Abstract
Serine protease autotransporters of the family Enterobacteriaceae (SPATE) comprise a family of virulence proteins secreted by enteric Gram-negative bacteria via the autotransporter secretion pathway. A SPATE polypeptide contains a C-terminal translocator domain that inserts into the bacterial outer membrane as a β-barrel structure and mediates secretion of the passenger domain to the extracellular environment. In the present study, we examined the role of conserved residues located in the SPATE β-barrel-forming region in passenger domain secretion. Thirty-nine fully conserved residues in Tsh were mutated by single-residue substitution, and defects in their secretion phenotypes were assessed by cell fractionation and immunochemistry. A total of 22 single mutants exhibited abnormal phenotypes in different cellular compartments. Most mutants affecting secretion are charged residues with side chains pointing into the β-barrel interior. Seven mutants showed notable abnormalities in processing (constructs with the E1231A, E1249A, and R1374A mutations) and β-barrel assembly or insertion into the outer membrane (constructs with the G1158Y, F1360A, Y1375A, and F1377A mutations). The phenotypes of the β-barrel assembly/insertion mutants and the presence of a processed Tsh passenger domain in the periplasm support the possibility that the translocator domain must undergo extensive folding prior to insertion into the outer membrane. Results from double-mutation experiments further demonstrate that F1360 and F1377 affect β-barrel insertion/assembly at different times. In light of these new data, a more refined model for the mechanism of SPATE secretion is presented.
In Gram-negative bacteria, the type V, or autotransporter (AT), pathway is among the most commonly utilized protein secretion pathways (14). A nascent AT polypeptide consists of an N-terminal signal sequence involved in inner membrane (IM) translocation, a passenger domain to be secreted to the extracellular environment, and a C-terminal translocator domain further comprised of an N-terminal α-helical linker and a C-terminal β-domain (14, 20). Following its IM translocation mediated by the Sec translocase and the removal of the signal peptide by the signal peptidase, an AT is released into the periplasm (14, 20). The extent of AT folding in the periplasm is still under investigation, although recent evidence suggests the presence of some tertiary structure in this compartment (2, 12, 16, 32) and the involvement of periplasmic chaperones in the secretion event (11, 25, 28). After an AT transits through the periplasm, the outer membrane (OM) assembly Bam (YaeT/Omp85) complex assists in an AT insertion into the OM (15, 37); there the AT translocator domain forms a pore-like β-barrel. In conventional ATs, only a single protein's translocator domain is required to form a complete β-barrel (14). In the OM, the β-barrel tethers the folded passenger domain via the flexible α-helical linker (6, 14, 20). In many cases, the tethered passenger domain is eventually cleaved and released from the cell surface (6, 14, 20).
Most ATs characterized to date possess virulence functions (6). Among the numerous conventional ATs is a subfamily named the serine protease ATs of the family Enterobacteriaceae (SPATE), which is a group of multifunctional ATs implicated in the pathogenicity of enteric bacterial pathogens (44). SPATE share many conserved elements: all of these ATs possess a requisite serine protease motif [GDSGS(P)] in the passenger domain and an α-helical linker motif (EVNNLNKRMGDLRD) in which the double asparagines serve as the cleavage site between the passenger and translocator (6, 18). Cleavage releases a SPATE into the extracellular environment, its final secretion destination (44). Considering the ubiquity of conventional ATs in pathogenic bacteria and their participation in disease development (6), a better understanding of their secretion mechanism might help improve current therapeutic means. To study the secretion mechanism of conventional ATs, we used the temperature-sensitive hemagglutinin (Tsh), a SPATE from avian-pathogenic Escherichia coli, as a model (24).
This study focused on a conventional AT's translocator domain, which has been shown to be essential for the secretion of the passenger domain (23). The crystal structure of the translocator domain of a SPATE protein, EspP, revealed a β-barrel formed by 12 antiparallel β-strands, with the α-helical linker plugging the interior of the β-barrel (1). While these features of the EspP translocator resemble those of the crystallized translocator domain of NalP (22), the processing sites between the passenger and translocator in these two ATs are remarkably different. In NalP the processing site is close to the opening of the β-barrel, and thus, the α-helical linker extends throughout the length of the channel (22). In contrast, cleavage in EspP is assisted by an aspartate residue located in the midlength of the β-barrel through autoproteolysis (3), hence leaving only half of the α-helical linker in the β-barrel (1). Autoproteolytic release of the passenger domain is unique, and the processing of other ATs requires OM proteases (5, 31), the GDSGS motif located in the passenger (8, 23), or other unknown factors.
Considering the different roles of the translocator domain in an AT's secretion process, it is possible that residues located in the translocator have different secretion functions. For instance, some residues might be the recognition sites for OM assembly factors or periplasmic chaperones; some might play a structural role stabilizing the β-barrel in the OM; and others might be responsible for promoting the processing of the passenger domain, as seen in EspP (3). Indeed, a number of residues within the conserved α-linker motif have been shown by this laboratory to be vital for passenger secretion (18). Here, we focus on the remaining region of the SPATE translocator, the β-domain. Mutations introduced into residues in this domain resulted in many defective phenotypes related to passenger secretion. Notably, several mutants impaired processing of the Tsh passenger domain, and some prevented proper assembly or insertion of the β-domain into the OM. Two key residues involved in the assembly/insertion of the β-barrel at different times were also identified. Based on our results, as interpreted in conjunction with recently published structural and biochemical data, we have proposed a more refined model for the SPATE secretion mechanism.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
All constructs were expressed in E. coli K-12 strain XL1-Blue (Stratagene, La Jolla, CA) and cultured at 37°C in Luria-Bertani (LB) broth containing 100 μg/ml ampicillin. Plasmid pYA3418 containing the wild-type tsh gene under the control of the lac promoter was previously constructed from plasmid pWKS30 (40), as described by Stathopoulos et al. (34).
Site-directed mutagenesis.
The site-directed mutagenesis technique was described elsewhere (18). The primers used are listed in Table S1 in the supplemental material. Mutations were confirmed by sequencing at the City of Hope DNA sequencing laboratory (Duarte, CA).
Cell fractionation.
Conditions for cell growth and protein induction, methods of cell harvesting, and techniques of culture medium and OM preparation by the sarkosine method were described previously (18). In addition to OM isolation using sarkosine, the OMs of E. coli XL1-Blue expressing pWKS30, wild-type Tsh, F1360A, and F1377A were also isolated by the sucrose gradient method (30), with some modifications, indicated below; no differences in OM phenotypes were observed by the two techniques (data not shown). For OM isolation by the sucrose gradient method, the total membrane samples were ultracentrifuged at 128,000 × g for 16 h cushioned by different concentrations of sucrose solutions, prepared in 25 mM Tris (pH 7.4)-5 mM EDTA. The OM samples were isolated using syringes and were diluted with Tris buffer to a sucrose concentration of <10%; the OM pellets were obtained by centrifugation at 140,000 × g for 1 h. Periplasmic extraction was performed using the osmotic shock method described previously (29), with some modifications. Briefly, periplasmic fractions were obtained from cell pellets resuspended in 0.75 mM sucrose in 10 mM Tris-HCl (pH 8.0) and incubated on ice for 30 min after the addition of 0.5 mg/ml lysozyme in 100 mM EDTA. Samples were centrifuged at 13,000 × g for 20 min at 4°C to obtain the periplasmic proteins in the supernatants.
