Abstract
ExoU is a potent effector protein that causes rapid host cell death upon injection by the type III secretion system of Pseudomonas aeruginosa. The N-terminal half of ExoU contains a patatin-like phospholipase A2 (PLA2) domain that requires the host cell cofactor superoxide dismutase 1 (SOD1) for activation, while the C-terminal 137 amino acids constitute a membrane localization domain (MLD). Previous studies had utilized insertion and deletion mutations to show that portions of the MLD are required for membrane localization and catalytic activity. Here we further characterize this domain by identifying six residues that are essential for ExoU activity. Substitutions at each of these positions resulted in abrogation of membrane targeting, decreased ExoU-mediated cytotoxicity, and reductions in PLA2 activity. Likewise, each of the six MLD residues was necessary for full virulence in cell culture and murine models of acute pneumonia. Purified recombinant ExoU proteins with substitutions at five of the six residues were not activated by SOD1, suggesting that these five residues are critical for activation by this cofactor. Interestingly, these same five ExoU proteins were partially activated by HeLa cell extracts, suggesting that a host cell cofactor other than SOD1 is capable of modulating the activity of ExoU. These findings add to our understanding of the role of the MLD in ExoU-mediated virulence.
Pseudomonas aeruginosa is an opportunistic human pathogen that is a common cause of infections in hospitalized patients and individuals with cystic fibrosis (24). One of the principal virulence determinants of P. aeruginosa is its type III secretion system, which is essential for the injection of four effector proteins into host cells (12, 14). Two of these effectors, ExoS and ExoT, are bifunctional enzymes that have N-terminal Rho GTPase-activating protein (Rho-GAP) domains as well as C-terminal ADP-ribosyltransferase (ADP-RT) domains (3). The Rho-GAP domains of both proteins function to reorganize the host cell actin cytoskeleton, while the ADP-RT domains act upon different substrates that lead to distinct effects. ExoS ADP-ribosylates Ras and Rab proteins in vivo, resulting in cell rounding and apoptosis (1, 2, 6, 13, 18), while ExoT acts as an anti-internalization factor by specifically targeting CT10 regulator of kinase (Crk) proteins I and II (36). The ADP-RT activities of these effectors require interaction with the eukaryotic scaffold protein 14-3-3, which functions as an activating cofactor for both ExoS and ExoT (5, 16, 22, 38). ExoY has adenylate cyclase activity that also requires interaction with a host cell cofactor, although the identity of this factor remains unknown (19, 37, 38).
The fourth effector protein of the P. aeruginosa type III secretion system, ExoU, has the greatest impact on infection severity and induces rapid cell lysis upon injection into host cells (11, 15, 33, 34). The N-terminal half of ExoU encompasses a patatin-like domain that has phospholipase A2 (PLA2) activity (23, 30). Substitutions of putative active-site residues within this domain abrogate ExoU phospholipase activity, cytotoxicity, and virulence (23, 25, 30, 31). PLA2 activity requires interaction with a host cofactor, which has recently been identified as superoxide dismutase 1 (SOD1) (4, 28).
Another feature of ExoU is its ability to localize to the plasma membrane of injected cells (23, 27, 35). The C-terminal 137 amino acids of ExoU, referred to as the membrane localization domain (MLD), constitute a domain that is sufficient for this process (27). We previously used linker-scanning mutagenesis to show that 5-amino-acid insertions within certain regions of the MLD not only abolished membrane localization but also eliminated PLA2 activity, implying that this domain may be critical for both appropriate localization and activation of ExoU (27).
In this study, we extend our previous findings and describe a detailed structure-function analysis of the MLD of ExoU. An error-prone mutagenesis screen identified six individual residues within the MLD that were essential for toxin localization and full PLA2 activity in vitro. Furthermore, substitution of these critical residues led to an attenuation of virulence relative to wild-type ExoU. Finally, in vitro PLA2 assays using purified proteins indicated that specific substitutions within the MLD abolish activation by SOD1 but not by HeLa cell lysates, suggesting that there are factors other than SOD1 that modulate ExoU within mammalian cells.
MATERIALS AND METHODS
Bacterial strains, media, and plasmids.
Escherichia coli strains XL10 Gold, S17.1, and BL21(DE3) Star were utilized for cloning experiments and were grown in Luria-Bertani broth (LB) (Table 1). When appropriate, medium was supplemented with 100 μg/ml ampicillin or 20 μg/ml tetracycline. P. aeruginosa strains (Table 1) were grown in LB or MINS medium (21). When appropriate, medium was supplemented with 100 μg/ml tetracycline, 500 μg/ml carbenicillin, or 100 μg/ml gentamicin. HeLa cervical carcinoma cells (ATCC, Manassas, VA) were grown in Eagle's minimal essential medium (ATCC) supplemented with 10% fetal bovine serum (FBS) (HyClone, Logan, UT).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Reference or source |
|---|---|---|
| E. coli strains | ||
| XL10 Gold | Tetr Δ(mcrA)183 Δ(mcrCB-hsdSMR-mrr)173 endA1 supE44 thi-1 recA1 gyrA96 relA1 lac Hte [F′ proAB lacIqZΔM15Tn10 (Tetr) Amy Camr] | Stratagene |
| BL21(DE3) Star | F−ompT hsdSB (rB− mB−) gal dcm (DE3) | Invitrogen |
| S17.1 | recA pro hsdR RP4-2-Tc::Mu-Km::Tn7 | ATCC |
| P. aeruginosa strains | ||
| PA99null | PA99 that secretes no known effectors | 34 |
| PA99null-ExoU | PA99 that secretes only ExoU | 34 |
| PA99null-S142A | PA99 that secretes only ExoU-S142A | 8 |
| PA99null-I609N | PA99 that secretes only ExoU-I609N | This study |
| PA99null-Q623R | PA99 that secretes only ExoU-Q623R | This study |
| PA99null-N627I | PA99 that secretes only ExoU-N627I | This study |
| PA99null-I654N | PA99 that secretes only ExoU-I654N | This study |
| PA99null-R661L | PA99 that secretes only ExoU-R661L | This study |
| PA99null-A678D | PA99 that secretes only ExoU-A678D | This study |
| Plasmids | ||
| pEGFP-F | Plasmid encoding GFP fused to the farnesylation sequence; used for farnesylation tag cloning | Clontech |
| pcDNA3.1-NT-GFP (pGFP) | Plasmid for expression in mammalian cells; Apr | Invitrogen |
| pcDNA3.1-NT-GFP-ExoU | exoU allele in pGFP; Apr | 25 |
| pcDNA3.1-NT-GFP-S142A | exoU allele encoding ExoU-S142A in pGFP; Apr | 25 |
| pcDNA3.1-NT-GFP-LS608 | exoU allele encoding ExoU-LS608 (MFKH inserted before residue 608) in pGFP; Apr | 27 |
| pcDNA3.1-NT-GFP-ExoU-Farn | Farnesylation sequence cloned at 3′ end of pGFP-ExoU; Apr | This study |
| pcDNA3.1-NT-GFP-LS608-Farn | Farnesylation sequence cloned at 3′ end of pGFP-LS608; Apr | This study |
| pcDNA3.1-NT-GFP-I609N | exoU allele encoding ExoU-I609N in pGFP; Apr | This study |
| pcDNA3.1-NT-GFP-Q623R | exoU allele encoding ExoU-Q623R in pGFP; Apr | This study |
| pcDNA3.1-NT-GFP-N627I | exoU allele encoding ExoU-N627I in pGFP; Apr | This study |
| pcDNA3.1-NT-GFP-I654N | exoU allele encoding ExoU-I654N in pGFP; Apr | This study |
| pcDNA3.1-NT-GFP-R661L | exoU allele encoding ExoU-R661L in pGFP; Apr | This study |
| pcDNA3.1-NT-GFP-A678D | exoU allele encoding ExoU-A678D in pGFP; Apr | This study |
| pcDNA3.1-NT-GFP-S142A-I609N | exoU allele encoding ExoU-S142A-I609N in pGFP; Apr | This study |
| pcDNA3.1-NT-GFP-S142A-Q623R | exoU allele encoding ExoU-S142A-Q623R in pGFP; Apr | This study |
| pcDNA3.1-NT-GFP-S142A-N627I | exoU allele encoding ExoU-S142A-N627I in pGFP; Apr | This study |
| pcDNA3.1-NT-GFP-S142A-I654N | exoU allele encoding ExoU-S142A-I654N in pGFP; Apr | This study |
| pcDNA3.1-NT-GFP-S142A-R661L | exoU allele encoding ExoU-S142A-R661L in pGFP; Apr | This study |
| pcDNA3.1-NT-GFP-S142A-A678D | exoU allele encoding ExoU-S142A-A678D in pGFP; Apr | This study |
Random mutagenesis of the MLD.
