, we found PARP1 to be expressed predominantly in the proliferative zone of the crypts (Fig. 4A). PARP1 expression and tissue distribution did not change markedly after 6 h of Salmonella infection (Fig. 4A), and PARP1 mRNA levels also remained constant (Fig. 4B). Also, at later time points, we did not observe changes in PARP1 mRNA or protein levels and distribution (data not shown). Moreover, although we were able to detect poly(ADP-ribose) in H2O2-treated AGS cells (Fig. 4C), we did not detect poly(ADP-ribose) synthesized by PARP1 in cecum cryosections 6 h to 10 h after Salmonella infection (Fig. 4D). Thus, neither PARP1 expression and tissue distribution nor the detectable levels of poly(ADP-ribose) were strongly affected by S. Typhimurium infection.
FIG. 4.
PARP1 is expressed in the proliferative zone of cecum crypts, and its expression and activity are not markedly affected by S. Typhimurium infection. (A) PARP1 immunofluorescence in cecum cryosections from uninfected wild-type (wt) and PARP1 knockout (ko) mice and from wild-type mice infected with S. Typhimurium SL1344 ΔinvG (S.tmavir) or wild-type S. Typhimurium (S.tm) for 6 h. Bar = 100 μm. (B) Quantitative RT-PCR for PARP1 at 6 h p.i. Values obtained for S. Typhimurium SL1344 ΔinvG-infected wild-type mice were set to 1. Reactions were performed in triplicate, and mean values ± SD are shown. (C) Poly(ADP-ribose) (PAR) immunofluorescence in AGS gastric epithelial cells after treatment for 10 min with 10 mM H2O2. Bar = 10 μm. (D) PAR immunofluorescence in cecum cryosections from wild-type and PARP1 knockout mice infected with S. Typhimurium SL1344 ΔinvG or wild-type S. Typhimurium for the indicated times. Bar = 100 μm.
PARP1 is required for efficient gene expression after Salmonella infection.
To obtain more molecular insights into the delayed pathophysiology observed in PARP1 knockout mice compared to that in wild-type mice, proinflammatory gene expression in cecum preparations was analyzed by quantitative RT-PCR. Remarkably, the relative expression of Cxcl9 and Gbp2 was robustly increased at 6 h and 8 h p.i. in ceca from wild-type mice but not in those from PARP1 knockout animals (Fig. 5A). In line with the histopathologic data, both genes were almost as efficiently transcribed in knockout animals as in wild-type controls at 10 h p.i. (Fig. 5A). Importantly, the proinflammatory gene expression at 6 h p.i. represents an early, primary host response of the mucosal epithelial cell layer to the invading pathogen, which precedes and is still independent of the infiltration of immune cells (Fig. 2). The induction of Cxcl9 and Gbp2 after S. Typhimurium infection was significantly reduced in PARP1 knockout animals at this early time point, although the basal expression levels of these proinflammatory genes in control strain-infected animals were unchanged (Fig. 5A). Furthermore, inflammation-unrelated genes, such as GAPDH, were expressed in a PARP1-independent manner (Fig. 5B). Together, these data strongly suggest that the immediate proinflammatory gene expression response of the host tissue was specifically impaired in mice lacking PARP1.
FIG. 5.