To confirm that there was no OM protein contamination in the periplasmic extracts, Western blotting was performed, in which an antibody recognizing OM protein BamA (laboratory collection) was used to probe the periplasmic extracts of several constructs (see Fig. S3A, lanes 8 to 10, in the supplemental material). The same antibody reacting with the OM fraction of E. coli XL1-Blue expressing wild-type Tsh served as the positive control (see Fig. S3A, lane 7, in the supplemental material). To confirm that there were no proteins from the culture medium contaminating the periplasmic extracts, an exogenous protein (YapA) was added into the culture of E. coli XL1-Blue expressing wild-type Tsh, prior to cell fractionation (see Fig. S3B in the supplemental material). YapA was obtained from the culture medium fraction of Yersinia pestis (43). Cell fractionation and preparation of proteins from each fraction were performed as described above.
Electrophoresis and immunoblot.
Samples were resolved by 10% SDS-PAGE and visualized by silver staining (45). For secreted protein samples, equivalent volumes of the culture medium were loaded. The OM and periplasmic sample loading volumes were normalized by estimating equal amounts of OM porins (21) and periplasmic protein β-lactamase, respectively. Western blotting was carried out by semidry electrotransfer (Bio-Rad, Hercules, CA), according to the manufacturer's instructions. Blocking was performed overnight at 4°C using 5% (wt/vol) nonfat milk in Tris-buffered saline containing 0.2% (vol/vol) Tween 20 (TTBS; Bio-Rad). The blots were incubated for 2 h at room temperature in a rabbit anti-Tsh antiserum (34) diluted 1:5,000 or in a rabbit anti-β-lactamase antibody (Millipore, Billerica, MA) diluted 1:4,000 and then incubated for 2 h at room temperature in a goat anti-rabbit horseradish peroxidase-conjugated IgG (Bio-Rad) diluted 1:20,000. Washing was done three times for 5 min each time in TTBS after each incubation period, and the blots were developed using chemiluminescence (Pierce, Rockford, IL), according to the manufacturer's instructions. Silver-stained gel and Western blot images were taken with a DE-500 MultiImage II light cabinet using FluorChem 5500 (version 4.0.1) software (Alpha Innotech Corporation, San Leandro, CA).
Fluorescence microscopy.
Bacterial cells were grown and induced as described above. Cells were harvested by centrifugation at 3,214 × g for 6 min and fixed in phosphate-buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4) containing 2.8% paraformaldehyde and 0.04% glutaraldehyde for 30 min. Samples subjected to permeabilization were incubated with 0.1% Triton X-100 in PBS, followed by washing three times with PBS for 45 min at room temperature and then treating the samples with 100 μg/ml of lysozyme and 5 mM EDTA in PBS for 45 min at room temperature. Blocking was performed with 0.1% bovine serum albumin (BSA; Fisher Scientific, Fair Lawn, NJ) for 1 h at 37°C. Primary incubation was performed with a rabbit anti-Tsh antibody (34) diluted to 1:1,000 or with a rabbit anti-β-lactamase (Millipore) diluted 1:500 (in PBS containing 0.1% BSA) for 1 h at 37°C. The cells were then incubated in the dark with a goat anti-rabbit rhodamine-conjugated antibody (Pierce) diluted to 1:100 in PBS containing 0.1% BSA for 1 h at 37°C. The cells were washed three times with PBS after fixation and three times with 0.05% Tween 20 in PBS after each antibody incubation step. One additional wash with PBS was carried out before the cells were mounted on slides coated with poly-l-lysine (Polysciences, Warrington, PA) and visualized with an Axioskop 40 fluorescence microscope using AxioVision (version 4.0) software (Carl Zeiss, Germany).
Analysis of fluorescence signals.
Fluorescence microscopy data were analyzed using ImageJ (version 1.41) software (26). For constructs that showed moderate to severe defects (Table 1, two and three asterisks), microscopy was performed and the constructs were imaged in three independent studies; for those that showed slightly defective phenotypes (Table 1, one asterisk), it was performed once. In each study, two separate takes or images of each mutant were analyzed and the average fluorescence intensities were obtained for each mutant. Using a bright-field image, at least 10 individual cells in the corresponding fluorescence image from the same area were selected to be measured; background intensities were subtracted. The relative intensities of all mutants were then normalized to those of the wild-type Tsh (intensity, 2.0) and pWKS30 (intensity, 0). These values were later combined to obtain the final average fluorescence intensities shown in Fig. S2 in the supplemental material.
TABLE 1.
Summary of phenotypes and ratings of defects in Tsh single mutant constructsa
| Conserved region | Residue orientation to β-barrel | Secretion into culture medium |
Surface localization | OM accumulation |
Periplasm localization |
Defect rating | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Processed Tsh | Unprocessed Tsh | Cell lysis | Unprocessed Tsh | Processed Tsh | Protein degradation | Processed Tsh | Unprocessed Tsh | Protein degradation | ||||
| Region 1 | ||||||||||||
| D1154A | In | +++ | ## | +U | + | ++ | * | |||||
| L1155Y | Out | +++ | No data | − | ++ | Wt | ||||||
| F1156A | In | +++ | No data | +U | ++ | Wt | ||||||
| T1157A | Out | +++ | No data | +U | ++ | Wt | ||||||
| G1158Y | In | ++ | ### | − | + | +U+U | dg | *** | ||||
| Region 2 | ||||||||||||
| H1224A | Out | +++ | No data | − | ++ | Wt | ||||||
| S1225A | In | +++ | No data | +U | ++ | Wt | ||||||
| Y1227A | In | + | ## | − | + | +++ | ** | |||||
| G1229Y | In | +++ | No data | − | + | ++ | Wt | |||||
| E1231A | In | + | #### | +U+U+U | + | + | *** | |||||
| G1233Y | In | ++ | ## | +U+U | ++ | + | ** | |||||
| Y1234A | Out | +++ | No data | − | + | Wt | ||||||
| R1235A | In | +++ | No data | − | + | Wt | ||||||
| Region 3 | ||||||||||||
| E1245A | In | ++ | ### | + | + | ** | ||||||
| P1246Y | Out | +++ | No data | +U | ++ | Wt | ||||||
| Q1247A | In | ++ | #### | +U | ++ | − | ** | |||||
| E1249A | In | − | #### | +U+U+U | − | *** | ||||||
| L1250Y | Out | +++ | ### | +U+U | dg | ++ | ** | |||||
| V1251Y | In | +++ | ### | +U+U | + | ++ | * | |||||
| G1253Y | In | +++ | − | − | − | * | ||||||
| Region 4 | ||||||||||||
| S1358A | In | +++ | No data | +U | ++ | Wt | ||||||
| A1359Y | Out | ++ | ### | +U+U+U | dg | ++ | ** | |||||
| F1360A | Loop | − | Lysis | # | +U+U+U | dg | − | +U | dg | *** | ||
| G1361Y | Loop | +++ | ### | +U+U | dg | ++ | * | |||||
| Y1363A | Loop | +++ | ### | +U+U | + | ++ | * | |||||
| N1364A | Out | +++ | #### | + | ++ | ** | ||||||
| D1366A | Out | ++ | #### | + | + | +U | (dg) | ** | ||||
| N1370A | In | ++ | #### | +U | + | + | ** | |||||
| A1371Y | Out | ++ | ### | +U+U | + | ++ | ** | |||||
| R1374A | In | + | #### | +U+U+U | + | *** | ||||||
| Y1375A | Out | ++ | Lysis | #### | +U+U+U | + | (dg) | *** | ||||
| F1377A | Out | + | +U | Lysis | # | +U+U+U | dg | − | +U | dg | *** | |
| Wt Tsh | +++ | ### | +U | ++ | ||||||||
| N1100A | − | ##### | +U+U+U | − | *** | |||||||
+, processed Tsh; +U, unprocessed Tsh; #, fluorescence signal; −, no signal detected; dg, degradation; (dg), degradation phenotypes less severe than dg; Wt, wild-type phenotype; *, slightly defective; **, moderately defective; ***, severely defective.
