Abstract
The pneumonic lesions and mortality caused by Mannheimia haemolytica in bighorn sheep (BHS; Ovis canadensis) are more severe than those in the related species, domestic sheep (DS; Ovis aries), under both natural and experimental conditions. Leukotoxin (Lkt) and lipopolysaccharide (LPS) are the most important virulence factors of this organism. One hallmark of pathogenesis of pneumonia is the influx of polymorphonuclear leukocytes (PMNs) into the lungs. Lkt-induced cytolysis of PMNs results in the release of cytotoxic compounds capable of damaging lung tissue. Interleukin-8 (IL-8) is a potent PMN chemoattractant. The objective of the present study was to determine if there is differential expression of IL-8 by the macrophages and PMNs of BHS and DS in response to M. haemolytica. Macrophages and PMNs of BHS and DS were stimulated with heat-killed M. haemolytica or LPS. IL-8 expression by the cells was measured by enzyme-linked immunosorbent assays and real-time reverse transcription-PCR (RT-PCR). The PMNs of BHS expressed severalfold higher levels of IL-8 than those of DS upon stimulation. Lesional lung tissue of M. haemolytica-infected BHS contained significantly higher levels of IL-8 than nonlesional tissue. The bronchoalveolar lavage (BAL) fluid of infected BHS also contained higher levels of IL-8 than that of infected DS. Depletion of IL-8 reduced migration of PMNs toward BAL fluid by approximately 50%, indicating that IL-8 is integral to PMN recruitment to the lung during M. haemolytica infection. Excessive production of IL-8, enhanced recruitment of PMNs, and PMN lysis by Lkt are likely responsible for the severity of the lung lesions in M. haemolytica-infected BHS.
Pneumonia caused by Mannheimia haemolytica is an important factor in the drastic decline of bighorn sheep (BHS; Ovis canadensis) populations in North America (3, 31). The pneumonic lesions and mortality caused by this organism in BHS are much more severe than those in the related species, domestic sheep (DS; Ovis aries), under both natural and experimental conditions (11, 12, 13, 24). The cellular and molecular basis underlying the differential pathogenicity exhibited by M. haemolytica in BHS and DS has not been elucidated yet.
M. haemolytica is a Gram-negative coccobacillus commonly found as a commensal bacterium in the nasopharynx of ruminants. In conjunction with stress factors and/or viral infections, the bacteria proliferate rapidly and enter the lungs. An acute fibrinonecrotic pleuropneumonia characterized by leukocyte influx into the alveoli, fibrin deposition, necrosis of alveolar walls, and capillary thrombosis ensues (14, 25). M. haemolytica produces several virulence factors, including a polysaccharide capsule, outer membrane proteins, adhesins, neuraminidase, endotoxin (lipopolysaccharide [LPS]), and an exotoxin (leukotoxin [Lkt]). Among these, Lkt and LPS are considered the most important virulence factors responsible for the pathogenesis of pneumonia (2, 7). Lkt is a 102-kDa protein which belongs to the RTX family of exotoxins produced by a group of Gram-negative bacteria including Escherichia coli and Actinobacillus actinomycetemcomitans (16, 30). All subsets of ruminant leukocytes are susceptible to the cytolytic activity of Lkt, but the polymorphonuclear leukocytes (PMNs) are the most susceptible subset (28). The PMNs of BHS are 4- to 8-fold more susceptible to Lkt than the PMNs of DS (27). At low concentrations, Lkt causes leukocyte activation, stimulating a respiratory burst, degranulation, secretion of cytokines, and apoptosis (19, 33). At high concentrations, Lkt causes rapid cell death by forming pores in the host cell membrane (6). The Lkt interaction with PMNs and other leukocytes is mediated by CD18, the β subunit of β2 integrins (1, 10, 18).
The LPS of M. haemolytica stimulates alveolar macrophages to produce proinflammatory cytokines, including interleukin-8 (IL-8) (17, 20, 23, 32), which is a highly potent chemoattractant for PMNs (5, 22). Significant levels of IL-8 can be detected in the bronchoalveolar lavage (BAL) fluid from animals infected with M. haeomolytica (4). Thus, there is a significant influx of PMNs into the lower respiratory tract of M. haemolytica-infected animals. PMNs produce highly reactive oxygen species (ROS), such as hydrogen peroxide, superoxide, the hydroxyl radical, and hypochlorous acid (8, 9), which are released into the phagolysosome to facilitate pathogen killing. However, on contact with Lkt, PMNs undergo lysis, releasing these cytotoxic compounds, which subsequently cause extensive damage to the lung tissue (6, 28). Both PMN degranulation and endothelial cell damage are significantly decreased when IL-8 is neutralized (21). Although the production of inflammatory cytokines such as IL-8 is essential for the clearance of infections, overproduction of these cytokines damages lung tissue through PMN degranulation and release of cytotoxic compounds. Therefore, we hypothesized that the enhanced lung pathology and mortality caused by M. haemolytica in BHS is due to the production of higher levels of proinflammatory cytokines in BHS in comparison with the levels in DS. The objective of the present study was to compare the levels of production of IL-8 by the macrophages and PMNs of BHS and DS and the IL-8-mediated chemotactic responses of these cells.
MATERIALS AND METHODS
Materials.
