Abstract
Mucosal immunity to gastrointestinal pathogens in early life has been studied only slightly. Recently, we developed an infection model in murine neonates using the gastroenteric pathogen Yersinia enterocolitica. Here, we report that oral infection of neonatal mice with low doses of virulent Y. enterocolitica leads to vigorous intestinal and systemic adaptive immunity. Y. enterocolitica infection promoted the development of anti-LcrV memory serum IgG1 and IgG2a responses of comparable affinity and magnitude to adult responses. Strikingly, neonatal mesenteric lymph node CD4+ T cells produced Yersinia-specific gamma interferon (IFN-γ) and interleukin-17A (IL-17A), exceeding adult levels. The robust T- and B-cell responses elicited in neonates exposed to Y. enterocolitica were associated with long-term protection against mucosal challenge with this pathogen. Using genetically deficient mice, we found that IFN-γ and CD4+ cells, but not B cells, are critical for protection of neonates during primary Y. enterocolitica infection. In contrast, adults infected with low bacterial doses did not require either cell population for protection. CD4-deficient neonatal mice adoptively transferred with CD4+ cells from wild-type, IFN-γ-deficient, or IL-17AF-deficient mice were equally protected from infection. These data demonstrate that inflammatory CD4+ T cells are required for protection of neonatal mice and that this protection may not require CD4-derived IFN-γ, IL-17A, or IL-17F. Overall, these studies support the idea that Y. enterocolitica promotes the development of highly inflammatory mucosal responses in neonates and that intestinal T-cell function may be a key immune component in protection from gastrointestinal pathogens in early life.
Host protection against microbial agents ultimately relies on the cooperative action of the innate and adaptive immune systems. In both human and murine neonates, adaptive immune responses are compromised compared to responses in developmentally mature hosts (5, 66). Factors that may contribute to the immunological immaturity reported during neonatal life include the following: the lack of antigen-specific immunological memory (5, 65), reduced levels of antigen presenting cells (APC) (46) and adaptive immune cells (21), delays in the development of lymph node germinal centers (57), and cell-intrinsic differences in immune responsiveness (4, 48, 67). Thus, neonatal immune responses following infection or vaccination often appear to be diminished compared to responses in adults. In particular, B-cell and CD4+ T helper (Th) responses to a variety of antigens may be reduced in magnitude, quality, and duration (5, 65). Neonatal immunization with prototypic protein vaccine antigens often leads to mixed Th1 and Th2 primary responses (2), but the development of Th1-associated memory (3) and production of Th1-associated IgG2a antibodies are often reduced compared to these responses in adults (9). However, adult-like Th1 immunity has been achieved in neonatal hosts after Mycobacterium bovis bacillus Calmette-Guérin (BCG) vaccination (53, 72), DNA vaccines (55, 62), or attenuated vaccinia-derived vectors (44). These observations led to the recognition that immune responsiveness during early life could be greatly enhanced by optimizing the conditions of antigen exposure using highly inflammatory treatments.
Activation of the neonatal immune system through microbe-associated molecular pattern receptors has demonstrated remarkable improvements in promoting effective immunity to vaccine antigens. For example, bacterially derived products such as mutated Escherichia coli enterotoxins LT-R192G (70) and LT-K63 (11, 14, 27, 34), CpG oligonucleotides (CpG) (8, 29, 31), and lyophilized bacterial extracts (12) have been described to markedly enhance neonatal vaccine responses. Another approach used to improve immune responses has been the delivery of specific antigens using live attenuated bacterial vectors such as Listeria monocytogenes (42, 50) and Salmonella species (16, 59). Both of these approaches have shown dramatic improvements in CD4+ and CD8+ IFN-γ production, mucosal IgA production, and systemic IgG1 and IgG2a antibodies to the delivered vaccine antigens. Recently, CD4+ Th17-mediated immunity has been studied in response to vaccination with rotavirus antigen in adjuvant (70) and to Mycobacterium tuberculosis antigens in the presence of non-CpG oligonucleotides (36) or cationic liposomes (37). These vaccines promoted interleukin-17A (IL-17A) levels of the same magnitude in neonatal and adult CD4+ cells (36, 37, 70). Altogether, it has become apparent that under the proper stimulation conditions, all arms of the neonatal adaptive immune system can be induced to generate adult-like responses. Importantly, some of these immunization regimens promoted protective immunity against infection with fully pathogenic bacteria (16, 29, 31, 34, 42, 59).
Despite the profound maturation of the neonatal immune system through vaccination with live attenuated L. monocytogenes and Salmonella vectors (16, 17, 42, 50, 59), neonatal immune responses to fully virulent pathogens are inefficient in controlling infection (15, 25, 31, 61). This exquisite susceptibility to infection during neonatal life includes both peripheral and mucosal routes of infection. In particular, neonatal animals succumb rapidly to pulmonary infection with Streptococcus pneumoniae (24) and gastrointestinal infection with enteropathogens including Vibrio cholerae (10), Aeromonas hydrophila (76), Shigella flexneri (23), enterotoxigenic E. coli (19), and Salmonella species (15, 61). Thus, mucosal immune responses to most pathogens studied to date are severely compromised in early life.
In contrast to the vast majority of experimental systems, we recently demonstrated (20) that murine neonates are highly resistant to oral infection with the Gram-negative enteropathogen Yersinia enterocolitica. The resistance of neonatal mice infected with Y. enterocolitica was associated with robust innate inflammation, characterized by the recruitment of high levels of neutrophils and macrophages into the intestinal tissue (20). We hypothesized that the vigorous innate responses in neonates may promote similarly robust adaptive immunity. Here, we have compared the development of Yersinia-specific B- and CD4+ T-cell immunity in neonatal and adult mice. We demonstrate that highly protective intestinal and systemic adaptive immunity can be induced in neonatal mice. Remarkably, neonatal mice developed greater Yersinia-specific Th1 and Th17 responses in the mesenteric lymph nodes (MLN) than did adults. Experiments using genetically deficient mice with or without adoptive transfer of donor cells showed that CD4+ T cells, but not B cells, appeared to be necessary for resistance of infected neonates. Thus, we extend our earlier studies to further demonstrate the unprecedented inflammatory potential of the neonatal gastrointestinal immune system in response to a fully virulent enteric pathogen.
MATERIALS AND METHODS
Mice.
BALB/c (Charles River, Wilmington, MA), C57BL/6, B-cell-deficient mice (μMT, B6.129s2-Igh-6-tm1cgn/J), gamma interferon (IFN-γ)-deficient (IFN-γ−/−, B6.129s7-ifng tm1Ts/J) (Jackson Laboratories, Bar Harbor, ME), and CD4-deficient mice (CD4−/−) (provided by Robert Levy, University of Miami, Miami, FL) were bred and housed under barrier conditions in the Division of Veterinary resources of the University of Miami Miller School of Medicine. Adult female IL-17AF-deficient mice (IL-17AF−/−, B6.Il17atm2Yiw/Il17ftm1Yiw) (30) were provided by Yoichiro Iwakura, University of Tokyo. Females from timed matings were monitored from days 19 to 21 of gestation; day 0 of life was the day of birth. Mouse colonies were periodically tested and found to be free of common infectious agents. Adult female mice (7 to 10 weeks old) received sterile food and water ad libitum prior to infection. Seven-day-old female mice from different litters were mixed prior to infection, and immediately thereafter they were returned to the dams. Infected neonatal mice were weaned at 24 days of age. All animal experiments were approved by the University of Miami Miller School of Medicine Animal Care and Use Committee.
Mouse infections.
Wild-type high-virulence Y. enterocolitica WA serotype 0:8/biotype IB was used in all experiments. Preparation of bacterial stocks and orogastric infection of 7-day-old neonatal and adult female mice have been previously described in detail (20). After infection, adult mice were monitored for signs of disease and distress, including ruffled coat, hunched posture, failure to defecate, and lethargy. Signs of disease in neonatal mice included abdominal distention, lethargy and unresponsiveness to stimuli, loose stools, excessive grooming or unkempt coat, and failure to gain weight. Adult mice that had signs of disease and had lost ≥25% of their body weight were euthanized. In neonates, the failure to gain weight or weight loss for 1 to 4 consecutive days in addition to the signs of disease listed above were used as surrogate markers of mortality.
Bacterial enumeration from infected mice.
To enhance the detection of viable Y. enterocolitica in fecal pellets, we used a cold enrichment procedure. One to four fecal pellets were collected aseptically into 1.5-ml tubes, and then 0.5 ml of LB medium was added, and tubes were placed overnight at 4°C. Pellets were disrupted by vortexing vigorously, and the insoluble matter was allowed to settle. Fifty microliters of the suspension was spread in duplicate on Yersinia selective agar (YSA) plates and incubated for 48 h at 27°C. The presence of Y. enterocolitica was scored as positive (growth) or negative (no growth) based on typical colony morphology on YSA plates. Bacterial titers were also measured from whole small intestines by homogenizing the tissue in cold Hank's balanced salt solution (HBSS) using a Seward Stomacher Biomaster 80 (Brinkmann, Westbury, NY), as previously described (20). In other experiments, Peyer's patches (PP) in the small intestine were excised aseptically from individual mice. Tissues were homogenized in 0.5 ml of HBSS for 1 to 2 min using pestles attached to a hand-held motorized Kontes homogenizer (Kimble-Chase, Vineland, NJ). Serial dilutions of the suspensions were plated in duplicate on YSA plates and incubated at 27°C for 48 h.
Cell staining, antibodies, and flow cytometry analysis.
Cell suspensions of MLN cells were prepared by mincing the tissue finely with scissors and pressing it with the bottom of a glass tube through 45-μm wire mesh, with extensive washing in cold HBSS containing 1% calf serum (Gibco), 10 mM HEPES (Gibco), and 4 mM sodium azide. MLN cells were stained with phycoerythrin (PE)-conjugated anti-CD8α (53-6.7), anti-major histocompatibility complex class II (MHC-II) I-A/I-E (2G9), allophycocyanin-conjugated anti-CD4 (RM4-5) and anti-CD11c (HL3), biotinylated anti-CD80 (16-10A1), and anti-CD86 (PO3) (all from BD Pharmingen). Fluorescein isothiocyanate (FITC) anti-CD44 (IM-7.8.1) and biotinylated anti-CD25 (PC61 5.3) were from Caltag Laboratories (Burlingame, CA). FITC-conjugated anti-IgM (μ chain) was from Jackson Immunoresearch. Cells stained with biotinylated antibodies were then stained with streptavidin-peridinin chlorophyll-a protein (PerCP), streptavidin-PE, or streptavidin-FITC antibodies (BD Pharmingen). For Foxp3 staining, cells were stained for CD4 and CD25 as described above, followed by fixation and permeabilization for 18 h using an intracellular staining kit (eBioscience, San Diego, CA). PE-conjugated anti-Foxp3 (FKJ-16S) was used for staining for 30 min using permeabilization buffer. All samples were analyzed on a BD LSR I flow cytometer, using CellQuest software.