RESULTS
Construction of single-residue substitution mutations in the highly conserved β-domain.
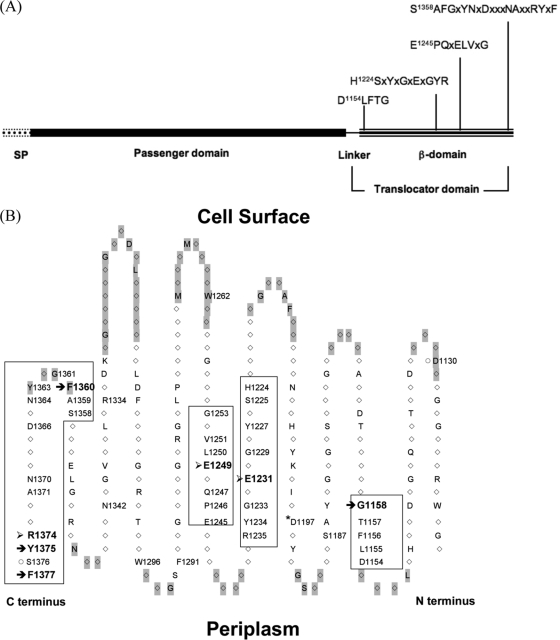
A previous study from this laboratory identified a 14-conserved-residue motif (EVNNLNKRMGDLRD) in the α-helical linker of the SPATE's translocator domain that is important for passenger secretion (18) and suggested that the SPATE might share a secretion mechanism (44). However, the role of conserved residues in the β-domain of the translocator is unclear, and it is assessed in this study by targeted mutagenesis. In the 262-residue β-domain, roughly 40%, or 104 residues, is fully conserved. We focused on four long conserved regions containing 32 fully conserved residues located in the 3rd, 6th, 7th, and primarily 12th β-strand of the β-domain (Fig. 1). A single substitution mutation was introduced into each of the 32 conserved positions: nonaromatic, hydrophobic residues were changed to tyrosine, and others were mutated to alanine (Table 1).
FIG. 1.
Four conserved regions of the SPATE β-domain. Residues are numbered on the basis of their positions in the Tsh primary structure. (A) Domains of Tsh. The four conserved sequences in the β-domain are specified. (B) Topology of the Tsh β-domain. The topology was constructed based on the crystal structure of the EspP translocator (1). Positions shaded in gray are loop localized. Only fully conserved positions are revealed (with one-letter amino acid symbols), and those accompanied by a position number are residues that have been mutated in this study. Exceptions are D1130 and S1376 (○), two nonconserved residues also mutated. The four conserved regions are boxed, within which seven residues that exhibited severe defects upon mutation are marked with arrows: those that affect β-barrel assembly/insertion (→) and those that influence processing (➢). Also marked is D1197 (*); its homologous position in EspP is responsible for assisting with complete autoprocessing of the passenger domain (3).
In addition, mutations were introduced into four fully conserved residues (W1262, F1291, W1296, and D1197). The rationales for and the results obtained with these mutations will be discussed below. Also mutated was the second to the last residue, S1376, located in the 4th conserved region. S1376 is part of the AT signature sequence (7) and is believed to interact with BamA (27). However, it is not a conserved position in SPATE, and its mutation to alanine produced wild-type phenotypes (data not shown). Lastly, four mutants (those with the D1130A, S1187A, R1334A, and N1342A mutations) were constructed on the basis of our molecular modeling data, which suggested their interactions with the α-helical linker. Cell fractionation results showed merely mild secretion defects in these mutants (data not shown) and thus prompted us to abandon this approach. In sum, 39 or 38% of the 104 fully conserved residues and two nonconserved residues in the Tsh β-domain were mutated (Fig. 1B).
All of the 32 mutant constructs expressed Tsh.
To make sure that the mutations affected Tsh secretion but not its expression, total Tsh protein produced by each of the 32 constructs in the four conserved regions was assessed by examining the amount of Tsh present in the whole-cell lysate and culture medium. In all of the constructs, Tsh could be found in one or combinations of three forms: unprocessed (140-kDa) and processed (106-kDa) forms in the cell lysate and processed Tsh (106 kDa) released to the culture medium (see Fig. S1 in the supplemental material). The results indicate that Tsh was expressed in all of the constructs analyzed. Surprisingly, for the mutant G1253Y construct, Tsh was detected in the culture medium fraction but not in the cell lysate (see Fig. S1, lane 31, in the supplemental material). An explanation for this observation could be that much of the mutated Tsh might be degraded due to misfolding. Thus, it is difficult to quantify the amount of total Tsh protein expression because the amount of Tsh degradation in each construct is unknown.
Cell fractionation revealed a variety of trafficking-defective phenotypes in mutants.
The profiles of protein expression (see Fig. S1 in the supplemental material) revealed secretion abnormalities in several mutants. To find out in which cellular compartment(s) these defects occurred, we performed cell fractionation studies on the Tsh mutants and assessed the level of Tsh secreted to the culture medium, OM, and periplasm. The level of Tsh secretion by each mutant was then compared to that by the wild type to determine the degree of the defect.