Mouse anti-bovine IL-8, rabbit anti-bovine IL-8, and sheep anti-rabbit IgG conjugated to biotin were obtained from AbD Serotec (Raleigh, NC). The density gradient centrifugation medium Ficoll-Paque Plus and CNBr-activated Sepharose 4B were obtained from GE Healthcare Biosciences (Piscataway, NJ). The iQ SYBR green supermix was purchased from Bio-Rad (Hercules, CA). Primers for real-time reverse transcription-PCR (RT-PCR) were purchased from IDT Technology Services (Coralville, IA). Recombinant BHS and DS IL-8 have been cloned, sequenced, and expressed in our laboratory (C. H. Herndon and S. Srikumaran, unpublished data). Lkt was prepared according to the method of Gentry and Srikumaran (15). LPS purified from M. haemolytica was generously provided by S. K. Maheswaran of the University of Minnesota. RPMI medium, Dulbecco modified Eagle medium, Trizol, and the pCR4-TOPO vector were purchased from Invitrogen (Carlsbad, CA). Random hexamers and Moloney murine leukemia virus (MMLV) reverse transcriptase were purchased from Promega (Madison, WI). The pET30a vector was purchased from Novagen (Gibbstown, NJ). Fluorescent cell migration assay kits were purchased from Millipore (Billerica, MA). Brain heart infusion (BHI) blood agar plates were obtained from Remel (Lenexa, KS). The QIAprep miniprep kit was purchased from Qiagen (Valencia, CA).
PMN isolation.
Peripheral blood was collected by venipuncture from six BHS and six DS. The PMNs were isolated by density gradient centrifugation on Ficoll-Paque Plus and hypotonic lysis of the red blood cell pellet. After isolation, the PMNs were resuspended in complete RPMI medium containing 10% fetal bovine serum, 2 mM l-glutamine, 500 U/ml penicillin, and 0.1 mg/ml streptomycin. The purity and viability of the PMN population were determined to be greater than 95% by flow cytometry. For stimulation prior to enzyme-linked immunosorbent assays (ELISAs), PMNs were plated in 96-well plates at 8 × 106 cells/ml. For stimulation prior to real-time RT-PCR, the PMNs were plated in 24-well plates at 8 × 106 cells/ml.
PMN culture and stimulation.
M. haemolytica was cultured in BHI broth overnight at 37°C on a rotary shaker with agitation at 200 rpm. On the following morning, the cultures were centrifuged at 13,000 × g for 15 min at room temperature. The bacterial pellets were resuspended in 50 ml BHI medium and cultured for an additional 4 h. The bacteria were then incubated at 72°C for 10 min and confirmed to be dead by the absence of growth on BHI medium plates incubated at 37°C overnight. PMNs were stimulated with heat-killed M. haemolytica at a ratio of 1:50. The PMNs were also stimulated separately with 1 μg/ml LPS purified from M. haemolytica. PMNs were incubated with stimulants at 37°C in 5% CO2 for 8 h or 16 h, and the culture supernatant was frozen at −20°C until it was assayed by ELISA. PMNs were stimulated for 1 h or 5 h prior to isolation of total RNA for real time RT-PCR.
MDM culture and stimulation.
Peripheral blood mononuclear cells (PBMCs) were separated from the blood of six BHS and six DS by density gradient centrifugation using Ficoll-Paque Plus. Monocytes were isolated from the PBMCs by adherence to tissue culture plates for 1.5 h, after which time the nonadherent cells were washed off. Monocytes were cultured in RPMI medium containing 10% fetal bovine serum, 25 mM HEPES, 2 mM l-glutamine, 500 U/ml penicillin, 0.1 mg/ml streptomycin, and 44 mM β-mercaptoethanol for 5 to 7 days before stimulation. On the day before stimulation, monocyte-derived macrophages (MDMs) were eluted by culturing them in plates for 1 h at 37°C with 0.5 mM EDTA, followed by gentle vortexing. The MDMs were then plated in 96-well plates at a concentration of 2 × 105 cells/ml and were stimulated for 8 h or 16 h with heat-killed M. haemolytica at a ratio of 1:50 or with LPS at 1 μg/ml. After stimulation, the plates were centrifuged at 600 × g for 5 min. The culture supernatant was collected and frozen at −20°C until it was assayed by ELISA.
ELISAs.
A standard sandwich ELISA was used to quantify the concentration of IL-8 in the culture supernatant of stimulated PMNs and MDMs. A mouse monoclonal antibody specific for ovine IL-8 was used as the capture antibody. The recombinant IL-8 or the IL-8 in the test samples captured by the mouse antibody was detected by the addition of polyclonal rabbit antibodies specific for ovine IL-8, followed by the addition of biotinylated sheep anti-rabbit IgG and then streptavidin-horseradish peroxidase (HRP) and the HRP substrate 2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid). All incubations were carried out for 45 min at 37°C. The concentrations of IL-8 in the test samples were extrapolated from a standard curve generated with known concentrations of recombinant BHS and DS IL-8. The detection limit of the assay was approximately 10 pg/ml.
RNA extraction and cDNA synthesis.
BHS and DS PMNs at a concentration of 8 × 106 cells/ml were stimulated with heat-killed M. haemolytica at a ratio of 1:50 or 1 μg/ml LPS. Total cellular RNA was extracted from the stimulated PMNs using Trizol, according to the manufacturer's protocol. Contaminating DNA was removed by DNase treatment in a 10-μl reaction mixture containing 1 μg total RNA, 1 μl 10× reaction buffer, 1 μl DNase I, and diethyl pyrocarbonate-treated water. The reaction mixtures were incubated at 37°C for 30 min, followed by the addition of 1 μl 25 mM EDTA and a 10-min incubation at 65°C. First-strand cDNA was synthesized with MMLV reverse transcriptase using 0.5 μg random hexamers and 1 μg total RNA in a 15-μl reaction mixture. The reaction mixtures were heated for 5 min at 70°C and immediately cooled on ice, followed by the addition of 5 μl MMLV reaction buffer, 5 μl 10 mM deoxynucleoside triphosphates (dNTPs), 1 μl RNasin RNase inhibitor, 1 μl MMLV reverse transcriptase, and nuclease-free water to a final volume of 25 μl. The reaction mixtures were incubated for 60 min at 37°C.
Generation of plasmids carrying ovine IL-8 and 18S rRNA.
