Abstract
Yersinia pestis, the causative agent of plague, evades host immune responses and rapidly causes disease. The Y. pestis adhesin Ail mediates host cell binding and is critical for Yop delivery. To identify the Ail receptor(s), Ail was purified following overexpression in Escherichia coli. Ail bound specifically to fibronectin, an extracellular matrix protein with the potential to act as a bridge between Ail and host cells. Ail expressed by E. coli also mediated binding to purified fibronectin, and Ail-mediated E. coli adhesion to host cells was dependent on fibronectin. Ail expressed by Y. pestis bound purified fibronectin, as did the Y. pestis adhesin plasminogen activator (Pla). However, a KIM5 Δail mutant had decreased binding to host cells, while a KIM5 Δpla mutant had no significant defect in adhesion. Furthermore, treatment with antifibronectin antibodies decreased Ail-mediated adhesion by KIM5 and the KIM5 Δpla mutant, indicating that the Ail-fibronectin interaction was important for cell binding. Finally, antifibronectin antibodies inhibited the KIM5-mediated cytotoxicity of host cells in an Ail-dependent fashion. These data indicate that Ail is a key adhesin that mediates binding to host cells through interaction with fibronectin on the surface of host cells, and this interaction is important for Yop delivery by Y. pestis.
The three species of Yersinia pathogenic for humans, Yersinia enterocolitica, Y. pseudotuberculosis, and Y. pestis, cause distinct diseases. Y. pseudotuberculosis and Y. enterocolitica typically cause acute gastroenteritis and mesenteric lymphadenitis. On the other hand, Y. pestis, the causative agent of the plague, is one of the most deadly human infectious diseases (8). Y. pestis is a close relative of Y. pseudotuberculosis, diverging only 1,500 to 20,000 years ago (1). To accommodate flea-borne transmission, Y. pestis has acquired two unique plasmids not harbored by enteropathogenic Yersinia species. All three pathogenic Yersinia species inject cytotoxic Yersinia outer proteins (Yops) into host cells via the Ysc type III secretion system (TTSS) to establish an infection (11). Host cell contact is essential for engagement of the TTSS and secretion of Yops (9, 54). Within the host cell, Yops effect actin rearrangements, inhibit phagocytosis, and block proinflammatory signals (4, 40, 42). Both Y. enterocolitica and Y. pseudotuberculosis express the well-studied adhesin molecules invasin (Inv) and YadA, capable of mediating Yop delivery (9, 54). However, Y. pestis does not express either adhesin due to an IS1541 element insertion within inv (58) and a frameshift mutation in yadA (44, 55). Y. pestis has a number of other adhesins capable of mediating host cell interaction. Both the pH 6 antigen (Psa [29, 63]) and plasminogen activator (Pla [28]) of Y. pestis have been shown to be adhesins. Psa is a tightly regulated pilus expressed at a pH of <6.7 and 37°C (52, 67) and is known to bind to β-linked galactosylated glycosphingolipids (46), low-density lipoprotein (31), and human IgG (69). Pla, expressed at 26°C but further induced at 37°C (49), is known to bind to several extracellular matrix components (23, 28, 30). The putative autotransporter, YapC, is also capable of mediating cell adhesion when it is expressed in Escherichia coli (15), as is the pilus encoded by the chaperone/usher system locus y0561-0563 (16), but neither yapC nor y0561-0563 results in significantly decreased adhesion when they are deleted from Y. pestis (15, 16).
Recently, an additional adhesin of Y. pestis, Ail (adherence and invasion locus), was determined to facilitate cell binding (14, 25). Ail (encoded by y1324) is a 21.5-kDa outer membrane protein of the OmpX family that is predicted to have eight transmembrane domains and four extracellular loops extending above the surface of the bacterium (17, 65). Ail homologues include OmpX of Escherichia coli (32) and Enterobacter cloacae (61), PagC in Salmonella (53), and Opa proteins from Neisseria (10). Ail from Y. enterocolitica has been studied previously and shown to have three activities: cell adhesion, cell invasion (36), and the ability to confer serum resistance (5, 51) by binding to complement regulatory proteins (24). The residues for all three activities have been mapped to particular amino acids in the surface-exposed loops (35). Y. pseudotuberculosis Ail also confers adhesion and invasion functions (T. M. Tsang and E. S. Krukonis, unpublished data) and serum resistance (68), although the two amino acid changes between Y. pseudotuberculosis Ail and Y. pestis Ail result in decreased adhesion and invasion mediated by the former (Tsang and Krukonis, unpublished). More recently, Y. pestis Ail was also shown to mediate cell adhesion (14, 25), autoaggregation (25), and serum resistance (3, 24, 25) and to facilitate Yop delivery to host cells (14). Furthermore, Y. pestis Ail is required for virulence, as a Y. pestis Δail mutant has a >3,000-fold increase in the 50% lethal dose (14). A Y. pestis Δail mutant shows reduced binding to both epithelial and phagocytic human-derived cell lines, and in a mouse model of infection, a Y. pestis KIM5 Δail mutant colonizes host tissue to much lower levels than the parental KIM5 strain (14). Over the course of 7 days, the Δail mutant is cleared from the host (14). Together, these data demonstrate that Ail is an important adhesin that contributes to colonization and virulence.
Cell adhesion is important for the establishment of a successful infection. Adhesion is also significant in Y. pestis pathogenesis because host cell contact is required for the production and translocation of the Yop effector proteins (48, 54). Bacteria can bind directly to host cell receptors (21) or use molecules like extracellular matrix (ECM) components to mediate attachment to host cells (12, 22, 30, 45, 57, 64). Common components of the cellular matrix that facilitate bacterial binding include fibronectin (22, 28, 64), collagen (23, 45), and laminin (28, 30, 45). Interactions between bacteria and ECM can lead to bridge-like attachments to host cells.
Fibronectin is a large glycoprotein that is a key structural component in many tissues. This ∼220-kDa protein is commonly found as a dimer that is linked by two disulfide bonds located near the C terminus. Fibronectin is a complex molecule made up of three types of modular repeating units (43, 47). Fibronectin can bind to many substrates, including collagen (13), integrin receptors on host cells (50, 56), and heparin (60). Additionally, fibronectin contains a binding site for several bacterial pathogens at the N-terminal end of the molecule (39, 59).
A number of fibronectin binding proteins on bacterial pathogens have been identified and studied, including SigB from Staphylococcus aureus (34), protein F from Streptococcus pyogenes (41), and YadA from Y. pseudotuberculosis (12, 19) and Y. enterocolitica (64). Binding of Y. pseudotuberculosis YadA to fibronectin allows Y. pseudotuberculosis to utilize β1 integrins on the surface of host cells for invasion (12). Given the key role of Y. pestis Ail in cell adhesion, Yop delivery, and virulence, we sought to determine the component on host cells to which Ail binds.
Although Ail has been studied extensively in other Yersinia species, the substrate on host cells with which Ail interacts is not known. In this study, we used a purified Y. pestis Ail to identify the extracellular matrix component, fibronectin, as a protein bound by Ail. Furthermore, Ail-mediated binding to host cells through fibronectin is important for the delivery of Yop effector proteins.
MATERIALS AND METHODS
Strains and culture conditions.
Y. pestis strains were cultivated in heart infusion broth (HIB) overnight or on heart infusion agar (HIA) for 48 h at 28°C. Escherichia coli strains were cultured in Luria-Bertani (LB) broth or LB agar at 28°C or 37°C. Antibiotics were used at the following concentrations: streptomycin (Sm), 100 μg/ml; chloramphenicol (Cm), 10 μg/ml; kanamycin (Kan), 30 μg/ml; and ampicillin (Amp), 100 μg/ml. Isopropyl-β-d-thiogalactopyranoside (IPTG) was used at a 100 μM concentration, unless otherwise noted (Table 1).
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Genotype or features | Drug resistance | Reference or source |
|---|---|---|---|
| Strains | |||
| E. coli | |||
| BL21(DE3) | F−ompT gal dcm lon hsdSB(rB− mB−) λ (DE3 [lacI lacUV5-T7 gene 1 ind1 Sam7 nin5]) | 62 | |
| AAEC185 | supE44 hsdR17 mcrA mcrB endA1 thi-1 ΔfimB-fimH ΔrecA | 7 | |
| Y. pestis | |||
| KIM5-3001 | pgm (referred to as KIM5 in text) | Smr | 29 |
| KIM5-3001 Δail mutant | pgm Δail | Smr | 14 |
| KIM5-3001 Δpla mutant | pgm Δpla | Smr | This study |
| KIM5-3001 Δail Δpla mutant | pgm Δail Δpla | Smr | This study |
| KIM5-3001 Δail Δpla ΔpsaA mutant | referred to as KIM5 Δ3, pgm Δail Δpla ΔpsaA | Smr | Felek et al., submitted |
| KIM5-3001 ΔyopB mutant | pgm ΔyopB | Smr | This study |
| Plasmids | |||
| pET30b+ | 5.4-kb C-terminal His6 tag vector | Kanr | Novagen |
| pET30b+-ail | 6.0 kb encoding ail | Kanr | This study |
| pMMB207 | 9.1 kb IPTG-inducible expression vector | Cmr | 38 |
| pMMB207-ail | 9.7 kb encoding ail | Cmr | 14 |
| pMMB207-psaABC | 13.1 kb encoding psaABC (pH 6 antigen locus) | Cmr | 15 |
HEp-2 cells were cultured at 5% CO2 (37°C) in modified Eagle's medium (Gibco) supplemented with 10% (vol/vol) fetal bovine serum (FBS; Gibco), 1% sodium pyruvate (Gibco), and 1% nonessential amino acids (Gibco). THP-1 cells were cultured at 5% CO2 (37°C) in RPMI medium supplemented with 10% (vol/vol) FBS (Gibco). THP-1 cells were activated by treatment with 10 pg/ml phorbol myristate acetate (PMA; Sigma) for 3 days to stimulate attachment to 24-well plates.
