Abstract
Enteropathogenic Escherichia coli (EPEC) strains are extracellular pathogens that generate actin-rich structures (pedestals) beneath the adherent bacteria as part of their virulence strategy. Pedestals are hallmarks of EPEC infections, and their efficient formation in vitro routinely requires phosphorylation of the EPEC effector protein Tir at tyrosine 474 (Y474). This phosphorylation results in the recruitment and direct attachment of the host adaptor protein Nck to Tir at Y474, which is utilized for actin nucleation through a downstream N-WASP-Arp2/3-based mechanism. Recently, the endocytic protein clathrin was demonstrated to be involved in EPEC pedestal formation. Here we examine the organization of clathrin in pedestals and report that CD2AP, an endocytosis-associated and cortactin-binding protein, is a novel and important component of EPEC pedestal formation that also utilizes Y474 phosphorylation of EPEC Tir. We also demonstrate the successive recruitment of Nck and then clathrin prior to actin polymerization at pedestals during the Nck-dependent pathway of pedestal formation. This study further demonstrates that endocytic proteins are key components of EPEC pedestals and suggests a novel endocytosis subversion strategy employed by these extracellular bacteria.
The extracellular bacterial pathogen enteropathogenic Escherichia coli (EPEC) causes serious diarrheal disease in humans and is a prevalent microbe involved in childhood mortality in the developing world. This microbe is part of a larger family of bacteria called the attaching and effacing (A/E) pathogens that also includes the human-specific pathogen enterohemorrhagic E. coli (EHEC) and the murine disease-causing bacterium Citrobacter rodentium. These bacteria attach to intestinal epithelial cells and use a type III secretion system to directly deliver effector proteins from the bacterial cytosol into the cytoplasm of host cells. Among other functions, these effectors harness the host cell's cytoskeleton (4) to generate actin-rich pedestals that are hallmarks of virulence for this class of pathogens (14). One of these effectors, the translocated intimin receptor (Tir), is key to pedestal formation. Following translocation into the host cell, Tir becomes embedded in the host cell plasma membrane, where its extracellular domain acts to firmly anchor the pathogen to the epithelial cell. In cultured cells, the intracellular cytoplasmic domain of EPEC Tir can become phosphorylated at tyrosine 474 (Y474) (6), where it recruits the adaptor protein Nck (7). These events all occur prior to actin filament polymerization beneath the attached bacteria via an N-WASP- and Arp2/3-based mechanism (7, 11). Although this is the prominent strategy used by EPEC to recruit actin to pedestals, a Y474-independent strategy also exists, but it occurs at a much lower frequency. During such instances, EPEC Tir becomes phosphorylated at Y454 and actin recruitment is independent of Nck (1).
Previous work highlighted a role for clathrin during some bacterial infections (19, 20). Although the role of clathrin during enteropathogenic E. coli infections was not investigated until recently (20), the finding of clathrin at the tips of EPEC pedestals, coupled with the discovery of dynamin-2, another protein known to be involved in endocytosis, associated within the actin stalk of EPEC pedestals (18), suggests a possible role for additional endocytosis-associated proteins and indicates that a unique mechanism is employed by EPEC to remain extracellular despite the presence of these endocytic components. Other proteins, including the actin-associated protein cortactin, are also prominent at these structures. Cortactin is found throughout EPEC pedestals as well as pedestals formed by other attaching and effacing pathogens (2, 3). Thus, in order to further examine other endocytosis-associated proteins during EPEC pedestal formation, we opted to immunolocalize the endocytosis-related protein CD2AP (CD-2-associated protein) during these infections. CD2AP is a clathrin-associated endocytosis protein that directly associates with cortactin in other systems (12, 17).
We discovered that CD2AP is present at EPEC pedestals and is a crucial component for their formation. Through the use of various host cell modification strategies, we subsequently explored the recruitment of the endocytosis-associated proteins at EPEC pedestals and found that during Nck-dependent pedestal formation, EPEC sequentially recruits Nck, clathrin, cortactin, and then CD2AP at the pedestal tip prior to the actin filament polymerization machinery at these sites.
MATERIALS AND METHODS
Chemicals.
All chemicals and reagents were obtained from Sigma-Aldrich Canada (Mississauga, Ontario, Canada), unless otherwise specified. Paraformaldehyde was purchased from Canmeco (Canton de Gore, Quebec, Canada), and NaCl was acquired from Fisher Scientific (Vancouver, British Columbia, Canada). Control immunoglobulins G (IgGs) and secondary antibodies conjugated to horseradish peroxidase were purchased from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA).
Bacterial strains.
EPEC strains that were used in this study included wild-type EPEC strain E2348/69 and wild-type EPEC strain JPN15, as well as previously used mutant strains in that background, including a Δtir mutant, a Δtir mutant complemented with EPEC tir, and a Δtir mutant complemented with EPEC tir(Y474F) (6). The JPN15 strain lacks bundle-forming pilus (BFP) expression (10). This enables the clear identification of individual pedestals and consequently allows for pedestals to be counted accurately. The EHEC strains used in this study included EHEC O157:H7 strain 86-24.