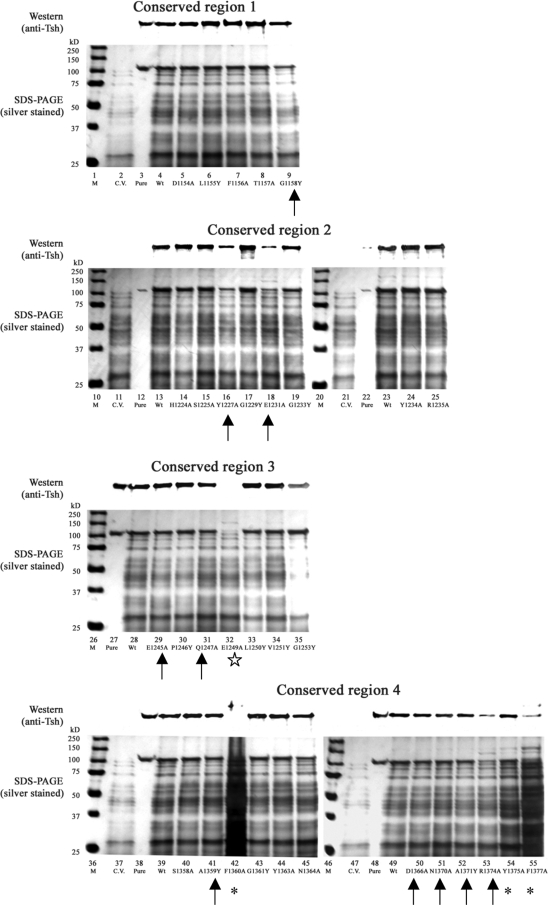
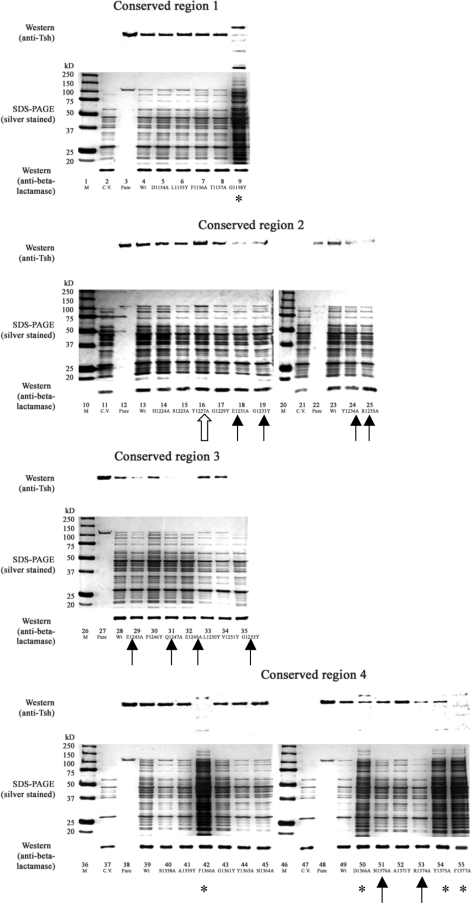
In the culture medium fractions (Fig. 2), three categories of defective phenotypes can be seen in 14 mutants: (i) cell lysis and reduction in Tsh secretion, shown by three mutants, those with the F1360A, Y1375A, and F1377A mutations (cell lysis is indicated by poor culture growth [data not shown] and more than normal amounts of proteins released into the culture medium) (Fig. 2, lanes 42, 54, and 55, respectively); (ii) abolished Tsh secretion shown in the E1249A construct (Fig. 2, lane 32); and (iii) reduction in Tsh secretion with no signs of lysis in 10 mutants, those with the G1158Y, Y1227A, E1231A, E1245A, Q1247A, A1359Y, D1366A, N1370A, A1371Y, and R1374A mutations (Fig. 2, lanes 9, 16, 18, 29, 31, 41, and 50 to 53, respectively). The level of Tsh secreted into the culture medium by each construct was quantified by ImageJ software (26).
FIG. 2.
Culture medium fractions of Tsh single mutant constructs. Shown are representative data from at least two independent experiments. Lanes: M, markers; C.V., cloning vector pWKS30; Pure, purified secreted Tsh protein (Tshs) (17), 106 kDa; Wt, wild-type Tsh. (Top of each panel) Western blotting was performed with anti-Tsh antibody; (bottom of each panel) silver-stained 10% SDS-polyacrylamide gels. Special symbols indicate mutants with the following notable defective phenotypes: cell lysis and reduction of Tsh secretion (*), abolished Tsh secretion (⋆), and reduction in secretion but no signs of lysis (↑).
In the OM fractions, a number of mutants showed the abnormal accumulation of Tsh in different forms (Fig. 3). For wild-type Tsh, only a trace of the unprocessed form (140 kDa) remained in the OM. However, there was the extra accumulation of the unprocessed form in this compartment for eight constructs: E1249A, L1250Y, A1359Y, F1360A, G1361Y, R1374A, Y1375A, and F1377A (Fig. 3, lanes 32 to 33, 41 to 43, and 53 to 55, respectively). Five constructs possessed only processed Tsh (106 kDa) in the OM: Y1227A, G1229Y, E1245A, N1364A, and D1366A (Fig. 3, lanes 16, 17, 29, 45, and 50, respectively), whereas eight constructs had incomplete processing: D1154A, E1231A, G1233Y, Q1247A, V1251Y, Y1363A, N1370A, and A1371Y (Fig. 3, lanes 5, 18, 19, 31, 34, 44, 51, and 52, respectively).
FIG. 3.
OM fractions of Tsh single mutant constructs. Shown are representative data from at least two independent experiments. Lanes: M, markers; C.V., cloning vector pWKS30; Pure, purified secreted Tsh protein (Tshs) (17), 106 kDa; Wt, wild-type Tsh. (Top of each panel) Results of Western blotting performed with anti-Tsh antibody; (bottom of each panel) silver-stained 10% SDS-polyacrylamide gels. The solid arrowheads point to the eight mutants with accumulation of unprocessed Tsh (140 kDa). The asterisks indicate the five mutants showing only processed Tsh (106 kDa). The hollow arrows point to the eight constructs possessing both the processed (106 kDa) and unprocessed (140 kDa) forms of Tsh. The position of the β-barrel in Tsh is also indicated by a long arrow.
We also attempted to assess the level of Tsh localized to the cell surface by fluorescence microscopy, performed using whole cells probed with anti-Tsh antibody (see the data and Fig. S2 in the supplemental material). Surprisingly, many mutants showed a compromised OM integrity with this approach (see Fig. S2 in the supplemental material), thus making it difficult to assess the amount of Tsh localized to the cell surface.
Analysis of the periplasmic fractions showed that 16 mutants exhibited defective phenotypes (Fig. 4) that can be divided into two categories: those that accumulated elevated levels of degradation proteins in the periplasm, as seen in mutants G1158Y, F1360A, D1366A, Y1375A, and F1377A (Fig. 4, lanes 9, 42, 50, 54, and 55, respectively), and those that showed reduced amount of processed Tsh, as seen in mutants E1231A, G1233Y, Y1234A, R1235A, E1245A, Q1247A, E1249A, G1253Y, N1370A, and R1374A (Fig. 4, lanes 18, 19, 24, 25, 29, 31, 32, 35, 51, and 53, respectively). In addition, Y1227A appeared to be the only mutant that had accumulated more Tsh in the periplasm than the wild type (Fig. 4, lane 16). The abnormal phenotypes observed here thus indicate that the defects might occur during Tsh transit through the periplasmic compartment.
FIG. 4.
Periplasmic fractions of Tsh single-mutant constructs. Shown are representative data from at least two independent experiments. Lanes: M, markers; C.V., cloning vector pWKS30; Pure, purified secreted Tsh protein (Tshs) (17), 106 kDa; Wt, wild-type Tsh. (Top of each panel) Results of Western blotting performed with anti-Tsh antibody; (middle of each panel) silver-stained 10% SDS-polyacrylamide gels; (bottom of each panel) results of Western blotting performed with anti-β-lactamase antibody. Symbols highlight mutants showing the following defects in the periplasm: accumulating degradation products (*), having a reduced amount of processed Tsh (↑), and having a higher than wild-type level of processed Tsh (⇑).