Purification of Ail-His6.
The coding region of the processed form of ail was PCR amplified with the 5′ primer 5′-GCGCTCTAGAAATAATTTTGTTTAACTTTAAGAAGGAGATATACATATGGAAGGCGAAAGCAGTATTTC-3′ and the 3′ primer 5′-GCGCGGTACCGAACCGGTAACCCGCGCC-3′. The amplified product was digested with KpnI and XbaI and ligated into the C-terminal His6-tag vector pET30b+ (Novagen) using the KpnI and XbaI sites. E. coli BL21(DE3) expressing the pET30b+ Ail-His6 construct was grown in LB-Kan medium at 37°C overnight. The next day, the culture was diluted 1:50 into LB-Kan medium, and the expression of Ail-His6 was induced with 10 μM IPTG. Ail-His6 was purified under denaturing conditions (8 M urea) from whole cells (including inclusion bodies). Ail-His6 was subjected to batch column affinity purification with nickel-nitrilotriacetic acid (Ni-NTA) Superflow beads (Qiagen) by following the manufacturer's protocol (Qiagen). Cells were lysed with lysis buffer (100 mM NaH2PO4, 10 mM Tris-Cl, 8 M urea, pH 8.0). Cell lysates were allowed to bind to the beads for 1 h at room temperature, and the beads were loaded onto a gravity-flow column. The column was washed twice with 5 ml wash buffer (100 mM NaH2PO4, 10 mM Tris-Cl, 8 M urea, pH 7.3). Elution from the column was achieved with stepwise lowering of the pH of the elution solutions, 100 mM NaH2PO4, 10 mM Tris-Cl, 8 M urea, pH 5.9 and pH 4.5, in three 0.5-ml fractions. Equal volumes of column fractions and SDS-polyacrylamide gel electrophoresis (PAGE) sample buffer were mixed. The samples were boiled for 5 min, microcentrifuged for 5 min at 13,200 × g, and then subjected to SDS-PAGE, followed by Coomassie blue staining. Fractions containing a single Ail-His6 band with similar concentrations were pooled. Ail-His6 was refolded with stepwise dialysis in 20 mM HEPES, pH 7.0, buffer with 1% dodecyl maltoside (DDM; Anatrace) against decreasing concentrations of urea. The final dialysis step was against 20 mM HEPES, pH 7.0, with 0.1% DDM. The concentration of each preparation was determined by the Bradford assay (Bio-Rad), and each preparation was stored at −20°C in 50-μl aliquots. The identity of purified Ail-His6 was confirmed by mass spectrometry (MS/MS) analysis.
While in the end Ail-His6 was purified under denaturing conditions, we also attempted to purify Ail-His6 from the outer membrane in its natural conformation (using a full-length Ail-His6 clone). The outer membranes of E. coli expressing the Ail-His6 were isolated; however, the Ail-His6 protein was not recovered upon purification on Ni-NTA Superflow beads. Thus, the denatured form of Ail-His6 was purified and slowly refolded in DDM detergent. We also tried the detergent N-dodecyl-N,N-dimethyl-3-ammonio-1-propanesulfonate, but Ail-His6 precipitated out of solution in this detergent.
Extracellular matrix binding assay.
Ninety-six-well plates (Pro-bind; BD Falcon) were coated overnight at 4°C with appropriate concentrations of plasma fibronectin (F2006; Sigma), cellular fibronectin (F2518; Sigma), collagen I (C5483; Sigma), and collagen IV (27663; Sigma) resuspended in phosphate-buffered saline (PBS). The wells were washed twice with PBS before they were blocked with Tris-buffered saline (TBS; 50 mM Tris 7.4, 150 mM NaCl) with 2% bovine serum albumin (BSA; blocking buffer) for 2 h at room temperature. Purified Ail-His6 was added to the ECM-coated wells at the appropriate concentration in TBS-0.05% Tween 20, and the plates were incubated for 3 h at 37°C or overnight at 4°C to allow binding. To detect Ail-His6 binding, rabbit anti-His6-tag polyclonal antibody (Biovision) was added at a dilution of 1:250 and allowed to bind to the wells at room temperature for 2 h. After unbound primary antibody was washed away, goat anti-rabbit antibody conjugated to alkaline phosphatase (Zymed) was added at a 1:3,000 dilution and the mixture was incubated for 1 h at room temperature. The wells were washed with PBS three times and once with 100 mM Tris, pH 8.0. The alkaline phosphatase substrate 4-nitrophenyl phosphate (PNPP; Fluka) was added at a concentration of 4 mg/ml in 100 mM Tris, pH 8.0. The A405 was then measured.
Bacterial binding assay and antibody blocking.
Ninety-six-well plates (Pro-bind; BD Falcon) were coated with the appropriate concentrations of plasma fibronectin, cellular fibronectin, collagen I, or collagen IV overnight at 4°C. The E. coli and Y. pestis derivatives were cultured overnight at 37°C in LB medium and at 28°C in HIB, respectively (IPTG and Cm were added for the strains containing pMMB207). On the following day, the wells were washed twice with PBS, before they were blocked with blocking buffer (TBS, 2% BSA) for 2 h at room temperature. The bacterial cells were normalized to an optical density at 600 nm (OD600) or OD620 (for E. coli and Y. pestis, respectively) of 1.2 in PBS-0.4% BSA. Fifty microliters of the bacterial suspension was added to the appropriate wells. The plate was incubated at 37°C for 2 h. The wells were washed three times with PBS, before they were fixed with 100 μl methanol. The bacteria bound to the wells were stained with 0.01% crystal violet. After the excess crystal violet was washed away, the bacterium-associated crystal violet stain was solubilized with ethanol-acetone solution. The A595 was then measured.
Antibody blocking assays were performed similarly to the bacteral binding assays, except that the polyclonal rabbit anti-human fibronectin antibody (F3648; Sigma) was diluted in 40 μl of PBS-0.4% BSA to the proper concentrations and was added to fibronectin-coated wells 1 h prior to the addition of 10 μl 5-fold-concentrated bacteria (i.e., OD600 or OD620 = 6.0).
Adhesion assays.
HEp-2 cells were cultured in 24-well tissue culture plates until they reached 100% confluence. THP-1 cells were plated at 1 × 105 cells/ml and activated with 10 pg/ml PMA (Sigma) for 3 days to stimulate attachment to 24-well plates. Y. pestis KIM5 or E. coli AAEC185 derivatives were cultured in HIB or LB medium overnight at 28°C and 37°C, respectively. Cm was added for the strains containing pMMB207 for complementation studies. Overnight E. coli cultures were diluted 1:10 in fresh LB medium with Cm and 100 μM IPTG to induce plasmid-based expression. The strains were allowed to grow for an additional 3 h at 37°C. The Y. pestis strains were diluted 1:10 in fresh HIB with Sm, Cm, and 100 μM IPTG to induce expression of genes encoded on the vector pMMB207, as required. The Y. pestis strains were allowed to grow for an additional 3 h at 28°C or 37°C, as indicated. Tissue culture cells were washed twice with 1 ml of serum-free minimal essential medium (MEM) for HEp-2 cells or RPMI medium for THP-1 cells. A polyclonal rabbit anti-human fibronectin antibody (F3648; Sigma) was diluted to the appropriate concentrations in tissue culture medium, without serum, with 20 mM HEPES, pH 7.0, and 0.4% BSA. Two hundred microliters of antibody was added to the cultured cells for 1 h prior to the addition of bacteria. For THP-1 cells, the cells were pretreated with 5 μg/ml cytochalasin D (Sigma) for 1 h prior to infection to inhibit phagocytosis. After the 1-h preincubation, 50 μl of bacteria was added to each well for final multiplicities of infection (MOIs) of 100 and 10 for E. coli and Y. pestis, respectively. The plates with the bacteria were incubated for 2 h at 37°C in 5% CO2. The cells were then washed two times with PBS, and the cell-associated bacteria were liberated by the addition of sterile H2O containing 0.1% Triton X-100 for 10 min. Serial dilutions of samples were plated onto HIA or LB agar for CFU analysis. In parallel wells, the entire population of bacteria was harvested and enumerated by dilution and CFU analysis to determine the total number of bacteria per well. Percent adhesion was calculated by dividing the number of bound CFU by the total number of bacteria in the well at the end of 2 h of incubation and then multiplying by 100.
Cytotoxicity assay.
HEp-2 cells and THP-1 cells were cultivated until they reached about 50% confluence in 24-well tissue culture plates. THP-1 cells were activated by treatment with 10 pg/ml PMA (Sigma) for 3 days to stimulate attachment to 24-well plates.