Cell infections and immunolocalization.
HeLa, Caco2, and T84 cells and murine fibroblasts (from a parental strain and an nck−/− mutant) (7) were grown on coverslips and infected with log-phase EPEC at a multiplicity of infection (MOI) of about 10 for 6 h. Infections that persisted for over 3 h were rinsed with either cell culture medium or modified Dulbecco's phosphate-buffered saline (DPBS) containing calcium and magnesium (Thermo Fisher Scientific, Nepean, Ontario, Canada) at 3 h postinfection and then allowed to continue for the additional time. Following the infections, the cells were washed three times to remove any unbound bacteria and then fixed using 3% paraformaldehyde (37°C) in PBS (150 mM NaCl, 5 mM KCl, 0.8 mM KH2PO4, 3.2 mM Na2HPO4, pH 7.3) for 15 min. For clathrin immunostaining, cells were washed once for 5 min with PBS and then treated for 5 min at room temperature with 0.2% Triton X-100 in PBS to permeabilize the cells. The cells were washed three times, for 10 min each wash, and then blocked with 5% normal goat serum prior to overnight incubations at 4°C in primary antibodies. For CD2AP immunolocalization studies, cells were washed for 5 min in PBS following fixation and then plunged into −20°C acetone for 5 min. Coverslips were air dried and rehydrated in 5% serum for 20 min. Antibodies consisted of a mouse anti-clathrin antibody (BD Biosciences, Mississauga, Ontario, Canada), used at 15.6 μg/ml; a rabbit anti-CD2AP antibody (Santa Cruz Biotechnology, Santa Cruz, CA), used at 4.0 μg/ml; a rabbit anti-Nck antibody, used at 5 mg/ml (BD Biosciences, Mississauga, Ontario, Canada); rabbit anti-Nck antibody clone Y531, used at a 1:100 dilution (Novus Biologicals, Littleton, CO); mouse anti-cortactin (p80/85) antibody clone 4F11 (Upstate/Millipore, Etobicoke, Ontario, Canada), used at 20 μg/ml; a rabbit anti-numb antibody (Cell Signaling Technology, Danvers, MA), used at 0.55 μg/ml; and a mouse anti-Tir antibody, used at a 1:1,000 dilution (5). The next day, all of the cells were washed three times in PBS containing 0.05% Tween 20 and 0.1% bovine serum albumin (TPBS-BSA) and then incubated with goat anti-mouse or goat anti-rabbit secondary antibodies conjugated to Alexa Fluor 488 or Alexa Fluor 546 (Invitrogen, Carlsbad, CA). Filamentous actin was labeled using phalloidin conjugated to Alexa 488, 546, or 568 (Invitrogen, Carlsbad, CA). The stain was made up in TPBS-BSA. Following 3 additional washes, coverslips were mounted with either Vectashield, Hard-Set Vectashield with DAPI (4′,6-diamidino-2-phenylindole) (Vector Laboratories, Burlingame, CA), or Fluoromount G (Interchim, Montluçon, France).
All immunostained material was imaged using a Zeiss Axiophot microscope (Carl Zeiss, Inc., Toronto, Ontario, Canada) with documentation to T-Max 400 film, a Zeiss Axiovert 135 microscope (Carl Zeiss, Inc., Toronto, Ontario, Canada) coupled to a charge-coupled device (CCD) camera (MicroMax 5Mhz; Princeton Instruments, Trenton, NJ), a Leica DMI4000B inverted microscope coupled to a Hamamatsu Orca R2 CCD camera (Hamamatsu, Japan) driven by Metamorph imaging system software (Universal Imaging Corp., Downingtown, PA), or a Zeiss AxioObserver microscope coupled to a Zeiss Axiocam camera.
Standard methods were used for Western blotting (8). Antibodies used in this study included a mouse anti-clathrin antibody (BD Biosciences, Mississauga, Ontario, Canada), used at a concentration of 0.623 μg/ml; mouse anti-CD2AP (Santa Cruz Biotechnology, Santa Cruz, CA), used at 0.4 μg/ml; a mouse anti-α-tubulin antibody (clone 12G10) (Developmental Studies Hybridoma Bank, Iowa City, IA), used at 0.186 mg/ml; a rabbit anti-calnexin antibody (Sigma-Aldrich, Mississauga, Ontario, Canada), used at 14 μg/ml; and a mouse anti-cortactin antibody, used at 20 ng/ml (Cell Signaling Technology, Danvers, MA).
RNAi.