The EZ clone mutagenesis system (Stratagene, La Jolla, CA) was utilized to generate a library of point mutations within the MLD-encoding portion of the exoU gene in the plasmid pcDNA 3.1-NT-GFP-ExoU (25). Briefly, the fragment of the exoU gene encoding the MLD was amplified using an error-prone polymerase by mixing 50 ng template DNA (pcDNA3.1-NT-GFP-ExoU [Table 1]), 1.5 μl (125 ng) MLD-specific forward and reverse primers (Table 2), 5 μl Mutazyme II buffer (supplied by the manufacturer), 1 μl deoxynucleoside triphosphate mixture (supplied by the manufacturer) and 1 μl Mutazyme II polymerase (supplied by the manufacturer) in distilled water (dH2O) (50-μl total volume). Amplification was performed using the following parameters: 1 cycle at 95°C for 2 min; followed by 30 cycles at 95°C for 30 s, 62°C for 30 s, and 72°C for 1 min; followed by 1 cycle at 72°C for 10 min. PCR products were then purified using the QIAquick PCR purification system (Qiagen, Valencia CA) and utilized as primers to incorporate the mutations into the parental pcDNA 3.1-NT-GFP-ExoU plasmid. To do so, 50 ng template DNA, 250 ng primer, 3 μl EZ clone solution (supplied by the manufacturer), and 25 μl 2X-EZclone enzyme mix (supplied by the manufacturer) were mixed in dH2O (50-μl total volume). Amplification was performed using the following parameters: 1 cycle at 95°C for 1 min; followed by 25 cycles at 95°C for 50 s, 60°C for 50 s, and 69°C for 16 min; followed by 1 cycle at 69°C for 15 min. Following amplification, the original template plasmid DNA was degraded by incubation with 1 μl DpnI restriction enzyme (10 U) at 37°C for 1.5 h. Mutated plasmids were transformed into XL10 Gold E. coli cells. Plasmid DNA was purified from transformants using the QIAprep plasmid purification system (Qiagen), and mutations were identified by nucleotide sequencing performed by the University of Chicago Cancer Research Center. Plasmids carrying mutations consisting of insertions, deletions, or premature stop codons were not analyzed further.
TABLE 2.
Primers used in this study
| Primer | Sequence |
|---|---|
| FarnesylNotI 5′ | AAAAAAAGCGGCCGCATGTCCGGACTCAGAT TCTAAGC |
| FarnesylPmeI 3′ | AAAAAAAGTTTAAACCGCAGCGTGACCGCTACAC |
| MLD-EZclone 5′ | CTGGCAGATACTCCGGAAC |
| MLD-EZclone 3′ | TGTGAACTCCTTATTCCGCCAA |
| ExoU-HN-HindIII-5′ | AAAAAGCTTATGCATATCCAATCGTTG |
| ExoU-HN-NotI-3′ | AAAGCGGCCGCCTGTGAACTCCTTATTCCG |
| I609N 5′ | GCGGTTATTGCCGAGAACAATCGTAAGGAAGTTATCTTCCCC |
| I609N 3′ | GGGGAAGATAACTTCCTTACGATTGTTCTCGGCAATAACCGC |
| Q623R 5′ | GTATCGCCCTGGCCGGCCGGATTCCAACG |
| Q623R 3′ | CGTTGGAATCCGGCCGGCCAGGGCGATAC |
| N627I 5′ | GCCGGATTCCATCGTAGCTCTGTTACG |
| N627I 3′ | CGTAACAGAGCTACGATGGAATCCGGC |
| I654N 5′ | CAATCAAGCGCTGAACGATAACGTCGACAACTACTCGGCACG |
| I654N 3′ | CGTGCCGAGTAGTTGTCGACGTTATCGTTCAGCGCTTGATTG |
| R661L 5′ | GTCGACAACTACTCGGCACTAGGCTTCCTGCGTTTCGGC |
| R661L 3′ | GCCGAAACGCAGGAAGCCTAGTGCCGAGTAGTTGTCGAC |
| A678D 5′ | GTTCGACTACCGTTGAGATGGATAAGGCTTGGCGGAATAAG |
| A678D 3′ | CTTATTCCGCCAAGCCTTATCCATCTCAACGGTAGTCGAAC |
Site-directed mutagenesis of the exoU gene.
The QuikChange II XL site-directed mutagenesis (Stratagene) method was utilized to generate single-nucleotide mutations in the exoU gene. Briefly, 50 ng of parental plasmid DNA (pcDNA3.1-NT-GFP-ExoU) was mixed with 1.5 μl (125 ng) forward and reverse primers (Table 2), 1 μl deoxynucleoside triphosphate mixture (supplied by the manufacturer), 5 μl reaction buffer (supplied by the manufacturer), 3 μl QuikSolution (supplied by the manufacturer) and 1 μl Pfu ultra-high-fidelity DNA polymerase (2.5 U) in dH2O (50-μl total volume). Amplification was performed using the following parameters: 1 cycle at 95°C for 1 min; followed by 18 cycles at 95°C for 50 s, 60°C for 50 s, and 68°C for 15 min; followed by 1 cycle at 68°C for 7 min. Following amplification, the original template DNA was degraded by incubation with 1 μl DpnI restriction enzyme (10 U) at 37°C for 1.5 h. Mutated plasmids were then transformed into XL10 Gold E. coli cells, and plasmid DNA was recovered using the QIAprep plasmid purification system. All mutations were verified by nucleotide sequencing.
Farnesylation of ExoU.
Farnesylated ExoU was generated by inserting the mammalian farnesylation-encoding sequence at the 3′ end of exoU. Briefly, the farnesylation sequence was amplified from the pEGFP-F vector (Clontech, Mountain View, CA) by PCR using the upstream primer ExoU-F-NotI-5′ and the downstream primer ExoU-F-PmeI-3′ (Table 2) under the following conditions: 1 cycle at 95°C for 1 min; followed by 30 cycles at 95°C for 1 min, 65°C for 1 min, and 72°C for 3 min; followed by 1 cycle at 72°C for 10 min. Amplified product as well as the plasmids pcDNA3.1-NT-GFP-ExoU and pcDNA3.1-NT-GFP-LS608 (Table 1) were each individually digested with NotI and PmeI and separated by gel electrophoresis through a 1% (wt/vol) agarose gel. Digested fragments were extracted from the agarose gels and purified using the QIAquick gel extraction method. Amplified products and plasmids were ligated and transformed into XL10 Gold competent cells. Plasmid DNA was recovered from transformants using the QIAprep plasmid purification system, and constructs were verified by nucleotide sequencing.
Mammalian cell cytotoxicity assays.
For transfection experiments, HeLa cells were seeded into 48-well tissue culture plates and grown to 80% confluence. Cells were then transiently transfected by adding 1 μl Fugene 6 (Roche, Madison, WI) and 250 ng of plasmid DNA in 25 μl Opti-MEM (Invitrogen) to each well. Cells were incubated at 37°C in 5% CO2. Cytotoxicity was quantified by measuring the release of lactate dehydrogenase (LDH) from HeLa cells using the Cytotox 96 cytotoxicity system (Promega, Madison WI) as follows. Medium overlying HeLa cells was collected at 20 h posttransfection and centrifuged at 180 × g for 5 min to pellet cell debris. A total of 50 μl of medium was then mixed with 50 μl of assay substrate and incubated at room temperature for 30 min, after which 50 μl of assay stop solution was added. The A490 was then measured using an Emax plate reader (Molecular Devices, Sunnyvale, CA). The cytotoxicities of individual mutants were measured in triplicate and normalized to cells transfected with a plasmid expressing wild-type ExoU using the following formula: 100 × (A490sample − A490untransfected cells)/(A490ExoU-transfected cells − A490untransfected cells).