Y. pestis KIM5 derivatives strain were cultured in HIB overnight at 28°C. Overnight cultures were diluted 1:10 in fresh HIB and incubated for 3 h at 28°C. Tissue culture plates were washed twice with PBS, and 0.5 ml serum-free tissue culture medium was added to each well. Bacteria were added to each well at an MOI of 10. The plates were incubated at 37°C in 5% CO2. As appropriate, cells were preincubated with dilutions of an antifibronectin (anti-Fn) antibody (F3648; Sigma) for 1 h prior to infection to inhibit Yop delivery. Cells were fixed with 0.5 ml methanol and stained with 0.76 mg/ml Geimsa stain at 4 h postinoculation. Rounding was observed, and pictures were taken with a phase-contrast microscope. Cytotoxicity was enumerated by counting all cells and the number of round dark purple (indicating a shrunken cytoplasm) cells experiencing cytotoxicity in three microscopic fields (∼75 cells/field). Percent cytotoxicity was calculated by dividing the number of rounded cells by the total number of cells.
Generation of anti-PsaE antibodies.
PsaE is a regulatory protein of Yersinia species that regulates Psa (pH 6 antigen) production. Anti-PsaE antibodies against a peptide derived from near the N terminus of PsaE, GDSRYALSKNEVLLLEC, were generated in rabbits by Proteintech Group Inc. (Chicago, IL). Following antibody generation, anti-PsaE antibodies were purified using the immunizing peptide. The final concentration of the purified antibody was 600 μg/ml.
RESULTS
Purified Ail specifically binds to fibronectin.
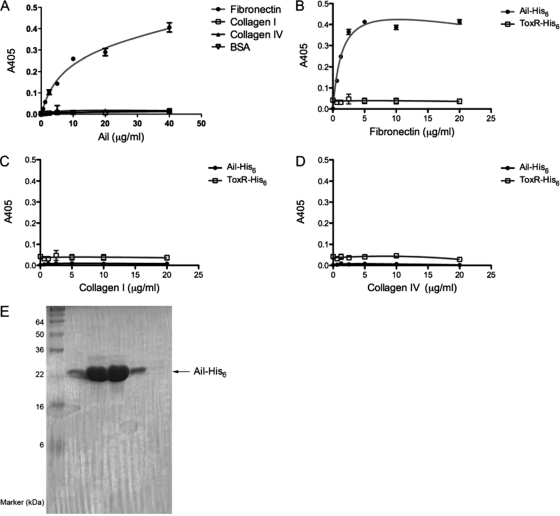
Several bacterial adhesins are known to bind to components of the ECM (22, 45). Since Ail-mediated binding to host cells shows many bacteria binding to regions between cells (14), we tested the binding capability of purified Ail-His6 (reconstituted in micelles of the nonionic detergent DDM) (Fig. 1 E) to the common ECM components fibronectin, collagen I, and collagen IV. Binding of Ail-His6 to the extracellular matrix was performed by coating plates with 20 μg/ml of ECM and adding purified Ail-His6 at increasing concentrations. The amount of purified Ail-His6 bound to fibronectin increased with increasing concentrations of purified Ail-His6 (Fig. 1A). By 40 μg/ml, the binding of Ail-His6 to fibronectin was approaching saturating levels, with a calculated KD (equilibrium dissociation constant) of 290 nM. This is comparable to the KD of the interaction between the classic fibronectin receptor α5β1 integrin and fibronectin, which is 800 nM (2). No binding of Ail-His6 was detected with collagen I, collagen IV, or the negative control, BSA. Thus, binding of Ail-His6 to fibronectin was specific. The Ail-His6-fibronectin interaction was also studied in the converse experiment, where increasing concentrations of ECM components were coated onto a microtiter plate and the amount of Ail-His6 was held constant. When 10 μg/ml Ail-His6 was added to increasing concentrations of fibronectin, there was increased binding which saturated at 5 μg/ml fibronectin (Fig. 1B). This binding was specific, as Ail-His6 never bound to collagen I or collagen IV at any concentration tested. Binding of Ail-His6 to fibronectin was not due to the His6 tag since a control His6-tagged protein, ToxR-His6 (a transcription factor of Vibrio cholerae [37]), did not bind to any ECM component tested (Fig. 1B to D).
FIG. 1.
Purified Ail-His6 binds to fibronectin. (A) Purified Ail-His6 was added at increasing concentrations to ECM and allowed to bind overnight at 4°C. Binding was detected using a mouse anti-His6 antibody in an enzyme-linked immunosorbent assay. (B to D) Increasing concentrations of fibronectin (B), collagen I (C), and collagen IV (D) were preabsorbed onto a 96-well plate. Ail-His6 was added at 10 μg/ml and allowed to bind overnight at 4°C. The anti-His6 enzyme-linked immunosorbent assay was performed again. Purified ToxR-His6, an unrelated DNA binding protein, was used as a negative control to demonstrate that the His6 tag played no role in binding. Shown are the results of a representative experiment (n = 3) of two trials. (E) Coomassie-stained purified Ail-His6 protein used for these studies, indicating that the protein is pure. The protein concentration in lanes 1 and 4 is 117 μg/ml, and that in lanes 2 and 3 is 1.1 mg/ml.
Bacteria expressing Ail bind to purified fibronectin.
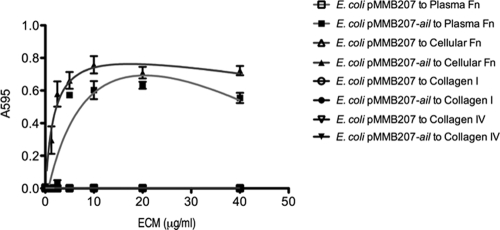
To determine whether Ail expressed on the surface of bacteria can mediate binding to fibronectin, an IPTG-inducible construct encoding ail (14) was expressed in E. coli AAEC185, a derivative of E. coli lacking type 1 fimbriae (6). E. coli expressing Ail was added to increasing concentrations of purified ECM components coated on microtiter plates. E. coli expressing Ail on its surface bound to both plasma and cellular fibronectin in a specific manner that saturated at concentrations higher than 5 μg/ml (Fig. 2). This binding was specific for fibronectin, since E. coli expressing Ail does not bind to collagen I or collagen IV (Fig. 2). Additionally, E. coli expressing the empty vector pMMB207 does not bind to any of the extracellular matrix components tested (Fig. 2).
FIG. 2.
E. coli expressing Ail binds to purified fibronectin. Purified plasma fibronectin, cellular fibronectin, collagen I, and collagen IV were preabsorbed onto 96-well plates. E. coli AAEC185 derivatives were added to the wells and allowed to bind at 37°C. Bound bacteria were stained with 0.01% crystal violet. The cells and crystal violet were solubilized, and the A595 of the solution was read. Shown are the results of a representative experiment (n = 3) of two trials.
Anti-fibronectin antibody blocks Ail-mediated interaction with purified fibronectin and host cells.
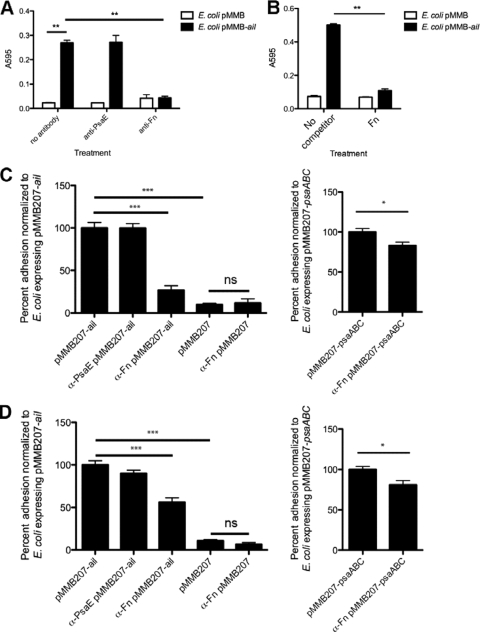
Having demonstrated that E. coli expressing Ail can bind to purified fibronectin, we used a polyclonal antibody against fibronectin to block Ail-mediated bacterial binding to purified fibronectin. Without addition of antibody or with addition of an irrelevant antibody against PsaE of Y. pestis, E. coli expressing Ail bound to purified fibronectin. Addition of a 1:50 dilution of anti-Fn polyclonal antibody resulted in a pronounced decrease in Ail-mediated E. coli binding to fibronectin (Fig. 3 A). E. coli expressing the empty vector control showed minimal binding to purified fibronectin, irrespective of treatment with antibody. In addition, pretreatment of E. coli expressing Ail on its surface with excess (40 μg/ml) cellular fibronectin resulted in inhibition of binding to wells coated with 10 μg/ml fibronectin (Fig. 3B).
FIG. 3.
Anti-human fibronectin antibody blocks Ail-mediated bacteria binding to purified fibronectin and cultured epithelial cells. (A) Fibronectin-coated wells (10 μg/ml) were pretreated with antifibronectin antibody at the indicated concentrations for 1 h, and E. coli AAEC185 derivatives were added and allowed to bind at 37°C. Bound bacteria were stained with 0.01% crystal violet. The cells and crystal violet were solubilized, and the A595 of the solution was read. (B) Binding of E. coli expressing Ail to wells coated with 10 μg/ml plasma fibronectin could be inhibited by prebinding the bacteria with 40 μg/ml soluble cellular fibronectin. HEp-2 cells (C) and THP-1 cells (D) were infected with E. coli AAEC185 derivatives. Percent adhesion was calculated by dividing the number of cell-associated CFU by the total number of bacteria in the well and multiplying by 100. A 1:50 dilution (A) or 1:25 dilution (∼24 μg/ml) (C and D) of a purified anti-Fn rabbit antibody was added where indicated. The adhesion of E. coli AAEC185 expressing Ail bound to cultured cells was set equal to 100% (HEp-2 cell average adhesion = 9.0%, THP-1 cell average adhesion = 6.8%). The adhesion of E. coli through Psa without antibody was set equal to 100% (HEp-2 cell average adhesion = 18.7%, THP-1 cell average adhesion = 19.0%). A control purified anti-PsaE rabbit antibody (used at the same protein concentration as the anti-Fn antibody) did not inhibit Ail-mediated binding. Data are from at least two independent experiments performed in triplicate (n = 6 to 15). *, P < 0.02; **, P < 0.0005; ***, P < 10−6; ns, not significant (P > 0.05). Significance was assessed using the Student t test.