Production of heat-killed Yersinia (HKY) and recombinant LcrV.
Y. enterocolitica was grown overnight at 27°C in LB broth; then the culture was diluted to an optical density at 620 nm (OD620) of 0.1 and incubated in a 27°C water bath until the culture reached an OD620 of ∼1.9. The cells were collected by centrifugation, resuspended in sterile distilled water, and heat inactivated for 20 min at 70°C in a shaking water bath set at ∼50 rpm. Recombinant LcrV was purified as a fusion protein of maltose binding protein and LcrV (MBP-LcrV) from a plasmid encoding a Yersinia pestis 195/P LcrV sequence (pKM763) (unpublished vector from David S. Waugh, National Cancer Institute, Frederick, MD). E. coli BL21(DE3) cells (Stratagene) were transformed with pKM763 and grown on LB-ampicillin (100 μg/ml) agar plates at 37°C. Transformants were grown overnight at 37°C in rich medium (1% tryptone, 0.5% yeast extract, 0.5% NaCl, 0.2% glucose, 100 μg/ml ampicillin; all from Sigma). Protein expression was induced in diluted cultures (OD620 of 0.4) using 0.1 mM isopropyl-β-d-thiogalactopyranoside, and cells were collected when the OD620 reached ∼1.8. Pellets were resuspended in cold column buffer (20 mM Tris-HCl, 200 mM NaCl, 1 mM EDTA, pH 7.6), followed by cell lysis using glass beads (particle size, ≤106 μm; Sigma) in an FP120 homogenizer (Bio101; Thermo Electron Corporation) set at 6 for 20 s at 4°C. Lysates were cleared by centrifugation and loaded onto high-flow amylose resin columns (New England BioLabs, Ipswich, MA), and the protein was eluted using 10 mM maltose (Sigma). The purity of the protein was visualized in 10% SDS-polyacrylamide gels followed by Brilliant Blue R-250 staining. Protein concentrations were quantified using a bicinchoninic acid protein assay kit (Pierce, Rockford, IL). Heat-killed cells and MBP-LcrV aliquots were stored at −80°C.
Serum ELISA and avidity index determination.
Blood was collected by submandibular bleeding using 4-mm animal lancets (Medipoint, Mineola, NY), and serum was aliquoted and frozen at −80°C. For traditional enzyme-linked immunosorbent assays (ELISAs), Nunc Maxisorp eight-well strips (Nunc, Naperville, IL) or Costar 96-well high-binding polystyrene plates (Corning Inc., Corning, NY) were coated overnight at 4°C with 0.5 μg per well of MBP-LcrV in 50 μl of phosphate-buffered saline (PBS). Plates were blocked with 200 μl of assay buffer (2% bovine serum albumin [BSA], 0.01% thimerosal in PBS, pH ∼7.2) for ≥2 h. All the washes were done using 0.2% Tween in PBS (PBS-Tw). Sera were serially diluted in PBS and assay buffer (1/150 for primary and 1/166 to 1/1,093,500 for secondary antibodies) and allowed to incubate in the wells overnight at room temperature. Monoclonal biotinylated anti-IgG1 (A85-1) and anti-IgG2a (R19-15) antibodies (BD Pharmingen, CA) were added to wells for ≥2 h, followed by incubation with streptavidin-horseradish peroxidase ([HRP] Jackson Immunoresearch, West Grove, PA) for ≥30 min. The reaction was developed with 3,3′,5,5′-tetramethylbenzidine substrate (Zymed Laboratories, San Francisco, CA) and stopped with 5.5 mM sulfuric acid. The OD was measured at 450 nm with a 550-nm reference filter. The relative antibody titers were estimated by using the OD450 values from the lowest dilution of control serum tested in parallel with immune serum as the cutoff value. The antibody titer was defined as the reciprocal of the dilution that was 2 standard deviations above the cutoff value. The avidity index of IgG1 was measured using a modified ELISA procedure as previously described, with some modifications (11). In brief, ELISA plates coated with MBP-LcrV were incubated overnight with diluted amounts of serum known to give an OD450 of ∼1.0. Then, all the wells were incubated for 15 min with either 100 μl of PBS-Tw or with 2-fold dilutions of 0.109 to 3.5 M potassium thiocyanate (KSCN) in PBS-Tw (Mallinckrodt Baker, Phillipsburg NJ), and the ELISA was completed as described above. The avidity index, i.e., the molar concentration of KSCN that reduced the OD reading by 50%, was calculated. The OD values from the ELISA were transformed as a percentage of the initial values (using the OD value in the well without KSCN as the 100% value). Then, the corresponding percentage was log10 transformed and plotted against the KSCN molarity using a nonlinear third-degree polynomial fit curve (GraphPad Prism, version 4.0). The avidity index was chosen as the KSCN concentration having a log10 value closest to 50% (1.699).
In vitro culture of MLN and CD4+ cells.
MLN were isolated, and single-cell suspensions were prepared in HBSS buffer with 1% calf serum and 10 mM HEPES (Gibco). A total of 4 × 105 cells per well were cultured in 96-well plates in 200 μl of culture medium with 10 μg/well HKY. Culture medium was RPMI 1640 medium (Gibco) containing 10% heat-inactivated fetal bovine serum ([FBS] HyClone, Logan, UT), 1 mM sodium pyruvate, 2 mM l-glutamine, 1% penicillin-streptomycin (Invitrogen), and 5 × 10−2 mM β-mercaptoethanol (Life Technologies, Grand Island, NY). MLN CD4+ cells were positively selected using a Miltenyi Biotec magnetic bead cell sorting (MACS) system (Miltenyi Biotec, Auburn, CA). CD4+ cell purity was ≥95% by flow cytometry. To deplete cells expressing CD4+ CD25+, purified CD4+ cells were incubated with anti-CD25 (MAb 7D4) plus rabbit complement (Cedarlane Laboratories, Burlington, NC). APC were prepared from spleens of naïve 10- to 12-week-old BALB/c mice. Splenocytes were treated with anti-Thy-1 (MAb 42-21) plus complement; then, 4 × 107 cells were incubated in 10 ml of culture medium containing 100 μg/ml HKY for 16 to 22 h. Cells were treated with 50 μg/ml mitomycin C for 20 min. A total of 2 × 105 purified CD4+ cells were stimulated with 4 × 105 APC pulsed with HKY and cultured for 72 h in a humidified 5% CO2 incubator at 37°C. IFN-γ and IL-4 were quantified using mouse-specific cytokine ELISA kits (Endogen, Rockford, IL). IFN-γ and IL-17A were also measured using monoclonal antibodies from BD Pharmingen and protein standards from eBioscience. The limits of detection were 250 pg/ml for IFN-γ, 20 pg/ml for IL-4, and 31 pg/ml for IL-17A.
Adoptive transfer of CD4+ adult lymph node cells.
Lymph node (mesenteric, inguinal, axillary, branchial, and cervical) CD4+ cells were prepared from 8- to 12-week-old wild-type C57BL/6, IFN-γ−/−, and IL-17AF−/− adult mice as described in the previous section. A total of 6 × 106 purified cells were resuspended in 50 μl of HBSS and injected via the facial vein into CD4−/− female mice (≤1 day old). Mice were maintained with their original dams and infected orogastrically with Y. enterocolitica on day 7 postbirth.
Statistical analysis.
Survival analysis was done two times with four to five adults and five neonates per group with doses ranging from 2 × 104 to 6 × 107 CFU of Y. enterocolitica. The 50% lethal dose (LD50) was calculated from individual experiments by probit analysis (Finney method) (BioStat 2009, version 5.7.4.0). Survival curves were generated by the Kaplan-Meier method, and survival kinetics were analyzed by the Mantel-Haenszel log rank test (GraphPad Prism, version 4) with significance at a P value of <0.05. For the majority of experiments, the data from two to six experiments were pooled for analysis and analyzed for normal distribution with the D'Agostino and Pearson omnibus normality test (GraphPad Prism, version 4). Data that followed a normal distribution were analyzed by an unpaired t test; otherwise, a Mann-Whitney test was used. Data are reported as ranges, as individual values (with the means indicated in graphs), or as means ± standard errors of the means (SEM).
RESULTS
Prolonged survival and antigen persistence in neonates infected with Y. enterocolitica.
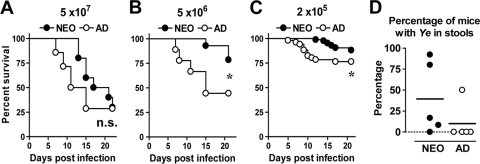
Our previous study demonstrated that neonates were highly resistant to the A127/90 strain of Y. enterocolitica (20). For these experiments, we calculated the orogastric LD50 to Y. enterocolitica WA, another highly virulent strain more commonly used in experimental infection with Y. enterocolitica. The orogastric LD50 in neonates ranged from 5.7 × 106 to 8.1 × 106 CFU (mean, 6.9 × 106 CFU) and in adults from 1.8 × 106 to 2.4 × 106 CFU (mean, 2.1 × 106 CFU). There was only a 3.3-fold change between the average LD50s of neonates and adults, which was not statistically significant (P = 0.07), demonstrating that neonatal mice are as resistant as adult mice to the WA strain of Y. enterocolitica. Infection with 5 × 107 CFU of Y. enterocolitica led to similar survival rates in both age groups (P = 0.36). However, infection with lower bacterial doses (Fig. 1 B and C) revealed that neonates survived for a prolonged period of time compared to adults. The mortalities in infected adults occurred between 5 and 15 days postinfection (p.i.), while most neonatal mice survived the infection throughout this period of time. The prolonged survival in neonates is consistent with an enhanced early innate response in neonates compared to adults, as we have previously demonstrated (20). Moreover, this extended survival period suggested that neonates may have an expanded window of opportunity for the development of adaptive responses.
FIG. 1.