For infection assays, HeLa cells were grown in 24-well tissue culture plates to 100% confluence. P. aeruginosa strains were grown overnight in LB with shaking at 37°C. On the day of infection, bacteria were diluted to 1 × 108 CFU/ml in serum-free Eagle's minimal essential medium (ATCC). A total of 100 μl of each bacterial suspension was then combined with 900 μl of serum-free Eagle's medium and was dispensed into wells containing the HeLa cells, resulting in a final bacterial concentration of 1 × 107 CFU/well (a multiplicity of infection [MOI] of approximately 10). Centrifugation at 750 × g was performed to synchronize the infections, and the culture plates were then incubated at 37°C in 5% CO2. At various time points, 100 μl of the overlying medium was removed from each well and processed as described above. Control wells were treated with 0.9% (vol/vol) Triton X-100 in serum-free Eagle's minimal essential medium to achieve 100% lysis of HeLa cells. The percentage of cell death was calculated using the following formula: 100 × (A490sample − A490uninfected cells)/(A490Triton X-100-treated cells − A490uninfected cells). All measurements were performed in triplicate.
Fluorescence microscopy.
HeLa cells were seeded onto glass coverslips in 24-well tissue culture plates at a density of 5 × 104 cells/well 1 day prior to transfection. Cells were transfected by adding 1.5 μl of Fugene 6 and 500 ng of plasmid DNA in 50 μl of Opti-MEM to each well. Cells were incubated at 37°C in 5% CO2. After 20 h, cells expressing ExoU variants were fixed with 3.7% (vol/vol) paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) in phosphate-buffered saline (PBS). Cells were then incubated with 10 μg/ml Hoechst 33342 stain (Molecular Probes, Eugene, OR) for 3 min for visualization of nuclei, and coverslips were then transferred to slides with 5 μl 1,4-diazabicyclo[2.2.2]octane (DABCO) (Fluka, Switzerland) and sealed with nail polish. Microscopy was performed using a Leica DMIREZ microscope powered by a 100-W mercury lamp and equipped with a Hamamatsu ORCA-ER camera. Pictures were taken at a magnification of ×1,000 using Openlab (5.01) software and deconvolved with Volocity 3.7 software.
Purification of recombinant ExoU proteins.
For protein purification, wild-type and mutated exoU alleles were cloned into the pE.coli 6x-C-terminal HN expression vector (Clontech). To accomplish this, alleles were amplified from the pcDNA3.1-NT-GFP-ExoU vector using the upstream primer ExoU HN-HindIII-5′ and the downstream primer ExoU HN-NotI-3′ (Table 2). Amplified products as well as the HN-tagging vector were digested with HindIII and NotI restriction enzymes and separated by gel electrophoresis on a 1% (wt/vol) agarose gel. Digested DNA was cut from the gel, purified using the QIAquick gel purification system, ligated, and transformed into BL21(DE3) Star competent cells (Invitrogen). ExoU proteins were purified from transformants using a tandem purification strategy consisting of nickel affinity chromatography followed by gel filtration. Briefly, 1 liter of LB supplemented with 100 μg/ml ampicillin was inoculated with 10 ml overnight culture of BL21 E. coli containing ExoU-HN constructs. Cultures were grown to an optical density at 600 nm (OD600) of approximately 0.6, induced with 1.5 mM IPTG (isopropyl-β-d-thiogalactopyranoside) (Fisher), and grown overnight with shaking at 25°C. Cultures were harvested by centrifugation at 6,000 × g for 10 min at 4°C. Cell pellets were resuspended in 100 ml lysis buffer (10 mM Tris [pH 8.3], 500 mM NaCl, 10% [vol/vol] glycerol, 0.01% [vol/vol] Igepal CA-630, and 5 mM β-mercaptoethanol) and lysed by sonication. Lysates were cleared of cellular debris by centrifugation at 10,000 × g at 4°C for 20 min and then purified using an AKTAxpress purification system (GE Healthcare, Piscataway, NJ) fitted with a HisTrap FF nickel column (GE Healthcare) and a HiPrep 26/10 desalting column (GE Healthcare). The resulting recombinant ExoU (rExoU)-containing fractions were concentrated using Vivaspin concentrators (Sartorius Stedim Biotech, Aubagne, France) and further purified by gel filtration using a HiLoadTM 26/60 Superdex 200 column (GE Healthcare). Protein was eluted in 3.5 ml fractions of loading buffer (10 mM Tris-HCl [pH 8.3], 500 mM NaCl, 5 mM β-mercaptoethanol), and ExoU-containing fractions were again concentrated as described above. Protein purity and stability were assessed by SDS-polyacrylamide gel electrophoresis with Coomassie blue staining.
PLA2 assays.
Catalytic activities of rExoU proteins were assessed using the cPLA2 assay approach (Cayman Chemicals, Ann Arbor, MI). First, HeLa cell lysates were generated by washing two 10-cm plates of HeLa cells (80% confluence) three times with cold PBS. Cells were collected into 1 ml cold PBS using a cell scraper (Sarstedt, Newton, NC) and lysed by repeated passage through a 27-gauge needle. Lysates were then cleared by centrifugation at 10,000 × g at 4°C for 10 min. A total of 5 μg of purified ExoU protein (66 pmol/liter) was incubated with 200 μl of the assay substrate (1.5 mM arachidonoyl thio-phosphatidylcholine) and 5 μl of a precleared HeLa cell lysate at 25°C. The assay was stopped at various time points by addition of 10 μl of 25 mM 5,5′-dithiobis(2-dinitrobenzoic acid) and activity quantified by measuring the A405. The PLA2 activity of ExoU was calculated using the following formula: A405/10.00(extinction coefficient for DTNB) × 1/(protein concentration [μg]). Assays using purified SOD1 were performed similarly except that 20 μg of purified ExoU (265 pmol/liter) was incubated with 40 μg (2.2 nmol/liter) purified bovine liver-derived SOD1 (Sigma-Aldrich). All measurements were performed in triplicate.
CD.
Purified rExoU proteins were diluted to a final concentration of approximately 0.225 mg/ml and pipetted into 1-mm-pathlength cuvettes. The circular dichroism (CD) spectrum of each sample was then measured over the wavelength range of 198 to 250 nm using a J-815 circular dichroism spectrometer outfitted with Spectra Manager software (Jasco, Easton, MD). Final spectra were presented as the average of nine separate readings. Structural assignments were performed using CD Pro software (Colorado State University, Fort Collins, CO).
Construction of P. aeruginosa mutants.
The parental strain for construction of P. aeruginosa mutants was PA99, a clinical isolate that carries the exoS, exoT, and exoU genes but not the exoY gene (9). PA99null, a derivative of PA99 that has an intact type III secretion system but disrupted exoS, exoT, and exoU genes, was used in this study to deliver ExoU proteins during infections. PA99null+ExoU and PA99null+ExoU-S142A were previously generated by inserting a single copy of the exoU or exoU-S142A gene, respectively, under the control of its endogenous promoter into a neutral site of the PA99null chromosome (Table 1). In this study, strains expressing and secreting ExoU-MLD variants were likewise generated in a similar manner. First, specific mutations were generated within the exoU gene of the plasmid mini-CTX1-ExoU using the QuikChange II XL site-directed mutagenesis method (Table 1). Each of the resulting plasmids was then transformed into the E. coli strain S17.1 and subsequently conjugated into PA99null. The exoU allele was then integrated into the PA99null chromosome at the attB locus using the method of Hoang et al. (17). The presence of the appropriate mutation was verified by nucleotide sequencing. Protein secretion was verified by growth in MINS medium (20) followed by immunoblot analysis of culture supernatants using ExoU-specific antibodies, as previously described (34).
Mouse model of acute pneumonia.
Studies of acute pneumonia were performed using the mouse aspiration method described by Comolli et al. (7). Briefly, P. aeruginosa strains were grown overnight in MINS medium at 37°C with shaking. On the day of infection, strains were diluted and regrown to exponential phase. Strains were then collected by centrifugation and diluted to the appropriate concentration in PBS. Six- to 8-week-old female BALB/c mice were anesthetized by intraperitoneal injection of a mixture of ketamine (100 mg/ml) and xylazine (20 mg/ml) and inoculated intranasally with 1.2 × 106 CFU of bacteria in 50 μl of PBS, as determined by optical density. Inocula were verified by dilution plating. Survival of mice was monitored over 7 days. Mice were euthanized when severe illness developed and were scored as dead. Animals were purchased from Harlan and housed in the containment ward of the Center for Comparative Medicine at Northwestern University. All experiments were carried out in compliance with the guidelines set forth by the Northwestern University Animal Care and Use Committee.
Statistical methods.
Student's t test was utilized to compare measurements in the cytotoxicity and PLA2 assays. The log rank test was used to compare survival in the mouse infections. A P value of <0.05 was considered significant.
RESULTS
Generation of a library of exoU alleles containing mutations in the MLD-encoding region.
Previous work had defined the MLD (residues 550 to 687) of ExoU as sufficient for localization to the host cell plasma membrane and necessary for cytotoxicity (27). Our plan was to further characterize the functional significance of the MLD by employing a mutagenesis approach to identify specific amino acids that were critical for activity.