A subset of Salmonella-induced genes requires PARP1 for efficient expression. (A) Quantitative RT-PCR analysis of the indicated genes at the indicated time points. Values obtained for S. Typhimurium SL1344 ΔinvG (S.tmavir)-infected wild-type mice were set to 1. Reactions were performed in triplicate, and mean values ± SD are shown. (B) Quantitative RT-PCR for GAPDH at 6 h p.i. Values obtained for S. Typhimurium SL1344 ΔinvG-infected wild-type mice were set to 1. Reactions were performed in triplicate, and mean values ± SD are shown. (C) Comparison of whole-mouse-genome microarray data. Salmonella-induced and Salmonella-repressed genes (wild-type S. Typhimurium over S. Typhimurium SL1344 ΔinvG; 10 h p.i.) are shown in gray. Salmonella-induced and Salmonella-repressed genes (wild-type S. Typhimurium over S. Typhimurium SL1344 ΔinvG; 10 h p.i.) whose expression was reduced at least 1.5-fold in S. Typhimurium-infected PARP1 knockout mice compared to S. Typhimurium-infected wild-type mice (6 h p.i.) are shown in black and are indicated by protein names. (D) Relative number of PARP1-dependent, Salmonella-induced genes. (E) Gene ontology (GO) analysis of PARP1-dependent, Salmonella-induced genes.
PARP1 is required for efficient expression of a subset of Salmonella-induced genes.
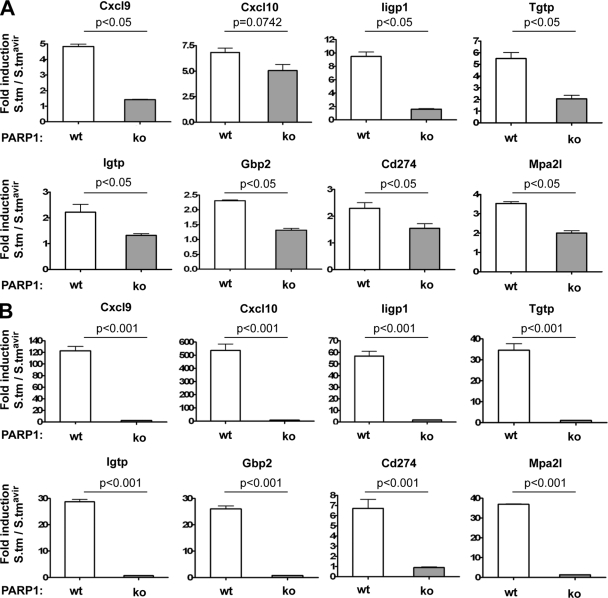
To determine the extent of proinflammatory genes which depend on PARP1 for efficient expression after Salmonella infection, whole-genome arrays for wild-type and PARP1 knockout mice infected with S. Typhimurium for 6 h were examined. Of 1,684 genes which showed an upregulation at 10 h p.i., 65 genes (3.86%) were dependent on PARP1 at 6 h p.i. (Fig. 5C and D; see Table S6 in the supplemental material). In contrast, only 4 genes were repressed by Salmonella infection and expressed less in PARP1 knockout mice than in wild-type controls (Fig. 5C). A gene ontology analysis revealed that many of these PARP1-dependent genes are involved in the immune response (Fig. 5E; see Table S7 in the supplemental material) and are related to interferon signaling, most prominently to gamma interferon (IFN-γ) signaling (see Table S8 in the supplemental material). Interferon signaling is highly upregulated after Salmonella infection and contributes greatly to host inflammation (8, 32). The observed defect in interferon signaling in PARP1 knockout mice might thus be responsible for the delayed onset of inflammation. We confirmed several PARP1-dependent genes at 6 h p.i. by quantitative RT-PCR and also extended the analysis to the 8-h time point (Fig. 6A and B). In line with the pathological differences between wild-type and PARP1 knockout mice at 8 h p.i., all genes were greatly reduced in knockout animals at this later time point (Fig. 6A and B; Table 1). Together, our data provide strong evidence that PARP1 is required for the efficient expression of a subset of Salmonella-induced proinflammatory genes in an in vivo mouse model. Efficient PARP1-dependent expression of these genes very likely contributes to the activation of resident immune cells and/or recruitment of inflammatory cells to trigger an inflammatory response. Thus, our data identify PARP1 as a host factor controlling the early phase of Salmonella-induced inflammation.
FIG. 6.