Note that wild-type Tsh and the majority of mutant Tsh constructs in the periplasmic fraction are in the 106-kDa processed form (Fig. 4). This is surprising and has not been observed in other SPATE or ATs. Thus, we checked the periplasmic fractions for the presence of OM and culture medium proteins to rule out the possibility of contamination during the periplasm extraction process (see Fig. S3 in the supplemental material). The periplasmic fractions from the controls did not show reactivity to anti-BamA antibody (see Fig. S3A, lanes 8 to 10, in the supplemental material), indicating that the periplasmic extracts were free of contamination from the OM. Similarly, the periplasmic fraction of wild-type Tsh did not show reactivity to anti-YapA antibody (see Fig. S3B, lane 5, in the supplemental material), indicating that the periplasmic extracts were also free of contamination from the culture medium.
Conserved residues in the β-domain play different roles in SPATE secretion.
The cumulative data from the fractionation experiments (Table 1) indicate that over half of the mutated positions in the β-domain are important for secretion. The diversity and severity of the defects are particularly remarkable in conserved region 4, in which nearly all of the mutations showed notable defects (Table 1). This observation differs from that for regions 1 to 3, in which mutants with side chains pointing into the β-barrel show more remarkable secretion defects than those with side chains pointing outwards (Table 1). The data thus suggest a unique role for the 12th β-strand, in that it might interact with factors other than the α-helical linker traversing the pore. Collectively, the results support the possibility that the β-domain carries out diverse functions and probably participates in different stages of Tsh trafficking through different cellular compartments.
Residues participate in protein processing.
To further analyze the roles of the conserved residues in secretion, we first directed our attention to E1249. Mutating this position to alanine abolished Tsh secretion into the culture medium and resulted in accumulation of only unprocessed Tsh in the OM (Fig. 2 and 3, lanes 32), signifying processing errors. Note that the E1249A construct also has no β-barrel in the OM, since the β-domain is not cleaved and remains part of the unprocessed protein. Analysis of the crystal structure (1) with modeling revealed that the side chain of E1249 inserts into the interior of the β-barrel and is in proximity to the cleavage site in the α-helical linker. Thus, its position puts the residue in a probable position to participate in the processing of Tsh.
In EspP, an aspartate (EspP D1120) rather than a glutamate can facilitate complete cleavage of its passenger domain (3). Thus, we wondered if mutating the homologous aspartate position in Tsh (Tsh D1197) could also abolish its release into the culture medium. The results indicate that mutating D1197 to alanine did abolish Tsh secretion (see Fig. S4A, lane 5, in the supplemental material), as in the E1249A construct, suggesting that both E1249 and D1197 participate in autoprocessing of Tsh.
Residues involved in proper β-barrel assembly or insertion into the OM.
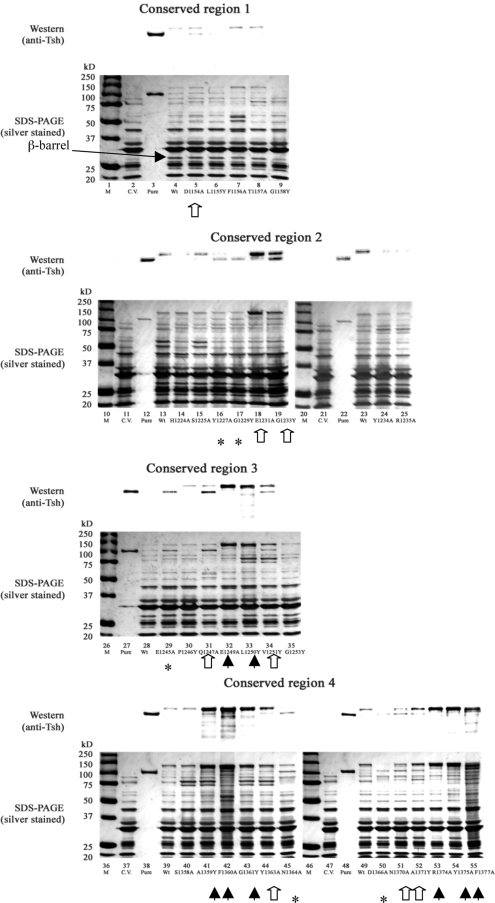
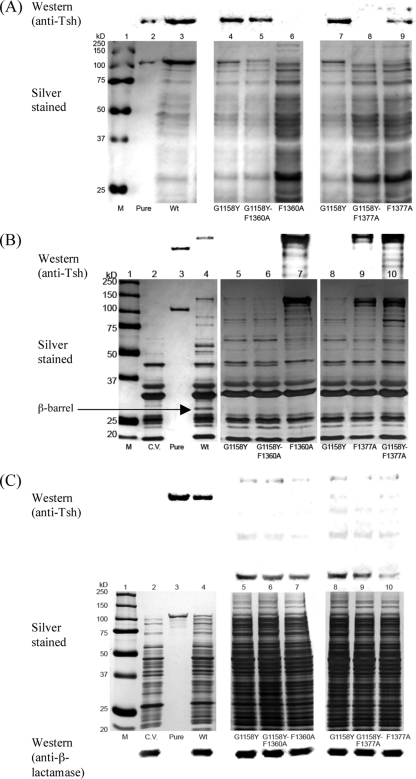
We are also interested in the G1158Y, F1360A, and F1377A mutations. All of the three mutants exhibited accumulation of degraded Tsh and other proteins in the periplasm (Fig. 4, lanes 9, 42, and 55, respectively), signifying defects that impact Tsh trafficking through this compartment. G1158Y secreted less Tsh protein into the culture medium than the wild type (Fig. 2, lane 9) and appeared to have significantly less β-barrel and passenger in the OM than the wild type (Fig. 3, lane 9; Fig. 5B, lanes 5 and 8). Thus, improper β-barrel assembly or insertion into the OM of the G1158Y mutant likely triggered the degradation condition of Tsh and other proteins observed in the periplasm.
FIG. 5.
Cell fractionation of the double-mutant constructs compared side by side to the single mutants. Lanes: M, markers; C.V., cloning vector pWKS30; Pure, purified secreted Tsh protein (Tshs) (17), 106 kDa; Wt, wild-type Tsh. (A) Culture medium fractions; (B) OM fractions. (Top of each panel) results of Western blotting performed with anti-Tsh antibody; (bottom of each panel) silver-stained 10% SDS-polyacrylamide gels. (C) Periplasmic fractions. (Top of panel) results of Western blotting with anti-Tsh antibody; (middle of panel) silver-stained 10% SDS-polyacrylamide gels; (bottom of panel) Western blotting performed with anti-β-lactamase-detecting periplasm-localized β-lactamase (as a loading control) from each sample.
Unlike the G1158Y mutant, the F1360A and F1377A mutants further showed cell lysis phenotypes in the culture medium fractions (Fig. 2, lanes 42 and 55, respectively) and accumulation of degraded Tsh products in the OM fractions (Fig. 3, lanes 42 and 55, respectively). Less β-barrel is also observed in the OMs of these two mutants than in the OM of the wild type (Fig. 3, lanes 42 and 55, respectively; Fig. 5B, lanes 7 and 9, respectively), suggesting that the defects are also related to either β-barrel misassembly or misinsertion into the OM. Since mutating F1360 and F1377 produced such severe lysis phenotypes and aromatic residues are abundant in OM proteins, we wondered if mutating other aromatic residues in the β-domain of Tsh would also impact secretion. Three conserved aromatic residues (W1262, F1291, W1296), located in the 7th, 8th, and 9th β-strands, respectively, were mutated to alanine. Their mutations resulted in wild-type phenotypes in all of the cell fractions (data not shown). In addition, mutations to other conserved aromatic residues, such as F1156, Y1234, and Y1363, produced wild-type or nearly wild-type phenotypes (Table 1). Furthermore, mutating F1360 to tyrosine produced a wild-type rather than a lysis phenotype (data not shown). Together, these results underscore the importance of F1360 and F1377 in Tsh secretion and signify the requirement of an aromatic residue in either position.