Error-prone PCR mutagenesis was utilized to generate a library of exoU alleles containing mutations within the MLD-encoding portion of the gene. Using this approach, 418 constructs were generated and characterized by nucleotide sequencing. Of the 418 constructs, 182 were found to encode no amino acid changes and 38 were found to carry either a premature stop codon or a frameshift mutation. These constructs were not analyzed further. The remaining 198 constructs contained mutations predicted to encode one or more amino acid substitutions within the MLD. Of these, 120 had a mutation resulting in a single amino acid substitution, 52 in two substitutions, and 26 in three or more substitutions (see Table S1 in the supplemental material). Twenty residues within the MLD were not substituted in this initial screen. Therefore, site-directed mutagenesis was utilized to generate nonconservative amino acid substitutions at these positions, allowing us to achieve complete coverage of the MLD. These 218 constructs were used to determine residues critical for the function of the MLD.
Specific amino acid residues within the ExoU MLD are critical for cytotoxicity.
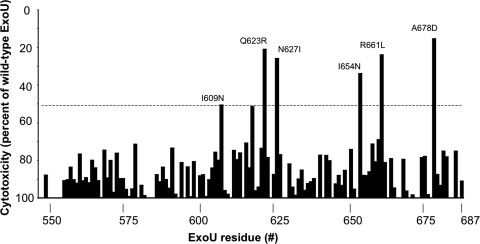
To identify residues within the MLD that are necessary for full cytotoxicity, the 218 constructs were expressed in HeLa cells by transient transfection, and cell death was quantified at 20 h posttransfection by measuring LDH release into the medium. The cytotoxicity of each ExoU variant was compared to that of wild-type ExoU, and a 50% reduction in cell death was arbitrarily defined as a threshold for a significant defect in killing (Fig. 1; see Table S1 in the supplemental material). Of the 218 constructs analyzed, 19 constructs containing a total of 45 distinct mutations resulted in a 50% or greater reduction in cell death (Table 3). No single amino acid substitution resulted in a complete abrogation of cytotoxicity.
FIG. 1.
Cytotoxicity associated with ExoU proteins containing amino acid substitutions within the MLD. MLD variants were expressed in HeLa cells by transient transfection, and LDH release into the medium was measured 20 h later. Results are presented as cytotoxicity relative to that of wild-type ExoU. For proteins with multiple substitutions, the cumulative cytotoxicity is depicted for each substituted residue. If substantial loss of cytotoxicity occurred and this defect could not be attributed to a single-amino-acid substitution, site-directed mutagenesis was employed to define which residue was critical for cytotoxicity, and the level of cytotoxicity associated with the single-amino-acid substitution is shown. All experiments were performed in triplicate. Data represents mean values. For clarity, standard deviations are not shown, but they were usually within ±10%.
TABLE 3.
Substitutions resulting in a substantial decrease in ExoU-mediated cytotoxicity
| Construct | Mutation(s) | Relative cytotoxicity (%) |
|---|---|---|
| MLD-14 | L594S, R632C, S643G | 49 |
| MLD-45 | N627D,a A634V | 39 |
| MLD-63 | A592G, V604D, N608K | 50 |
| MLD-74 | A597V, N608I | 52 |
| MLD-79 | W560R, Q575Pa | 28 |
| MLD-118 | A603V, F615FI, I654Na | 29 |
| MLD-128 | R599S, I609Na | 45 |
| MLD-150 | R610P,a P644Q, S671G, T673A | 42 |
| MLD-156 | Q575R, A603S, V613F | 48 |
| MLD-179 | Q623Ra | 23 |
| MLD-202 | N627Ia | 26 |
| MLD-241 | I614V, R632S, V655D | 47 |
| MLD-277 | A568V, I614F, L630Pa | 25 |
| MLD-282 | A593S, N627K,a A641P | 19 |
| MLD-287 | A593V, Y619N | 22 |
| MLD-297 | W560R, A562V, V628L, K668I | 14 |
| MLD-370 | V571A, P624L, L630Pa | 30 |
| MLD-394 | D578E, A678Da | 19 |
| MLD-396 | R661La | 24 |
Substitution which alone resulted in a reduction in cytotoxicity of at least 50%.
Many of these 19 constructs were not informative, as they contained more than one amino acid substitution or a proline substitution, which had a high likelihood of inducing major folding changes. For this reason, site-directed mutagenesis was used to generate single-amino-acid substitutions in each of the 45 candidate residues identified in the initial screen, and LDH release assays were performed to quantify the cytotoxicity associated with each construct. Substitutions in 6 of the 45 candidate residues were found to decrease cytotoxicity by at least 50%. Either conservative or nonconservative base substitutions at position 623, 627, or 661 resulted in at least a 50% defect in ExoU-mediated killing, whereas only nonconservative changes at positions 609, 654, and 678 were found to result in cytotoxicity defects of this magnitude (Fig. 1; see Table S1 in the supplemental material). ExoU mutants containing the following single-residue substitutions were therefore chosen for further analysis: I609N, Q623R, N627I, I654N, R661L, and A678D. These proteins will be referred to as ExoU-I609N, ExoU-Q623R, etc.
Substitution of critical residues within the ExoU MLD do not disrupt overall protein folding.
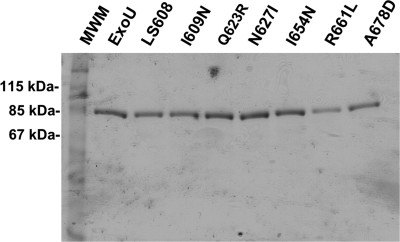
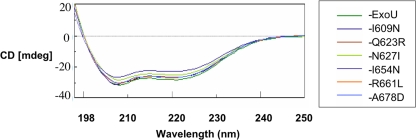
To determine whether cytotoxicity defects in ExoU-I609N, ExoU-Q623R, ExoU-N627I, ExoU-I654N, ExoU-R661L, and ExoU-A678D were the result of protein misfolding, recombinant HN-tagged forms of each protein were overexpressed in E. coli and purified using nickel column chromatography and gel filtration (see Materials and Methods). We had previously shown that a linker-scanning insertion variant of ExoU with five amino acids inserted at position N608 of the MLD was noncytotoxic (25, 27). A recombinant version of this protein, referred to as rExoU-LS608, was also purified and used as a control in subsequent experiments. SDS-polyacrylamide gel electrophoresis followed by Coomassie blue staining indicated that these proteins were >95% pure (Fig. 2). Proteins were then subjected to CD, and the resulting spectra were compared to that of wild-type ExoU. The far-UV spectra of each of the single-amino-acid substitution proteins were very similar (Fig. 3), and the structural composition prediction for each protein suggested no discernible difference in the α-helical, β-strand, or disordered-region content relative to wild-type ExoU (data not shown). Likewise, the CD spectrum of rExoU-LS608 was nearly identical to that of wild-type ExoU (data not shown). These results indicated that the overall structure of each of the recombinant ExoU protein variants was intact and that defects in cytotoxicity were therefore due to direct roles played by these residues in the mechanism of ExoU-mediated killing.
FIG. 2.
SDS-polyacrylamide gel electrophoresis of purified recombinant ExoU proteins containing single-residue substitutions in their MLDs. C-terminal HN-tagged ExoU proteins were purified by nickel column chromatography followed by gel filtration. LS608 refers to rExoU-LS608, etc. MWM, molecular weight marker.
FIG. 3.
Circular dichroism spectra of recombinant ExoU proteins. I609N refers to rExoU-I609N, etc.
Single amino acid substitutions within the MLD abolish membrane localization.
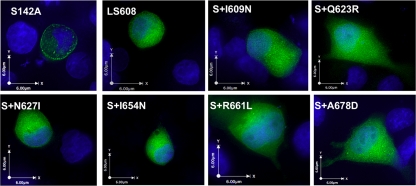
To verify whether MLD substitutions that diminished ExoU-mediated cell killing also resulted in defects in membrane targeting, we attempted to visualize green fluorescent protein (GFP)-tagged ExoU variants expressed from transfection constructs within HeLa cells by fluorescence microscopy. The residual cytotoxicity of each of these ExoU variants lysed transfected cells and prevented visualization (data not shown), so it was necessary to use catalytically inactive forms of ExoU. This was accomplished by replacing the catalytic serine residue at position 142 with an alanine (25, 31). Constructs encoding each of the GFP-tagged noncatalytic ExoU variants were then expressed within HeLa cells by transient transfection. At approximately 20 h posttransfection, cells were fixed, incubated with Hoechst stain to visualize nuclei, and analyzed by fluorescence microscopy. Catalytically inactive ExoU with an intact MLD (GFP-ExoU-S142A) was used as a positive control. In accordance with previous observations, this protein was observed in a punctate pattern, especially around the cell periphery (Fig. 4). GFP-ExoU-LS608, the linker-scanning insertion variant of ExoU known to be defective in localization, was used as a negative control. As expected, this protein was distributed homogenously throughout the cytosol (Fig. 4). All of the proteins with substitutions in the MLD were distributed in a homogenous pattern throughout the cytosol as well (Fig. 4). Thus, residues I609, Q623, N627, I654, R661, and A678 of the MLD are necessary for appropriate localization of ExoU to the plasma membrane.