Impaired gene induction in PARP knockout mice at 6 h p.i. and at 8 h p.i. (A) Quantitative RT-PCR analysis of the indicated genes at 6 h p.i. Fold inductions over S. Typhimurium SL1344 ΔinvG-infected controls are presented. Reactions were performed in triplicate, and mean values ± SD are shown. (B) Quantitative RT-PCR analysis of the indicated genes at 8 h p.i. Fold inductions over S. Typhimurium SL1344 ΔinvG-infected controls are presented. Reactions were performed in triplicate, and mean values ± SD are shown.
TABLE 1.
Summary of gene expression analysis for selected genes and time points after Salmonella infectiona
| Gene no. | Name |
Salmonella induction (wild-type strain/mutant strain) |
PARP1 dependence (knockout mice/ wild-type mice) |
||||
|---|---|---|---|---|---|---|---|
| Fold induction by array data at 10 h p.i. | Rank | Fold induction by qRT-PCR at 6 h p.i. | Fold reduction by array at 6 h p.i. | Fold reduction by qRT-PCR at: |
|||
| 6 h p.i. | 8 h p.i. | ||||||
| 1 | Cxcl9 | 309.33 | 2 | 4.84 | 2.33 | 3.43 | 45.23 |
| 2 | Iigp1 | 149.09 | 8 | 9.48 | 3.46 | 5.23 | 35.70 |
| 3 | Cxcl10 | 98.23 | 15 | 6.83 | 1.72 | 1.35 | 81.95 |
| 4 | Tgtp | 93.33 | 17 | 5.50 | 1.56 | 2.69 | 33.54 |
| 5 | Gbp2 | 61.07 | 37 | 2.31 | 2.91 | 1.76 | 34.16 |
| 6 | Mpa2l | 59.90 | 38 | 3.53 | 2.32 | 1.77 | 29.87 |
| 7 | Socs1 | 47.60 | 46 | 1.54 | 2.10 | 0.92 | 19.11 |
| 8 | Igtp | 27.75 | 80 | 2.22 | 3.52 | 1.68 | 42.80 |
| 9 | Gbp6 | 24.85 | 87 | ND | 2.00 | ND | ND |
| 10 | Gbp3 | 22.79 | 100 | ND | 2.37 | ND | ND |
| 11 | Iigp2 | 16.52 | 139 | ND | 2.61 | ND | ND |
| 12 | Cd274 | 7.93 | 295 | 2.30 | 2.48 | 1.49 | 7.56 |
| 13 | Stat1 | 7.56 | 308 | 1.63 | 1.73 | 1.10 | 11.71 |
| 14 | Il18bp | 7.20 | 321 | ND | 2.23 | ND | ND |
Gene expression data obtained by whole-mouse-genome arrays and quantitative RT-PCR (qRT-PCR) are presented. ND, not determined.
DISCUSSION
Here we show for the first time that the nuclear protein PARP1 is directly linked to salmonellosis. In an in vivo mouse model of Salmonella-induced colitis, we demonstrated that PARP1 is a novel host factor whose absence delays inflammation after Salmonella infection. PARP1 is predominantly expressed in the proliferative zone of the crypts and is required for the efficient early expression of several Salmonella-induced proinflammatory genes. Reduced early proinflammatory gene expression in mice lacking PARP1 correlates with and may be causally responsible for delayed infiltration of immune cells and, consequently, delayed onset of inflammation. Together, our findings reveal that PARP1 is required for an efficient early innate immune response against Salmonella infection.