Two general techniques were used to knock down the targeted genes. The first used Lipofectamine 2000 (Invitrogen, Carlsbad, CA) to knock down clathrin. The other used Oligofectamine (Invitrogen, Carlsbad, CA) to knock down clathrin, CD2AP, and cortactin. Both techniques were performed according to the manufacturer's procedures. The small interfering RNAs (siRNAs) consisted of the following. For cortactin, an On-Target Smart pool against human cortactin (Thermo Fisher Scientific/Dharmacon, Nepean, Ontario, Canada) was used for the clathrin heavy chain, an On-Target Smart pool against the clathrin heavy chain (Thermo Fisher Scientific/Dharmacon, Nepean, Ontario, Canada) or the sequence GGCCCAGGUGGUAAUCAUUtt was used (the lowercase letters represent 2 thymine residues that were added to the siRNA to stabilize it). The CD2AP siRNA sequence used was GGAAUGUGAAAAAGCUACAtt (Thermo Fisher Scientific/Dharmacon, Nepean, Ontario, Canada). Control siRNAs for SMARTpool RNA interference (RNAi) included the siCONTROL nontargeting siRNA pool (Thermo Fisher Scientific/Dharmacon, Nepean, Ontario, Canada). All individual sequence RNAi was controlled using siCONTROL nontargeting siRNA 1 (Thermo Fisher Scientific/Dharmacon, Nepean, Ontario, Canada).
Statistical analysis.
Bacteria attached to infected cells were counted, and the presence of staining was assessed at points of contact for cells that were treated with either nontargeting siRNA (control RNAi) or targeting siRNA (RNAi). Host cells that were RNAi treated were counted only if they had undetectable levels of protein within them, as determined by fluorescence microscopy, to ensure that the proteins were knocked down within those cells. Results are presented as means ± standard errors of the means (SEM). The n in the figure legends refers to the number of host cells counted. Statistical significance was determined by unpaired, two-tailed t tests, using GraphPad Prism 4.0b.
Lysate preparation and Western blotting.
HeLa cells were grown on 150-mm tissue culture dishes and infected at an MOI of about 10 for 3 to 6 h, as described above. Cells were then washed five times with PBS containing 1 mM CaCl2 and 1 mM MgCl2 to remove any unbound bacteria.
Western blots of RNAi- or control RNAi-treated cells (as described above) were performed in the absence of bacterial infections, and cells were lysed in RIPA lysis buffer (150 mM NaCl, 50 mM Tris, pH 7.4, 5 mM EDTA, 1% Nonidet P-40, 1% deoxycholic acid [sodium salt], 10% SDS) for 10 min on ice. Standard Western blotting procedures were used (20).
RESULTS
Previous studies have demonstrated that the host proteins Nck and clathrin are important for efficient EPEC pedestal formation and that their recruitment to the apical tips of these structures requires the phosphorylation of Tir at tyrosine 474 (Y474). Additionally, cortactin is a prominent host protein that is recruited to both the pedestal tip and stalk (2, 3).
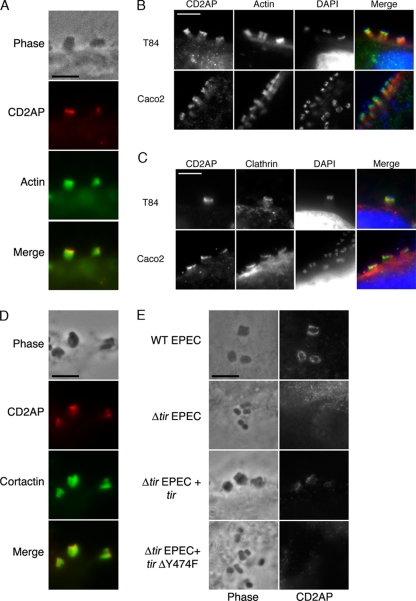
To further investigate the role of endocytosis-related proteins in pedestal formation, we examined the localization of CD2AP (CD-2-associated protein) at pedestals. Like clathrin, CD2AP was concentrated at the pedestal tip in wild-type EPEC-infected HeLa, Caco2, and T84 cells (Fig. 1; see Fig. S1 in the supplemental material). CD2AP colocalized with cortactin at the pedestal tip but did not localize with the cortactin within the pedestal stalk (Fig. 1; see Fig. S1 in the supplemental material). The colocalization of CD2AP with cortactin only at the tips of these structures is not unique, as F-actin-rich tails generated during particle transport in cells expressing a mutant of ARF6 which is defective in GTP hydrolysis also localized cortactin along their entire length but recruited CD2AP only to the point of particle contact (16). Based on the different localization patterns of CD2AP and cortactin at these sites, this suggested that these proteins likely do not associate throughout the pedestal stalk. To study whether CD2AP recruitment was specific, we used the localization of another protein involved in endocytosis, numb, as a negative control. Numb is known to interact with endocytosis-related proteins and to localize with internalized receptors to endocytic organelles (15). Additionally, fragments of numb are known to impede clathrin-mediated endocytosis, acting in a dominant-negative manner (15). Numb was never found at EPEC pedestals (see Fig. S2 in the supplemental material).