Neonatal mice show prolonged resistance to orogastric Y. enterocolitica infection and prolonged antigen persistence compared to adult mice. Seven-day-old neonatal (NEO) and adult (AD) BALB/c mice were infected orogastrically with 5 × 107 CFU (A), 5 × 106 CFU (B), or 2 × 105 CFU (C) of Y. enterocolitica. Survival curves were generated by Kaplan-Meier Survival analysis. The survival curves depict pooled data from three experiments (10 neonates and 14 adults) (A), two experiments (9 neonates and 10 adults) (B), or 6 experiments with 78 neonates and 51 adults (C). The survival kinetics were compared using the Mantel-Haenszel log rank test. n.s., not significant; *, P ≤ 0.046. (D) Fecal pellets from individual mice infected orogastrically with 2 × 105 CFU of Y. enterocolitica (Ye) were collected at 19 days p.i. and enriched for the presence of Y. enterocolitica bacteria. Each symbol represents the percentage of mice with stools positive for Y. enterocolitica from five separate experiments with 5 to 13 neonates per experiment (total of 48 mice) and 3 to 9 adults per experiment (total of 31 mice). Symbols on the dashed line are from experiments with samples with undetectable bacteria, and the line indicates the mean for the pooled data.
Next, we analyzed whether neonatal mice efficiently cleared the bacteria from intestinal tissues after infection with a low dose (2 × 105 CFU of Y. enterocolitica) by analyzing the excretion of viable Y. enterocolitica in the stool. Although neonatal mice survived the infection, we found that by 19 days p.i., a greater proportion of neonates (39.6%) than adults (12.9%) harbored viable bacteria in the intestine (Fig. 1D). Neonates stopped shedding viable Y. enterocolitica from the intestine at 25 to 30 days after infection. These data suggest that neonatal mice eliminated Y. enterocolitica at a lower rate from the intestine than adult mice infected with the same dose. This is of special consideration because antigen persistence may be crucial for priming of adaptive immunity (75).
Oral Y. enterocolitica exposure in neonatal life leads to increased levels of antigen-presenting cells, B cells, and CD4+ cells with an activated phenotype in the MLN.
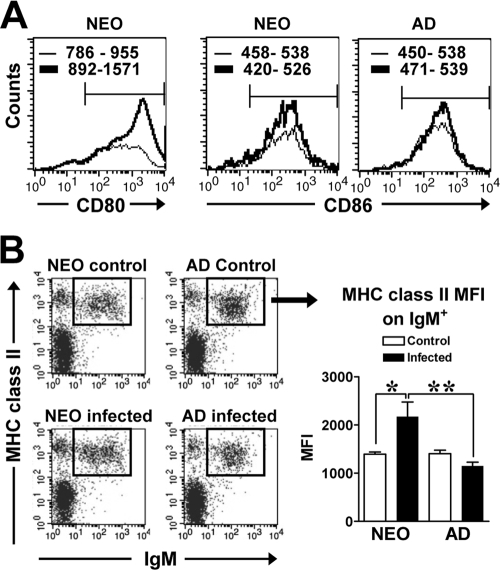
We assessed whether Y. enterocolitica infection induced any phenotypic changes in the MLN, one of the main sites for the priming of adaptive immunity in the intestinal tissue (52). Neonatal mice infected with 2 × 105 CFU of Y. enterocolitica had increased cellularity in the MLN at 19 to 20 days p.i. compared to naïve age-matched mice (2.4-fold) (P = 0.013) (Table 1 ), while infected adults did not undergo any changes in cell numbers (Table 1). Flow cytometric analysis revealed multiple differences in the cellular composition of the MLN in infected neonates and adults. First, unlike responses in adults, there was a reduction in the percentage of CD4+ T cells in infected neonates compared to naïve neonates (Table 1). In spite of the decrease in the percentage of CD4+ cells, there were greater absolute numbers of CD4+ cells in infected neonates than in naïve controls. In fact, infection of neonates with Y. enterocolitica induced CD4+ T-cell numbers to reach adult levels (P = 0.43). Strikingly, CD4+ neonatal T cells had increased expression levels of CD44 relative to naïve controls or infected adults, demonstrating activation of these cells during infection (Table 1). Second, the percentages of cells expressing CD8α were not different between control and infected neonates or adults (Table 1). Similar to CD4+ cells, the absolute number of CD8α+ cells in infected neonates increased to adult levels (Table 1). Third, in infected neonates, the reduction in the percentage of CD4+ cells was associated with a proportional increase in the percentage of MHC-II-positive (MHC-II+) cells. Importantly, the absolute number of CD11c+ MHC-II+ cells increased in the MLN of infected neonates (Table 1). In addition, these cells expressed high levels of costimulatory molecules CD80 and CD86 (Fig. 2 A); however, the mean fluorescence intensities (MFI) of CD80 and CD86 expression were not different between control and infected neonatal mice (P ≤ 0.91). The MFIs of CD86 expression were also not significantly different between control and adult mice (P = 0.61). Moreover, the majority of cells expressing MHC-II were B cells (IgM+), resembling the phenotype of inflammatory intestinal B cells (64) (Table 1 and Fig. 2B). The percentage of IgM+ MHC-II+ cells increased in infected neonates compared to the percentage in infected adults (Table 1), and their absolute cell numbers reached adult levels (P = 0.16). In addition, neonatal IgM+ MHC-II+ cells expressed significantly higher levels of MHC-II than cells of either naïve neonates or infected adults (Fig. 2B). Altogether, these data demonstrate that infection of neonatal mice with Y. enterocolitica induces increased levels of APC and CD4+ cells in the MLN, resembling the cellular composition of adult mice. However, neonatal, but not adult, MHC-II+ and CD4+ T cells appeared to have undergone phenotypical changes indicative of functional maturation.
TABLE 1.
Phenotypic analysis of the MLN of infected neonatal mice demonstrates high levels of APC, B cells, and CD4+ T cells with activated phenotypes
| Groupa | No. of MLN cellsb | Phenotypic analysis of the indicated MLN cell populationc |
|||||
|---|---|---|---|---|---|---|---|
| CD4+ population |
CD8∝+ population (percentage of positive cells [no.]) | APC population (percentage of positive cells [no.]) |
|||||
| Percentage of positive cells (no.) | CD44 MFId | MHC-II+ | CD11c+ MHC-II+ | IgM+ MHC-II+ | |||
| Control neonates | 1.14 × 107 ± 1.79 × 106 | 53.30 ± 1.07 (5.66 × 106 ± 1.08 × 106) | 34.99 ± 2.09 | 20.16 ± 0.69 (2.39 × 106 ± 3.27 × 105) | 23.63 ± 1.35 (3.17 × 106 ± 4.58 × 105) | 0.50 ± 0.01 (8.59 × 104 ± 9.29 × 103) | 20.14 ± 1.37 (2.29 × 106 ± 5.65 × 105) |
| Infected neonates | 2.74 × 107 ± 4.37 × 106e | 40.85 ± 2.61e,f (1.09 × 107 ± 1.34 × 106)e | 50.14 ± 4.69e,f | 18.26 ± 0.91 (4.00 × 106 ± 4.47 × 105)e | 29.45 ± 3.15f (8.81 × 106 ± 1.95 × 106)e | 0.49 ± 0.03 (1.25 × 105 ± 8.9 × 103)e | 30.89 ± 3.32e,f (1.00 × 107 ± 2.69 × 106)e |
| Control adults | 2.56 × 107 ± 2.62 × 106 | 58.16 ± 2.14 (1.29 × 107 ± 1.48 × 106) | 33.76 ± 1.51 | 16.95 ± 0.29 (4.32 × 106 ± 4.33 × 105) | 24.25 ± 1.83 (6.31 × 106 ± 8.65 × 105) | 0.97 ± 0.17 (2.72 × 105 ± 6.23 × 104) | 19.91 ± 1.52 (5.29 × 106 ± 7.93 × 105) |
| Infected adults | 2.25 × 107 ± 2.08 × 106 | 56.57 ± 0.75 (1.23 × 107 ± 1.17 × 106) | 34.59 ± 0.89 | 16.64 ± 0.33 (3.69 × 106 ± 3.32 × 105) | 21.88 ± 2.01 (4.85 × 106 ± 6.79 × 105) | 0.77 ± 0.13 (1.78 × 105 ± 3.92 × 104) | 20.87 ± 0.83 (4.49 × 106 ± 6.02 × 105) |
Neonatal and adult BALB/c mice were infected orogastrically with 2 × 105 CFU of Y. enterocolitica.
MLN from age-matched uninfected and infected mice were collected at 19 to 21 days p.i.
MLN cells were stained with anti-CD4, anti-CD8α, anti-CD44, anti-MHC-II, anti-CD11c, and anti-IgM antibodies and analyzed by flow cytometry. The data from two to five experiments were pooled for 5 to 13 mice per group.
MFI of CD44 expression within CD4+ cells.
P < 0.05 between control and infected mice of each age group, as analyzed by the Mann-Whitney test.
P < 0.05 between infected neonates and infected adult mice, as analyzed by the Mann-Whitney test.
FIG. 2.
APC in the MLN of infected mice express activation and costimulatory markers. Neonatal and adult BALB/c mice were infected orogastrically with 5 × 105 CFU of Y. enterocolitica. (A) MLN from individual control (dashed line) and infected (solid line) mice were collected at 15 days p.i., and cells stained were with anti-CD11c, anti-CD80, or anti-CD86 antibody and analyzed by flow cytometry. The expression levels of CD80 and CD86 within CD11c+ cells were overlaid for each age group. The range in the MFIs of CD80 or CD86 for each group (n = 3 to 6 mice per group) is shown within each graph. (B) At 21 days p.i., MLN cells from mice infected with 2 × 105 CFU of Y. enterocolitica were stained with anti-IgM and anti-MHC-II antibodies. A representative dot plot profile of IgM+ MHC-II+ cells is shown and the MFI of MHC-II within IgM+ cells for control (□) and infected (▪) mice is shown in panel B (right). The data from two experiments were pooled for five to seven uninfected controls and six infected neonates or four infected adults. Differences were analyzed by the Mann-Whitney test. *, P = 0.017; **, P = 0.0095.
Development of vigorous systemic Yersinia-specific T-cell-dependent antibody responses.