FIG. 4.
Intracellular localization of ExoU proteins with MLD substitutions. ExoU proteins containing an N-terminal GFP tag were expressed in HeLa cells by transient transfection. At 20 h posttransfection, cells were fixed, treated with Hoechst stain to label nuclei (blue), and visualized by fluorescence microscopy. All constructs except GFP-ExoU-LS608 contain the S142A substitution, which eliminates cytotoxicity, so that proteins could be visualized. Representative images are shown. S142A, GFP-ExoU-S142A; LS608, GFP-ExoU-LS608; S+I609N, GFP-ExoU-S142A-I609N, etc.
Membrane localization of ExoU is not sufficient for activation.
Our results indicated that key residues within the MLD of ExoU are essential both for targeting to the plasma membrane and for cytotoxicity. One explanation for these findings is that membrane localization is a prerequisite for cytotoxicity. In other words, ExoU must first reach the plasma membrane before it can kill cells. To address this possibility, we attempted to restore membrane localization to an ExoU MLD variant protein by artificially targeting it to the plasma membrane. We used GFP-ExoU-LS608 for this purpose because it is minimally cytotoxic, and disruption of its patatin-like PLA2 domain is not necessary to allow for visualization within host cells. To artificially target GFP-ExoU-LS608 to the plasma membrane, we used the membrane-targeting farnesylation sequence. Farnesylation, a mechanism by which many mammalian cell proteins (e.g., Ras and Rab) are targeted to the plasma membrane, is a posttranslational modification by which a 15-carbon isoprenoid (farnesyl) group is attached to a protein containing a C-terminal CAAX box motif (39). We therefore fused a CAAX box to the C terminus of GFP-ExoU-LS608 and then assessed the ability of the fusion protein to localize to the cell periphery and induce cell death.
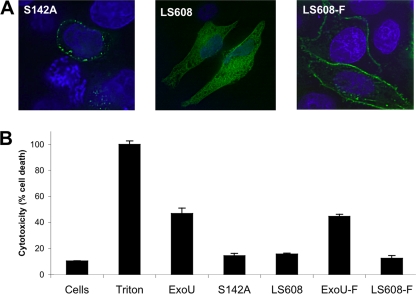
To verify that the farnesylation sequence indeed restored localization of ExoU to the plasma membrane, GFP-ExoU-LS608 with a farnesylation site (designated GFP-ExoU-LS608-F) was expressed constitutively within HeLa cells by transient transfection. GFP-ExoU-S142A, which has an intact MLD, was used as a positive control for membrane localization, and GFP-ExoU-LS608, which has a disrupted MLD and lacks a farnesylation site, was used as a negative control (23, 25, 29, 30, 35). In agreement with previous observations, GFP-ExoU-LS608 was distributed throughout the cytoplasm, whereas GFP-ExoU-S142A was visualized in a punctate pattern around the periphery of the cell (Fig. 5 A) (27). GFP-ExoU-LS608-F was also observed around the cell periphery, although in a less punctate pattern than GFP-ExoU-S142A (Fig. 5A), demonstrating that the farnesylation tag was sufficient to target ExoU to the plasma membrane.
FIG. 5.
Localization and cytotoxicity of farnesylated ExoU proteins. To test the effects of farnesylation on ExoU function, GFP-tagged ExoU proteins were expressed in HeLa cells by transient transfection. At 20 h posttransfection, cells were analyzed for ExoU localization and for cytotoxicity. (A) To assess localization, cells were fixed, treated with Hoechst stain to label nuclei (blue), and visualized by fluorescence microscopy. Comparison of unfarnesylated protein (GFP-ExoU-S142A and GFP-ExoU-LS608) and farnesylated protein (GFP-ExoU-LS608-F) demonstrated that farnesylation resulted in localization of protein to the plasma membrane. Representative images are shown. (B) The cytotoxicity of ExoU variants was quantified by measuring LDH release. Triton was used to cause 100% cell lysis. Experiments were performed in triplicate; data represent means ± standard deviations. Cells, no transfection; LS608-F, GFP-ExoU-LS608 containing a C-terminal farnesylation sequence; ExoU, GFP-ExoU; S142A, GFP-ExoU-S142A, etc.
We next assessed whether farnesylation-mediated targeting of ExoU to the plasma membrane was sufficient to restore cytotoxicity. We reasoned that if the sole role of the MLD was to target ExoU to the plasma membrane, then the MLD would be dispensable when an alternate targeting mechanism such as the farnesylation sequence was provided. However, expression of GFP-ExoU-LS608-F in HeLa cells resulted in only 12% cell killing, which was comparable to the degree of cytotoxicity observed in cells expressing the inactive ExoU variants GFP-ExoU-S142A (14%) and GFP-ExoU-LS608 (16%) (Fig. 5B). Conversely, expression of GFP-ExoU and GFP-ExoU-F resulted in the death of 47% and 45% of cells, respectively, indicating that the addition of a C-terminal farnesylation tag did not prevent ExoU from killing cells (Fig. 5B). These findings suggest that the role of the MLD is more complex than simply targeting ExoU to the plasma membrane. For example, the MLD may be essential for specific targeting of this toxin to a distinct factor or compartment within the plasma membrane, which itself may be necessary for downstream steps leading to cytotoxicity. Alternatively the MLD may play a more direct role in the catalytic mechanism of ExoU that is distinct from its membrane localization function. To address the latter possibility, we examined whether the PLA2 activity of each of the MLD variants of ExoU was intact.
Single-residue substitutions within the MLD cause defects in PLA2 activity.
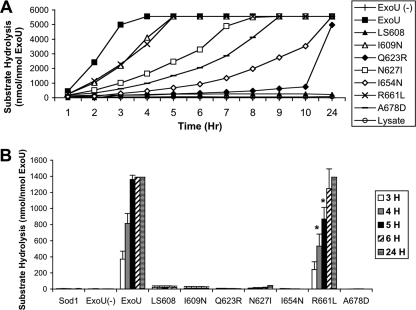
Although the MLD (residues 550 to 687) is separated from the patatin-like PLA2 domain (residues 107 to 357), previous studies examining ExoU with insertions or deletions in the MLD had demonstrated defects in ExoU enzymatic activity (10, 25, 26, 35). Therefore, we determined whether the six ExoU proteins with single-residue substitutions in the MLD were compromised in their catalytic activity. We performed in vitro PLA2 activity assays using a purified lipid substrate (arachidonoyl thio-phosphatidylcholine). Hydrolysis of the sn2 arachidonoyl thioester bond from this substrate releases a “free thiol” group that is detected by a change in absorbance, allowing quantification of PLA2 activity. rExoU-I609N, rExoU-Q623R, rExoU-N627I, rExoU-I654N, rExoU-R661L, and rExoU-A678D were each tested with this substrate. Recombinant wild-type ExoU protein was used as a positive control and rExoU-LS608 as a negative control. Importantly, in this assay the lipid substrate is not incorporated into membranes, so membrane targeting would not affect the results. As previously described, ExoU protein alone was unable to cleave phospholipids, due to the requirement of a host cell cofactor (Fig. 6) (31, 35). However, addition of cleared HeLa cell lysates resulted in substantial catalytic activation of ExoU, reaching the maximal detection limit of the assay within 4 h. Conversely, minimal catalytic activation was seen with rExoU-LS608 even upon incubation for 24 h with HeLa cell lysates, supporting our previous findings (26). As an additional control, HeLa cell lysate alone was not sufficient to achieve substrate hydrolysis (Fig. 6A). When incubated with HeLa cell lysates, all of the ExoU-MLD variant proteins were defective in phospholipase activity to various degrees (Fig. 6A). rExoU-I609N and rExoU-R661L were the most active, exhibiting 44% and 46% of wild-type activity, respectively, after 3 h of incubation. rExoU-N627I and rExoU-A678D exhibited approximately 20% and 12%, respectively, of wild-type activity at this time point, while rExoU-I654N and rExoU-Q623R demonstrated the weakest activities, possessing only 6% and 4%, respectively, of wild-type activity after 3 h of incubation (Fig. 6A). Unlike rExoU-LS608, all of the MLD variants were capable of cleaving nearly maximal amounts of substrate after 24 h of incubation, indicating that catalysis was intact but proceeded at a lower rate in these variants. These results indicate that residues I609, Q623, N627, I654, R661, and A678 of the MLD are critical not only for membrane targeting but for wild-type levels of catalytic activity as well.