The function of PARP1 as a transcriptional coregulator of NF-κΒ is now well established (1, 15-17). PARP1 directly interacts with both subunits of NF-κΒ (p65 and p50) inside the nucleus, where it most likely functions as a bridging factor or adapter molecule to connect NF-κΒ with the basal transcription machinery (15, 17). Interestingly, this function was reported to be independent of PARP1 DNA binding and enzymatic activity (13). In line with this report, we did not observe detectable amounts of poly(ADP-ribose) formation in S. Typhimurium-infected animals (Fig. 4C and D), indicating that polymers of ADP-ribose are not required for the NF-κB coactivator function of PARP1. We cannot completely exclude the possibility, however, that undetectable short chains of poly(ADP-ribose) or mono(ADP-ribose) were synthesized by PARP1 following S. Typhimurium infection or that poly(ADP-ribose) levels below the detection limit were generated. PARP inhibitors are commercially available and could be used in future studies to block ADP-ribosylation in Salmonella-infected mice. It should be noted, however, that PARP inhibitors show only moderate specificity toward PARP1 and also inhibit other ADP-ribosylating enzymes, a fact which poses an important drawback to this approach.
Besides NF-κB, other transcription factors also require PARP1 as a cofactor (20, 28, 31, 38). PARP1-dependent genes typically are not completely silenced in cells lacking PARP1, but instead show a 30 to 70% reduced expression (our unpublished observation). Moreover, PARP1 seems to be more important for induced gene expression after cell stimulation than for basal gene expression under nonstimulated conditions. In a study by Saenz et al., only 93 genes (0.66%) were found to be expressed differentially in unstimulated wild-type and PARP1 knockout T cells, while 203 genes (1.44%) were found to be expressed differentially after anti-CD3/anti-CD28 stimulation (30). In addition to its transcriptional coactivator function, PARP1 was very recently also described to indirectly promote the mRNA stability of the single cytokine IP-10 through the activation of p38 (7). Although the underlying molecular mechanism is currently not clear, reduced cytokine mRNA stability may also contribute to the reduced mRNA levels observed in this study of PARP1-deficient mice.
IFN-γ is the sole type II interferon and is often found among the cytokines whose expression is most prominently induced during Salmonella infection (8, 21, 32). Production of IFN-γ in the early phase of intestinal inflammation contributes to antimicrobial responses in the intestinal mucosa (29) and may be required to amplify initial responses generated by bacterium-host-cell interaction (32). Although a gene ontology analysis of S. Typhimurium-induced genes revealed that many of the PARP1-dependent genes are IFN-γ target genes, IFN-γ gene expression was not significantly different in wild-type and PARP1 knockout animals (see Tables S1 and S6 in the supplemental material; also data not shown), suggesting that IFN-γ levels per se are not the primary cause for the impaired expression of IFN-γ-related genes in PARP1 knockout mice. IFN-γ priming was reported to increase TLR expression and to promote NF-κB-mediated gene expression (21). Moreover, many of the identified genes, including those encoding Cxcl9, Cxcl10, Iigp1, and Cd274, are reported NF-κB target genes or contain κB sites and play important roles in inflammation (39). In line with the notion that NF-κB may be involved in the regulation of PARP1-dependent IFN-γ response genes, STAT-1 alone, the primary transcription factor downstream of IFN-γ signaling, is often not sufficient for transcription of target genes (6, 33, 35). Thus, the impaired expression of IFN-γ-related genes in PARP1 knockout mice after S. Typhimurium infection might be explained by the combined presence of STAT-1 and NF-κB at the promoter elements of responsive genes and PARP1 regulating the induced gene expression. Such a mechanism may not necessarily be limited to IFN-γ signaling. In fact, several PARP1-dependent genes are also related to type I interferon signaling (see Tables S6 and S8 in the supplemental material), and since there is some overlap between type I and type II interferon-induced genes, PARP1 deficiency may result in a more general defect in interferon signaling.
Interestingly, we found PARP1 expressed predominantly in the highly proliferative zone of cecum crypts. Differential expression of PARP1 within the tissue was also observed in mouse spleen sections by immunofluorescence microscopy (data not shown), and we are currently investigating whether PARP1 expression is also restricted to proliferating cells in this tissue. In the proliferative zone of the cecum crypts, PARP1-expressing, proliferating cells most likely represent absorptive enterocytes and/or goblet cells (see references 27 and 36). A region of high cell turnover may be especially vulnerable to pathogenic insults, because proliferative cells such as migrating enterocytes have to coordinate rapid DNA replication with the transcription of proinflammatory mediators. Note that PARP1 has been implicated in cell proliferation before (5, 25, 31).