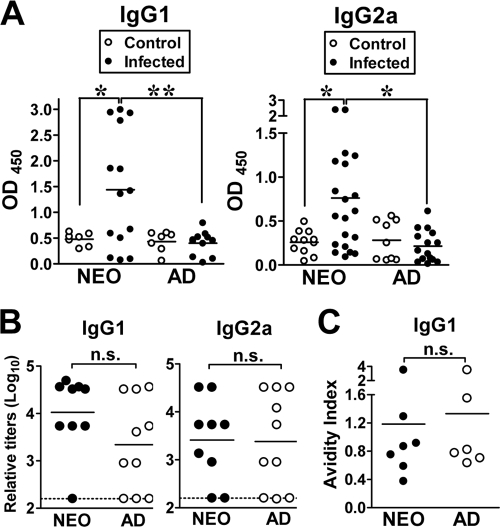
The phenotypic changes in the neonatal MLN provided evidence that T- and B-cell-associated immunity may have been induced in neonatal mice. To study the development of Yersinia-specific adaptive immunity, neonates and adults were infected with 2 × 105 CFU of Y. enterocolitica, and serum antibody responses to the immunogenic Yersinia protein LcrV were compared. The levels of IgG1 and IgG2a were assessed as in vivo markers of Th1 (IgG2a)-, Th2 (IgG1)-, or Th17 (IgG1)-mediated immunity. During primary infection (19 to 20 days p.i.), neonatal mice had increased serum levels of LcrV-reactive IgG1 and IgG2a antibodies compared to infected adults (Fig. 3 A). IgG1 was detectable over uninfected control levels in 9 out of 14 of infected neonates, whereas only 1 out of 11 infected adults had detectable IgG1. The proportion of neonates with detectable IgG2a was also higher (10 out of 20) than in infected adults (1 out of 15). In adult mice, increasing the infectious dose to 2 × 106 CFU induced levels of anti-LcrV IgG1 (OD450 of 1.33 ± 0.47) similar to those of neonatal mice infected with 2 × 105 CFU of Y. enterocolitica. However, the levels of anti-LcrV IgG2a antibodies did not change (OD450 of 0.36 ± 0.37) relative to uninfected adults. Therefore, even with a 10-fold greater dose of bacteria, adults underproduced IgG2a relative to infected neonates. These data demonstrate that the neonatal B-cell compartment was efficiently activated during primary infection, leading to the development of robust Yersinia-specific serum IgG1 and IgG2a antibody responses. To measure the development of long-lived systemic immunity, we compared memory serum antibody levels. Nine to 11 weeks following primary infection, mice were boosted orogastrically with 5 × 106 CFU of Y. enterocolitica, and anti-LcrV antibodies were measured 21 days postboost. When serum samples were individually titrated for IgG1 and IgG2a levels, there were three distinct patterns of response evident in both neonates and adults. The group of mice with higher levels of IgG1 than IgG2a was the predominant type (neonates, 62.5%; adults, 50%), followed by mice with higher levels of IgG2a than IgG1 (neonates, 25%; adults, 37.5%) and, to a lesser extent, mice with equal IgG1/IgG2a levels (neonates, 12.5%; adults, 12.5%). While the serum antibody responses of mice primed as neonates and adults appeared to be heterogeneous, there were no statistically significant differences in the relative amounts of anti-LcrV IgG1 (P = 0.09) (Fig. 3B, left) or IgG2a (P = 0.9) antibodies (Fig. 3B, right) between mice primed as neonates or adults.
FIG. 3.
Infection of neonatal mice with Y. enterocolitica leads to development of greater primary Yersinia-specific serum antibody responses and to secondary antibody responses similar in quantity and quality to adult responses. (A to C) BALB/c neonates and adults were infected orogastrically with 2 × 105 CFU of Y. enterocolitica. (A) Serum levels of Yersinia-specific IgG1 and IgG2a antibodies using purified MBP-LcrV were measured at 19 to 20 days p.i. from individual uninfected (○) and infected (•) neonates and adults using serum samples diluted 1/150. OD450 values are shown in the graphs (n = 7 to 11 controls and 11 to 20 infected mice). In the experiments shown in panels B and C, mice initially infected as neonates (NEO) or adults (AD) were boosted orogastrically at 9 to 11 weeks p.i. with 5 × 106 CFU of Y. enterocolitica, and sera were collected at 21 days postboost and serially diluted. (B) The relative levels of IgG1 and IgG2a for 9 neonates (•) and 10 adults (○) were pooled for analysis from two independent experiments. Undetectable antibody titers were plotted at the dashed line. (C) The avidity of memory IgG1 antibodies to LcrV for 7 neonates and 6 adults was tested in a serum ELISA using increasing amounts of KSCN (0.1 to 3.5 M). The amount of KSCN that reduced the OD450 by 50% is defined as the avidity index. Differences were calculated by the Mann-Whitney test. n.s., not significant; *, P ≤ 0.03; **, P = 0.007.
We next compared the quality of these antibodies by measuring the avidity index or overall strength of the antibody-antigen interaction, using the destabilizing anion potassium thiocyanate (KSCN) in a modified ELISA (11). Mice primed as neonates and adults with Y. enterocolitica showed similar avidities of anti-LcrV IgG1 (Fig. 3C), demonstrating that anti-LcrV antibodies of adult-like quality can be achieved in neonatal mice through orogastric infection with Y. enterocolitica. Altogether, these data provided evidence that neonates and adults developed long-lived systemic antibody responses of similar magnitudes and quality following Y. enterocolitica infection.
Oral exposure to Y. enterocolitica during neonatal life leads to long-term, protective mucosal immunity.
The observation that Y. enterocolitica efficiently mobilized the adaptive immune system for both primary and secondary T-cell-mediated antibody responses prompted us to investigate whether protective immunological memory developed in neonates. Mice initially infected as neonates or adults were challenged in adulthood with a high bacterial dose (2 × 108 CFU of Y. enterocolitica), and weight loss was used as a surrogate marker for mortality. Both neonatal and adult mice orogastrically primed with Y. enterocolitica showed enhanced survival (Fig. 4 A) after challenge in adulthood compared to naïve age-matched mice. To assess indirectly whether mucosal memory responses may have accounted for this enhanced protection, we measured Y. enterocolitica titers in the Peyer's patches (PP), one of the main sites for bacterial invasion beyond the intestinal lumen. The bacterial titers in the PP were reduced by 10- to 100-fold in both groups compared to naïve mice at 7 days p.i. (Fig. 4B). In addition, 40% of primed neonates had sterile PP at this time point. These data demonstrate that mice initially infected as neonates were at least as resistant to a bacterial challenge as were adults and indicate that neonatal mice exposed to Y. enterocolitica through the gastrointestinal tract developed adaptive memory responses that were protective at the mucosal interface.
FIG. 4.
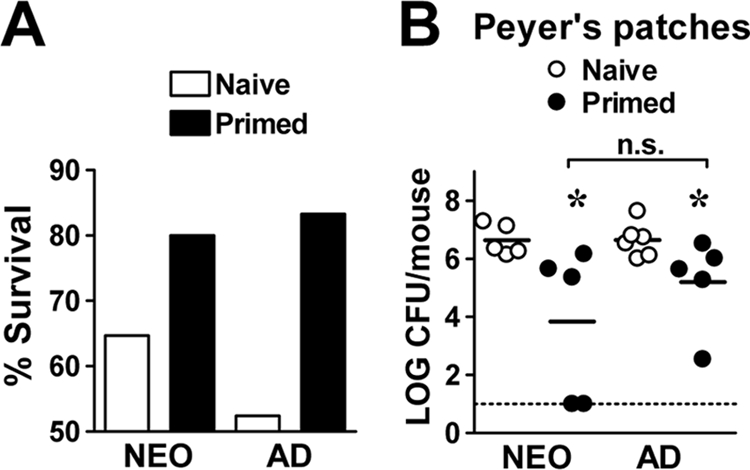
Mice infected orogastrically as neonates are protected against challenge in adulthood with a high dose of Y. enterocolitica. Neonatal and adult BALB/c mice were infected orogastrically with 2 × 105 CFU of Y. enterocolitica. Nine to 11 weeks p.i., mice were orogastrically infected with 2 × 108 CFU of Y. enterocolitica in parallel with age-matched naïve mice. (A) Weight loss was used as a surrogate marker for mortality; mice that lost over 25% of their body weight were deemed moribund. The bar graphs show the percent survival at 7 days postchallenge of naïve age-matched and primed mice pooled from three independent experiments (17 to 21 mice per group). (B) At 7 days postchallenge, the PP from naïve and primed mice (n = 5 to 6 mice per group) were analyzed for Y. enterocolitica titers. The dotted line is at the limit of detection. Differences were analyzed by the Mann-Whitney test. n.s., not significant; *, P ≤ 0.03 between naïve and primed mice in each group.
Neonatal infection with Y. enterocolitica polarizes mucosal CD4+ T cells toward the Th1 and Th17 pathways.
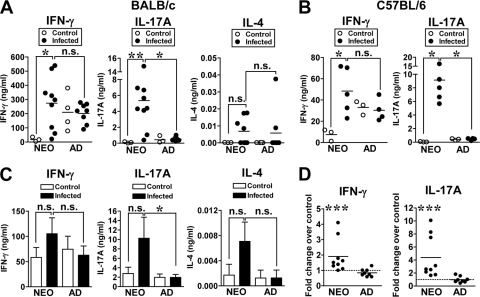
Given that robust T-cell-dependent antibody responses were generated, we next compared the T-cell cytokine profiles from infected neonates and adults during primary infection. The MLN from mice were collected at 19 to 20 days p.i., and cells were restimulated with heat-killed Yersinia (HKY). MLN cells from 89% of infected neonates produced higher levels of IFN-γ than uninfected controls (Fig. 5 A). The amount of IFN-γ from infected neonatal cells was comparable (P = 0.32) to the amount of cytokine produced by infected adult cells. In contrast, there was no increase in IFN-γ in infected adults compared to levels in uninfected animals (P = 0.32) (Fig. 5A). These data suggest that the IFN-γ in adults may not be specific to Yersinia antigens as it could have been produced through nonspecific stimulation of MLN cells or by cross-reactivity of Yersinia antigens with commensal bacteria antigens. Strikingly, IL-17A levels were markedly increased in 100% of infected neonates compared to naïve controls or infected adults (Fig. 5A). However, IL-4 was undetectable in the majority of mice (level of detection, 0.020 ng/ml) under these culture conditions (Fig. 5A). The increased neonatal levels of IFN-γ and IL-17A were not limited to the BALB/c mouse strain as similar cytokine profiles were detected in C57BL/6 mice infected with 1 × 107 CFU of Y. enterocolitica (Fig. 5B), a dose at which over 67% of infected mice survived the infection.