To determine whether fibronectin is the predominant Ail substrate on the surface of host cells, the polyclonal anti-Fn antibody was used to block bacterial adhesion to cultured HEp-2 (human epithelial-like) cells and THP-1 (human monocyte-like) cells (Fig. 3C and D, respectively). The percentage of HEp-2 adhesion by E. coli expressing Ail was normalized to 100% to compare adhesion across multiple experiments. The adhesion of E. coli expressing Ail to HEp-2 cells decreased 4-fold when the anti-Fn antibody was used at a 1:25 dilution, while E. coli expressing the empty vector (pMMB207) bound poorly to HEp-2 cells and the binding was not affected by the addition of anti-Fn antibody (Fig. 3C). This indicates that the anti-Fn antibody is specific to the Ail-fibronectin interaction. Again, the anti-PsaE antibody (present at the same concentration as the anti-Fn antibody) had no effect on Fn binding. Cell binding by the anti-Fn antibody was only weakly affected in E. coli expressing the Y. pestis adhesin pH 6 antigen (Psa), encoded by psaABC. Psa is known to interact with several cellular receptors and substrates and serves as a negative control for an adhesin whose function should be largely independent of anti-Fn antibody treatment (18, 46; S. Felek and E. S. Krukonis, unpublished results).
Ail-mediated adhesion to THP-1 cells was also affected by the anti-Fn antibody, although the decrease in binding was only 2-fold with this cell line (Fig. 3D). Again, the empty vector control directed very little binding to THP-1 cells, and the anti-Fn antibody did not affect background binding. Psa also mediated binding to THP-1 cells, and this adhesion was modestly decreased in the presence of anti-Fn antibody. These data indicate that fibronectin is a substrate for Ail-mediated adhesion of E. coli on both phagocytic and nonphagocytic human-derived host cells. Taken together, the anti-Fn antibody can inhibit Ail-mediated binding to purified fibronectin and host cells.
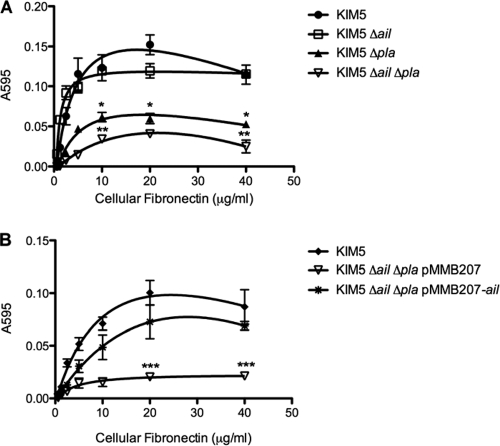
Y. pestis binding to fibronectin is mediated through Ail and Pla.
Having demonstrated that Ail expressed on the surface of E. coli can mediate specific binding to fibronectin, we wanted to determine whether Ail expressed in its natural context, on the surface of Y. pestis, can also bind to fibronectin. Again, wells were coated with purified fibronectin at increasing concentrations. Y. pestis KIM5 binding to fibronectin increased with increasing concentrations of fibronectin (Fig. 4 A). However, a KIM5 Δail strain also bound to fibronectin (Fig. 4A), suggesting that another Y. pestis adhesin may also bind to fibronectin. Since Pla is known to bind to several ECM components, (23, 27, 30), we tested a KIM5 Δpla mutant for fibronectin binding. The KIM5 Δpla mutant had decreased adhesion to fibronectin (Fig. 4A), and a KIM5 Δail Δpla double mutant displayed a further reduction in binding to fibronectin (P < 0.04 for KIM5 Δail Δpla binding relative to KIM5 Δpla binding). To determine whether Ail can restore fibronectin binding to the KIM5 Δail Δpla double mutant, we complemented the strain with the pMMB207-ail plasmid. Fibronectin binding was restored upon induction with 100 μM IPTG, while the KIM5 Δail Δpla strain harboring pMMB207 alone bound fibronectin poorly (Fig. 4B). Thus, Y. pestis KIM5 can bind to fibronectin via both Ail and Pla.
FIG. 4.
Ail and plasminogen activator (Pla) of Y. pestis bind to fibronectin. Purified cellular fibronectin was preabsorbed onto 96-well plates. Y. pestis KIM5 and derivatives were added to the wells and allowed to bind at 37°C. Bound bacteria were stained with 0.01% crystal violet. The cells and crystal violet were solubilized, and the A595 of the solution was read. *, P < 0.03 (relative to the results for KIM5); **, P < 0.04 (relative to the results for KIM5 Δpla); ***, P < 0.04 (relative to the results for KIM5 Δail Δpla with pMMB207-ail). Significance was assessed using the Student t test.
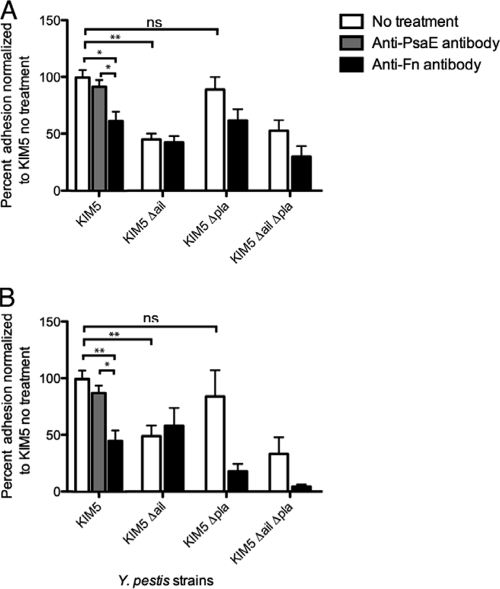
Anti-Fn antibody blocks Y. pestis Ail-mediated binding to host cells.
We have shown that fibronectin is the primary substrate on host cells for the Ail-mediated adhesion of E. coli. In addition, Y. pestis can bind to purified fibronectin via Ail and Pla. Therefore, we wanted to determine whether Y. pestis adhesion to cultured host cells is also dependent upon fibronectin interactions and what role Ail and Pla play in binding to fibronectin deposited by host cells. Y. pestis KIM5 and various mutant derivatives were tested for their abilities to adhere to HEp-2 cells (Fig. 5 A) and THP-1 cells (Fig. 5B) in the absence and presence of the polyclonal anti-Fn antibody. The level of Y. pestis KIM5 adhesion to HEp-2 cells without anti-Fn antibody was normalized to 100%, and the level of adhesion of the KIM5 Δail mutant (Pla dependent) to HEp-2 cells (Fig. 5A) was 50% of that of parental strain KIM5. However, the adhesion by the KIM5 Δpla mutant (Ail dependent) was similar to that of parental strain KIM5. The KIM5 Δail Δpla double-deletion mutant also showed a 50% decrease in adhesion compared to that of KIM5 (Fig. 5A). After pretreatment with a 1:25 dilution of anti-Fn antibody, adhesion by the parental KIM5 strain was reduced by 40%. The anti-Fn antibody did not affect Pla-dependent adhesion (KIM5 Δail). There was a trend toward decreased Ail-mediated adhesion in the Δpla mutant in the presence of anti-Fn antibody, although this decrease did not reach statistical significance (P = 0.085). Treatment of KIM5 with a control anti-PsaE antibody did not affect adhesion to HEp-2 cells.
FIG. 5.
Antifibronectin antibody inhibits adhesion of Y. pestis to host cells. HEp-2 cells (A) and THP-1 cells (B) were infected with Y. pestis KIM5 and derivatives. Percent adhesion was calculated by dividing the numbers of cell-associated CFU by the total number of bacteria in the well (bound and unbound) and multiplying by 100. The adhesion of KIM5 to HEp-2 or THP-1 cells in the absence of antibody was set equal to 100% (HEp-2 cell average adhesion = 12.0%, THP-1 cell average adhesion = 9.13%). Anti-Fn and anti-PsaE antibodies were used at a 1:25 dilution (24 μg/ml). Data are from three independent experiments performed in triplicate (n = 9). *, P < 0.02; **, P < 0.0006; ns, not significant (P > 0.05). Significance was assessed using the Student t test.