Primers were designed to amplify ovine IL-8 and 18S rRNA on the basis of GenBank sequences (NM_001009401 and AY753190, respectively) using IDT primer design software (Table 1). DS PBMCs were plated at 1 × 106 cells/ml and stimulated for 4 h with 1 μg/ml LPS. Total cellular RNA was then isolated from the stimulated cells using Trizol, according to the manufacturer's protocol. First-strand cDNA was generated as described above and used to amplify ovine IL-8 and 18S rRNA in 50-μl-volume PCR mixtures containing 50 ng DNA, 5 μl PCR buffer, 0.5 μl 50 nM dNTPs, 200 ng of each primer, and 1 U Taq polymerase. The cycling conditions were as follows: activation at 94°C for 3 min, followed by 35 cycles of denaturation at 94°C for 45 s, annealing at 55°C for 30 s, and extension at 72°C for 90 s, with a final cycle of extension at 72°C for 10 min. The PCR products were purified from the agarose gels and cloned into a pCR4-TOPO vector for 18S rRNA and a pET30a vector for IL-8, according to the manufacturers' protocols. The PCR products were then transformed into TOP10 E. coli cells, and the colonies were screened according to the manufacturer's protocol. Plasmid DNA was isolated using a QIAprep Miniprep kit, according to the manufacturer's protocol.
TABLE 1.
Oligonucleotide sequences of upstream (5′) and downstream (3′) primers of target genes
| Target gene | Oligonucleotide sequence |
|
|---|---|---|
| 5′ primer | 3′ primer | |
| IL-8a | CCGGAATTCCGGGTTCTGTCAAGAATGAGTACAGA | CCGCTCGAGCGGTCATGGATCTTGCTTCTC |
| IL-8b | TTCCAAGCTGGCTGTTGCTCTCTT | GCATTGGCATCGAAGTTCTGTACTC |
| 18S rRNAa | CGCTCCTCTCCTACTTGGATAACT | TCCTTGGATGTGGTAGCCGTTTCT |
| 18S rRNAb | TCAACTTTCGATGGTAGTCGCCGT | TCCTTGGATGTGGTAGCCGTTTCT |
Primer used for cloning and designed with IDT primer design software on the basis of the GenBank sequences of ovine IL-8 and 18S rRNA (accession numbers NM_001009401 and AY753190, respectively).
Primer used for real-time RT-PCR and designed with IDT primer design software on the basis of the GenBank sequences of ovine IL-8 and 18S rRNA (accession numbers NM_001009401 and AY753190, respectively).
Real time RT-PCR.
For the quantification of IL-8 transcripts in stimulated PMNs, first-strand cDNA was amplified by real-time RT-PCR using iQ SYBR green supermix with the primer pair sets listed in Table 1. Real time RT-PCR was performed with a Bio-Rad iCycler apparatus. Each 25-μl reaction mixture contained 12.5 μl supermix, 6.5 μl double-distilled H2O, 5 μl cDNA or plasmid DNA, and 400 nmol each primer. The cycling conditions were as follows: activation at 95°C for 3 min, followed by 45 cycles of denaturation at 95°C for 30 s, annealing at 55°C for 30 s, and extension at 72°C for 30 s, with a final cycle of extension at 72°C for 5 min. Melting curve analysis was performed at the conclusion of the PCR assay. Gene expression was quantified on the basis of plasmid standard curves using iCycler iQ optical system software, edition 3.1. The results were expressed as the ratio of the IL-8 mRNA copy number to the 18S rRNA copy number. Negative controls consisted of a no-template control and a no-primer control for each experiment.
Growth of M. haemolytica serotype A2.
M. haemolytica serotype A2 strain WSU-1 was streaked onto BHI blood agar plates, and the plates were incubated at 37°C overnight. On the following day, 4 ml colorless RPMI medium was added to the plates and colonies were removed with gentle scraping. The optical density at 600 nm (OD600) of the solution was adjusted to 0.4 with colorless RPMI medium. M. haemolytica was cultured for 1.5 h at 37°C until the OD600 reached 0.6. The M. haemolytica culture was then diluted to acquire a concentration of 1 × 108 CFU/ml, and 1 ml bacteria was combined with 4 ml colorless RPMI medium.
Animal inoculation.
All DS were 2- to 3-year-old ewes and were purchased from the University of Idaho Sheep Center. All the BHS were hand raised as part of the captive herd maintained at Washington State University. Seven of the BHS were female and two were male, and all BHS were between the ages of 2 and 3 years. None of the animals used in this study had a history of respiratory disease. Nine BHS and nine DS were inoculated intratracheally with the bacteria and then 5 ml colorless RPMI medium. Three, two, and four animals per species were humanely euthanized at 4, 12, and 18 h postinfection (hpi), respectively. A larger number of animals (n = 4) was used for the late time point (18 h) because previous studies by other research groups have evaluated the IL-8 response at 18 h. Therefore, we anticipated that any possible species difference was likely to be observed at this time point. Fewer animals were used for the early time points (4 h and 12 h) because of the limited number of BHS available for this study.
Collection of BAL fluid and lung tissue.
After euthanization, the lungs of each animal were removed and BAL was performed with 200 ml sterile 1× phosphate-buffered saline (PBS). The BAL fluid was centrifuged at 400 × g for 10 min at 4°C, and the supernatant was frozen at −20°C until it was analyzed by ELISA. Tissue samples were removed from the right cranial lobe of each BHS and DS, with both lesional and nonlesional tissue samples being taken where applicable. The tissue samples (0.5 g tissue per sample) were minced and homogenized in 1 ml of 0.05% Tween-PBS. Tissue sample homogenates were then centrifuged at 15,000 × g for 10 min, and the supernatant was frozen at −20°C until it was assayed by ELISA.
Depletion of IL-8 from BAL fluid.