FIG. 1.
(A) CD2AP colocalization with actin in EPEC pedestals during EPEC (strain JPN15) infections in HeLa cells. (B) CD2AP colocalization with actin during EPEC (strain JPN15) infections in T84 and Caco2 human intestinal epithelial cells. (C) CD2AP colocalization with clathrin during EPEC (strain JPN15) infections in T84 and Caco2 human intestinal epithelial cells. (D) CD2AP colocalization with cortactin in EPEC pedestals. (E) CD2AP immunolocalization on HeLa cells infected with wild-type EPEC, EPEC Δtir, EPEC Δtir complemented with EPEC tir (Δtir EPEC + tir), and EPEC Δtir complemented with tir that has a point mutation at tyrosine 474 (Δtir + tir Y474F). Bar = 5 μm.
Because clathrin and Nck recruitment to EPEC pedestals requires the phosphorylation of Y474 of EPEC Tir (7, 20), we examined if this effector, and specifically the Y474 phosphorylation site, was also needed for CD2AP localization at EPEC pedestals. We found that CD2AP recruitment to pedestals also required Tir and the Y474 phosphorylation site of Tir, as EPEC Δtir (Fig. 1) and Tir mutants lacking the Y474 phosphorylation site did not recruit CD2AP to these sites (Fig. 1), despite Tir localizing at points of EPEC-host-cell attachment (see Fig. S3 in the supplemental material), thus paralleling the requirements for clathrin and Nck recruitment.
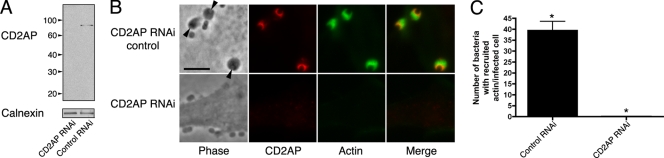
CD2AP acts as a scaffold between the endocytic and actin machineries in other systems (17). Thus, in order to examine the importance of CD2AP during EPEC pedestal formation, we knocked down CD2AP in HeLa cells (Fig. 2; see Fig. S4 in the supplemental material) and assessed the ability of EPEC to form actin-rich pedestals. In cells with CD2AP knockdown, actin pedestals were absent despite Tir being delivered into the host cells (Fig. 2; see Fig. S4, S5, and S6 in the supplemental material), demonstrating that CD2AP is required for EPEC pedestal formation and lies upstream of actin filament polymerization during these infections.
FIG. 2.
CD2AP RNAi-treated HeLa cells infected with EPEC (strain JPN15). (A) Western blot of CD2AP and control RNAi-treated HeLa cells probed for CD2AP. Blots were stripped and reprobed for calnexin to demonstrate loading levels. Molecular masses are in kilodaltons. (B) Control RNAi-treated and CD2AP knockdown HeLa cells infected with wild-type EPEC. Cells were stained for CD2AP and actin. Arrowheads point to EPEC at pedestals. Bar = 5 μm. (C) Recruitment of F-actin to pedestals in infected host cells treated with either nontargeting siRNA (control RNAi; n = 49) or CD2AP-targeting siRNA (CD2AP RNAi; n = 55). *, statistically significant difference (P < 0.0001).
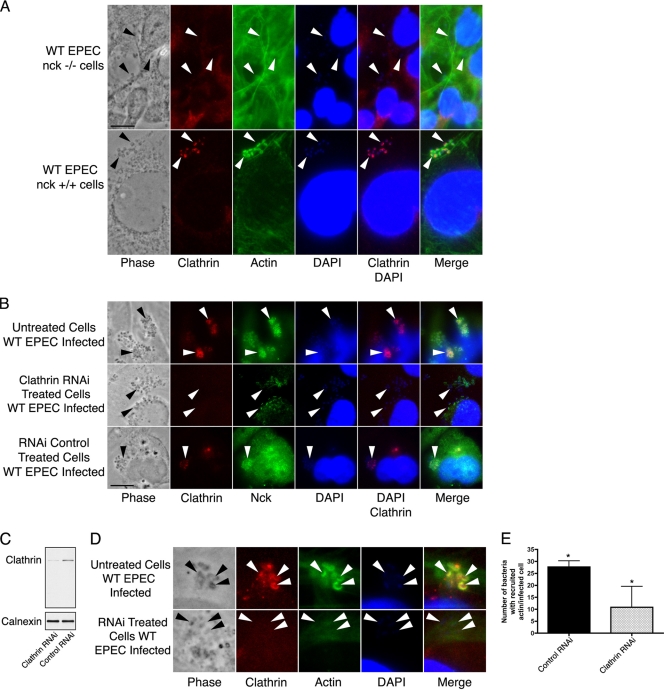
Because EPEC generally remains extracellular during pathogenesis and because endocytic proteins are recruited to the pedestal tips, we sought to determine the order of recruitment of the endocytosis-associated proteins at EPEC pedestals by coupling the use of cells devoid of certain host proteins and RNAi. Based on previous data indicating that Nck binds directly to phosphorylated Y474 of EPEC Tir during Nck-dependent pedestal formation (7), we hypothesized that clathrin was positioned downstream of Nck and upstream of actin filament polymerization in EPEC pedestals. To address this issue, we infected Nck null murine embryonic fibroblasts (7) or the corresponding Nck+/+ cells with wild-type EPEC. Clathrin was localized at EPEC pedestals when Nck was present in the cells but was absent when the Nck null cells were infected (Fig. 3). This indicates that clathrin lies downstream of Nck during pedestal formation.