Although our analyses focused on the very early response of the host tissue directly in contact with the pathogen, we cannot completely rule out that PARP1 also regulates functions in other cell types besides the infected cecum (e.g., circulating lymphocytes), which may also contribute to Salmonella-induced inflammation. One way to address this point would be the use of conditional PARP1 knockout mice, which, unfortunately, are not available at present. Importantly, the wild-type and PARP1 knockout mice used in this study showed comparable cecum compositions, as revealed by microscopy, showed no differences in the basal expression of proinflammatory genes (Fig. 5A), and had comparable colonization levels after Salmonella infection (Fig. 3), all indicating that the intestinal barrier function of PARP1 knockout mice was intact. It was reported earlier that treatment of interleukin-10 (IL-10)-deficient mice with the unspecific PARP inhibitor 3-aminobenzamide attenuates chronic colitis, in part by altering colonic permeability (23). Although we cannot formally rule out that PARP1 knockout mice have an altered intestinal permeability, the observation that Salmonella colonization in the mLN and spleen was similar in wild-type and PARP1 knockout animals (Fig. 3) indicates that differences in permeability and, consequently, in Salmonella invasion are most likely not responsible for the delayed inflammation in PARP1 knockout mice.
While we observed a significantly reduced proinflammatory response in PARP1 knockout animals at early time points after Salmonella infection, PARP1 knockout mice also developed severe colitis later. Therefore, the innate immune system does not completely rely on PARP1-dependent mechanisms, but instead, it can compensate for the lack of PARP1 at later time points. We speculate that the immune system senses the dampened response to Salmonella infection in the absence of PARP1 and then triggers delayed, compensatory, PARP1-independent mechanisms to achieve an adequate host response. Thus, the physiological function of PARP1 may be to boost the early innate immune response to invading pathogens in order to achieve a rapid and efficient host defense to parry the insult. Whether the late adaptive inflammatory response in the absence of PARP1 is functionally equivalent to or differs from the response in wild-type animals requires future studies.
In conclusion, our data directly link PARP1 to the pathogenesis of Salmonella-induced inflammation and provide a mechanistic explanation for how the absence of PARP1 delays colitis. These findings increase our understanding of how the nuclear multifunctional protein PARP1 is involved in proinflammatory gene expression and how it is implicated in proinflammatory diseases. Our results also shed light on the regulation of the early host response after Salmonella infection and may prove beneficial for the design of new therapeutic strategies to combat salmonellosis.
Supplementary Material
Acknowledgments
We are grateful to Alexander Bürkle (University of Konstanz, Germany) for the generous gift of anti-PAR antibody 10H. Paul Hassa (IVBMB, University of Zurich, Switzerland) kindly coordinated animal breedings. Andrea Patrignani (Functional Genomics Center Zurich, Switzerland) is acknowledged for excellent technical support. We gratefully acknowledge the excellent support of our animal experiments by the staff of the animal facility RCHCI (ETH Zurich, Switzerland), in particular, Thomas Weber, Susanne Freedrich, and Jörg Fehr.
This work was supported by grants to W.-D.H. from the Swiss National Science Foundation (310000-113623/1) and the European Union (SavinMucoPath grant 032296). Research on ADP-ribosyltransferases in the laboratory of M.O.H. is supported by the Swiss National Science Foundation (SNF 31-122421).