Polyclonal anti-BHS IL-8 antibodies were produced in mice. Recombinant BHS IL-8 produced in E. coli was used as the antigen. Complete and incomplete Freund's adjuvants were used for priming and boosting, respectively. The antiserum was purified by ammonium sulfate precipitation. The anti-BHS IL-8 antibodies (12 mg) were coupled to CNBr-activated Sepharose 4B (2 g), according to the manufacturer's instructions. The BAL fluid of an animal infected with M. haemolytica A2 was depleted of IL-8 by incubation with Sepharose-coupled anti-IL-8 antibodies overnight with agitation at 4°C. After absorption, the BAL fluid was tested by ELISA to confirm the absence of IL-8.
Neutrophil migration assay.
The migration of BHS PMNs toward IL-8 in BAL fluid from BHS infected with M. haemolytica A2 was measured by a fluorescent migration assay, according to the manufacturer's instructions. The PMNs from five BHS were allowed to migrate for 90 min at 37°C in 5% CO2.
Data analysis.
All the data were analyzed using the nonparametric Kluskal-Wallis test, and results were considered significant when the P value was <0.05.
RESULTS
MDMs of BHS and DS express similar levels of IL-8.
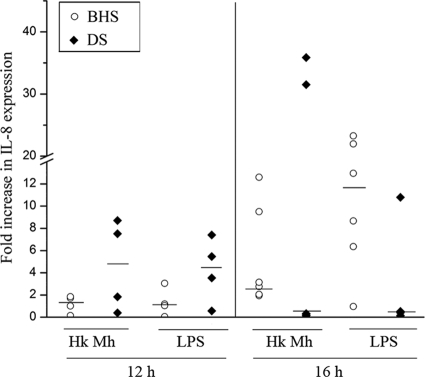
To compare the production of IL-8 by the MDMs of BHS and DS in response to M. haemolytica infection, MDMs of six BHS and six DS were stimulated with heat-killed M. haemolytica or LPS for 12 h or 16 h, and the IL-8 concentration in the culture supernatant was determined by sandwich ELISA. Although the production of IL-8 by the MDMs increased with time, there was no significant difference in the levels of IL-8 production by the MDMs of the two species in response to heat-killed M. haemolytica or LPS (Fig. 1). Similar results were observed with different concentrations of stimulants and MDMs and at multiple time points.
FIG. 1.
IL-8 production by MDMs of BHS and DS. MDMs from BHS and DS (n = 6) were stimulated with heat-killed M. haemolytica or LPS from M. haemolytica for 12 h or 16 h, and the culture supernatant was assayed for the IL-8 concentration by a sandwich ELISA. IL-8 production is expressed as the fold increase in expression by stimulated MDMs over that by unstimulated MDMs. Each data point represents the IL-8 production by MDMs from one animal. Medians are indicated by horizontal lines. The P values were >0.05 when the results for BHS and DS were compared by the Kruskal-Wallis test. Hk Mh, heat-killed M. haemolytica; LPS, lipopolysaccharide derived from M. haemolytica.
PMNs of BHS express higher levels of IL-8 than those of DS.
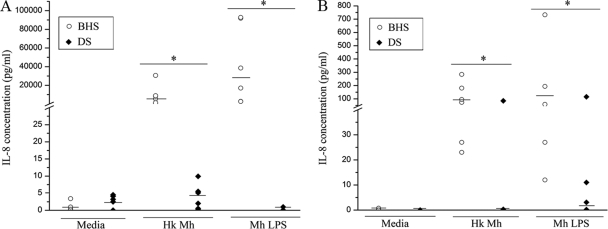
The PMNs of six BHS and six DS were stimulated with heat-killed M. haemolytica or purified LPS for 8 h or 16 h. The concentrations of IL-8 produced by BHS PMNs in response to heat-killed M. haemolytica and LPS were approximately 10,000- and 35,000-fold higher, respectively, than those produced by DS PMNs. At 16 h, the concentrations of IL-8 produced by BHS PMNs in response to heat-killed M. haemolytica and LPS were approximately 100- and 150-fold higher, respectively, than those produced by DS PMNs (Fig. 2). The difference in the level of IL-8 production by BHS and DS PMNs in response to stimulation with LPS was greater than that in response to heat-killed M. haemolytica.
FIG. 2.
IL-8 production by PMNs of BHS and DS stimulated with heat-killed M. haemolytica or LPS from M. haemolytica. PMNs from BHS and DS (n = 6) were stimulated with heat-killed M. haemolytica or LPS for 8 h (A) or 16 h (B). The culture supernatant was assayed for the IL-8 concentration by a sandwich ELISA. Each data point represents the level of IL-8 production by the PMNs from one animal, and medians are indicated by horizontal lines. Where significance is indicated (*), P was <0.05 when the results for BHS were compared to those for DS by the Kruskal-Wallis test. Hk Mh, heat-killed M. haemolytica; LPS, lipopolysaccharide derived from M. haemolytica.
BHS PMNs contain higher IL-8 mRNA transcript levels than DS PMNs.
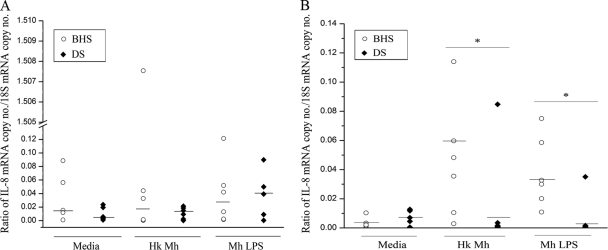
Real time RT-PCR was employed to determine if the differential levels of IL-8 production by the PMNs of BHS and DS were due to the differential transcription of IL-8 mRNA. At 1 h poststimulation, the IL-8 mRNA levels in the PMNs of BHS and DS were not significantly different from each other or from those in unstimulated PMNs (Fig. 3A). However, at 5 h poststimulation, the IL-8 mRNA levels in BHS PMNs stimulated with heat-killed M. haemolytica or LPS were approximately 6-fold higher than those in DS PMNs (Fig. 3B). Thus, BHS PMNs had higher levels of IL-8 than DS PMNs at both the transcript and protein levels.