FIG. 3.
(A) Wild-type (WT) EPEC (strain E2348/69)-infected Nck null murine embryonic fibroblasts and wild-type EPEC-infected cells from the parental strain, stained for clathrin, actin, and DAPI. Bar = 10 μm. Arrowheads indicate the locations of some of the attached bacteria. (B) Untreated, clathrin RNAi-pretreated, and control RNAi-pretreated cells were infected with wild-type EPEC (strain E2348/69) and stained for clathrin and Nck. Arrowheads indicate the locations of some of the attached bacteria. (C) Western blot of uninfected clathrin RNAi- and control RNAi-treated cells. Blots were stripped and probed with anti-calnexin antibodies to determine the protein loading level in each lane. (D) Untreated and clathrin RNAi-pretreated cells were infected with wild-type EPEC (strain E2348/69) and stained for clathrin and filamentous actin. Arrowheads indicate the locations of some of the attached bacteria. (E) Recruitment of F-actin to pedestals in infected host cells treated with either nontargeting siRNA (control RNAi; n = 50) or clathrin-targeting siRNA (clathrin RNAi; n = 13). *, statistically significant difference (P = 0.0140).
This result predicted that if clathrin was knocked down, Nck would still be associated beneath the adherent bacteria but actin filaments would not be recruited to pedestals. We used an siRNA approach to knock down clathrin expression. In clathrin knockdown cells that expressed undetectable levels of clathrin, Nck remained at sites of bacterial attachment (Fig. 3; see Fig. S7 in the supplemental material). However, the absence of clathrin resulted in a significant decrease in the number of actin-rich pedestals at sites of bacterial contact (Fig. 3) (20). In contrast, clathrin and actin pedestals were associated beneath EPEC in wild-type cells (Fig. 3). Thus, clathrin is utilized during EPEC pedestal formation and functions downstream of Nck but upstream of actin filament polymerization during the Nck-dependent pedestal generation process.
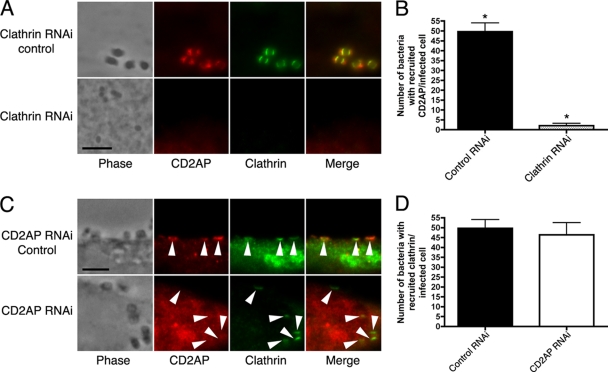
To investigate CD2AP in relation to clathrin at EPEC pedestals, we used RNAi to knock down these proteins in HeLa cells prior to infection and tested for the presence of CD2AP. In cells with undetectable levels of clathrin, CD2AP was also absent (Fig. 4; see Fig. S8 in the supplemental material). However, clathrin RNAi did not alter CD2AP levels (see Fig. S9 in the supplemental material). When CD2AP RNAi was performed, clathrin remained beneath the attached bacteria (Fig. 4; see Fig. S8 in the supplemental material), demonstrating that clathrin is recruited to EPEC pedestals prior to CD2AP and that both of these proteins are important for pedestal formation.
FIG. 4.
(A) Control RNAi-treated and clathrin knockdown HeLa cells infected with wild-type EPEC (strain JPN15). Cells were stained for CD2AP and clathrin. (B) Recruitment of CD2AP to pedestals in infected host cells treated with either nontargeting siRNA (control RNAi; n = 27) or clathrin-targeting siRNA (clathrin RNAi; n = 22). *, statistically significant difference (P < 0.0001). (C) CD2AP knockdown HeLa cells infected with wild-type EPEC (strain JPN15). Cells were stained for CD2AP and clathrin. Arrowheads point to some of the attached bacteria. Bar = 5 μm. (D) Recruitment of clathrin to pedestals in infected host cells treated with either nontargeting siRNA (control RNAi; n = 27) or CD2AP-targeting siRNA (CD2AP RNAi; n = 12). Results are not statistically significantly different (P = 0.6770).