Similar results were observed with adhesion to THP-1 cells. Again, when the level of adhesion of the parental Y. pestis strain, KIM5, was set equal to 100%, the level of adhesion by the KIM5 Δail mutant (Pla dependent) was about 50% of that of parental strain KIM5, but the level of adherence by the KIM5 Δpla mutant (Ail dependent) was similar to the level of adherence by parental strain KIM5 (Fig. 5B). The Δail Δpla double-deletion strain appeared to show a further decreased level of adhesion relative to that of the Δail mutant, but this difference was not significant (P = 0.38). In the presence of anti-Fn antibody, the parental KIM5 strain showed 50% adhesion to THP-1 cells (Fig. 5B). When the fibronectin on THP-1 cells was blocked with anti-Fn antibody, the Pla-dependent adhesion (KIM5 Δail) was not affected, but there was a significant decrease in the level of Ail-mediated (KIM5 Δpla) adhesion with treatment by the anti-Fn antibody (Fig. 5B). The adhesion of the KIM5 Δail Δpla double-deletion mutant was also reduced in the presence of anti-Fn antibody, although this trend toward reduced adhesion did not reach statistical significance (P = 0.066). These data indicate that Y. pestis KIM5 expressing Ail can engage host cells via Ail and that this interaction is bridged by fibronectin, since treatment with anti-Fn antibody blocks Ail-mediated interactions with HEp-2 cells to nearly the same level as the KIM5 Δail mutant does (Fig. 5A) and to the same level as the KIM5 Δail mutant does for THP-1 cells (Fig. 5B). As with HEp-2 cells, addition of the anti-PsaE control antibody had no effect on adhesion to THP-1 cells. While Pla expressed on the surface of Y. pestis can mediate binding to purified fibronectin (Fig. 4A), it does not bind to fibronectin deposited by host cells as efficiently as Ail (Fig. 5A and B).
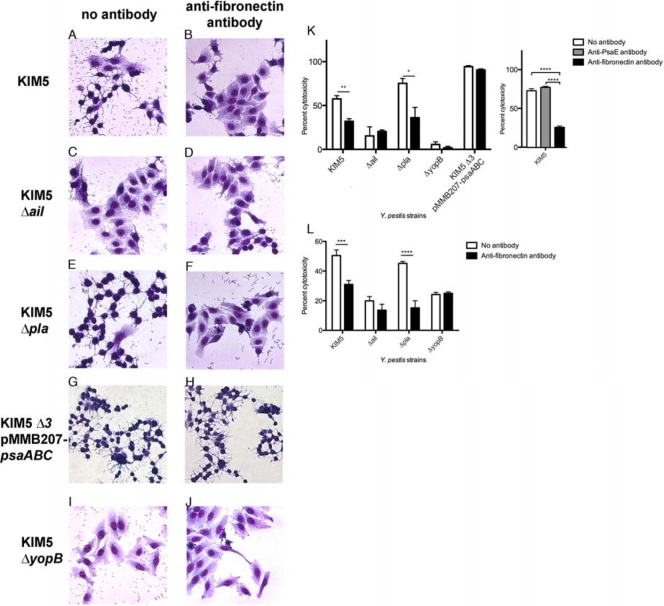
Anti-Fn antibody blocks Ail-mediated Yop delivery.
Previous studies have shown Ail to be a primary mediator of Yop delivery in Y. pestis KIM5 (14). Given that Ail can mediate bacterial binding to host cells in a fibronectin-dependent manner (Fig. 3 and 5) and Y. pestis-expressed Ail can bind to purified fibronectin (Fig. 4B), we determined whether anti-Fn antibodies could inhibit Yop delivery by Y. pestis. Cytotoxicity (cell rounding) was used as a measure of Yop delivery. The anti-Fn antibody was added to HEp-2 cells 1 h prior to the addition of Y. pestis KIM5 and derivatives. Y. pestis strains were incubated with host cells for 4 h prior to visualization of cell rounding. Sixty percent of the HEp-2 cells showed cytotoxicity upon interaction with the parental KIM5 strain in the absence of antibody treatment (Fig. 6 A and K). The KIM5 Δail mutant mediated reduced levels of cytotoxicity (15%), which is consistent with a previous report and which suggests that Ail is a key mediator of Yop delivery (14) (Fig. 6C and K). The deletion of pla resulted in levels of cytotoxicity similar to the level mediated by the KIM5 parental strain (Fig. 6E and K), indicating that Pla is not a key mediator of Yop delivery under these conditions. After the addition of the anti-Fn antibody, the level of KIM5-mediated cytotoxicity was reduced to 32% (Fig. 6A, B, and K). In the presence anti-Fn antibody, the level of cytotoxicity of the KIM5 Δail mutant for host cells was not affected (Fig. 6C, D, and K), indicating that fibronectin-dependent cytotoxicity is mediated by Ail. The KIM5 Δpla mutant (expressing Ail) showed a 2-fold reduction in cytotoxicity in the presence of anti-Fn antibody, similar to the result for the parental KIM5 strain (Fig. 6E, F, and K). Thus, in the presence of anti-Fn antibody, the levels of Yop delivery in both KIM5 and KIM5 Δpla were significantly reduced, while Yop delivery was not affected in the KIM5 Δail strain. Furthermore, the levels of Yop-mediated cytotoxicity for KIM5 and KIM5 Δpla in the presence of anti-Fn antibody approached the levels of cytotoxicity elicited by the KIM5 Δail strain. The anti-Fn antibody does not generally decrease Yop delivery, since Yop delivery in the Y. pestis KIM5 Δail Δpla ΔpsaA mutant (KIM5 Δ3) expressing Psa (pH 6 antigen) from a plasmid is not inhibited (Fig. 6G, H, and K). A KIM5 ΔyopB control strain (unable to deliver Yops due to lack of a component of the translocation apparatus [11]) (Fig. 6I and K), showed very little cytotoxicity, indicating that the cytotoxicity being measured is Yop dependent. HEp-2 cell cytotoxicity was evaluated by counting three fields with ∼100 cells/field (Fig. 6K). As with the adhesion experiments, treatment of HEp-2 cells with a control anti-PsaE antibody had no effect on cytotoxicity.
FIG. 6.
Antifibronectin antibody blocks Ail-dependent Yop delivery to HEp-2 and THP-1 cells. Y. pestis KIM5 and derivatives were added to cells at an MOI of 10. Photos were taken at 4 h postinfection for HEp-2 cell infection. Giemsa staining reveals a shrunken cytoplasm, which is indicative of Yop-mediated cytotoxicity (A). Cytotoxicity of host cells was enumerated and plotted for HEp-2 cells (K) and THP-1 cells (L). Anti-Fn and anti-PsaE antibodies were used at a 1:25 dilution (24 μg/ml). KIM5 Δ3, = KIM5 Δail Δpla ΔpsaA (G and H). *, P < 0.05; **, P < 0.01; ***, P < 0.02; ****, P < 0.004. Significance was assessed using the Student t test.
To determine whether Yop delivery through the Y. pestis Ail interaction with fibronectin was specific to HEp-2 cells, we also analyzed the ability of the anti-Fn antibody to block cytotoxic effects on THP-1 cells. Similar to the HEp-2 cell experiment, THP-1 cells were pretreated with the antifibronectin antibody for 1 h prior to the addition of Y. pestis KIM5 derivatives. The proportion of cells exhibiting cytotoxicity is presented in Fig. 6L. The percentage of KIM5-infected THP-1 cells displaying cytotoxicity was about 50%, while the KIM5 Δail mutant exhibited about 20% cytotoxicity (Fig. 6L). The KIM5 Δpla mutant induced similar levels of cytotoxicity as the parental KIM5 strain (Fig. 6L). These data are in agreement with the data obtained using HEp-2 cells and show that Ail is important for the cytotoxicity caused by Y. pestis. Infection by the KIM5 ΔyopB mutant induced about 20% of the THP-1 cells to show cytotoxicity, indicating that most of the KIM5-mediated cytotoxicity was due to the delivery of the Yop effector proteins (Fig. 6L). With the treatment with anti-Fn antibody, the cytotoxicity caused by the parental KIM5 strain dropped from 50% to about 30%, while the anti-Fn antibody reduced the levels of cytotoxicity caused by the KIM5 Δpla mutant from 45% to about 15% (Fig. 6L). This shows that the Ail-mediated cytotoxicity of the KIM5 Δpla mutant can be inhibited by blocking fibronectin. Finally, the anti-Fn antibody had little or no effect on the KIM5 Δail mutant, demonstrating that the fibronectin-dependent cytotoxicity was Ail mediated (Fig. 6L). The fact that the ΔyopB mutant still mediated significant cytotoxicity (20% in 3 h) may indicate that some amount of Yops gains access to phagocytic cells even in the absence of the YopBD translocation apparatus. This may occur within phagocytic vacuoles in a YopB/D-independent manner via an as yet uncharacterized mechanism. Alternatively, this residual cytotoxicity in THP-1 cells may be Yop independent.
These results confirm that Ail is a key mediator of Yop delivery to human-derived nonphagocytic (HEp-2) and phagocytic (THP-1) cells (14) and demonstrate that blocking the interaction between Ail and fibronectin on the surface of host cells decreases Yop delivery. Thus, fibronectin acts as a bridging molecule between Y. pestis Ail and host cells to facilitate docking of the TTSS apparatus.
DISCUSSION
A critical step in the process of infection with Y. pestis is adhesion to host cells, the first step in engagement of the Ysc TTSS and delivery of cytotoxic Yop effector proteins. One Y. pestis adhesin, Ail, has been shown to mediate cell binding, serum resistance, and Yop delivery (3, 14, 25). However, the component on the surface of host cells to which Ail interacts has not been identified. In the study described in this paper, we sought to identify the host cell receptor for Y. pestis Ail. Using purified Ail and Ail expressed on the surface of E. coli and Y. pestis, we demonstrated that Ail interacts with fibronectin, leading to cell binding and delivery of Yop effector proteins.
While our findings demonstrated Ail binding to fibronectin and not collagen I or collagen IV, there may be additional substrates/receptors for Ail. However, the Ail-fibronectin interaction that we characterized is important for cell binding and Yop delivery to host cells.