FIG. 3.
IL-8 mRNA transcript levels from PMNs stimulated with M. haemolytica or LPS from M. haemolytica. PMNs from BHS and DS (n = 6) were stimulated with heat-killed M. haemolytica or LPS for 1 h (A) or 5 h (B). The mRNA levels were quantified using real-time RT-PCR and were normalized against the 18S rRNA copy numbers. IL-8 mRNA levels are expressed as the ratio of the IL-8 mRNA copy number to the 18S rRNA copy number. Each data point represents the IL-8 mRNA level in the PMNs from one animal. Medians are indicated by horizontal lines. Where significance is indicated (*), P was <0.05 when the results for BHS and DS were compared by the Kruskal-Wallis test. Hk Mh, heat-killed M. haemolytica; LPS, lipopolysaccharide derived from M. haemolytica.
BHS infected with M. haemolytica have higher levels of IL-8 in lung tissue than infected DS.
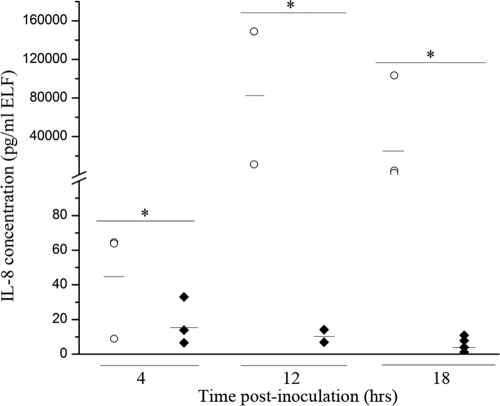
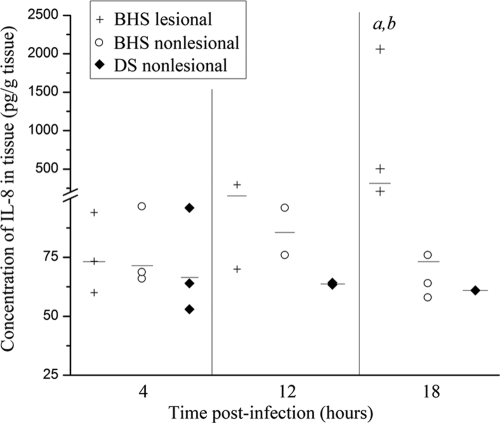
To determine if the differential expression of IL-8 by the PMNs of BHS and DS observed in vitro occurs in vivo as well, each of nine BHS and nine DS were inoculated intratracheally with 1 × 107 CFU of M. haemolytica serotype A2. Sheep were euthanized and a necropsy was performed at 4, 12, or 18 hpi. Prior to necropsy, none of the BHS or DS displayed clinical symptoms. At the time of necropsy, BAL was performed and tissue samples were taken from the lesional and nonlesional right cranial lung of each animal. In the absence of lesions, only nonlesional tissue was collected. The IL-8 concentration in the BAL fluid samples from each animal was measured by sandwich ELISA. Since different volumes of BAL fluid were recovered from each animal, IL-8 concentrations were normalized to the volume of epithelial lining fluid (ELF) according to the urea method (26). At all time points analyzed, the IL-8 concentration was significantly higher in the BAL fluid of infected BHS than in that of infected DS (Fig. 4). The IL-8 levels in the DS BAL fluid were very low at all time points tested. The IL-8 concentration in the lung tissue was also measured. At 4 hpi, the IL-8 concentrations in both lesional and nonlesional BHS lung tissue, as well as DS lung tissue, were comparable to one another (Fig. 5). By 12 hpi, the IL-8 concentration in lesional lung tissue was slightly higher than that in both nonlesional BHS tissue and nonlesional DS tissue. However, this difference was not statistically significant. By 18 hpi, the IL-8 concentration in the lesional tissue of infected BHS was significantly higher than that in nonlesional BHS and DS tissue.
FIG. 4.
IL-8 concentrations in the BAL fluid of M. haemolytica-infected BHS and DS. The concentrations of IL-8 in the BAL fluid of BHS and DS were measured by a sandwich ELISA and were normalized to the ELF volume at 4, 12, or 18 hpi. Each data point represents the IL-8 concentration in the BAL fluid from one animal. Medians are indicated by horizontal lines. Where significance is indicated (*), P was <0.05 when the results for BHS and DS were compared by the Kruskal-Wallis test.
FIG. 5.
IL-8 concentrations in the lung tissue of BHS and DS inoculated with M. haemolytica A2. Lung tissue samples were collected from animals necropsied at 4, 12, or 18 hpi. The IL-8 concentration was measured by a sandwich ELISA. Each data point represents the IL-8 concentration in the lung tissue sample from one animal. Medians are indicated by horizontal lines. BHS lesional, IL-8 concentration in the lung tissue sample collected from areas of BHS lungs exhibiting pneumonic lesions; BHS and DS nonlesional, IL-8 concentrations in lung tissue samples collected from areas of BHS or DS lungs devoid of pneumonic lesions; a, the IL-8 concentrations in BHS lesional tissue are significantly different from those in BHS nonlesional tissue (P < 0.05, Kruskal-Wallis test); b, the IL-8 concentrations in BHS lesional tissue are significantly different from those in DS nonlesional tissue (P < 0.05, using the Kruskal-Wallis test).
IL-8 is a critical mediator of PMN migration.