FIG. 5.
The inflammatory cytokines IFN-γ and IL-17A are produced by MLN cells from infected neonates upon restimulation in vitro with Yersinia antigens. (A and C) Neonatal and adult BALB/c mice were infected orogastrically with 2 × 105 CFU of Y. enterocolitica. (A) At 19 to 20 days p.i., individual MLN were harvested from three to four control and eight to nine infected mice, and 4 × 105 MLN cells were cultured in vitro with 10 μg/well of HKY for 72 h. The amounts of IFN-γ, IL-17A, and IL-4 quantified by ELISA from three experiments are shown, with the horizontal line indicating the mean. (B) Neonatal and adult C57BL/6 mice were infected orogastrically with 1 × 107 CFU of Y. enterocolitica. At 20 days p.i., MLN cells from individual mice were cultured as described in panel A. (C) At 20 to 21 days p.i., MLN CD4+ T cells were isolated from BALB/c mice, and 2 × 105 cells were stimulated with 4 × 105 splenic APC pulsed with HKY for 72 h. IFN-γ, IL-17A, and IL-4 were quantified by ELISA. The pooled data from three experiments are shown in the bar graphs. (D) The fold difference in IFN-γ and IL-17A levels between age-matched control and infected mice was calculated from each experiment in panel C and pooled for analysis (nine infected neonates and eight infected adults). Fold difference values greater than 1 are above the dotted line. In all experiments, differences between groups were analyzed by the Mann-Whitney test. n.s., not significant; *, P ≤ 0.035; **, P ≤ 0.0048; ***, P ≤ 0.002.
Studies in adult mice infected orogastrically with Y. enterocolitica have measured only CD4+ T cells with a Th1 profile (IFN-γ producers) (7). Although IL-17 mRNA has been detected in the PP and MLN of adult mice early after infection with Y. enterocolitica (28), CD4+ Th17 cells and their signature cytokine IL-17A, however, have not been described during Y. enterocolitica infection. Since both IFN-γ and IL-17 can be made by multiple cell types (45, 63), we next investigated whether CD4+ T cells were a cellular source of IFN-γ and IL-17A. MLN CD4+ T cells were stimulated in vitro with splenic antigen APC loaded with HKY. MLN CD4+ T cells from control and infected mice produced IFN-γ under these culture conditions; we could not detect significant differences in the total amounts of cytokine produced (Fig. 5C). However, IL-17A levels were more consistently detected from infected neonatal CD4+ T cells than from adult CD4+ T cells (Fig. 5C). As for stimulated MLN cells, IL-4 levels were negligible from infected neonatal and adult CD4+ T cells (Fig. 5C). To better compare the levels of IFN-γ and IL-17A produced by CD4+ cells from infected mice, we analyzed the fold increase in cytokine production between infected and control mice in each age group (Fig. 5D). Remarkably, 77.8% of infected neonates produced greater amounts of IFN-γ than uninfected mice while only 12.5% of infected adults did (Fig. 5D). A similar pattern was observed for IL-17A: 100% of neonatal CD4+ T cells produced specific IL-17A while 25% of adult CD4+ T cells did. Increasing the infectious dose in adult mice to 2 × 106 CFU of Y. enterocolitica led to increased mortality, but in surviving mice the levels of CD4+ T-cell-derived IFN-γ and IL-17A were increased 3.15- ± 2.28-fold and 9.66- ± 8.56-fold, respectively, suggesting that adult CD4+ T cells might be similarly activated as long as the antigenic load is higher.
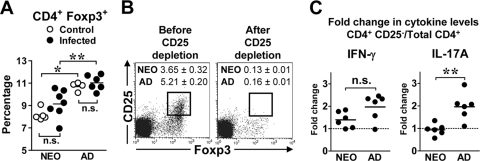
It has become apparent that inducible T regulatory (Treg) Foxp3+ cells and Th17 cells have a common developmental pathway because they mature in response to differential levels of IL-6 and transforming growth factor β (TGF-β) (43, 78). Studies on the infection of neonatal mice with rotavirus (39) and herpes simplex virus type 2 (HSV-2) (22) have demonstrated that CD4+ CD25+ Foxp3+ Treg cells are involved in attenuating inflammatory cytokine production in vivo, particularly Th1 function. To address whether the reduced in vitro Th1/Th17 response of adults was due to high levels of Treg cells, we compared the levels of Treg cells in the MLN of infected mice. Phenotypic analysis of the MLN revealed similar percentages of CD4+ Foxp3+ cells in control and infected mice of either age group (Fig. 6 A). However, neonatal mice had lower percentages of CD4+ Foxp3+ cells in the MLN than adults (Fig. 6A). To test for a functional activity of Treg cells in suppressing cytokine production in vitro, we depleted cells expressing the IL-2 receptor α chain (CD25) by antibody treatment to target cells expressing Foxp3 intracellularly. We confirmed the loss of the majority of CD25+ Foxp3+ cells in isolated MLN CD4+ T cells after anti-CD25 treatment (Fig. 6B). Depletion of CD25+ Foxp3+ cells led to increased production of IFN-γ by both neonatal and adult cells. However, the fold change in IFN-γ levels was greater (>2-fold), albeit not statistically significant (P = 0.12), in four out of six adult samples (Fig. 6C). In contrast, the levels of IL-17A produced by total CD4+ and CD4+ CD25− cells were similar in neonates. In adults, the fold change in IL-17A was greater (P = 0.0043) in five out of six samples than in neonatal cells (Fig. 6C). Therefore, the presence of Foxp3+ cells leads to preferential suppression of adult Th1 and Th17 function.
FIG. 6.
The levels of CD4+ Foxp3+ T cells in the MLN of infected adults are increased compared to infected neonates, and their presence affects cytokine production in vitro. (A to C) Neonatal and adult BALB/c mice were infected orogastrically with 2 × 105 CFU of Y. enterocolitica. (A) MLN were harvested from control and infected mice at 21 days p.i.; total MLN or purified CD4+ cells were stained with anti-CD4 and anti-Foxp3 antibodies. The percentage of CD4+ cells expressing Foxp3 was quantified by flow cytometry. Each dot represents the percentage of CD4+ Foxp3+ cells from three to four individual mice in each group or CD4+ cells pooled from two to four mice. Differences were analyzed by the Mann-Whitney test. n.s., not significant; *, P = 0.016; **, P = 0.0047. (B) At 21 to 23 days p.i., CD4+ MLN cells were isolated, followed by depletion of CD25+ cells using monoclonal 7D4 antibody. CD4+ cells were stained with anti-CD25 and anti-Foxp3 antibodies before and after CD25 depletion. A representative flow cytometry profile from an infected adult is shown with the percentage ± SEM of CD25+ Foxp3+ cells from six samples in each age group. (C) Total CD4+ and CD4+ CD25− cells were cultured in vitro with splenic APC pulsed with HKY. Supernatants were collected 72 h after culture, and the levels of IFN-γ and IL-17A were quantified by ELISA. The fold change in cytokine production between CD4+ CD25− cells and total CD4+ cells for each age group is shown, and the horizontal line indicates the mean from two independent experiments (n = 6 in each group). Fold difference values greater than 1 are above the dotted line. Differences in fold change were analyzed by the Mann-Whitney test. n.s., not significant; **, P = 0.0043.
CD4+ T cells are required for long-term protection of neonatal mice following orogastric infection.
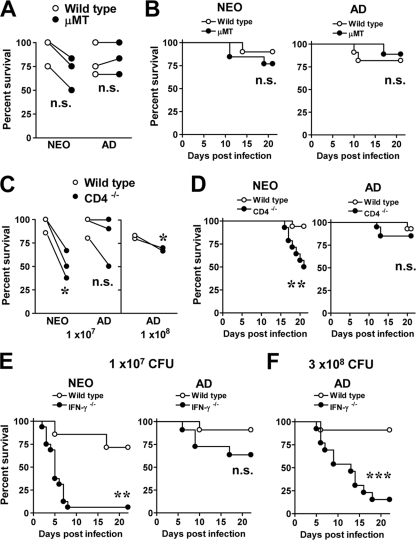
The evidence that both B- and T-cell-associated immunity was generated during neonatal life led us to assess the roles of both cell populations during infection. First, to test whether B cells had a beneficial role during primary infection of neonatal mice, we compared the susceptibility of mice deficient in B lymphocytes (μMT mice). These mice are impaired in normal development of B2 B cells and have no detectable IgG antibodies in serum (40). For these studies we used a dose at which over 67% of neonatal and adult C57BL/6 mice survived the infection. Infected μMT neonates showed a consistent decrease in resistance to infection (50 to 83.3% survival) compared to wild-type infected neonates (75 to 100% survival) (Fig. 7 A). However, the difference in survival rates between groups was not statistically significant (P = 0.41) (Fig. 7B). Infected wild-type and μMT adults also showed similar survival proportions (66.7 to 100%) (Fig. 7A), and the survival rates were also not significantly different (P = 0.52) (Fig. 7B). These experiments suggest that B2 B cells do not play a major role in the protection of either neonatal or adult mice to primary infection with Y. enterocolitica.
FIG. 7.
Neonatal mice deficient in CD4+ cells (CD4−/−) and IFN-γ (IFN-γ−/−) are more susceptible to orogastric infection, while the absence of B cells only modestly increases their susceptibility to Y. enterocolitica. (A and B) Wild-type C57BL/6 and μMT neonates and adults were infected orogastrically with 1 × 107 CFU of Y. enterocolitica. The percent survival from three experiments for each age group is depicted with a connecting line (n = 2 to 6 neonates and n = 3 to 6 adults of each strain per experiment). Differences between groups were analyzed by an unpaired t test. n.s., not significant. (B) The data in panel A were used to generate survival curves for neonates (10 wild-type and 13 μMT mice) and adults (11 wild type and 18 μMT mice). Survival curves were analyzed by the Mantel-Haenszel log rank test. n.s., not significant. (C) Wild-type C57BL/6 and CD4−/− neonatal and adult mice were infected orogastrically with 1 × 107 CFU of Y. enterocolitica (left) or 1 × 108 CFU of Y. enterocolitica (right). The percent survival from three experiments is connected with a line. Differences between groups were analyzed by an unpaired t test. *, P = 0.04. (D) The data shown in panel C were pooled for neonates (17 wild-type and 14 CD4−/− mice) and adults (15 wild-type and 20 CD4−/− mice) and analyzed by the Mantel-Haenszel log rank test. n.s., not significant; **, P = 0.0052. (E) Neonatal and adult C57BL/6 (wild type)and IFN-γ−/− mice were infected orogastrically with 1.4 × 107 CFU of Y. enterocolitica. Survival was recorded from two experiments with 7 wild-type and 16 IFN-γ−/− neonates and 11 wild-type and 13 IFN-γ−/− mice adults. (F) Adult mice were also infected with 3 × 108 CFU of Y. enterocolitica, and survival curves were generated from 10 wild-type and 13 IFN-γ−/− mice. Data were analyzed by the Mantel-Haenszel log rank test. n.s., not significant; **, P = 0.0014; ***, P = 0.0005.