FIG. 6.
PLA2 activity of purified recombinant ExoU proteins. ExoU proteins were incubated with the arachidonoyl thio-phosphatidylcholine substrate in the presence of HeLa cell lysate (A) or purified SOD1 (B) and cleavage of substrate measured. Experiments were performed in triplicate; data represent mean values. Error bars, which represent ± standard deviation, standard deviations are not shown in panel A for clarity but were usually within ±100 to 500 nmol. In panel A, each of the single-residue MLD variants except rLS608 was statistically different from wild-type ExoU and from lysate (P < 0.05). LS608, rExoU-LS608, etc.; Lysate, HeLa cell lysate without ExoU protein; ExoU (−), rExoU without lysate; Sod1, SOD1 without ExoU protein. *, statistically different from rExoU (P < 0.05).
Amino acid substitutions within the MLD alter activation by SOD1.
A possible explanation for the decreased phospholipase activity associated with substitutions within the MLD is that these residues are important for the binding or activation of ExoU by its cofactor. Since cofactor interaction is a prerequisite for PLA2 activity, amino acid substitutions that prevented this interaction would be expected to decrease catalytic activity. Previous studies have demonstrated that SOD1 is a cofactor of ExoU in vitro (4, 28). To test whether this cofactor was sufficient for catalytic activation of the MLD variants, we repeated the in vitro PLA2 assays using purified SOD1 in place of HeLa cell lysates. As previously reported (4, 28), incubation of wild-type ExoU with SOD1 resulted in substantial activation, and the maximal detection limit of the assay was reached within 5 h (Fig. 6B). Surprisingly, only one of the six MLD variants was activated to any degree by SOD1. At 5 h, substrate cleavage by rExoU-R661L was approximately 64% of that observed with recombinant wild-type ExoU. By 6 h of incubation, cleavage by rExoU-R661L had reached maximal levels. None of the other MLD variants exhibited appreciable catalytic activity even after 24 h of coincubation with SOD1 (Fig. 6B). Since these same proteins exhibited substantial PLA2 activity following incubation with HeLa cell lysates (Fig. 6A), these results suggest that a second cofactor (other than SOD1) is present in HeLa cell lysates and directly activates or facilitates activation of ExoU. In addition, residues I609, Q623, N627, I654, and A678 may play critical roles in SOD1 binding to ExoU or subsequent activation of ExoU by SOD1. In contrast, a substitution in residue R661 did not abolish SOD1-mediated activation, suggesting that this residue plays a distinct role in cytotoxicity.
Identified MLD residues are important for the pathogenic effect of ExoU during infection.
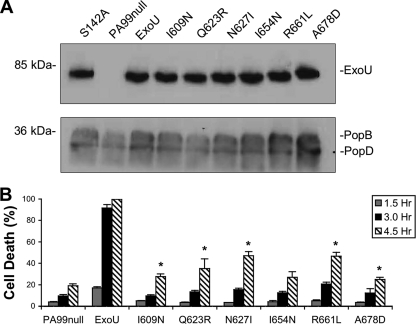
We have successfully identified MLD residues that are critical for ExoU localization and catalytic activity in vitro and in transfected cells. To establish a biological relevance for these defects, P. aeruginosa strains secreting each of these six ExoU-MLD variants were generated. PA99null, which has an intact type III secretion system but disrupted effector-encoding genes, was utilized to deliver the ExoU proteins into host cells. A single copy of each mutated exoU allele was inserted into a neutral site of the PA99null chromosome to generate strains that secreted wild-type ExoU (PA99null-ExoU) or one of the MLD variants (designated PA99null-I609N, PA99null-Q623R, PA99null-N627I, PA99null-I654N, PA99null-R661L, and PA99null-A678D). Strains were then grown under conditions permissive for secretion of ExoU, and culture supernatants were examined by immunoblot analysis to verify that individual strains secreted similar amounts of ExoU protein (Fig. 7 A). Each strain was used to infect HeLa cell monolayers, and cell death was quantified by LDH release. As expected, the strain secreting no type III effectors exhibited little cell killing even after 4.5 h, whereas the strain secreting wild-type ExoU killed greater than 90% of the total cells within 3 h (Fig. 7B). Strains secreting MLD variants were severely attenuated in killing (Fig. 7B). Infection with PA99-N627I and PA99-R661L resulted in approximately 50% cell death after 4.5 h, but no other strain killed greater than 40% of the cells at this time point. Each of the MLD variants was associated with a statistically significant decrease in cell killing relative to wild-type ExoU (Student's t test, P < 0.05), but each also caused more killing than PA99null, except for PA99null-I654N (Student's t test, P < 0.05) (Fig. 7B). These data demonstrate that residues I609, Q623, N627, I654, R661, and A678 of the MLD are essential for normal levels of ExoU-mediated cytotoxicity during cell culture infections.
FIG. 7.
Cytotoxicity of P. aeruginosa strains secreting ExoU protein with MLD substitutions following infection of HeLa cells. (A) P. aeruginosa strains were grown under type III secretion-inducing conditions, and immunoblotting was performed on culture supernatants using an ExoU-specific antibody as well as antibodies against the translocon proteins PopB and PopD. (B) HeLa cells were infected at an MOI of 10, and cytotoxicity was determined by measuring LDH release at 1.5, 3.0, and 4.5 h postinfection. PA99null, which does not secrete ExoU, was utilized as a negative control. Experiments were performed in triplicate; data represent means ± standard deviations. I609N, PA99null+ExoU-I609N, etc. All strains secreting MLD variants were significantly different from ExoU (P < 0.01). *, statistically different from PA99null (P < 0.05).
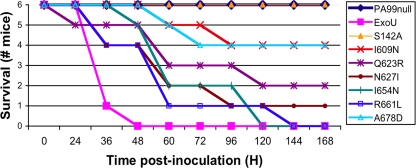
To determine whether decreased killing of cells corresponded to reductions in virulence, the P. aeruginosa strains were used to infect mice in a murine model of acute pneumonia. Mice were infected intranasally with an inoculum equivalent to twice the 50% lethal dose (LD50) of PA99null-ExoU, and survival was assessed over a 7-day time course. A total of six mice were infected per strain. As expected, all mice infected with PA99null and PA99null-S142A survived for the full 7 days of the experiment, while mice infected with PA99null-ExoU succumbed to the infection within 48 h (Fig. 8). Similar to the results of our cell culture infection assay, all strains secreting MLD variants were more virulent than PA99null in the mouse model. Additionally, each of these strains except PA99null-R661L was also attenuated relative to PA99null-ExoU (log rank test, P < 0.05) (Fig. 8). These results demonstrate that single-amino-acid substitutions within the MLD of ExoU are capable of attenuating the virulence of strains secreting these proteins. However, they also indicate that even the residual levels of PLA2 activity associated with each of these variants were sufficient to cause substantial illness in a mouse model of pneumonia.
FIG. 8.
Virulence of P. aeruginosa strains secreting MLD variants in a mouse model of acute pneumonia. Six mice per group were anesthetized, intranasally infected with 1.2 × 106 CFU bacteria, and monitored for survival over the subsequent 7 days. Each of the MLD variants was statistically different from ExoU-S142A (P < 0.05). Each MLD variant except ExoU-R661L was also statistically different from wild-type ExoU (P < 0.05). ExoU, PA99null-ExoU; S142A, PA99null-S142A, etc.