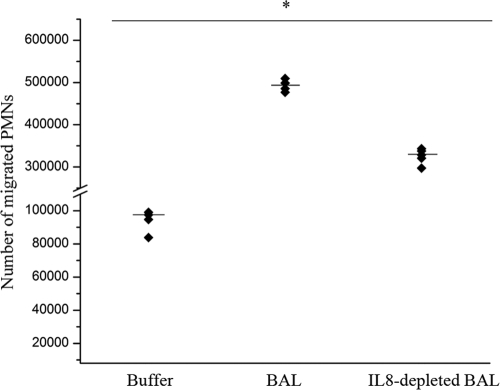
The chemoattractant property of IL-8 likely overlaps with that of other mediators, such as platelet-activating factor, leukotriene B4, and interleukin-1β. To determine the biological significance of IL-8 as a PMN chemoattractant in the lungs of infected BHS, migration of PMNs in vitro toward pneumonic BAL fluid was measured before and after depletion of IL-8 by immunoabsorption. Depletion of IL-8 from the BAL fluid of BHS infected with M. haemolytica reduced the migration of the PMNs of five BHS toward the BAL fluid by approximately 50% (Fig. 6). These results indicate that IL-8 is an important mediator of migration of BHS PMNs toward inflamed lung tissue.
FIG. 6.
Effect of depletion of IL-8 from BAL fluid on migration of PMNs. BAL fluid was collected at necropsy from BHS infected with M. haemolytica serotype A2. The IL-8 in BAL fluid was depleted by overnight incubation with mouse anti-IL-8 polyclonal antibodies coupled to CNBr-activated Sepharose 4B. Migration of PMNs from BHS (n = 5) was measured by a fluorescent chemotaxis assay, as described in Materials and Methods. Each data point represents the number of migrated PMNs from each animal, and medians are indicated by horizontal lines. All treatments were significant at a P value of <0.05 by the Kruskal-Wallis test.
DISCUSSION
The cellular and molecular basis for the induction of severe pneumonic lesions and mortality caused by M. haemolytica in BHS is not clear. We hypothesized that the enhanced lung pathology and mortality caused by M. haemolytica in BHS is due to the production of higher levels of proinflammatory cytokines in BHS than in DS. In an effort to test this hypothesis, in this study we compared the production of IL-8 by the MDMs and PMNs of BHS and DS and the IL-8-mediated chemotactic response of the PMNs of these two species. Previously, the production of inflammatory cytokines in M. haemolytica infection has largely been attributed to resident alveolar macrophages (23, 32). The results of this study have revealed that the MDMs of BHS and DS produce similar levels of IL-8 in response to heat-killed M. haemolytica or purified LPS. In contrast, the PMNs of BHS produced higher levels of IL-8 than the PMNs of DS at both time points tested. The standard curves developed with known concentrations of recombinant BHS and DS IL-8 were identical. Therefore, the differences in IL-8 concentrations observed in the culture supernatants of the PMNs of BHS and DS are not due to a difference in the avidities of the antibodies for the IL-8 of these species. Furthermore, the amino acid sequences of BHS and DS IL-8 are 100% identical (Herndon and Srikumaran, unpublished), negating the possibility that sequence differences led to differential binding in the sandwich ELISAs. The large variation in cytokine production by PMNs from the individual BHS is very likely due to different degrees of differentiation and activation of these cells. The IL-8 mRNA levels in BHS PMNs stimulated with heat-killed M. haemolytica or LPS were also higher than those of DS PMNs. However, the fold difference in the IL-8 protein levels between BHS and DS PMNs was much higher than that in the IL-8 mRNA levels. It is possible that the IL-8 protein of DS is less stable than that of BHS, giving rise to low concentrations in the culture fluid to be detected by the ELISA.
The IL-8 concentrations in the BHS BAL fluid were significantly higher than those in the DS BAL fluid at as early as 4 hpi, and this difference continued to the final time point of 18 hpi. Higher concentrations of IL-8 were found in lesional lung tissue of BHS than in nonlesional lung tissue of BHS and DS by 18 hpi. The presence of higher IL-8 concentrations in lesional lung tissue is consistent with the results from similar studies of cattle (5) and implicates IL-8 in the lung pathology observed in pneumonia. The presence of higher levels of IL-8 in the BAL fluid and the lung tissue of M. haemolytica-infected BHS than in those of DS indicates that the observed production of higher levels of IL-8 by BHS PMNs in vitro likely occurs in vivo as well. The presence of high levels of IL-8 in the lungs of BHS would draw large numbers of PMNs into the lungs. Given the ability of IL-8 to induce PMN activation, degranulation, and endothelial cell damage (19, 21, 23), a large population of PMNs producing high levels of IL-8 at the site of inflammation is likely responsible for the severe lung damage observed in M. haemolytica-infected BHS in comparison to that observed in DS.
Depletion of IL-8 in the BAL fluid of infected BHS reduced the migration of PMNs by approximately 50%, which is consistent with the observation that instillation of a modest dose of IL-8 into the lungs of calves induced a significant influx of PMNs within 18 h (5). However, the finding that BHS PMNs also migrated significantly toward IL-8-depleted BAL fluid suggests that other chemoattractants and possibly other inflammatory mediators were present, and some of these might also be substantially upregulated in BHS. Enhanced IL-8 expression is perhaps representative of an exaggerated and detrimental inflammatory response by BHS infected with M. haemolytica.
The role of IL-8 is not limited to the induction of PMN migration. Neutralization of IL-8 also reduced the degrees of both pulmonary arterial endothelial cell (PAEC) damage and PMN degranulation in a coculture system containing bovine PAEC and PMNs (21). Taken together, these results clearly implicate IL-8 as an important mediator of both PMN influx and host cell damage during M. haemolytica infection. Thus, the production of higher levels of IL-8 by the PMNs of BHS is likely to be a significant cause of the enhanced lung pathology in BHS.