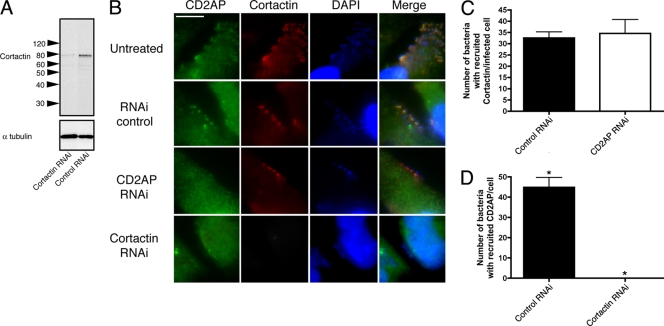
CD2AP is associated with cortactin in other systems, and both proteins colocalize at the pedestal tip (Fig. 1). Consequently, we explored the recruitment of cortactin in relation to CD2AP at the pedestal tip by using RNAi. When CD2AP was knocked down to undetectable levels, cortactin remained localized beneath the attached EPEC cells; however, when cortactin was knocked down, CD2AP was not found underlying the attached bacteria (Fig. 5; see Fig. S10 in the supplemental material). This suggests that at the pedestal tip, cortactin is recruited upstream of CD2AP.
FIG. 5.
CD2AP and cortactin RNAi-treated HeLa cells infected with EPEC (strain JPN15). (A) Western blot of cortactin RNAi- and control RNAi-treated HeLa cells probed for cortactin. Blots were stripped and reprobed for α-tubulin to demonstrate loading levels. Molecular masses are in kilodaltons. (B) Immunolocalization of CD2AP, cortactin, and DAPI on HeLa cells that were either untreated or treated with control RNAi, CD2AP RNAi, or cortactin RNAi and infected with EPEC (strain JPN15). CD2AP was absent during cortactin RNAi treatments, but cortactin remained at pedestals during CD2AP RNAi. Bar = 5 μm. (C) Recruitment of cortactin to pedestals in infected host cells treated with either nontargeting siRNA (control RNAi; n = 33) or CD2AP-targeting siRNA (CD2AP RNAi; n = 22). Results are not statistically significantly different (P = 0.7426). (D) Recruitment of CD2AP to pedestals in infected host cells treated with either nontargeting siRNA (control RNAi; n = 21) or cortactin-targeting siRNA (cortactin RNAi; n = 21). *, statistically significant difference (P < 0.0001).
DISCUSSION
There is increasing evidence that actin is involved in clathrin-mediated endocytosis (9, 17). Although the majority of this work arises from the Saccharomyces cerevisiae system, there is still limited evidence that similar mechanisms take place in mammalian cells. Nevertheless, many of the proteins involved in the nucleation of actin filaments at the plasma membrane during endocytosis are found at EPEC pedestals, including N-WASP and Arp2/3. However, their organization at pedestals does not mimic exactly the arrangement proposed for vesicles undergoing clathrin-mediated endocytosis. Most of the models of clathrin-mediated endocytosis involve the invagination of the plasma membrane to internalize a particle. Those models tend to nucleate actin (likely by N-WASP) adjacent to clathrin and then exploit actin dynamics to transport the forming vesicle into the cell (reviewed in reference 9). During EPEC pedestal formation, we suggest that the actin recruiting machinery is positioned downstream of clathrin and could potentially use clathrin as a scaffold for other proteins prior to the recruitment of actin filaments to these sites. Consequently, the organization of endocytic proteins in relation to actin-associated proteins could influence pedestal formation over endocytosis and may represent a novel strategy for these microbes to avoid internalization.
Dynamin-2 was also recently identified at EPEC pedestals (18). During endocytosis, dynamin-2 has a dual function in both formation of the endocytic vesicle and pinching off from the membrane. This protein also has the ability to interact with a variety of proteins, including cortactin (13) and Nck (23). Although it is detected in EPEC pedestals, the exact positioning of dynamin during pedestal generation remains elusive. It was demonstrated that dynamin recruitment required Tir, Nck, and N-WASP (18). However, cells with dynamin knockdown prevented the recruitment of N-WASP, Arp3, and cortactin (18). The precise arrangement of dynamin-2 either upstream or downstream of N-WASP requires further clarification. We attempted to assess this but were unable to accurately localize dynamin at pedestals. This was likely due to the different antibodies that were used by Unsworth and coworkers (18) and those that we could acquire commercially.