DISCUSSION
Previously it had been shown that the MLD was required for cytotoxicity of ExoU. Here we extended those studies to identify specific amino acid residues within the MLD that are critical for this process. Using a HeLa cell transfection system, we found that residues I609, Q623, N627, I654, R661, and A678 of the MLD play a critical role in cytotoxicity. Interestingly, these residues are distributed across the entire MLD, suggesting that no one portion of this domain by itself is sufficient for these activities. The identification of these residues is consistent with previous reports describing regions of the MLD important in cell killing. For example, previously we had used a linker-scanning mutagenesis approach to show that 5-amino-acid insertions at residues 605, 608, and 619 abolished cytotoxicity and membrane targeting of ExoU (27). In a recent report, Schmalzer and colleagues utilized a similar method to demonstrate that 5-amino-acid insertions at residues 606, 608, and 622 abolished ExoU-mediated cytotoxicity, while insertions at residues 572 and 618 resulted in intermediate levels of cytotoxicity and PLA2 activity (32). Several investigators have observed that C-terminal deletions beginning at residue 634, 657, 661, or 668 and extending to the end of the protein abolished cytotoxicity (10, 15, 23, 25, 26, 31). Stirling and colleagues used serial deletions to demonstrate that residues 663 to 683 were required for full cytotoxicity of ExoU (35).
Of note, substitution of no single amino acid completely abolished cytotoxicity, although combinations of single-amino-acid substitutions did result in this phenotype (data not shown). Thus, a certain amount of redundancy or tolerance to alteration is present within the MLD.
Structural studies indicated that substitutions at the identified critical residues did not cause overall misfolding of ExoU, indicating that these residues themselves likely play an important role in intermolecular interactions or in inducing local conformations that allow other residues to do so. Interestingly, Q623, N627, and R661 are polar amino acids, consistent with their surface exposure. Indeed, secondary structure predictions of the ExoU MLD suggest that residues Q623, N627, and R661 are contained within two separate loop structures (data not shown), consistent with the hypothesis that they interact with other factors. In contrast, I609, I654, and A678 are predicted to be within three distinct α-helices (data not shown). Location within α-helices may explain why substitutions with polar amino acids were not tolerated at these residues. These α-helices may play critical roles in intermolecular interactions or maintenance of local structure.
Our mutagenesis screen did not identify any substitutions that substantially reduced cytotoxicity without also affecting membrane localization. This observation is consistent with previous reports, which have also noted a link between cytotoxicity and membrane localization within the MLD (27, 35). One possible explanation for these findings is that ExoU targeting to the plasma membrane must first be achieved before cytotoxicity can occur. For example, proximity to the phospholipids in the plasma membrane may be essential for ExoU to degrade these phospholipids and cause cell lysis. Our results, however, suggest that the role of the MLD is more complex, since providing membrane localization by the addition of a farnesylation sequence did not restore cytotoxicity to ExoU containing a disrupted MLD. Furthermore, in agreement with previous reports (35), we found that substitutions within the MLD abrogated PLA2 activity in vitro, in the absence of host cell membranes, indicating that the MLD plays a more direct role in catalysis. For example, the MLD may affect the ability of ExoU to interact with its lipid substrate or a host cell cofactor essential for catalysis. Since the same residues were required for both full cytotoxicity and localization, these findings suggest that these two functions of the MLD are intrinsically linked. For example, if the role of the MLD in PLA2 activity is to bind a host cell cofactor, binding of this same cofactor may also be associated with membrane localization.
Analysis of the role of the critical MLD residues in PLA2 activity led to the somewhat unexpected finding that a second host cell factor may participate in activation of ExoU. When HeLa cell lysates were supplied as a source of host cell cofactor, recombinant forms of each of the MLD variants were partially active in the cleavage of a synthetic lipid substrate. However, when these assays were repeated using purified SOD1 as a cofactor, only one of the six ExoU MLD variants (rExoU-R661L) demonstrated substantial activity. The remaining five ExoU MLD variants were devoid of detectable activity, even following 24 h of incubation. These results support the hypothesis that the MLD plays a critical role in activation by a host cell cofactor. Furthermore, the fact that some of the MLD variants demonstrated PLA2 activity in the presence of a HeLa cell extract but not purified SOD1 suggests that a second host factor that modulates ExoU activity is present within HeLa cells. The existence of a second cofactor is also consistent with our inability to identify a single-amino-acid substitution within the MLD that completely abrogated ExoU activity. If distinct portions of the MLD bind to distinct cofactors and each cofactor suffices for partial activation, no one MLD region would be required for PLA2 activity. These ExoU variants could therefore be useful tools for identifying the second cofactor and characterizing the portions of ExoU with which it interacts.
The assays described above were all performed in vitro using purified protein or by overexpression systems in cell culture, making it difficult to assess the relevance of the observed defects to actual infections. To address this question, P. aeruginosa strains secreting MLD variants were utilized in both cell culture infection assays and mouse experiments. Just as strains secreting MLD variants caused intermediate levels of cytotoxicity and PLA2 activity in vitro, they were also associated with partial virulence in these infection models. While mice infected with bacterial strains secreting no ExoU or catalytically inactive ExoU survived for the full course of the experiment, mice infected with each of the strains secreting MLD variants showed some degree of mortality. Interestingly, however, mortality in the mouse infection model did not always correlate with levels of PLA2 activity in our in vitro assays. For example, while rExoU-I609N was one of the most active variants in the PLA2 assay, it was among the least virulent strains in our mouse model of infection. Conversely, PA99null-Q623R exhibited intermediate levels of virulence in comparison to strains secreting other MLD variants despite the fact that it exhibited the lowest PLA2 activity in vitro. Additionally, virulence in the mouse model did not correspond with catalytic activation by SOD1. For example, while PA99null-R661L exhibited a high level of virulence in the mouse pneumonia model and was the only MLD variant activated by SOD1 in vitro, PA99null-N627I and PA99null-I654N exhibited similar levels of virulence in vivo despite being inactive when coincubated with SOD1 in vitro. These results suggest that the second cofactor's role in the activation of ExoU is biologically relevant. While our findings demonstrated that even relatively small amounts of cytotoxicity and PLA2 activity were sufficient for substantial virulence during infection, they also suggest that in vitro catalytic activity does not fully reflect the potential of individual ExoU variants to cause severe disease in vivo.
Together these observations indicate that the MLD functions in capacities beyond simply targeting ExoU to the plasma membrane. In the absence of a crystal structure of ExoU, which has proved difficult to obtain, continued use of genetic and biochemical approaches will be necessary to further define the mechanism by which this important region of ExoU contributes to infection.
Supplementary Material
Acknowledgments
We thank James Winsor for technical assistance with protein purification, Arabela Grigorescu for assistance and advice with the circular dichroism experiments, and Greg Smith for suggesting the farnesylation experiment. We also acknowledge the Keck Biophysics Facility for use of their circular dichroism spectrometer and Colorado State University for use of their CDPro software.
This work was supported by the National Institute of Health (grants AI053674, AI065615, and AI075191 to A.R.H.) and by the American Heart Association (grant 09PRE2130036 to J.L.V.). The Center for Structural Genomics of Infectious Diseases project has been funded in whole or in part with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under contract no. HHSN272200700058C (to W.F.A.).