To our knowledge, this is the first report of a marked difference in IL-8 production by the PMNs of two closely related species. The mechanism for increased IL-8 production in BHS PMNs is currently unknown. CD14 is a component of the host cell receptor complex for LPS. The association of LPS-bound CD14 with Toll-like receptor 4 and the accessory protein MD-2 triggers the eventual production of effector proteins such as IL-8. Sohn et al. (29) have demonstrated a correlation between the levels of soluble CD14 and IL-8 production. Higher levels of CD14 shedding from the surface of PMNs correlated with decreased production of IL-8 by these cells (29). Preliminary studies in our laboratory indicate that BHS PMNs constitutively express higher levels of CD14 on the cell surface than DS PMNs. A difference in either cell surface expression or shedding of CD14 may be responsible for the differential production of IL-8 by the PMNs of BHS and DS.
In summary, the PMNs of BHS produced higher levels of IL-8 than those of DS in response to M. haemolytica and LPS. Lung tissue and BAL fluid from M. haemolytica-infected BHS also contained higher IL-8 levels than those of DS. Depletion of IL-8 reduced migration of PMNs toward BAL fluid by approximately 50%, indicating that IL-8 is integral to PMN recruitment to the lung during M. haemolytica infection. Excessive production of IL-8, enhanced recruitment of PMNs, and PMN lysis by Lkt are likely responsible for the severity of the lung lesions in M. haemolytica-infected BHS.
Acknowledgments
Funding for this work was provided by the Wild Sheep Foundation, its state chapters, and the Rocky Mountain Bighorn Society. Caroline Herndon was partially supported by an Achievement Rewards for College Scientists fellowship.
We gratefully acknowledge the technical assistance and valuable suggestions of Debby Alperin, Rohana Dassanayake, Kevin Lahmers, Crystal Montoya, Sudarvili Shanthalingam, and Renuka Subramaniam.
Editor: A. J. Bäumler
Footnotes
Published ahead of print on 1 June 2010.
REFERENCES
- 1.Ambagala, T. C., A. P. Ambagala, and S. Srikumaran. 1999. The leukotoxin of Pasteurella haemolytica binds to beta(2) integrins on bovine leukocytes. FEMS Microbiol. Lett. 179:161-167. [DOI] [PubMed] [Google Scholar]
- 2.Berggren, K. A., C. S. Baluyut, R. R. Simonson, W. J. Bemrick, and S. K. Maheswaran. 1981. Cytotoxic effects of Pasteurella haemolytica on bovine neutrophils. Am. J. Vet. Res. 42:1383-1388. [PubMed] [Google Scholar]
- 3.Buechner, H. K. 1960. The bighorn sheep in the United States, its past, present, and future. In Wildlife monographs 4. Wildlife Society, Bethesda, MD.
- 4.Caswell, J. L., D. M. Middleton, S. D. Sorden, and J. R. Gordon. 1998. Expression of the neutrophil chemoattractant interleukin-8 in the lesions of bovine pneumonic pasteurellosis. Vet. Pathol. 35:124-131. [DOI] [PubMed] [Google Scholar]
- 5.Caswell, J. L., D. M. Middleton, and J. R. Gordon. 2001. The importance of interleukin-8 as a neutrophil chemoattractant in the lungs of cattle with pneumonic pasteurellosis. Can. J. Vet. Res. 65:229-232. [PMC free article] [PubMed] [Google Scholar]
- 6.Clinkenbeard, K. D., D. A. Mosier, and A. W. Confer. 1989. Transmembrane pore size and role of cell swelling in cytotoxicity caused by Pasteurella haemolytica leukotoxin. Infect. Immun. 57:420-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Confer A. W., R. J. Panciera, K. D. Clinkenbeard, and D. A. Mosier. 1990. Molecular aspects of virulence of Pasteurella haemolytica. Can. J. Vet. Res. 54(Suppl.):S48-S52. [PubMed] [Google Scholar]
- 8.Czuprynski, C. J., H. L. Hamilton, and E. J. Noel. 1987. Ingestion and killing of Pasteurella haemolytica A1 by bovine neutrophils in vitro. Vet. Microbiol. 14:61-74. [DOI] [PubMed] [Google Scholar]
- 9.Czuprynski, C. J., F. Leite, M. Sylte, C. Kuckleburg, R. Schultz, T. Inzana, E. Behling-Kelly, and L. Corbeil. 2004. Complexities of the pathogenesis of Mannheimia haemolytica and Haemophilus somnus infections: challenges and potential opportunities for prevention? Anim. Health Res. Rev. 5:277-282. [DOI] [PubMed] [Google Scholar]
- 10.Dassanayake, R. P., W. Liu, W. C. Davis, W. J. Foreyt, and S. Srikumaran. 2008. Bighorn sheep beta2-integrin LFA-1 serves as a receptor for Mannheimia haemolytica leukotoxin. J. Wildl. Dis. 44:743-747. [DOI] [PubMed] [Google Scholar]
- 11.Dassanayake, R. P., S. Shanthalingam, C. N. Herndon, P. K. Lawrence, F. E. Cassirer, K. A. Potter, W. J. Foreyt, K. D. Clinkenbeard, and S. Srikumaran. 2009. Mannheimia haemolytica serotype A1 exhibits differential pathogenicity in two related species, Ovis canadensis and Ovis aries. Vet. Microbiol. 133:366-371. [DOI] [PubMed] [Google Scholar]
- 12.Foreyt, W. J., and D. A. Jessup. 1982. Fatal pneumonia of bighorn sheep following association with domestic sheep. J. Wildl. Dis. 18:163-168. [DOI] [PubMed] [Google Scholar]
- 13.Foreyt, W. J., K. P. Snipes, and R. W. Kasten. 1994. Fatal pneumonia following inoculation of healthy bighorn sheep with Pasteurella haemolytica from healthy domestic sheep. J. Wildl. Dis. 30:137-145. [DOI] [PubMed] [Google Scholar]
- 14.Frank, G. H. 1989. Pasteurella and pasteurellosis, p. 197-222. Academic Press, New York, NY.