When the Y. pestis parental strain, KIM5, was tested, we identified two Y. pestis adhesins capable of binding to purified fibronectin, Ail and Pla (Fig. 4). The KIM5 Δail mutant binds to purified fibronectin at nearly wild-type levels, while the KIM5 Δpla mutant is defective for binding to purified fibronectin. This suggests that Pla is a better mediator of binding to purified fibronectin. However, complementation experiments confirmed that Ail can restore fibronectin binding activity to the KIM Δail Δpla double mutant. Pla has been reported to bind to collagen IV (23), laminin, and Matrigel (28, 30), while binding to both plasma and cellular fibronectin was poor (28). It is unclear why previous studies detected such poor binding to fibronectin, as we show a binding curve that becomes saturated at 5 μg/ml (100 ng/well), while previous studies appear to have used an even higher concentration of fibronectin for their binding experiments (about 1 μg/well [28, 66]). In any event, this is the first study to report Pla-mediated binding to fibronectin.
Although Pla can mediate Y. pestis binding to purified fibronectin, adhesion to host cells is primarily mediated through Ail, since the KIM5 Δail mutant has only half the adhesive ability of the parental KIM5 strain, while the KIM5 Δpla mutant has the same level of binding as KIM5 (Fig. 5; the result for KIM5 was not statistically different from that for KIM5 Δpla for HEp2 cells [P = 0.4] or THP-1 cells [P = 0.5]). Ail-dependent adhesion was observed with both the HEp-2 and the THP-1 cell lines. These data indicate that while Pla can mediate efficient binding to purified fibronectin, Ail is the primary adhesin for host cell binding. It is unclear why Ail prefers cell-deposited fibronectin for cell adhesion and Pla is more efficient for binding to purified fibronectin. It is possible that host cells arrange the deposited fibronectin matrix such that it is a better substrate for Ail rather than Pla (20). Alternatively, other cell-deposited proteins may also bind to fibronectin and interfere with the Pla binding site on fibronectin, but not the Ail binding site.
In order to test whether the Ail-fibronectin interaction is important for Y. pestis host cell adhesion, we used a polyclonal anti-Fn antibody to inhibit cell adhesion. Treatment with the anti-Fn antibody led to a 40 to 50% decrease in adhesion of the KIM5 strain and a decrease in Ail-mediated adhesion with the KIM5 Δpla mutant. However, the anti-Fn antibody did not inhibit Pla-dependent adhesion in the KIM5 Δail mutant (Fig. 5). This indicates that blocking fibronectin can inhibit adhesion of the Y. pestis strains expressing Ail to host cells but not strains expressing Pla. Interestingly, the adhesion of the KIM5 Δail Δpla double-deletion mutant appears to be reduced with treatment with the anti-Fn antibody. This difference is not significant, but there is a trend toward inhibition with both HEp-2 and THP-1 cells. It is possible that an additional adhesin may be responsible for this background fibronectin binding activity. Why the KIM5 Δail mutant did not demonstrate any reduced adhesion to fibronectin in the presence of the anti-Fn antibody due to this other uncharacterized adhesin is not clear. It is possible that the proteolytic activity of Pla leads to cleavage of this undefined adhesive factor in the Ail-negative and Pla-positive (KIM5 Δail) strain. One noteworthy trend of KIM5 binding to HEp-2 cells was that the addition of the anti-Fn antibody did not inhibit the level of Ail-mediated cell adhesion to the level for the Δail mutant, although the difference did not reach statistical significance (Fig. 5A; for KIM5 with anti-Fn versus KIM5 Δail, P = 0.10; for KIM5 Δpla with anti-Fn versus KIM5 Δail, P = 0.16). This may indicate that Ail can bind to additional cellular receptors, aside from fibronectin. If this is the case, such a receptor does not appear to reside on THP-1 cells, as the anti-Fn antibody was able to abrogate Ail-mediated cell adhesion to THP-1 cells to the same level as the Δail mutant (Fig. 5B). The fact that anti-Fn antibodies could nearly completely abolish binding of the KIM5 Δpla and KIM5 Δail Δpla mutants to THP-1 cells does not appear to be due to the reduced levels of fibronectin produced by THP-1 cells compared to the levels produced by HEp-2 cells (reduced fibronectin levels might have allowed more complete antibody inhibition of binding). Boiling-SDS extraction of HEp-2 and THP-1 cells demonstrated that THP-1 cells produce more extractable fibronectin than HEp-2 cells (data not shown).
To further characterize the importance of the Y. pestis Ail interaction with fibronectin, we demonstrated that when the Ail-fibronectin interaction is disrupted by anti-Fn antibodies, the cytotoxicity of the HEp-2 cells is reduced (Fig. 6). Significant cell rounding, indicating Yop-mediated cytotoxicity, can be visualized with the parental KIM5 strain and the KIM5 Δpla strain, while the majority of host cells infected with the KIM5 Δail mutant appear normal. Thus, Ail-mediated adhesion is the key facilitator of Yop delivery. This cytotoxicity is due to the delivery of the Yop effector proteins, as a yopB (a component of the Ysc translocon apparatus) mutant is defective for cell rounding. Upon treatment with the anti-Fn antibody, the percentage of HEp-2 cells that appear to have experienced cytotoxic effects is reduced in those strains expressing Ail (KIM5 and KIM5 Δpla) (Fig. 6K). This suggests that when fibronectin is no longer available for binding, Y. pestis is defective for delivery of Yops and that the critical molecule for Y. pestis interaction with host fibronectin is Ail. Similar effects on cytotoxicity in THP-1 cells (phagocytic) were observed in the presence of anti-Fn antibodies (Fig. 6L).
To ensure that the anti-Fn antibody was not blocking general bacterial adhesion, independent of Ail, or perturbing cell signaling pathways and preventing Yop delivery (33), we expressed Psa (pH 6 antigen) both in the E. coli heterologous system and in Y. pestis. Psa is known to bind to cultured cells and facilitate secretion of cytotoxic effectors (Fig. 3 and 6 [63; Felek and Krukonis, unpublished). E. coli expressing Psa bound to both HEp-2 and THP-1 cells. Treatment with the antifibronectin antibody resulted in only a slight decrease in bacterial adhesion. This slight decrease may be due to the ability of Psa to mediate binding to ECM proteins (Felek and Krukonis, unpublished), in addition to its other characterized receptors β1-linked galactosylated glycosphingolipids (46) and phosphatidylcholine (18).
Bacterial adhesion to host cells is a critical step in the infection process. Some bacteria can bind to the host cell surface directly by binding to cell receptors such as integrins (21); other bacteria bind to ECM components found in tissues (22, 45). These ECM components can serve as a bridge for binding to the host cell surface (12). Likewise, Ail-mediated Y. pestis adhesion to host cells through fibronectin appears to use fibronectin as a bridging molecule.
A number of bacterial pathogens bind to fibronectin, often at the N-terminal end of fibronectin (26, 39, 59). Fibronectin is a dimer held together by a disulfide bond at the C-terminal end of the molecule. Many of the cellular binding domains along the molecule have been well studied (43). Fibronectin is an important component of the tissues within the host. Besides the well-studied arginine-glycine-aspartate (RGD) cell-binding domain, there are a number of other integrin-binding sites along the length of fibronectin (43). Therefore, bacteria can bind to one region of fibronectin while allowing fibronectin to also engage host cell receptors from a different region.
We conclude that binding of Ail to fibronectin is an important event for Yop delivery to host cells under our experimental conditions, while Pla plays a less prominent role. We can envision a few scenarios to explain why Ail is the predominant adhesin capable of mediating the delivery of Yop effector proteins under our assay conditions. We hypothesize that Ail engages fibronectin in a unique manner that facilitates the most efficient interaction of the Ysc TTSS. This suggests that Ail and Pla may have different binding domains on the fibronectin molecule. Preliminary studies suggest that the Ail binding site within fibronectin is distinct from that for Pla (Tsang and Krukonis, unpublished). Another possible explanation for efficient Yop delivery by Ail may be that Ail facilitates more receptor clustering through multiple Ail molecules with the surface of host cells. Ail is highly expressed in the parental KIM5 strain (3, 14). Therefore, it is possible to envision that host cell binding through multiple Ail molecules leads to a more efficient cell signaling and Yop delivery. The role of efficient cell signaling in Yop delivery was recently demonstrated by Mejia et al., who showed that avid binding of invasin to β1 integrins leads to intracellular signaling and Yop delivery and that inhibitors blocking those signaling pathways disrupt Yop delivery (33). Thus, receptor clustering through multiple Ail molecules and fibronectin may lead to a robust signaling cascade in host cells and maximally efficient Yop delivery.
Acknowledgments
We thank the University of Michigan Proteomics Consortium for performing mass spectrometry analysis on our purified Ail-His6 protein. We thank Yvonne Kapila for helpful discussions regarding analysis of Ail binding to fibronectin. We thank David Thanassi and Susan Buchanan for helpful discussions on solubilization of the Ail-His6 protein. We thank Vern Carruthers for critical reading of the manuscript.
These studies were made possible by funding from the University of Michigan Biomedical Research Council (BMRC), Office of the Vice President of Research (OVPR), the Rackham Graduate School, and the Frances Wang Chin Endowed Fellowship from the University of Michigan Department of Microbiology and Immunology (to T.M.T.).
Editor: J. B. Bliska
Footnotes
Published ahead of print on 24 May 2010.