We declare no conflicts of interest.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 1 June 2010.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Aguilar-Quesada, R., J. A. Munoz-Gamez, D. Martin-Oliva, A. Peralta-Leal, R. Quiles-Perez, J. M. Rodriguez-Vargas, M. Ruiz de Almodovar, C. Conde, A. Ruiz-Extremera, and F. J. Oliver. 2007. Modulation of transcription by PARP-1: consequences in carcinogenesis and inflammation. Curr. Med. Chem. 14:1179-1187. [DOI] [PubMed] [Google Scholar]
- 2.Akira, S., and K. Takeda. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4:499-511. [DOI] [PubMed] [Google Scholar]
- 3.Akira, S., S. Uematsu, and O. Takeuchi. 2006. Pathogen recognition and innate immunity. Cell 124:783-801. [DOI] [PubMed] [Google Scholar]
- 4.Barthel, M., S. Hapfelmeier, L. Quintanilla-Martinez, M. Kremer, M. Rohde, M. Hogardt, K. Pfeffer, H. Russmann, and W. D. Hardt. 2003. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect. Immun. 71:2839-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carbone, M., M. N. Rossi, M. Cavaldesi, A. Notari, P. Amati, and R. Maione. 2008. Poly(ADP-ribosyl)ation is implicated in the G0-G1 transition of resting cells. Oncogene 27:6083-6092. [DOI] [PubMed] [Google Scholar]
- 6.Chan, E. D., and D. W. Riches. 2001. IFN-gamma + LPS induction of iNOS is modulated by ERK, JNK/SAPK, and p38(MAPK) in a mouse macrophage cell line. Am. J. Physiol. Cell Physiol. 280:C441-C450. [DOI] [PubMed] [Google Scholar]
- 7.Galbis-Martinez, M., L. Saenz, P. Ramirez, P. Parrilla, and J. Yelamos. 2010. Poly(ADP-ribose) polymerase-1 modulates interferon-gamma-inducible protein (IP)-10 expression in murine embryonic fibroblasts by stabilizing IP-10 mRNA. Mol. Immunol. 47:1492-1499. [DOI] [PubMed] [Google Scholar]
- 8.Godinez, I., T. Haneda, M. Raffatellu, M. D. George, T. A. Paixao, H. G. Rolan, R. L. Santos, S. Dandekar, R. M. Tsolis, and A. J. Baumler. 2008. T cells help to amplify inflammatory responses induced by Salmonella enterica serotype Typhimurium in the intestinal mucosa. Infect. Immun. 76:2008-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hapfelmeier, S., K. Ehrbar, B. Stecher, M. Barthel, M. Kremer, and W. D. Hardt. 2004. Role of the Salmonella pathogenicity island 1 effector proteins SipA, SopB, SopE, and SopE2 in Salmonella enterica subspecies 1 serovar Typhimurium colitis in streptomycin-pretreated mice. Infect. Immun. 72:795-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hapfelmeier, S., and W. D. Hardt. 2005. A mouse model for S. typhimurium-induced enterocolitis. Trends Microbiol. 13:497-503. [DOI] [PubMed] [Google Scholar]
- 11.Hapfelmeier, S., A. J. Muller, B. Stecher, P. Kaiser, M. Barthel, K. Endt, M. Eberhard, R. Robbiani, C. A. Jacobi, M. Heikenwalder, C. Kirschning, S. Jung, T. Stallmach, M. Kremer, and W. D. Hardt. 2008. Microbe sampling by mucosal dendritic cells is a discrete, MyD88-independent step in DeltainvG S. Typhimurium colitis. J. Exp. Med. 205:437-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hapfelmeier, S., B. Stecher, M. Barthel, M. Kremer, A. J. Muller, M. Heikenwalder, T. Stallmach, M. Hensel, K. Pfeffer, S. Akira, and W. D. Hardt. 2005. The Salmonella pathogenicity island (SPI)-2 and SPI-1 type III secretion systems allow Salmonella serovar Typhimurium to trigger colitis via MyD88-dependent and MyD88-independent mechanisms. J. Immunol. 174:1675-1685. [DOI] [PubMed] [Google Scholar]