- 15.Gentry, M. J., and S. Srikumaran. 1991. Neutralizing monoclonal antibodies to Pasteurella haemolytica leukotoxin affinity-purify the toxin from crude culture supernatants. Microb. Pathog. 10:411-417. [DOI] [PubMed] [Google Scholar]
- 16.Kolodrubetz, D., T. Dailey, J. Ebersole, and E. Kraig. 1989. Cloning and expression of the leukotoxin gene from Actinobacillus actinomycetemcomitans. Infect. Immun. 57:1465-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lafleur, R. L., C. Malazdrewich, S. Jeyaseelan, E. Bleifield, M. S. Abrahamsen, and S. K. Maheswaran. 2001. Lipopolysaccharide enhances cytolysis and inflammatory cytokine induction in bovine alveolar macrophages exposed to Pasteurella (Mannheimia) haemolytica leukotoxin. Microb. Pathog. 30:347-357. [DOI] [PubMed] [Google Scholar]
- 18.Li, J., K. D. Clinkenbeard, and J. W. Ritchey. 1999. Bovine CD18 identified as a species specific receptor for Pasteurella haemolytica leukotoxin. Vet. Microbiol. 67:91-97. [DOI] [PubMed] [Google Scholar]
- 19.Maheswaran, S. K., D. J. Weiss, M. S. Kannan, E. L. Townsend, K. R. Reddy, L. O. Whiteley, and S. Srikumaran. 1992. Effects of Pasteurella haemolytica A1 leukotoxin on bovine neutrophils: degranulation and generation of oxygen-derived free radicals. Vet. Immunol. Immunopathol. 33:51-68. [DOI] [PubMed] [Google Scholar]
- 20.Malazdrewich, C., T. R. Ames, M. S. Abrahamsen, and S. K. Maheswaran. 2001. Pulmonary expression of tumor necrosis factor alpha, interleukin-1 beta, and interleukin-8 in the acute phase of bovine pneumonic pasteurellosis. Vet. Pathol. 38:297-310. [DOI] [PubMed] [Google Scholar]
- 21.McClenahan, D. J., O. A. Evanson, and D. J. Weiss. 2002. In vitro evaluation of the role of platelet-activating factor and interleukin-8 in Mannheimia haemolytica-induced bovine pulmonary endothelial cell injury. Am. J. Vet. Res. 63:394-401. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell, G. B., B. N. Albright, and J. L. Caswell. 2003. Effect of interleukin-8 and granulocyte colony-stimulating factor on priming and activation of bovine neutrophils. Infect. Immun. 71:1643-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morsey, M. A., A. G. Van-Kessel, Y. Mori, Y. Popowych, D. Godson, M. Campos, and L. A. Babiuk. 1999. Cytokine profiles following interaction between bovine alveolar macrophages and Pasteurella haemolytica. Microb. Pathog. 26:325-331. [DOI] [PubMed] [Google Scholar]
- 24.Onderka, D. K., S. A. Rawluk, and W. D. Wishart. 1988. Susceptibility of Rocky Mountain bighorn sheep and domestic sheep to pneumonia induced by bighorn and domestic livestock strains of Pasteurella haemolytica. Can. J. Vet. Res. 52:439-444. [PMC free article] [PubMed] [Google Scholar]
- 25.Rehmtulla, A. J., and R. G. Thomson. 1981. A review of the lesions in shipping fever of cattle. Can. Vet. J. 22:1-8. [PMC free article] [PubMed] [Google Scholar]
- 26.Rennard, S. I., G. Basset, D. Lecossier, K. M. O'Donnell, P. Pinkston, P. G. Martin, and R. G. Crystal. 1986. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J. Appl. Physiol. 60:532-538. [DOI] [PubMed] [Google Scholar]
- 27.Silflow, R. M., W. J. Foreyt, and R. W. Leid. 1993. Pasteurella haemolytica cytotoxin-dependent killing of neutrophils from bighorn and domestic sheep. J. Wildl. Dis. 29:30-35. [DOI] [PubMed] [Google Scholar]
- 28.Slocombe, R. F., J. Malark, R. Ingersoll, F. J. Derksen, and N. E. Robinson. 1985. Importance of neutrophils in the pathogenesis of acute pneumonic pasteurellosis in calves. Am. J. Vet. Res. 46:2253-2258. [PubMed] [Google Scholar]
- 29.Sohn, E. J., M. J. Paape, D. D. Bannerman, E. E. Connor, R. H. Fetterer, and R. R. Peters. 2007. Shedding of sCD14 by bovine neutrophils following activation with bacterial lipopolysaccharide results in down-regulation of IL-8. Vet. Res. 38:95-108. [DOI] [PubMed] [Google Scholar]
- 30.Strathdee, C. A., and R. Y. Lo. 1989. Cloning, nucleotide sequence, and characterization of genes encoding the secretion function of the Pasteurella haemolytica leukotoxin determinant. J. Bacteriol. 171:916-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valdez, R., and P. R. Krausman. 1999. Mountain sheep of North America. University of Arizona Press, Tucson, AZ.
- 32.Yoo, H. S., S. K. Maheswaran, G. Lin, E. L. Townsend, and T. R. Ames. 1995. Induction of inflammatory cytokines in bovine alveolar macrophages following stimulation with Pasteurella haemolytica lipopolysaccharide. Infect. Immun. 63:381-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoo, H. S., B. S. Rajagopal, S. K. Maheswaran, and T. R. Ames. 1995. Purified Pasteurella haemolytica leukotoxin induces expression of inflammatory cytokines from bovine alveolar macrophages. Microb. Pathog. 18:237-252. [DOI] [PubMed] [Google Scholar]