REFERENCES
- 1.Achtman, M., G. Morelli, P. Zhu, T. Wirth, I. Diehl, B. Kusecek, A. J. Vogler, D. M. Wagner, C. J. Allender, W. R. Easterday, V. Chenal-Francisque, P. Worsham, N. R. Thomson, J. Parkhill, L. E. Lindler, E. Carniel, and P. Keim. 2004. Microevolution and history of the plague bacillus, Yersinia pestis. Proc. Natl. Acad. Sci. U. S. A. 101:17837-17842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akiyama, S. K., and K. M. Yamada. 1985. The interaction of plasma fibronectin with fibroblastic cells in suspension. J. Biol. Chem. 260:4492-4500. [PubMed] [Google Scholar]
- 3.Bartra, S. S., K. L. Styer, D. M. O'Bryant, M. L. Nilles, B. J. Hinnebusch, A. Aballay, and G. V. Plano. 2008. Resistance of Yersinia pestis to complement-dependent killing is mediated by the Ail outer membrane protein. Infect. Immun. 76:612-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black, D. S., and J. B. Bliska. 2000. The RhoGAP activity of the Yersinia pseudotuberculosis cytotoxin YopE is required for antiphagocytic function and virulence. Mol. Microbiol. 37:515-527. [DOI] [PubMed] [Google Scholar]
- 5.Bliska, J., and S. Falkow. 1992. Bacterial resistance to complement killing mediated by the Ail protein of Yersinia enterocolitica. Proc. Natl. Acad. Sci. U. S. A. 89:3561-3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blomfield, I. C., M. S. McClain, and B. I. Eisenstein. 1991. Type 1 fimbriae mutants of Escherichia coli K12: characterization of recognized afimbriate strains and construction of new fim deletion mutants. Mol. Microbiol. 5:1439-1445. [DOI] [PubMed] [Google Scholar]
- 7.Blomfield, I. C., M. S. McClain, J. A. Princ, P. J. Calie, and B. I. Eisenstein. 1991. Type 1 fibriation and fimE mutants in Escherichia coli K-12. J. Bacteriol. 173:5298-5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyd, A. P., and G. R. Cornelis. 2001. Yersinia, p. 227-264. In E. A. Groisman (ed.), Principles of bacterial pathogenesis. Academic Press, San Diego, CA.
- 9.Boyd, A. P., N. Grosdent, S. Totemeyer, C. Geuijen, S. Bleves, M. Iriarte, I. Lambermont, J.-N. Octave, and G. R. Cornelis. 2000. Yersinia enterocolitica can deliver Yop proteins into a wide range of cell types: Development of a delivery system for heterologous proteins. Eur. J. Cell Biol. 79:659-671. [DOI] [PubMed] [Google Scholar]
- 10.Brooks, G. F., L. Olinger, C. J. Lammel, K. S. Bhat, C. A. Calvello, M. L. Palmer, J. S. Knapp, and R. S. Stephens. 1991. Prevalence of gene sequences coding for hypervariable regions of Opa (protein II) in Neisseria gonorrhoeae. Mol. Microbiol. 5:3063-3072. [DOI] [PubMed] [Google Scholar]
- 11.Cornelis, G. R., A. Boland, A. P. Boyd, C. Geuijen, M. Iriarte, C. Neyt, M.-P. Sory, and I. Stainier. 1998. The virulence plasmid of Yersinia, an antihost genome. Microbiol. Mol. Biol. Rev. 62:1315-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eitel, J., and P. Dersch. 2002. The YadA protein of Yersinia pseudotuberculosis mediates high-efficiency uptake into human cells under environmental conditions in which invasin is repressed. Infect. Immun. 70:4880-4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engvall, E., and E. Ruoslahti. 1977. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int. J. Cancer 20:1-5. [DOI] [PubMed] [Google Scholar]
- 14.Felek, S., and E. S. Krukonis. 2009. The Yersinia pestis Ail protein mediates binding and Yop delivery to host cells required for plague virulence. Infect. Immun. 77:825-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Felek, S., M. B. Lawrenz, and E. S. Krukonis. 2008. The Yersinia pestis autotransporter YapC mediates host cell binding, autoaggregation and biofilm formation. Microbiology 154:1802-1812. [DOI] [PubMed] [Google Scholar]
- 16.Felek, S., L. M. Runco, D. G. Thanassi, and E. S. Krukonis. 2007. Characterization of six novel chaperone/usher systems in Yersinia pestis. Adv. Exp. Med. Biol. 603:97-105. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez, C., C. Hilty, S. Bonjour, K. Adeishvili, K. Pervushin, and K. Wuthrich. 2001. Solution NMR studies of the integral membrane proteins OmpX and OmpA from Escherichia coli. FEBS Lett. 504:173-178. [DOI] [PubMed] [Google Scholar]
- 18.Galvan, E. M., H. Chen, and D. M. Schifferli. 2007. The Psa fimbriae of Yersinia pestis interact with phosphatidylcholine on alveolar epithelial cells and pulmonary surfactant. Infect. Immun. 75:1272-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heise, T., and P. Dersch. 2006. Identification of a domain in Yersinia virulence factor YadA that is crucial for extracellular matrix-specific cell adhesion and uptake. Proc. Natl. Acad. Sci. U. S. A. 103:3375-3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hocking, D. C., J. Sottile, and P. J. McKeown-Longo. 1998. Activation of distinct alpha5beta1-mediated signaling pathways by fibronectin's cell adhesion and matrix assembly domains. J. Cell Biol. 141:241-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Isberg, R. R., and J. M. Leong. 1990. Multiple beta 1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell 60:861-871. [DOI] [PubMed] [Google Scholar]
- 22.Joh, D., E. R. Wann, B. Kreikemeyer, P. Speziale, and M. Hook. 1999. Role of fibronectin-binding MSCRAMMs in bacterial adherence and entry into mammalian cells. Matrix Biol. 18:211-223. [DOI] [PubMed] [Google Scholar]
- 23.Kienle, Z., L. Emody, C. Svanborg, and P. O'Toole. 1992. Adhesive properties conferred by the plasminogen activator of Yersinia pestis. J. Gen. Microbiol. 138:1679-1687. [DOI] [PubMed] [Google Scholar]
- 24.Kirjavainen, V., H. Jarva, M. Biedzka-Sarek, A. M. Blom, M. Skurnik, and S. Meri. 2008. Yersinia enterocolitica serum resistance proteins YadA and Ail bind the complement regulator C4b-binding protein. PLoS Pathog. 4:e1000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolodziejek, A. M., D. J. Sinclair, K. S. Seo, D. R. Schnider, C. F. Deobald, H. N. Rohde, A. K. Viall, S. S. Minnich, C. J. Hovde, S. A. Minnich, and G. A. Bohach. 2007. Phenotypic characterization of OmpX, an Ail homologue of Yersinia pestis KIM. Microbiology 153:2941-2951. [DOI] [PubMed] [Google Scholar]
- 26.Konkel, M. E., S. G. Garvis, S. L. Tipton, D. E. Anderson, Jr., and W. Cieplak, Jr. 1997. Identification and molecular cloning of a gene encoding a fibronectin-binding protein (CadF) from Campylobacter jejuni. Mol. Microbiol. 24:953-963. [DOI] [PubMed] [Google Scholar]
- 27.Lahteenmaki, K., M. Kukkonen, and T. K. Korhonen. 2001. The Pla surface protease/adhesin of Yersinia pestis mediates bacterial invasion into human endothelial cells. FEBS Lett. 504:69-72. [DOI] [PubMed] [Google Scholar]
- 28.Lahteenmaki, K., R. Virkola, A. Saren, L. Emody, and T. K. Korhonen. 1998. Expression of plasminogen activator Pla of Yersinia pestis enhances bacterial attachment to the mammalian extracellular matrix. Infect. Immun. 66:5755-5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindler, L., M. Klempner, and S. Straley. 1990. Yersinia pestis pH 6 antigen: genetic, biochemical, and virulence characterization of a protein involved in the pathogenesis of bubonic plague. Infect. Immun. 58:2569-2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lobo, L. A. 2006. Adhesive properties of the purified plasminogen activator Pla of Yersinia pestis. FEMS Microbiol. Lett. 262:158-162. [DOI] [PubMed] [Google Scholar]
- 31.Makoveichuk, E., P. Cherepanov, S. Lundberg, A. Forsberg, and G. Olivecrona. 2003. pH 6 antigen of Yersinia pestis interacts with plasma lipoproteins and cell membranes. J. Lipid Res. 44:320-330. [DOI] [PubMed] [Google Scholar]
- 32.Mecsas, J., R. Welch, J. Erickson, and C. Gross. 1995. Identification and characterization of an outer membrane protein, OmpX, in Escherichia coli that is homologous to a family of outer membrane proteins including Ail of Yersinia enterocolitica. J. Bacteriol. 177:799-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mejia, E., J. B. Bliska, and G. I. Viboud. 2008. Yersinia controls type III effector delivery into host cells by modulating Rho activity. PLoS Pathog. 4:e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Menzies, B. 2003. The role of fibronectin binding proteins in the pathogenesis of Staphylococcus aureus infections. Curr. Opin. Infect. Dis. 16:225-229. [DOI] [PubMed] [Google Scholar]