- 13.Hassa, P. O., M. Covic, S. Hasan, R. Imhof, and M. O. Hottiger. 2001. The enzymatic and DNA binding activity of PARP-1 are not required for NF-kappa B coactivator function. J. Biol. Chem. 276:45588-45597. [DOI] [PubMed] [Google Scholar]
- 14.Hassa, P. O., S. S. Haenni, M. Elser, and M. O. Hottiger. 2006. Nuclear ADP-ribosylation reactions in mammalian cells: where are we today and where are we going? Microbiol. Mol. Biol. Rev. 70:789-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hassa, P. O., and M. O. Hottiger. 1999. A role of poly (ADP-ribose) polymerase in NF-kappaB transcriptional activation. Biol. Chem. 380:953-959. [DOI] [PubMed] [Google Scholar]
- 16.Hassa, P. O., and M. O. Hottiger. 2008. The diverse biological roles of mammalian PARPS, a small but powerful family of poly-ADP-ribose polymerases. Front. Biosci. 13:3046-3082. [DOI] [PubMed] [Google Scholar]
- 17.Hassa, P. O., and M. O. Hottiger. 2002. The functional role of poly(ADP-ribose)polymerase 1 as novel coactivator of NF-kappaB in inflammatory disorders. Cell. Mol. Life Sci. 59:1534-1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayden, M. S., and S. Ghosh. 2008. Shared principles in NF-kappaB signaling. Cell 132:344-362. [DOI] [PubMed] [Google Scholar]
- 19.Hoiseth, S. K., and B. A. Stocker. 1981. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature 291:238-239. [DOI] [PubMed] [Google Scholar]
- 20.Hossain, M. B., P. Ji, R. Anish, R. H. Jacobson, and S. Takada. 2009. Poly(ADP-ribose) polymerase 1 interacts with nuclear respiratory factor 1 (NRF-1) and plays a role in NRF-1 transcriptional regulation. J. Biol. Chem. 284:8621-8632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu, X., and L. B. Ivashkiv. 2009. Cross-regulation of signaling pathways by interferon-gamma: implications for immune responses and autoimmune diseases. Immunity 31:539-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ilg, K., K. Endt, B. Misselwitz, B. Stecher, M. Aebi, and W. D. Hardt. 2009. O-antigen-negative Salmonella enterica serovar Typhimurium is attenuated in intestinal colonization but elicits colitis in streptomycin-treated mice. Infect. Immun. 77:2568-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jijon, H. B., T. Churchill, D. Malfair, A. Wessler, L. D. Jewell, H. G. Parsons, and K. L. Madsen. 2000. Inhibition of poly(ADP-ribose) polymerase attenuates inflammation in a model of chronic colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 279:G641-G651. [DOI] [PubMed] [Google Scholar]
- 24.Kaniga, K., J. C. Bossio, and J. E. Galan. 1994. The Salmonella typhimurium invasion genes invF and invG encode homologues of the AraC and PulD family of proteins. Mol. Microbiol. 13:555-568. [DOI] [PubMed] [Google Scholar]
- 25.Kun, E., E. Kirsten, P. I. Bauer, and C. P. Ordahl. 2006. Quantitative correlation between cellular proliferation and nuclear poly (ADP-ribose) polymerase (PARP-1). Int. J. Mol. Med. 17:293-300. [PubMed] [Google Scholar]
- 26.Mogensen, T. H. 2009. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 22:240-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muller, A. J., C. Hoffmann, M. Galle, A. Van Den Broeke, M. Heikenwalder, L. Falter, B. Misselwitz, M. Kremer, R. Beyaert, and W. D. Hardt. 2009. The S. Typhimurium effector SopE induces caspase-1 activation in stromal cells to initiate gut inflammation. Cell Host Microbe 6:125-136. [DOI] [PubMed] [Google Scholar]