F1360 and F1377 participate in different stages of β-barrel assembly/insertion.
Although both the F1360A and the F1377A mutants exhibited similar phenotypes, cell lysis in the F1360A mutant was consistently more severe than that in the F1377A mutant. No processed form of Tsh was ever noted in the culture medium of the F1360A mutant, but a very small amount was consistently observed in the F1377A mutant (Fig. 2, lanes 42 and 55, respectively). The slight differences in their phenotypes thus imply that each residue might have a unique function in relation to β-barrel assembly/insertion. To further distinguish the roles of F1360 and F1377 in this aspect, we constructed double mutants involving the two phenylalanines and each phenylalanine mutation with G1158Y, another mutation affecting β-barrel assembly. Double mutant F1360A-F1377A exhibited a cell lysis phenotype similar to that observed in the single mutants (data not shown). Because it was difficult to interpret such data, it was not pursued further. Cell fractionation results for the G1158Y-F1360A mutant indicate that the double mutant exhibited the phenotype of the G1158Y mutant rather than that of the F1360A mutant in the culture medium and OM fractions (Fig. 5A, lanes 4 to 6; Fig. 5B, lanes 5 to 7). Particularly evident in the culture medium fraction of mutant G1158Y-F1360A (Fig. 5A, lane 5) was that the cell lysis phenotype exhibited by F1360A alone (Fig. 5A, lane 6) appeared to be alleviated by the presence of G1158Y. In contrast, double mutant G1158Y-F1377A exhibited additive phenotypes of the single mutants, mainly noticeable in the culture medium and OM fractions (Fig. 5A, lanes 7 to 9; Fig. 5B, lanes 8 to 10). Together, these data indicate that F1360 and F1377 have distinct functions and suggest that they participate in different stages of β-barrel assembly or insertion into the OM.
DISCUSSION
An AT's translocator domain is believed to mediate the translocation of its passenger domain across the OM (6, 14). However, the exact mechanism is unclear and might involve accessory factors in the periplasm and OM, since BamA (15, 37) and periplasmic chaperones (11, 25, 28) have been shown to be essential for the secretion of several ATs. The Bam complex is believed to assist in protein integration into the bacterial OM (37, 41). The role of chaperones in AT secretion has been considered only recently. Any function carried out by known periplasmic chaperones is possible, including protein degradation (35), folding assistance (19), and OM targeting (33). In addition to facilitating OM translocation, a translocator domain can also have a role in the processing of the passenger domain, as observed in EspP and a non-SPATE AT, BrkA (3).
Considering the multiple functions of a SPATE translocator, we wondered which residues in this domain might be important for different stages of secretion and in what cellular compartments the functions were exerted. We thus focused on residues that are fully conserved in 20 SPATE. Using Tsh as a model, the secretion-related functions of 39 conserved residues were examined. Mutations to 39 conserved residues of the β-domain resulted in 22 mutants with mutations located in four conserved regions showing a variety of secretion-related defective phenotypes, with seven residues exhibiting the most notable defects (Fig. 1B).
Three mutant constructs, E1231A, E1249A, and R1374A, exhibited phenotypes suggestive of Tsh processing errors, namely, diminished (E1231A and R1374A constructs) or abolished (E1249A construct) Tsh secretion into the culture medium and accumulation of unprocessed Tsh in the OM. The side chains of all of these residues insert into the interior of the β-barrel and can serve either to mediate passenger cleavage or to properly position the α-helical linker for cleavage. In addition to mutating E1249, mutating D1197 in Tsh (its homologous residue in EspP is responsible for fully processing the passenger) abolished passenger secretion into the culture medium (see Fig. S4, lane 5, in the supplemental material). The data strongly suggest that both residues, which have similar carboxyl side chains, participate in the cleavage process. Mutation to E1231, located in proximity to E1249 (Fig. 1B), also severely impaired Tsh release into the culture medium but did not abolish it (Fig. 2, lanes 18 and 32). It is unknown whether E1231 directly participates in passenger processing. Mutating R1374 in Tsh produced similar defects; however, the side chain of the arginine is farther away from the cleavage site, and its guanidino group is less likely to carry out the same proton-abduct task as the carboxyl group in autoprocessing. Therefore, the role of R1374 might be positioning the α-helix inside the β-barrel pore rather than directly participating in proteolysis.
Mutating F1360A, Y1375A, and F1377A in Tsh resulted in cell lysis. Aromatic residues are abundant in β-barrel structures, and in particular, the aromatic-X-aromatic motifs are potential sites in OM proteins recognized by periplasmic chaperones (9, 28, 39, 42). F1377A and Y1375A, located in the last and third to the last of the C-terminal positions, respectively, are also part of the classic secretion signal for several OM proteins (27, 36), although their role in SPATE secretion has not been previously demonstrated. Apparently, the specificity of secretion signals differs among OM proteins, because mutating five C-terminal residues in Hap, a conventional AT from Haemophilus influenzae, produced no secretion defects (8), and deleting the final C-terminal phenylalanine in OM protein PorA did not affect its interaction with BamA either (27).
In addition to aromatic residues, an abundance of glycine residues is also present in β-barrels, and those most likely have structural importance (13). It has been noted that all of the glycines mutated in this study, by substitution with a bulky tyrosine, resulted in trafficking defects. In the G1158Y construct, the defects are less obvious in the culture medium, cell surface, and OM fractions; however, the abundant accumulation of degraded Tsh and other proteins in the periplasmic fraction is remarkable. Analysis of the OM fraction from the G1158Y mutant construct indicates a reduction in the β-barrel amount (Fig. 5B, lanes 5 and 8), which probably resulted from perturbation to the β-barrel structure that eventually led to proteolytic clearing by proteases/chaperones. In contrast to the G1158Y mutant, the G1253Y mutant appeared to have nearly normal secretion into the culture medium (Fig. 2). However, no Tsh was detected in the OM or periplasm, and no abnormal protein accumulation was observed in the periplasm (Fig. 3 to 4). The β-domain of the G1253Y mutant was inserted into the OM (Fig. 3), although a misfolded β-domain in the OM might be enough to trigger the process that led to degradation of the passenger domain before it folded into a proteolysis-resistant native structure.