How does clathrin and the endocytic machinery mediate pedestal formation and not bacterial internalization? Clues to this question may come from understanding the detailed organization of proteins in the pedestals. Nck, clathrin, CD2AP, and N-WASP localize to the pedestal tip directly beneath the attached bacteria, and Arp2/3 and actin are concentrated primarily in the stalks of EPEC pedestals, while cortactin is found both at the tip and throughout the stalk. The evidence that clathrin lies downstream of Nck when associated at pedestals reveals a novel type of clathrin recruitment at the level of the receptor and correlates well with the unique properties of the EPEC receptor, the Tir protein. Indeed, the situation is different from that described for the Listeria InlB protein, which interacts with a receptor known to be endocytosed by clathrin-mediated endocytosis. Nevertheless, what drives the enwrapping of the membrane around bacteria in the case of Listeria and does not in the case of EPEC remains elusive.
Clathrin is crucial to EPEC pedestal formation but is not recruited to EHEC pedestals (see Fig. S11 in the supplemental material). This could be due to the differences in strategies that these microbes use to recruit host proteins to Tir. EHEC does not utilize a Y474 phosphorylation mechanism and accordingly does not recruit Nck to Tir. Instead, it hijacks the host proteins IRSp53 and IRTKS and then uses a second EHEC effector, EspFu, to recruit the actin polymerization machinery (21, 22). Thus, we propose that the endocytic proteins at EPEC pedestals act as a scaffold for other pedestal components.
In summary, we can now frame the organization of endocytic proteins in EPEC pedestals in relation to other well-established EPEC pedestal components as well as demonstrate the presence of the endocytosis-associated protein CD2AP at these sites. The altered organization of clathrin and other associated pedestal components suggests that the extracellular localization of EPEC may result from a modification in the arrangement of endocytic components at pedestals compared to that proposed for clathrin-mediated endocytosis. Additionally, this altered organization suggests that clathrin may act as a scaffold for other pedestal proteins.
Supplementary Material
Acknowledgments
We thank A. Wayne Vogl and the Finlay lab members for their reviews of the manuscript.
Funding was provided through operating grants from the CIHR, NSERC, and the Howard Hughes Medical Institute (HHMI). B.B.F. is a Canadian Institutes of Health Research (CIHR) distinguished investigator and the UBC Peter Wall Distinguished Professor. J.A.G. is a CIHR new investigator. E.V. is a holder of a Ramon y Cajal contract from the Spanish Ministry of Science and Innovation. A.E.L. is funded through a CIHR CGS grant and the MSFHR. B.B.F. and P.C. are international research scholars of the HHMI.
Editor: A. Camilli
Footnotes
Published ahead of print on 1 June 2010.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Campellone, K. G., and J. M. Leong. 2005. Nck-independent actin assembly is mediated by two phosphorylated tyrosines within enteropathogenic Escherichia coli Tir. Mol. Microbiol. 56:416-432. [DOI] [PubMed] [Google Scholar]
- 2.Cantarelli, V. V., T. Kodama, N. Nijstad, S. K. Abolghait, T. Iida, and T. Honda. 2006. Cortactin is essential for F-actin assembly in enteropathogenic Escherichia coli (EPEC)- and enterohaemorrhagic E. coli (EHEC)-induced pedestals and the alpha-helical region is involved in the localization of cortactin to bacterial attachment sites. Cell. Microbiol. 8:769-780. [DOI] [PubMed] [Google Scholar]
- 3.Cantarelli, V. V., A. Takahashi, I. Yanagihara, Y. Akeda, K. Imura, T. Kodama, G. Kono, Y. Sato, T. Iida, and T. Honda. 2002. Cortactin is necessary for F-actin accumulation in pedestal structures induced by enteropathogenic Escherichia coli infection. Infect. Immun. 70:2206-2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caron, E., V. F. Crepin, N. Simpson, S. Knutton, J. Garmendia, and G. Frankel. 2006. Subversion of actin dynamics by EPEC and EHEC. Curr. Opin. Microbiol. 9:40-45. [DOI] [PubMed] [Google Scholar]
- 5.Deng, W., B. A. Vallance, Y. Li, J. L. Puente, and B. B. Finlay. 2003. Citrobacter rodentium translocated intimin receptor (Tir) is an essential virulence factor needed for actin condensation, intestinal colonization and colonic hyperplasia in mice. Mol. Microbiol. 48:95-115. [DOI] [PubMed] [Google Scholar]