Editor: J. B. Bliska
Footnotes
Published ahead of print on 17 May 2010.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Alaoui-El-Azher, M., J. Jia, W. Lian, and S. Jin. 2006. ExoS of Pseudomonas aeruginosa induces apoptosis through a Fas receptor/caspase 8-independent pathway in HeLa cells. Cell. Microbiol. 8:326-338. [DOI] [PubMed] [Google Scholar]
- 2.Barbieri, A. M., Q. Sha, P. Bette-Bobillo, P. D. Stahl, and M. Vidal. 2001. ADP-ribosylation of Rab5 by ExoS of Pseudomonas aeruginosa affects endocytosis. Infect. Immun. 69:5329-5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbieri, J. T., and J. Sun. 2004. Pseudomonas aeruginosa ExoS and ExoT. Rev. Physiol. Biochem. Pharmacol. 152:79-92. [DOI] [PubMed] [Google Scholar]
- 4.Benson, M. A., K. M. Schmalzer, and D. W. Frank. 2010. A sensitive fluorescence-based assay for the detection of ExoU-mediated PLA(2) activity. Clin. Chim Acta 411:190-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coburn, J., A. V. Kane, L. Feig, and D. M. Gill. 1991. Pseudomonas aeruginosa exoenzyme S requires a eukaryotic protein for ADP-ribosyltransferase activity. J. Biol. Chem. 266:6438-6446. [PubMed] [Google Scholar]
- 6.Coburn, J., R. T. Wyatt, B. H. Iglewski, and D. M. Gill. 1989. Several GTP-binding proteins, including p21c-H-ras, are preferred substrates of Pseudomonas aeruginosa exoenzyme S. J. Biol. Chem. 264:9004-9008. [PubMed] [Google Scholar]
- 7.Comolli, J. C., A. R. Hauser, L. Waite, C. B. Whitchurch, J. S. Mattick, and J. N. Engel. 1999. Pseudomonas aeruginosa gene products PilT and PilU are required for cytotoxicity in vitro and virulence in a mouse model of acute pneumonia. Infect. Immun. 67:3625-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diaz, M. H., C. M. Shaver, J. D. King, S. Musunuri, J. A. Kazzaz, and A. R. Hauser. 2008. Pseudomonas aeruginosa induces localized immunosuppression during pneumonia. Infect. Immun. 76:4414-4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feltman, H., G. Schulert, S. Khan, M. Jain, L. Peterson, and A. R. Hauser. 2001. Prevalence of type III secretion genes in clinical and environmental isolates of Pseudomonas aeruginosa. Microbiology 147:2659-2669. [DOI] [PubMed] [Google Scholar]
- 10.Finck-Barbancon, V., and D. W. Frank. 2001. Multiple domains are required for the toxic activity of Pseudomonas aeruginosa ExoU. J. Bacteriol. 183:4330-4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finck-Barbancon, V., J. Goranson, L. Zhu, T. Sawa, J. P. Wiener-Kronish, S. M. Fleiszig, C. Wu, L. Mende-Mueller, and D. W. Frank. 1997. ExoU expression by Pseudomonas aeruginosa correlates with acute cytotoxicity and epithelial injury. Mol. Microbiol. 25:547-557. [DOI] [PubMed] [Google Scholar]
- 12.Frank, D. W. 1997. The exoenzyme S regulon of Pseudomonas aeruginosa. Mol. Microbiol. 26:621-629. [DOI] [PubMed] [Google Scholar]
- 13.Ganesan, A. K., D. W. Frank, R. P. Misra, G. Schmidt, and J. T. Barbieri. 1998. Pseudomonas aeruginosa exoenzyme S ADP-ribosylates Ras at multiple sites. J. Biol. Chem. 273:7332-7337. [DOI] [PubMed] [Google Scholar]
- 14.Hauser, A. R. 2009. The type III secretion system of Pseudomonas aeruginosa: infection by injection. Nat. Rev. Microbiol. 7:654-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hauser, A. R., P. J. Kang, and J. N. Engel. 1998. PepA, a secreted protein of Pseudomonas aeruginosa, is necessary for cytotoxicity and virulence. Mol. Microbiol. 27:807-818. [DOI] [PubMed] [Google Scholar]
- 16.Henriksson, M. L., U. Troller, and B. Hallberg. 2000. 14-3-3 proteins are required for the inhibition of Ras by exoenzyme S. Biochem. J. 349 Pt. 3:697-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoang, T. T., A. J. Kutchma, A. Becher, and H. P. Schweizer. 2000. Integration-proficient plasmids for Pseudomonas aeruginosa: site-specific integration and use for engineering of reporter and expression strains. Plasmid 43:59-72. [DOI] [PubMed] [Google Scholar]
- 18.Kaufman, M. R., J. Jia, L. Zeng, U. Ha, M. Chow, and S. Jin. 2000. Pseudomonas aeruginosa mediated apoptosis requires the ADP-ribosylating activity of ExoS. Microbiology 146:2531-2541. [DOI] [PubMed] [Google Scholar]
- 19.Lee, V. T., R. S. Smith, B. Tummler, and S. Lory. 2005. Activities of Pseudomonas aeruginosa effectors secreted by the type III secretion system in vitro and during infection. Infect. Immun. 73:1695-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicas, T. I., and B. H. Iglewski. 1985. The contribution of exoproducts to virulence of Pseudomonas aeruginosa. Can. J. Microbiol. 31:387-392. [DOI] [PubMed] [Google Scholar]
- 21.Nicas, T. I., and B. H. Iglewski. 1984. Isolation and characterization of transposon-induced mutants of Pseudomonas aeruginosa deficient in production of exoenzyme S. Infect. Immun. 45:470-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ottmann, C., L. Yasmin, M. Weyand, J. L. Veesenmeyer, M. H. Diaz, R. H. Palmer, M. S. Francis, A. R. Hauser, A. Wittinghofer, and B. Hallberg. 2007. Phosphorylation-independent interaction between 14-3-3 and exoenzyme S: from structure to pathogenesis. EMBO J. 26:902-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phillips, R. M., D. A. Six, E. A. Dennis, and P. Ghosh. 2003. In vivo phospholipase activity of the Pseudomonas aeruginosa cytotoxin ExoU and protection of mammalian cells with phospholipase A2 inhibitors. J. Biol. Chem. 278:41326-41332. [DOI] [PubMed] [Google Scholar]
- 24.Pier, G. 2005. Application of vaccine technology to prevention of Pseudomonas aeruginosa infections. Expert Rev. Vaccines 4:645-656. [DOI] [PubMed] [Google Scholar]
- 25.Rabin, S. D., and A. R. Hauser. 2005. Functional regions of the Pseudomonas aeruginosa cytotoxin ExoU. Infect. Immun. 73:573-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rabin, S. D., and A. R. Hauser. 2003. Pseudomonas aeruginosa ExoU, a toxin transported by the type III secretion system, kills Saccharomyces cerevisiae. Infect. Immun. 71:4144-4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rabin, S. D., J. L. Veesenmeyer, K. T. Bieging, and A. R. Hauser. 2006. A C-terminal domain targets the Pseudomonas aeruginosa cytotoxin ExoU to the plasma membrane of host cells. Infect. Immun. 74:2552-2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sato, H., J. B. Feix, and D. W. Frank. 2006. Identification of superoxide dismutase as a cofactor for the pseudomonas type III toxin, ExoU. Biochemistry 45:10368-10375. [DOI] [PubMed] [Google Scholar]
- 29.Sato, H., J. B. Feix, C. J. Hillard, and D. W. Frank. 2005. Characterization of phospholipase activity of the Pseudomonas aeruginosa type III cytotoxin, ExoU. J. Bacteriol. 187:1192-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sato, H., and D. W. Frank. 2004. ExoU is a potent intracellular phospholipase. Mol. Microbiol. 53:1279-1290. [DOI] [PubMed] [Google Scholar]
- 31.Sato, H., D. W. Frank, C. J. Hillard, J. B. Feix, R. R. Pankhaniya, K. Moriyama, V. Finck-Barbancon, A. Buchaklian, M. Lei, R. M. Long, J. Wiener-Kronish, and T. Sawa. 2003. The mechanism of action of the Pseudomonas aeruginosa-encoded type III cytotoxin, ExoU. EMBO J. 22:2959-2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmalzer, K. M., M. A. Benson, and D. W. Frank. 2010. Activation of ExoU phospholipase activity requires specific C-terminal regions. J. Bacteriol. 192:1801-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schulert, G. S., H. Feltman, S. D. Rabin, C. G. Martin, S. E. Battle, J. Rello, and A. R. Hauser. 2003. Secretion of the toxin ExoU is a marker for highly virulent Pseudomonas aeruginosa isolates obtained from patients with hospital-acquired pneumonia. J. Infect. Dis. 188:1695-1706. [DOI] [PubMed] [Google Scholar]
- 34.Shaver, C. M., and A. R. Hauser. 2004. Relative contributions of Pseudomonas aeruginosa ExoU, ExoS, and ExoT to virulence in the lung. Infect. Immun. 72:6969-6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stirling, F. R., A. Cuzick, S. M. Kelly, D. Oxley, and T. J. Evans. 2006. Eukaryotic localization, activation and ubiquitinylation of a bacterial type III secreted toxin. Cell. Microbiol. 8:1294-1309. [DOI] [PubMed] [Google Scholar]
- 36.Sun, J., and J. T. Barbieri. 2003. Pseudomonas aeruginosa ExoT ADP-ribosylates CT10 regulator of kinase (Crk) proteins. J. Biol. Chem. 278:32794-32800. [DOI] [PubMed] [Google Scholar]
- 37.Vallis, A. J., V. Finck-Barbancon, T. L. Yahr, and D. W. Frank. 1999. Biological effects of Pseudomonas aeruginosa type III-secreted proteins on CHO cells. Infect. Immun. 67:2040-2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yahr, T. L., A. J. Vallis, M. K. Hancock, J. T. Barbieri, and D. W. Frank. 1998. ExoY, an adenylate cyclase secreted by the Pseudomonas aeruginosa type III system. Proc. Natl. Acad. Sci. U. S. A. 95:13899-13904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang, F. L., and P. J. Casey. 1996. Protein prenylation: molecular mechanisms and functional consequences. Annu. Rev. Biochem. 65:241-269. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.