We next studied the requirement of CD4+ cells for protection using CD4-deficient mice (CD4−/−). A greater proportion of CD4−/− neonates (33 to 62%) succumbed to infection than wild-type neonates (0 to 13%) (Fig. 7C). Adult CD4−/− mice infected with the same dose (1 × 107 CFU) did not appear to be more susceptible than wild-type mice (P = 0.63) (Fig. 7C), but infection with a 10-fold-higher dose (1 × 108 CFU) led to a small but significant increase in susceptibility of CD4−/− adults (Fig. 7C). Infected CD4−/− neonates displayed signs of disease as early as 13 days p.i., and the onset of mortality started around day 17 p.i., leading to more rapid kinetics of death (P = 0.005) than in infected wild-type neonates (Fig. 7D). The survival kinetics were not different (P = 0.65) between wild-type and CD4−/− adults (Fig. 7D). These data demonstrate that in neonatal mice, CD4+ T cells are required for protection from orogastric infection with a relatively low bacterial dose; however, in adult mice, the requirement for CD4+ T cells for protection may be dependent upon exposure to high bacterial burdens.
It is widely accepted that protection against Y. enterocolitica infection in adult mice is dependent on IFN-γ and Th1 immunity (6). In light of the susceptibility of neonatal CD4−/− mice to orogastric Y. enterocolitica infection, we infected IFN-γ−/− neonates and adults in a C57BL/6 background with ∼1.4 × 107 CFU of Y. enterocolitica and analyzed the ability of mice to survive infection. IFN-γ−/− neonates succumbed very rapidly following infection with this dose (Fig. 7E). In contrast, the survival rates of adult IFN-γ−/− mice infected with the same dose was not significantly different (P = 0.127) from survival rates of wild-type adult mice (Fig. 7E). Previous studies in adult mice used higher bacterial titers to study the role of IFN-γ; therefore, adult mice were infected with 3 × 108 CFU of Y. enterocolitica. As expected, 85% of IFN-γ−/− adult mice succumbed to infection relative to wild-type adults (Fig. 7F). Comparison of the survival rates between IFN-γ−/− neonates infected with ∼1 × 107 CFU of Y. enterocolitica and IFN-γ−/− adults infected with 3 × 108 CFU of Y. enterocolitica revealed that neonatal mice died with faster kinetics (P = 0.0049). While 50% of adult mice succumbed by 13 days p.i., 50% of neonatal mice succumbed by day 5. These data demonstrate that IFN-γ plays a particularly crucial role in the early immune response of neonatal mice leading to long-term protection from a sublethal bacterial dose. However, the rapid death kinetics of infected IFN-γ−/− neonates contrasts with the death kinetics of infected CD4−/− neonates, suggesting that CD4+ cells may not be the main cellular source of IFN-γ in neonates.
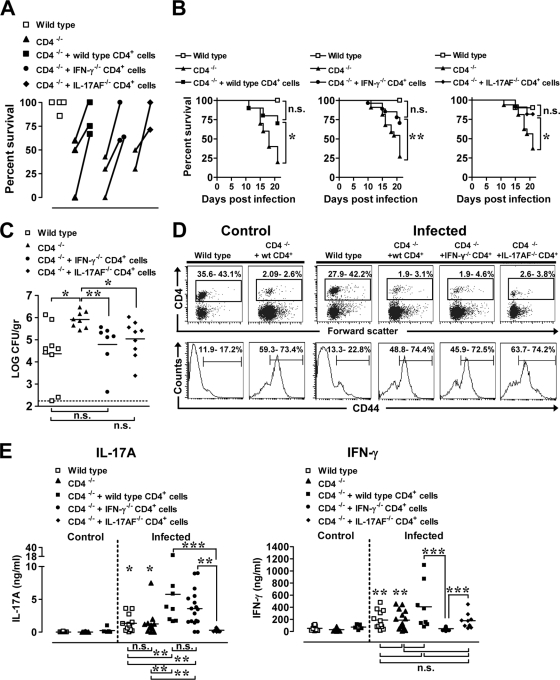
To test a functional role in vivo for CD4+ T cells, we adoptively transferred 6 × 106 adult lymph node CD4+ T cells into 1-day-old CD4−/− neonatal mice prior to infection and asked whether the transferred T cells were sufficient to restore survival of CD4−/− neonates to wild-type levels. On the day of infection, approximately 6.3% ± 3.3% CD4+ T cells colonized the MLN of CD4−/− neonatal mice reconstituted with adult CD4+ cells. Transferring wild-type CD4+ T cells into CD4−/− neonatal mice prior to orogastric infection improved long-term survival relative to infected CD4−/− mice (Fig. 8 A and B). Although the percent survival did not reach wild-type levels in all experiments (Fig. 8A), these results clearly indicated that adult CD4+ cells were capable of exerting a protective role in a neonatal host. Importantly, adoptively transferred adult IFN-γ−/− or IL-17AF−/− CD4+ T cells also improved survival of infected CD4−/− neonatal mice (Fig. 8A and B), and the survival rates between infected CD4−/− mice that received adult wild-type, IFN-γ−/− CD4+, or IL-17AF−/− CD4+ cells did not differ (P ≤ 0.99), suggesting that T-cell-derived IFN-γ or IL-17A or IL-17F may not be required for protection. The protective effect of the transferred adult IFN-γ−/− or IL-17AF−/− CD4+ T cells was associated with a reduction in the amount of Y. enterocolitica in the small intestine (Fig. 8C), demonstrating a potent antibacterial role for the adoptively transferred CD4+ cells. After 21 days of infection, the percentage of CD4+ cells in the MLN of infected reconstituted mice was low (Fig. 8D), but the percentage of CD4+ cells that expressed CD44 was high compared to percentages in wild-type infected mice (Fig. 8D), indicating that injected adult CD4+ cells had acquired an activated phenotype, as previously demonstrated in neonatal mice (56). To determine whether the transferred CD4+ cells responded to Yersinia antigens, MLN cells from control and infected wild-type, CD4−/−, and CD4+-reconstituted CD4−/− mice were restimulated with HKY. MLN cells from uninfected CD4−/− neonates injected with wild-type CD4+ T cells did not produce IFN-γ and IL-17A upon restimulation with HKY (Fig. 8E), demonstrating the Yersinia-specific nature of the cytokine production. IFN-γ and IL-17A levels were not significantly different (P = 0.73) between infected wild-type and infected CD4−/− mice (Fig. 8E); MLN cells from both groups produced larger amounts of IFN-γ and IL-17A than control wild-type (P ≤ 0.017) and control CD4−/− (P = 0.014) MLN cells. Surprisingly, despite the low numbers of CD4+ cells in the MLN of CD4+-reconstituted CD4−/− mice, IL-17A levels were markedly higher in MLN of CD4−/− neonates that received adult wild-type and IFN-γ−/− CD4+ cells than in MLN from infected wild-type and CD4−/− mice (Fig. 8E, left). MLN cells from infected mice reconstituted with CD4+ IL-17AF−/− cells did not produce IL-17A (Fig. 8E, left). In contrast, all infected mice except for CD4−/− neonates that received IFN-γ−/− CD4+ cells by adoptive transfer produced IFN-γ upon stimulation, but the levels of IFN-γ were not statistically significant between groups (Fig. 8E, right). These data demonstrate that adoptively transferred adult CD4+ cells are capable of attaining high-level Yersinia-specific IL-17A production in the neonatal environment.
FIG. 8.
Adoptively transferred wild-type, IFN-γ−/− or IL-17AF−/− CD4+ T cells improve survival of neonatal CD4−/− mice following infection with Y. enterocolitica, and MLN cells from these mice produce inflammatory cytokines upon in vitro stimulation with Y. enterocolitica antigens. Lymph node CD4+ T cells (6 × 106) from adult C57BL/6, IFN-γ−/−, or IL-17AF−/− mice were injected intravenously into 1-day-old CD4−/− mice. (A) Seven-day-old wild-type C57BL/6, CD4−/−, CD4−/− mice injected with wild type CD4+ cells, CD4−/− mice injected with IFN-γ−/− CD4+ cells, and CD4−/− mice injected with IL-17AF−/− CD4+ cells were infected orogastrically with 1 × 107 CFU of Y. enterocolitica. The percent survival values from two to three individual experiments are connected with a line. (B) The data from the experiments in panel A were pooled to generate survival curves and analyzed by the Mantel-Haenszel log rank test. n.s., not significant; *, P ≤ 0.03; **, P = 0.0025 (for CD4−/− versus CD4−/− mice injected with CD4+ cells). (C) The small intestines were collected at 25 days p.i. from infected wild-type, CD4−/−, CD4−/− mice injected with IFN-γ−/− CD4+ cells, or CD4−/− mice injected with IL-17AF−/− CD4+ cells, and tissues analyzed for Yersinia titers. The dashed line is at the limit of detection. The data from two experiments were pooled (n = 7 to 9 mice per group) and analyzed by the Mann-Whitney test. n.s., not significant; *, P = 0.01; **, P = 0.0012. (D) MLN from mice that survived the infection at 21 to 25 days p.i. were collected, and cell suspensions were stained with anti-CD4 and anti-CD44 antibodies. Representative flow cytometry profiles show the percentages of CD4+ (top) and CD44+ cells within CD4+ cells (bottom), with the range of cells from three to four experiments (n = 5 to 17 mice). (E) MLN cells from individual mice described in panel D were cultured for 72 h with 10 μg/well of HKY. MLN cells from uninfected C57BL/6 (n = 8), CD4−/− (n = 25), and CD4−/− mice injected with wild-type CD4+ cells (n = 5) were stimulated along with cells from infected CD4−/− (n = 15), CD4−/− mice injected with wild-type CD4+ cells (n = 8), CD4−/− mice injected with IFN-γ−/− CD4+ cells (n = 17), and CD4−/− mice injected with IL-17AF−/− CD4+ cells (n = 9). Supernatants were tested for IFN-γ and IL-17A by ELISA. Differences were analyzed by the Mann-Whitney test. n.s., not significant; For IL-17A, significance is indicated as follows: *, P ≤ 0.013 between infected CD4−/− plus IL-17AF−/− CD4+ cells versus infected wild-type or infected CD4−/− mice; **, P ≤ 0.0049, ***P ≤ 0.0004 (between selected groups). For IFN-γ, significance is indicated as follows: **, P ≤ 0.0025 between infected CD4 −/− plus IFN-γ−/− CD4+ cells versus infected wild-type or infected CD4−/− mice; ***, P ≤ 0.0004 (betwen selected groups).