- 28.Olabisi, O. A., N. Soto-Nieves, E. Nieves, T. T. Yang, X. Yang, R. Y. Yu, H. Y. Suk, F. Macian, and C. W. Chow. 2008. Regulation of transcription factor NFAT by ADP-ribosylation. Mol. Cell. Biol. 28:2860-2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rhee, S. J., W. A. Walker, and B. J. Cherayil. 2005. Developmentally regulated intestinal expression of IFN-gamma and its target genes and the age-specific response to enteric Salmonella infection. J. Immunol. 175:1127-1136. [DOI] [PubMed] [Google Scholar]
- 30.Saenz, L., J. J. Lozano, R. Valdor, A. Baroja-Mazo, P. Ramirez, P. Parrilla, P. Aparicio, L. Sumoy, and J. Yelamos. 2008. Transcriptional regulation by poly(ADP-ribose) polymerase-1 during T cell activation. BMC Genomics 9:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakamaki, J., H. Daitoku, K. Yoshimochi, M. Miwa, and A. Fukamizu. 2009. Regulation of FOXO1-mediated transcription and cell proliferation by PARP-1. Biochem. Biophys. Res. Commun. 382:497-502. [DOI] [PubMed] [Google Scholar]
- 32.Santos, R. L., M. Raffatellu, C. L. Bevins, L. G. Adams, C. Tukel, R. M. Tsolis, and A. J. Baumler. 2009. Life in the inflamed intestine, Salmonella style. Trends Microbiol. 17:498-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schroder, K., P. J. Hertzog, T. Ravasi, and D. A. Hume. 2004. Interferon-gamma: an overview of signals, mechanisms and functions. J. Leukoc. Biol. 75:163-189. [DOI] [PubMed] [Google Scholar]
- 34.Shall, S., and G. de Murcia. 2000. Poly(ADP-ribose) polymerase-1: what have we learned from the deficient mouse model? Mutat. Res. 460:1-15. [DOI] [PubMed] [Google Scholar]
- 35.Singh, K., J. L. Balligand, T. A. Fischer, T. W. Smith, and R. A. Kelly. 1996. Regulation of cytokine-inducible nitric oxide synthase in cardiac myocytes and microvascular endothelial cells. Role of extracellular signal-regulated kinases 1 and 2 (ERK1/ERK2) and STAT1 alpha. J. Biol. Chem. 271:1111-1117. [DOI] [PubMed] [Google Scholar]
- 36.Stecher, B., A. J. Macpherson, S. Hapfelmeier, M. Kremer, T. Stallmach, and W. D. Hardt. 2005. Comparison of Salmonella enterica serovar Typhimurium colitis in germfree mice and mice pretreated with streptomycin. Infect. Immun. 73:3228-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valdez, Y., G. A. Grassl, J. A. Guttman, B. Coburn, P. Gros, B. A. Vallance, and B. B. Finlay. 2009. Nramp1 drives an accelerated inflammatory response during Salmonella-induced colitis in mice. Cell. Microbiol. 11:351-362. [DOI] [PubMed] [Google Scholar]
- 38.Valdor, R., V. Schreiber, L. Saenz, T. Martinez, A. Munoz-Suano, M. Dominguez-Villar, P. Ramirez, P. Parrilla, E. Aguado, F. Garcia-Cozar, and J. Yelamos. 2008. Regulation of NFAT by poly(ADP-ribose) polymerase activity in T cells. Mol. Immunol. 45:1863-1871. [DOI] [PubMed] [Google Scholar]
- 39.Vallabhapurapu, S., and M. Karin. 2009. Regulation and function of NF-kappaB transcription factors in the immune system. Annu. Rev. Immunol. 27:693-733. [DOI] [PubMed] [Google Scholar]
- 40.Wang, Z. Q., B. Auer, L. Stingl, H. Berghammer, D. Haidacher, M. Schweiger, and E. F. Wagner. 1995. Mice lacking ADPRT and poly(ADP-ribosyl)ation develop normally but are susceptible to skin disease. Genes Dev. 9:509-520. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.