Because the G1158Y, F1360A, and F1377A mutants have common phenotypes in the periplasm, we constructed double mutants to investigate whether F1360 and F1377 have distinct functions involving β-barrel formation/insertion. In G1158Y-F1360A, cell lysis of the F1360A construct was alleviated. However, the same effect was not seen in the G1158Y-F1377A construct, implying that the function of F1377 is different from that of F1360. We speculate that one phenylalanine (F1377) could be affecting β-barrel formation, whereas the other (F1360) could affect its subsequent insertion into the OM. We think that the alleviation of cell lysis observed in the G1158Y-F1360A construct is perhaps the result of a damaged barrel (G1158Y) never being inserted into the OM (phenotype of the F1360Y construct); cell lysis persists in the F1377A-G1158Y double mutant because both mutations affect β-barrel formation and two mutations have compounded the effects. Remarkably, when screening the mutants' periplasmic fractions for defective phenotypes, we noticed that only the processed form of Tsh was observed, suggesting that the β-barrel has obtained a substantial tertiary structure to enable processing of the passenger domain. The data thus support a secretion model in which the Bam complex facilitates AT insertion into the OM by accommodating a considerably folded translocator domain (4, 10, 38). Alternatively, a population of Tsh could have its translocator inserted into the OM in an opposite orientation, thus releasing the passenger into the periplasm instead of the extracellular space.
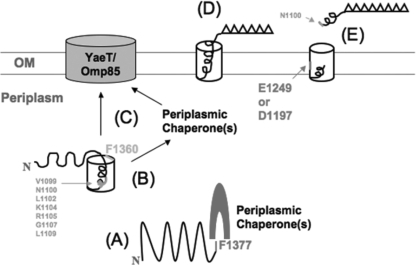
Based on the interpretations of the results, a mechanism of SPATE secretion explaining the roles of these residues has been proposed (Fig. 6). We propose that F1377 is needed for β-barrel formation, a function with which exogenous factors such as periplasmic chaperones likely assist. After the β-domain folds into a proto-β-barrel-like structure (12), it might be stabilized by the formation of the α-helical linker in the β-barrel (18). Once the folding of a β-barrel-like structure is achieved in the periplasm, the SPATE is targeted to and later inserted into the OM through facilitation by F1360. If a β-barrel fails to form properly, as seen in the G1158 mutant, then the protein will be mistargeted to or misinserted into the OM, and degradation in the periplasm or in the OM will occur. The targeting/insertion function of F1360 likely requires other secretion factors, such as BamA and/or periplasmic chaperones. Afterwards, the passenger domain folds into a tertiary conformation and is cleaved from the translocator, assisted by E1249 and D1197.
FIG. 6.
Proposed mechanism of the possible roles of the conserved residues located in the translocator domain of a SPATE. Residues shown in a larger font are essential for β-barrel insertion/assembly or passenger processing, as demonstrated in this study; those shown in a smaller font affect secretion, on the basis of the findings of a previous study (18). (A) The folding of the β-domain into a prototertiary structure (12) requires F1377. This process likely involves exogenous secretion factors such as periplasmic chaperones. (B) The folded intermediates are probably stabilized by the formation of the α-helical linker inside the β-barrel. (C) F1360 is required for the proper insertion of the folded translocator domain into the OM, an event also involving exogenous factors such as the Bam complex and, possibly, periplasmic chaperones. (D) Once the translocator domain is properly inserted, the passenger domain folds into its native conformation. (E) E1249 and D1197 then facilitate the cleavage of the passenger domain between N1100 and N1101 in the α-helical linker, resulting in the release of a functional AT into the extracellular environment.
Supplementary Material
Acknowledgments
We are grateful to Maria Kostakioti for critical reading of the manuscript and Peter Oeschlaeger for helpful discussions.
Editor: S. M. Payne
Footnotes
Published ahead of print on 1 June 2010.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Barnard, T. J., N. Dautin, P. Lukacik, H. D. Bernstein, and S. K. Buchanan. 2007. Autotransporter structure reveals intra-barrel cleavage followed by conformational changes. Nat. Struct. Mol. Biol. 14:1214-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brandon, L. D., and M. B. Goldberg. 2001. Periplasmic transit and disulfide bond formation of the autotransported Shigella protein IcsA. J. Bacteriol. 183:951-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dautin, N., T. J. Barnard, D. E. Anderson, and H. D. Bernstein. 2007. Cleavage of a bacterial autotransporter by an evolutionarily convergent autocatalytic mechanism. EMBO J. 26:1942-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dautin, N., and H. D. Bernstein. 2007. Protein secretion in gram-negative bacteria via the autotransporter pathway. Annu. Rev. Microbiol. 61:89-112. [DOI] [PubMed] [Google Scholar]
- 5.Egile, C., H. d'Hauteville, C. Parsot, and P. J. Sansonetti. 1997. SopA, the outer membrane protease responsible for polar localization of IcsA in Shigella flexneri. Mol. Microbiol. 23:1063-1073. [DOI] [PubMed] [Google Scholar]
- 6.Henderson, I. R., F. Navarro-Garcia, M. Desvaux, R. C. Fernandez, and D. Ala'Aldeen. 2004. Type V protein secretion pathway: the autotransporter story. Microbiol. Mol. Biol. Rev. 68:692-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henderson, I. R., F. Navarro-Garcia, and J. P. Nataro. 1998. The great escape: structure and function of the autotransporter proteins. Trends Microbiol. 6:370-378. [DOI] [PubMed] [Google Scholar]
- 8.Hendrixson, D. R., M. L. de la Morena, C. Stathopoulos, and J. W. St. Geme III. 1997. Structural determinants of processing and secretion of the Haemophilus influenzae Hap protein. Mol. Microbiol. 26:505-518. [DOI] [PubMed] [Google Scholar]
- 9.Hennecke, G., J. Nolte, R. Volkmer-Engert, J. Schneider-Mergener, and S. Behrens. 2005. The periplasmic chaperone SurA exploits two features characteristic of integral outer membrane proteins for selective substrate recognition. J. Biol. Chem. 280:23540-23548. [DOI] [PubMed] [Google Scholar]
- 10.Hodak, H., and F. Jacob-Dubuisson. 2007. Current challenges in autotransport and two-partner protein secretion pathways. Res. Microbiol. 158:631-637. [DOI] [PubMed] [Google Scholar]
- 11.Ieva, R., and H. D. Bernstein. 2009. Interaction of an autotransporter passenger domain with BamA during its translocation across the bacterial outer membrane. Proc. Natl. Acad. Sci. U. S. A. 106:19120-19125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ieva, R., K. M. Skillman, and H. D. Bernstein. 2008. Incorporation of a polypeptide segment into the beta-domain pore during the assembly of a bacterial autotransporter. Mol. Microbiol. 67:188-201. [DOI] [PubMed] [Google Scholar]
- 13.Jackups, R., Jr., and J. Liang. 2005. Interstrand pairing patterns in beta-barrel membrane proteins: the positive-outside rule, aromatic rescue, and strand registration prediction. J. Mol. Biol. 354:979-993. [DOI] [PubMed] [Google Scholar]
- 14.Jacob-Dubuisson, F., R. Fernandez, and L. Coutte. 2004. Protein secretion through autotransporter and two-partner pathways. Biochim. Biophys. Acta 1694:235-257. [DOI] [PubMed] [Google Scholar]