DISCUSSION
These studies demonstrate that neonatal mice exposed to Y. enterocolitica through the gastrointestinal tract mount robust B-cell and inflammatory CD4+ Th responses associated with protective immunity. This contrasts with our earlier finding (20) that neonates are highly susceptible to Y. enterocolitica introduced parenterally. Therefore, the neonatal intestinal immune system may be far better equipped than the systemic immune system to respond effectively to this pathogen. Exposure at mucosal surfaces, in general, may elicit vigorous immunity since vaccines administered through mucosal routes can more readily induce mature immune responses during neonatal life than vaccines administered through systemic routes (11, 12, 34). For the first time, we demonstrate the development of both high Th1 and Th17 function in the neonatal intestine to a fully virulent infectious bacterium in the absence of additional interventions. Based on the current understanding that immune responsiveness during the neonatal period of life can be modulated extensively by targeting the mucosal immune system (1, 8, 11, 12, 27, 29, 34, 35, 59, 70) and by using strong immunomodulatory agents (31, 36, 51), we favor the idea that robust immunity to Y. enterocolitica is a function of both the inflammatory potential of this pathogen and an intrinsic capacity of the neonatal intestine to develop high-level inflammation. These data support the idea that the development of inflammatory CD4+ Th responses may contribute substantially to protection of neonatal mice from gastrointestinal infection.
It is noteworthy that live attenuated bacterial vectors such as L. monocytogenes and Salmonella species are highly efficacious at enhancing all arms of the neonatal immune system to the vaccine antigens they deliver (16, 17, 42, 50, 59). However, when neonates encounter either virulent pathogen in the absence of vaccination, their responses are suboptimal and inefficient in eradicating the pathogen (15, 25, 31, 61). In contrast, neonatal innate and adaptive immunity to fully virulent Y. enterocolitica does not appear to be compromised. This suggests that intrinsic differences between pathogenic L. monocytogenes and Salmonella species and Y. enterocolitica may play a role in the enhanced immune response elicited in neonates to Y. enterocolitica. One potential explanation for these differences may lie in the ability of Y. enterocolitica to survive extracellularly, unlike L. monocytogenes and Salmonella species, which replicate intracellularly within myelomonocytic cells. This may provide a protective niche for these pathogens as hijacking innate cells, recognized to be important in the initiation of immune responses, may hinder the development of protective immunity in neonates. Given that Y. enterocolitica replicates primarily extracellularly (73), improvements in both the innate and adaptive arms of the immune system would render the bacteria more prone to be recognized by cellular and humoral immune components.
In adult mice, it is widely accepted that elimination of Y. enterocolitica relies on Th1 immunity (7) and IFN-γ (6). In our studies, IFN-γ−/− and CD4−/− neonates were particularly sensitive to infection with a low bacterial dose. While, CD4+ cells were important after the second week of infection, IFN-γ was required during the acute phase of the immune response. This observation is in keeping with the essential role of IFN-γ and IFN-γ-responsive chemokines and cytokines during neonatal infection with Salmonella enterica serovar Typhimurium (61). We hypothesize that shortly after Y. enterocolitica infection, immune cells other than CD4+ cells may serve as intestinal sources of IFN-γ. In neonatal rats, natural killer (NK) and NKT cells are abundant in the intestine during the first weeks of life (54), and there is evidence that intraepithelial NK cells (38) and CD8+ T cells (45, 69) produce IFN-γ in response to pathogens or Toll-like receptor (TLR) ligands. Hence, the predominance of innate cells capable of rapidly producing IFN-γ might serve as the first line of defense against pathogens in the intestinal mucosa, in the absence of established adaptive immunity.
Adoptive transfer of adult CD4+ cells into CD4−/− neonates protected infected mice from mortality and reduced Y. enterocolitica levels in the small intestine. However, this protection appeared to be independent of the ability of transferred Th CD4+ cells to produce either IFN-γ or IL-17AF in vivo. Although in these adoptive transfer studies we cannot exclude the possibility of a protective role for IL-17AF in the absence of IFN-γ and vice versa, our studies underscore a crucial role for inflammatory CD4+ T cells in mediating protection against this pathogen during neonatal life. It is conceivable that the ability to mount a protective response to Y. enterocolitica in neonates may rely on other molecules produced by the transferred IFN-γ−/− or IL-17AF−/− CD4+ cells. This possibility is in agreement with the potential function of cytokines such as IL-22, which may be produced by CD4+ CD3− lymphoid tissue inducer cells (68), Th1, and Th17 cells (49), in promoting antibacterial molecules in the gut mucosa (77).
Studies in neonatal mice have found that reduced levels of T-cell-derived IFN-γ were associated with expansion of CD4+ CD25+ Foxp3+ Treg cells following infection with rotavirus (39) and HSV-2 (22). Infection with Y. enterocolitica did not appear to increase the levels of CD4+ Foxp3+ Treg cells in the MLN of infected neonates and adults above those of uninfected mice. However, CD4+ CD25+ cells were functionally competent in vitro. The loss of CD25+ Foxp3+ cells within neonatal and adult CD4+ cells enhanced the production of IFN-γ; IL-17A production by adult cells was consistently increased relative to neonatal cells. It is possible that in neonates, the natural delay in the development of CD4+ CD25+ Foxp3+ cells may favor the development of pathogen-specific Th17 cells. During neonatal life the levels of CD4+ Th17 cells in the lamina propria are low (33), and their expansion during ontogeny may be related to the presence of specific symbiotic bacteria in the intestine (32, 33). It is likely that Y. enterocolitica targets similar signaling pathways as those induced naturally by the resident intestinal microbial species. Moreover, our results suggest that Y. enterocolitica readily induces Th17-promoting cytokines in the neonatal gut, providing indirect evidence in vivo for the preferential production in vitro of IL-6 (41, 47, 74) and IL-23 (41, 71) by neonatal APC relative to adult APC. Currently, we are focusing on identifying the cell types, the bacterially derived products, and the signaling pathways that may be at play in the neonatal immune response to this pathogen.
The proportion of mice that excreted the bacteria in the stools was greater in neonatal than in adult mice. These data indicate that neonates are exposed for a longer period of time to a higher bacterial burden in the intestinal tissue than adults. This prolonged antigen exposure may provide a window of opportunity for development of adaptive immunity in neonates. T- and B-cell immunity to live attenuated Salmonella vectors is enhanced by increasing the bacterial levels during neonatal immunization (17). In our studies, T- and B-cell immunity was reduced in adults infected with a bacterial dose similar to that used to prime neonates. Once adult mice were infected with higher bacterial titers, the inflammatory capacity of adult MLN CD4+ Th cells and the ability to produce IgG1 improved. This phenomenon had been previously observed in adult mice infected with L. monocytogenes-expressing ovalbumin (OVA) and treated with antibiotics to reduce the bacterial burden (75). Therefore, stimulation of the gastrointestinal immune system may require high antigenic loads or longer exposure for development of potent T- and B-cell immunity.
Robust B-cell immunity was induced in neonates, as assessed by high-level systemic LcrV-reactive antibodies during the primary infection and memory IgG antibodies similar in quality and quantity to those of adults. These results are unprecedented in the context of infection of neonatal animals with a virulent pathogen. Although high levels of antibody production in neonates can be generated following mucosal vaccination (11, 16, 59), our studies underscore the great potential of the neonatal intestinal immune system to promote systemic antibodies that may equal or even exceed adult levels in response to infection. To the best of our knowledge, anti-LcrV responses have been reported only in vaccinated adult mice (13, 18) or after intranasal delivery to neonatal mice with a vector expressing LcrV (60). Here, we demonstrate that LcrV is also immunogenic through the gastrointestinal tract in response to natural infection.
We believe that this neonatal infection model will be invaluable for expanding our understanding of the development of intestinal immunological networks in early life. Importantly, it provides a biological platform to discover novel Yersinia-derived molecules with adjuvant properties that could be used for development of more effective mucosal vaccines aimed at preventing gastrointestinal disease in the pediatric population.
Acknowledgments
We thank David S. Waugh, National Cancer Institute, Frederick, MD, for providing the vector used for expression of MBP-LcrV, Yoichiro Iwakura, University of Tokyo, for providing the IL-17AF−/− mice, and Patricia Guevara for technical assistance in the adoptive transfer experiments.
This work was supported by grant R01 AI44923 from the National Institute of Allergy and Infectious Diseases (NIAID) (B.A.), grant R01 AI53459 from the NIAID (K.S.), and the Department of Microbiology and Immunology, University of Miami Miller School of Medicine, Miami, FL.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 1 June 2010.