- 6.DeVinney, R., J. L. Puente, A. Gauthier, D. Goosney, and B. B. Finlay. 2001. Enterohaemorrhagic and enteropathogenic Escherichia coli use a different Tir-based mechanism for pedestal formation. Mol. Microbiol. 41:1445-1458. [DOI] [PubMed] [Google Scholar]
- 7.Gruenheid, S., R. DeVinney, F. Bladt, D. Goosney, S. Gelkop, G. D. Gish, T. Pawson, and B. B. Finlay. 2001. Enteropathogenic E. coli Tir binds Nck to initiate actin pedestal formation in host cells. Nat. Cell Biol. 3:856-859. [DOI] [PubMed] [Google Scholar]
- 8.Guttman, J. A., Y. Li, M. E. Wickham, W. Deng, A. W. Vogl, and B. B. Finlay. 2006. Attaching and effacing pathogen-induced tight junction disruption in vivo. Cell. Microbiol. 8:634-645. [DOI] [PubMed] [Google Scholar]
- 9.Kaksonen, M., C. P. Toret, and D. G. Drubin. 2006. Harnessing actin dynamics for clathrin-mediated endocytosis. Nat. Rev. Mol. Cell. Biol. 7:404-414. [DOI] [PubMed] [Google Scholar]
- 10.Knutton, S., J. Adu-Bobie, C. Bain, A. D. Phillips, G. Dougan, and G. Frankel. 1997. Down regulation of intimin expression during attaching and effacing enteropathogenic Escherichia coli adhesion. Infect. Immun. 65:1644-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lommel, S., S. Benesch, K. Rottner, T. Franz, J. Wehland, and R. Kuhn. 2001. Actin pedestal formation by enteropathogenic Escherichia coli and intracellular motility of Shigella flexneri are abolished in N-WASP-defective cells. EMBO Rep. 2:850-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lynch, D. K., S. C. Winata, R. J. Lyons, W. E. Hughes, G. M. Lehrbach, V. Wasinger, G. Corthals, S. Cordwell, and R. J. Daly. 2003. A cortactin-CD2-associated protein (CD2AP) complex provides a novel link between epidermal growth factor receptor endocytosis and the actin cytoskeleton. J. Biol. Chem. 278:21805-21813. [DOI] [PubMed] [Google Scholar]
- 13.Merrifield, C. J., D. Perrais, and D. Zenisek. 2005. Coupling between clathrin-coated-pit invagination, cortactin recruitment, and membrane scission observed in live cells. Cell 121:593-606. [DOI] [PubMed] [Google Scholar]
- 14.Mundy, R., T. T. MacDonald, G. Dougan, G. Frankel, and S. Wiles. 2005. Citrobacter rodentium of mice and man. Cell. Microbiol. 7:1697-1706. [DOI] [PubMed] [Google Scholar]
- 15.Santolini, E., C. Puri, A. E. Salcini, M. C. Gagliani, P. G. Pelicci, C. Tacchetti, and P. P. Di Fiore. 2000. Numb is an endocytic protein. J. Cell Biol. 151:1345-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schafer, D. A., C. D'Souza-Schorey, and J. A. Cooper. 2000. Actin assembly at membranes controlled by ARF6. Traffic 1:892-903. [DOI] [PubMed] [Google Scholar]
- 17.Smythe, E., and K. R. Ayscough. 2006. Actin regulation in endocytosis. J. Cell Sci. 119:4589-4598. [DOI] [PubMed] [Google Scholar]
- 18.Unsworth, K. E., P. Mazurkiewicz, F. Senf, M. Zettl, M. McNiven, M. Way, and D. W. Holden. 2007. Dynamin is required for F-actin assembly and pedestal formation by enteropathogenic Escherichia coli (EPEC). Cell. Microbiol. 9:438-449. [DOI] [PubMed] [Google Scholar]
- 19.Veiga, E., and P. Cossart. 2005. Listeria hijacks the clathrin-dependent endocytic machinery to invade mammalian cells. Nat. Cell Biol. 7:894-900. [DOI] [PubMed] [Google Scholar]
- 20.Veiga, E., J. A. Guttman, M. Bonazzi, E. Boucrot, A. Toledo-Arana, A. E. Lin, J. Enninga, J. Pizarro-Cerda, B. B. Finlay, T. Kirchhausen, and P. Cossart. 2007. Invasive and adherent bacterial pathogens co-opt host clathrin for infection. Cell Host Microbe 2:340-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vingadassalom, D., A. Kazlauskas, B. Skehan, H. C. Cheng, L. Magoun, D. Robbins, M. K. Rosen, K. Saksela, and J. M. Leong. 2009. Insulin receptor tyrosine kinase substrate links the E. coli O157:H7 actin assembly effectors Tir and EspF(U) during pedestal formation. Proc. Natl. Acad. Sci. U. S. A. 106:6754-6759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weiss, S. M., M. Ladwein, D. Schmidt, J. Ehinger, S. Lommel, K. Stading, U. Beutling, A. Disanza, R. Frank, L. Jansch, G. Scita, F. Gunzer, K. Rottner, and T. E. Stradal. 2009. IRSp53 links the enterohemorrhagic E. coli effectors Tir and EspFU for actin pedestal formation. Cell Host Microbe 5:244-258. [DOI] [PubMed] [Google Scholar]
- 23.Wunderlich, L., A. Farago, and L. Buday. 1999. Characterization of interactions of Nck with Sos and dynamin. Cell. Signal. 11:25-29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.