- 15.Jain, S., and M. B. Goldberg. 2007. Requirement for YaeT in the outer membrane assembly of autotransporter proteins. J. Bacteriol. 189:5393-5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jong, W. S., C. M. ten Hagen-Jongman, T. den Blaauwen, D. Jan Slotboom, J. R. Tame, D. Wickstrom, J. W. de Gier, B. R. Otto, and J. Luirink. 2007. Limited tolerance towards folded elements during secretion of the autotransporter Hbp. Mol. Microbiol. 63:1524-1536. [DOI] [PubMed] [Google Scholar]
- 17.Kostakioti, M., and C. Stathopoulos. 2004. Functional analysis of the Tsh autotransporter from an avian pathogenic Escherichia coli strain. Infect. Immun. 72:5548-5554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kostakioti, M., and C. Stathopoulos. 2006. Role of the alpha-helical linker of the C-terminal translocator in the biogenesis of the serine protease subfamily of autotransporters. Infect. Immun. 74:4961-4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lazar, S. W., and R. Kolter. 1996. SurA assists the folding of Escherichia coli outer membrane proteins. J. Bacteriol. 178:1770-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newman, C. L., and C. Stathopoulos. 2004. Autotransporter and two-partner secretion: delivery of large-size virulence factors by gram-negative bacterial pathogens. Crit. Rev. Microbiol. 30:275-286. [DOI] [PubMed] [Google Scholar]
- 21.Nikaido, H., and M. Vaara. 1987. Outer membrane, p. 7-22. In F. Neidhardt (ed.), Escherichia coli and Salmonella typhimurium: cellular and molecular biology, vol. 1. ASM Press, Washington, DC. [Google Scholar]
- 22.Oomen, C. J., P. van Ulsen, P. van Gelder, M. Feijen, J. Tommassen, and P. Gros. 2004. Structure of the translocator domain of a bacterial autotransporter. EMBO J. 23:1257-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pohlner, J., R. Halter, K. Beyreuther, and T. F. Meyer. 1987. Gene structure and extracellular secretion of Neisseria gonorrhoeae IgA protease. Nature 325:458-462. [DOI] [PubMed] [Google Scholar]
- 24.Provence, D. L., and R. Curtiss III. 1994. Isolation and characterization of a gene involved in hemagglutination by an avian pathogenic Escherichia coli strain. Infect. Immun. 62:1369-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Purdy, G. E., C. R. Fisher, and S. M. Payne. 2007. IcsA surface presentation in Shigella flexneri requires the periplasmic chaperones DegP, Skp, and SurA. J. Bacteriol. 189:5566-5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rasband, W. S. 2008 1997. ImageJ. National Institutes of Health, Bethesda, MD.
- 27.Robert, V., E. B. Volokhina, F. Senf, M. P. Bos, P. Van Gelder, and J. Tommassen. 2006. Assembly factor Omp85 recognizes its outer membrane protein substrates by a species-specific C-terminal motif. PLoS Biol. 4:e377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruiz-Perez, F., I. R. Henderson, D. L. Leyton, A. E. Rossiter, Y. Zhang, and J. P. Nataro. 2009. Roles of periplasmic chaperone proteins in the biogenesis of serine protease autotransporters of Enterobacteriaceae. J. Bacteriol. 191:6571-6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rutherford, N., M. E. Charbonneau, F. Berthiaume, J. M. Betton, and M. Mourez. 2006. The periplasmic folding of a cysteineless autotransporter passenger domain interferes with its outer membrane translocation. J. Bacteriol. 188:4111-4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schnaitman, C. A. 1970. Protein composition of the cell wall and cytoplasmic membrane of Escherichia coli. J. Bacteriol. 104:890-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shere, K. D., S. Sallustio, A. Manessis, T. G. D'Aversa, and M. B. Goldberg. 1997. Disruption of IcsP, the major Shigella protease that cleaves IcsA, accelerates actin-based motility. Mol. Microbiol. 25:451-462. [DOI] [PubMed] [Google Scholar]
- 32.Skillman, K. M., T. J. Barnard, J. H. Peterson, R. Ghirlando, and H. D. Bernstein. 2005. Efficient secretion of a folded protein domain by a monomeric bacterial autotransporter. Mol. Microbiol. 58:945-958. [DOI] [PubMed] [Google Scholar]
- 33.Soto, G. E., K. W. Dodson, D. Ogg, C. Liu, J. Heuser, S. Knight, J. Kihlberg, C. H. Jones, and S. J. Hultgren. 1998. Periplasmic chaperone recognition motif of subunits mediates quaternary interactions in the pilus. EMBO J. 17:6155-6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stathopoulos, C., D. L. Provence, and R. Curtiss III. 1999. Characterization of the avian pathogenic Escherichia coli hemagglutinin Tsh, a member of the immunoglobulin A protease-type family of autotransporters. Infect. Immun. 67:772-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strauch, K. L., K. Johnson, and J. Beckwith. 1989. Characterization of degP, a gene required for proteolysis in the cell envelope and essential for growth of Escherichia coli at high temperature. J. Bacteriol. 171:2689-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Struyve, M., M. Moons, and J. Tommassen. 1991. Carboxy-terminal phenylalanine is essential for the correct assembly of a bacterial outer membrane protein. J. Mol. Biol. 218:141-148. [DOI] [PubMed] [Google Scholar]
- 37.Voulhoux, R., M. P. Bos, J. Geurtsen, M. Mols, and J. Tommassen. 2003. Role of a highly conserved bacterial protein in outer membrane protein assembly. Science 299:262-265. [DOI] [PubMed] [Google Scholar]
- 38.Voulhoux, R., and J. Tommassen. 2004. Omp85, an evolutionarily conserved bacterial protein involved in outer-membrane-protein assembly. Res. Microbiol. 155:129-135. [DOI] [PubMed] [Google Scholar]
- 39.Walsh, N. P., B. M. Alba, B. Bose, C. A. Gross, and R. T. Sauer. 2003. OMP peptide signals initiate the envelope-stress response by activating DegS protease via relief of inhibition mediated by its PDZ domain. Cell 113:61-71. [DOI] [PubMed] [Google Scholar]
- 40.Wang, R. F., and S. R. Kushner. 1991. Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene 100:195-199. [PubMed] [Google Scholar]
- 41.Wu, T., J. Malinverni, N. Ruiz, S. Kim, T. J. Silhavy, and D. Kahne. 2005. Identification of a multicomponent complex required for outer membrane biogenesis in Escherichia coli. Cell 121:235-245. [DOI] [PubMed] [Google Scholar]
- 42.Xu, X., S. Wang, Y. X. Hu, and D. B. McKay. 2007. The periplasmic bacterial molecular chaperone SurA adapts its structure to bind peptides in different conformations to assert a sequence preference for aromatic residues. J. Mol. Biol. 373:367-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yen, Y. T., A. Karkal, M. Bhattacharya, R. C. Fernandez, and C. Stathopoulos. 2007. Identification and characterization of autotransporter proteins of Yersinia pestis KIM. Mol. Membr. Biol. 24:28-40. [DOI] [PubMed] [Google Scholar]
- 44.Yen, Y. T., M. Kostakioti, I. R. Henderson, and C. Stathopoulos. 2008. Common themes and variations in serine protease autotransporters. Trends Microbiol. 16:370-379. [DOI] [PubMed] [Google Scholar]
- 45.Yen, Y. T., and C. Stathopoulos. 2007. Identification of autotransporter proteins secreted by type v secretion systems in gram-negative bacteria. Methods Mol. Biol. 390:33-46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.