- 35.Miller, V. L., K. B. Beer, G. Heusipp, B. M. Young, and M. R. Wachtel. 2001. Identification of regions of Ail required for the invasion and serum resistance phenotypes. Mol. Microbiol. 41:1053-1062. [DOI] [PubMed] [Google Scholar]
- 36.Miller, V. L., and S. Falkow. 1988. Evidence for two genetic loci in Yersinia enterocolitica that can promote invasion of epithelial cells. Infect. Immun. 56:1242-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller, V. L., and J. J. Mekalanos. 1984. Synthesis of cholera toxin is positively regulated at the transcriptional level by ToxR. Proc. Natl. Acad. Sci. U. S. A. 81:3471-3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morales, V. M., A. Backman, and M. Bagdasarian. 1991. A series of wide-host-range low-copy-number vectors that allow direct screening for recombinants. Gene 97:39-47. [DOI] [PubMed] [Google Scholar]
- 39.Mosher, D. F., and R. A. Proctor. 1980. Binding and factor XIIIa-mediated cross-linking of a 27-kilodalton fragment of fibronectin to Staphylococcus aureus. Science 209:927-929. [DOI] [PubMed] [Google Scholar]
- 40.Orth, K., L. E. Palmer, Z. Q. Bao, S. Stewart, A. E. Rudolph, J. B. Bliska, and J. E. Dixon. 1999. Inhibition of the mitogen-activated protein kinase kinase superfamily by a Yersinia effector. Science 285:1920-1923. [DOI] [PubMed] [Google Scholar]
- 41.Ozeri, V., A. Tovi, I. Burstein, S. Natanson-Yaron, M. G. Caparon, K. M. Yamada, S. K. Akiyama, I. Vlodavsky, and E. Hanski. 1996. A two-domain mechanism for group A streptococcal adherence through protein F to the extracellular matrix. EMBO J. 15:989-998. [PMC free article] [PubMed] [Google Scholar]
- 42.Palmer, L. E., S. Hobbie, J. E. Galan, and J. B. Bliska. 1998. YopJ of Yersinia pseudotuberculosis is required for the inhibition of macrophage TNF-alpha production and downregulation of the MAP kinases p38 and JNK. Mol. Microbiol. 27:953-965. [DOI] [PubMed] [Google Scholar]
- 43.Pankov, R., and K. M. Yamada. 2002. Fibronectin at a glance. J. Cell Sci. 115:3861-3863. [DOI] [PubMed] [Google Scholar]
- 44.Parkhill, J., B. W. Wren, N. R. Thomson, R. W. Titball, M. T. G. Holden, M. B. Prentice, M. Sebaihia, K. D. James, C. Churcher, K. L. Mungall, S. Baker, D. Basham, S. D. Bentley, K. Brooks, A. M. Cerdeno-Tarraga, T. Chillingworth, A. Cronin, R. M. Davies, P. Davis, G. Dougan, T. Feltwell, N. Hamlin, S. Holroyd, K. Jagels, A. V. Karlyshev, S. Leather, S. Moule, P. C. F. Oyston, M. Quail, K. Rutherford, M. Simmonds, J. Skelton, K. Stevens, S. Whitehead, and B. G. Barrell. 2001. Genome sequence of Yersinia pestis, the causative agent of plague. Nature 413:523-527. [DOI] [PubMed] [Google Scholar]
- 45.Patti, J. M., B. L. Allen, M. J. McGavin, and M. Hook. 1994. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu. Rev. Microbiol. 48:585-617. [DOI] [PubMed] [Google Scholar]
- 46.Payne, D., D. Tatham, E. D. Williamson, and R. W. Titball. 1998. The pH 6 antigen of Yersinia pestis binds to beta 1-linked galactosyl residues in glycosphingolipids. Infect. Immun. 66:4545-4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petersen, T. E., H. C. Thogersen, K. Skorstengaard, K. Vibepedersen, P. Sahl, L. Sottrupjensen, and S. Magnusson. 1983. Partial primary structure of bovine plasma fibronectin-3 types of internal homology. Proc. Natl. Acad. Sci. U. S. A. 80:137-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pettersson, J., R. Nordfelth, E. Dubinina, T. Bergman, M. Gustafsson, K. E. Magnusson, and H. Wolf-Watz. 1996. Modulation of virulence factor expression by pathogen target cell contact. Science 273:1231-1233. [DOI] [PubMed] [Google Scholar]
- 49.Pieper, R., S. T. Huang, J. M. Robinson, D. J. Clark, H. Alami, P. P. Parmar, R. D. Perry, R. D. Fleischmann, and S. N. Peterson. 2009. Temperature and growth phase influence the outer-membrane proteome and the expression of a type VI secretion system in Yersinia pestis. Microbiology 155:498-512. [DOI] [PubMed] [Google Scholar]
- 50.Pierschbacher, M. D., E. G. Hayman, and E. Ruoslahti. 1981. Location of the cell-attachment site in fibronectin with monoclonal antibodies and proteolytic fragments of the molecule. Cell 26:259-267. [DOI] [PubMed] [Google Scholar]
- 51.Pierson, D., and S. Falkow. 1993. The ail gene of Yersinia enterocolitica has a role in the ability of the organism to survive serum killing. Infect. Immun. 61:1846-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Price, S., M. Freeman, and K. Yeh. 1995. Transcriptional analysis of the Yersinia pestis pH 6 antigen gene. J. Bacteriol. 177:5997-6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pulkkinen, W. S., and S. I. Miller. 1991. A Salmonella typhimurium virulence protein is similar to a Yersinia enterocolitica invasion protein and a bacteriophage lambda outer membrane protein. J. Bacteriol. 173:86-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rosqvist, R., A. Forsberg, M. Rimpilainen, T. Bergman, and H. Wolf-Watz. 1990. The cytotoxic protein YopE of Yersinia obstructs the primary host defence. Mol. Microbiol. 4:657-667. [DOI] [PubMed] [Google Scholar]
- 55.Rosqvist, R., M. Skurnik, and H. Wolf-Watz. 1988. Increased virulence of Yersinia pseudotuberculosis by two independent mutations. Nature 334:522-524. [DOI] [PubMed] [Google Scholar]
- 56.Ruoslahti, E., and E. G. Hayman. 1979. Two active sites with different characteristics in fibronectin. FEBS Lett. 97:221-224. [DOI] [PubMed] [Google Scholar]
- 57.Schwarz-Linek, U., M. Hook, and J. R. Potts. 2006. Fibronectin-binding proteins of gram-positive cocci. Microb. Infect. 8:2291-2298. [DOI] [PubMed] [Google Scholar]
- 58.Simonet, M., B. Riot, N. Fortineau, and P. Berche. 1996. Invasin production by Yersinia pestis is abolished by insertion of an IS200-like element within the inv gene. Infect. Immun. 64:375-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sottile, J., J. Schwarzbauer, J. Selegue, and D. F. Mosher. 1991. Five type I modules of fibronectin form a functional unit that binds to fibroblasts and Staphylococcus aureus. J. Biol. Chem. 266:12840-12843. [PubMed] [Google Scholar]
- 60.Stathakis, N. E., and M. W. Mosesson. 1977. Interactions among heparin, cold insoluble globulin, and fibrinogen in formation of the heparin precipitable fraction of plasma. J. Clin. Invest. 60:855-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stoorvogel, J., M. J. van Bussel, J. Tommassen, and J. A. van de Klundert. 1991. Molecular characterization of an Enterobacter cloacae outer membrane protein (OmpX). J. Bacteriol. 173:156-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Studier, F. W., A. H. Rosenberg, J. J. Dunn, and J. W. Dubendorff. 1990. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 185:60-89. [DOI] [PubMed] [Google Scholar]
- 63.Sundin, C., M. C. Wolfgang, S. Lory, A. Forsberg, and E. Frithz-Lindsten. 2002. Type IV pili are not specifically required for contact dependent translocation of exoenzymes by Pseudomonas aeruginosa. Microb. Pathog. 33:265-277. [DOI] [PubMed] [Google Scholar]
- 64.Tertti, R., M. Skurnik, T. Vartio, and P. Kuusela. 1992. Adhesion protein YadA of Yersinia species mediates binding of bacteria to fibronectin. Infect. Immun. 60:3021-3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vogt, J., and G. E. Schulz. 1999. The structure of the outer membrane protein OmpX from Escherichia coli reveals possible mechanisms of virulence. Structure 7:1301-1309. [DOI] [PubMed] [Google Scholar]
- 66.Westerlund, B., P. Kuusela, J. Risteli, L. Risteli, T. Vartio, H. Rauvala, R. Virkola, and T. K. Korhonen. 1989. The O75X adhesin of uropathogenic Escherichia coli is a type IV collagen-binding protein. Mol. Microbiol. 3:329-337. [DOI] [PubMed] [Google Scholar]
- 67.Yang, Y., and R. R. Isberg. 1997. Transcriptional regulation of the Yersinia pseudotuberculosis pH 6 antigen adhesin by two envelope-associated components. Mol. Microbiol. 24:499-510. [DOI] [PubMed] [Google Scholar]
- 68.Yang, Y., J. Merriam, J. Mueller, and R. Isberg. 1996. The psa locus is responsible for thermoinducible binding of Yersinia pseudotuberculosis to cultured cells. Infect. Immun. 64:2483-2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zav'yalov, V. P., V. M. Abramov, P. G. Cherepanov, G. V. Spirina, T. V. Chernovskaya, A. M. Vasiliev, and G. A. Zav'yalova. 1996. pH6 antigen (PsaA protein) of Yersinia pestis, a novel bacterial Fc-receptor. FEMS Immun. Med. Microbiol. 14:53-57. [DOI] [PubMed] [Google Scholar]