REFERENCES
- 1.Abolhassani, M., M. Lagranderie, I. Caminshi, F. Romain, A. M. Balazuc, M. C. Wagner, M. Tanguy, L. Fiette, I. Sobhani, G. Milon, and G. Marchal. 2006. Similar functional activity of dendritic cells recruited to the mesenteric lymph nodes of newborn and adult mice after the rectal delivery of Mycobacterium bovis BCG. Microbes Infect. 8:2341-2351. [DOI] [PubMed] [Google Scholar]
- 2.Adkins, B., Y. Bu, E. Cepero, and R. Perez. 2000. Exclusive Th2 primary effector function in spleens but mixed Th1/Th2 function in lymph nodes of murine neonates. J. Immunol. 164:2347-2353. [DOI] [PubMed] [Google Scholar]
- 3.Adkins, B., Y. Bu, and P. Guevara. 2001. The generation of Th memory in neonates versus adults: prolonged primary Th2 effector function and impaired development of Th1 memory effector function in murine neonates. J. Immunol. 166:918-925. [DOI] [PubMed] [Google Scholar]
- 4.Adkins, B., Y. Bu, and P. Guevara. 2002. Murine neonatal CD4+ lymph node cells are highly deficient in the development of antigen-specific Th1 function in adoptive adult hosts. J. Immunol. 169:4998-5004. [DOI] [PubMed] [Google Scholar]
- 5.Adkins, B., C. Leclerc, and S. Marshall-Clarke. 2004. Neonatal adaptive immunity comes of age. Nat. Rev. Immunol. 4:553-564. [DOI] [PubMed] [Google Scholar]
- 6.Autenrieth, I. B., V. Kempf, T. Sprinz, S. Preger, and A. Schnell. 1996. Defense mechanisms in Peyer's patches and mesenteric lymph nodes against Yersinia enterocolitica involve integrins and cytokines. Infect. Immun. 64:1357-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Autenrieth, I. B., A. Tingle, A. Reske-Kunz, and J. Heesemann. 1992. T lymphocytes mediate protection against Yersinia enterocolitica in mice: characterization of murine T-cell clones specific for Y. enterocolitica. Infect. Immun. 60:1140-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrier, M., S. Lacroix-Lamande, R. Mancassola, G. Auray, N. Bernardet, A. M. Chausse, S. Uematsu, S. Akira, and F. Laurent. 2006. Oral and intraperitoneal administration of phosphorothioate oligodeoxynucleotides leads to control of Cryptosporidium parvum infection in neonatal mice. J. Infect. Dis. 193:1400-1407. [DOI] [PubMed] [Google Scholar]
- 9.Barrios, C., P. Brawand, M. Berney, C. Brandt, P. H. Lambert, and C. A. Siegrist. 1996. Neonatal and early life immune responses to various forms of vaccine antigens qualitatively differ from adult responses: predominance of a Th2-biased pattern which persists after adult boosting. Eur. J. Immunol. 26:1489-1496. [DOI] [PubMed] [Google Scholar]
- 10.Baselski, V., R. Briggs, and C. Parker. 1977. Intestinal fluid accumulation induced by oral challenge with Vibrio cholerae or cholera toxin in infant mice. Infect. Immun. 15:704-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bjarnarson, S. P., H. Jakobsen, G. Del Giudice, E. Trannoy, C. A. Siegrist, and I. Jonsdottir. 2005. The advantage of mucosal immunization for polysaccharide-specific memory responses in early life. Eur. J. Immunol. 35:1037-1045. [DOI] [PubMed] [Google Scholar]
- 12.Bowman, L. M., and P. G. Holt. 2001. Selective enhancement of systemic Th1 immunity in immunologically immature rats with an orally administered bacterial extract. Infect. Immun. 69:3719-3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Branger, C. G., A. Torres-Escobar, W. Sun, R. Perry, J. Fetherston, K. L. Roland, and R. Curtiss III. 2009. Oral vaccination with LcrV from Yersinia pestis KIM delivered by live attenuated Salmonella enterica serovar Typhimurium elicits a protective immune response against challenge with Yersinia pseudotuberculosis and Yersinia enterocolitica. Vaccine 27:5363-5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brynjolfsson, S. F., S. P. Bjarnarson, E. Mori, G. Del Giudice, and I. Jonsdottir. 2008. Neonatal immune response and serum bactericidal activity induced by a meningococcal conjugate vaccine is enhanced by LT-K63 and CpG2006. Vaccine 26:4557-4562. [DOI] [PubMed] [Google Scholar]
- 15.Burns-Guydish, S. M., I. N. Olomu, H. Zhao, R. J. Wong, D. K. Stevenson, and C. H. Contag. 2005. Monitoring age-related susceptibility of young mice to oral Salmonella enterica serovar Typhimurium infection using an in vivo murine model. Pediatr. Res. 58:153-158. [DOI] [PubMed] [Google Scholar]
- 16.Burns-Guydish, S. M., H. Zhao, D. K. Stevenson, and C. H. Contag. 2007. The potential Salmonella aroA− vaccine strain is safe and effective in young BALB/c mice. Neonatology 91:114-120. [DOI] [PubMed] [Google Scholar]
- 17.Capozzo, A. V., L. Cuberos, M. M. Levine, and M. F. Pasetti. 2004. Mucosally delivered Salmonella live vector vaccines elicit potent immune responses against a foreign antigen in neonatal mice born to naive and immune mothers. Infect. Immun. 72:4637-4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daniel, C., F. Sebbane, S. Poiret, D. Goudercourt, J. Dewulf, C. Mullet, M. Simonet, and B. Pot. 2009. Protection against Yersinia pseudotuberculosis infection conferred by a Lactococcus lactis mucosal delivery vector secreting LcrV. Vaccine 27:1141-1144. [DOI] [PubMed] [Google Scholar]
- 19.Duchet-Suchaux, M., C. Le Maitre, and A. Bertin. 1990. Differences in susceptibility of inbred and outbred infant mice to enterotoxigenic Escherichia coli of bovine, porcine and human origin. J. Med. Microbiol. 31:185-190. [DOI] [PubMed] [Google Scholar]
- 20.Echeverry, A., K. Schesser, and B. Adkins. 2007. Murine neonates are highly resistant to Yersinia enterocolitica following orogastric exposure. Infect. Immun. 75:2234-2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fadel, S., and M. Sarzotti. 2000. Cellular immune responses in neonates. Int. Rev. Immunol. 19:173-193. [DOI] [PubMed] [Google Scholar]
- 22.Fernandez, M. A., F. K. Puttur, Y. M. Wang, W. Howden, S. I. Alexander, and C. A. Jones. 2008. T regulatory cells contribute to the attenuated primary CD8+ and CD4+ T cell responses to herpes simplex virus type 2 in neonatal mice. J. Immunol. 180:1556-1564. [DOI] [PubMed] [Google Scholar]
- 23.Fernandez, M. I., A. Thuizat, T. Pedron, M. Neutra, A. Phalipon, and P. J. Sansonetti. 2003. A newborn mouse model for the study of intestinal pathogenesis of shigellosis. Cell Microbiol. 5:481-491. [DOI] [PubMed] [Google Scholar]
- 24.Garvy, B. A., and A. G. Harmsen. 1996. The importance of neutrophils in resistance to pneumococcal pneumonia in adult and neonatal mice. Inflammation 20:499-512. [DOI] [PubMed] [Google Scholar]
- 25.Genovese, F., G. Mancuso, M. Cuzzola, C. Biondo, C. Beninati, D. Delfino, and G. Teti. 1999. Role of IL-10 in a neonatal mouse listeriosis model. J. Immunol. 163:2777-2782. [PubMed] [Google Scholar]
- 26.Reference deleted.
- 27.Hale, C., I. R. Humphreys, T. Hussell, F. Bowe, S. Clare, D. Pickard, A. Preston, G. Del Giudice, and G. Dougan. 2004. Mucosal immunisation of murine neonates using whole cell and acellular pertussis vaccines. Vaccine 22:3595-3602. [DOI] [PubMed] [Google Scholar]
- 28.Handley, S. A., P. H. Dube, and V. L. Miller. 2006. Histamine signaling through the H2 receptor in the Peyer's patch is important for controlling Yersinia enterocolitica infection. Proc. Natl. Acad. Sci. U. S. A. 103:9268-9273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang, C. F., C. C. Wang, T. C. Wu, K. G. Wu, C. C. Lee, and H. J. Peng. 2008. Neonatal sublingual vaccination with Salmonella proteins and adjuvant cholera toxin or CpG oligodeoxynucleotides induces mucosal and systemic immunity in mice. J. Pediatr. Gastroenterol. Nutr. 46:262-271. [DOI] [PubMed] [Google Scholar]
- 30.Ishigame, H., S. Kakuta, T. Nagai, M. Kadoki, A. Nambu, Y. Komiyama, N. Fujikado, Y. Tanahashi, A. Akitsu, H. Kotaki, K. Sudo, S. Nakae, C. Sasakawa, and Y. Iwakura. 2009. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity 30:108-119. [DOI] [PubMed] [Google Scholar]
- 31.Ito, S., K. J. Ishii, M. Gursel, H. Shirotra, A. Ihata, and D. M. Klinman. 2005. CpG oligodeoxynucleotides enhance neonatal resistance to Listeria infection. J. Immunol. 174:777-782. [DOI] [PubMed] [Google Scholar]
- 32.Ivanov, I. I., K. Atarashi, N. Manel, E. L. Brodie, T. Shima, U. Karaoz, D. Wei, K. C. Goldfarb, C. A. Santee, S. V. Lynch, T. Tanoue, A. Imaoka, K. Itoh, K. Takeda, Y. Umesaki, K. Honda, and D. R. Littman. 2009. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139:485-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ivanov, I. I., L. Frutos Rde, N. Manel, K. Yoshinaga, D. B. Rifkin, R. B. Sartor, B. B. Finlay, and D. R. Littman. 2008. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe 4:337-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jakobsen, H., S. Bjarnarson, G. Del Giudice, M. Moreau, C. A. Siegrist, and I. Jonsdottir. 2002. Intranasal immunization with pneumococcal conjugate vaccines with LT-K63, a nontoxic mutant of heat-labile enterotoxin, as adjuvant rapidly induces protective immunity against lethal pneumococcal infections in neonatal mice. Infect. Immun. 70:1443-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jupelli, M., M. N. Guentzel, P. A. Meier, G. Zhong, A. K. Murthy, and B. P. Arulanandam. 2008. Endogenous IFN-γ production is induced and required for protective immunity against pulmonary chlamydial infection in neonatal mice. J. Immunol. 180:4148-4155. [DOI] [PubMed] [Google Scholar]
- 36.Kamath, A. T., A.-F. Rochat, M. P. Valenti, E. M. Agger, K. Lingnau, P. Andersen, P.-H. Lambert, and C.-A. Siegrist. 2008. Adult-like anti-mycobacterial T cell and in vivo dendritic cell responses following neonatal immunization with Ag85B-ESAT-6 in the IC31 adjuvant. PLoS One 3:e3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kamath, A. T., A. F. Rochat, D. Christensen, E. M. Agger, P. Andersen, P. H. Lambert, and C. A. Siegrist. 2009. A Liposome-based mycobacterial vaccine induces potent adult and neonatal multifunctional T cells through the exquisite targeting of dendritic cells. PLoS One 4:e5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keilbaugh, S. A., M. E. Shin, R. F. Banchereau, L. D. McVay, N. Boyko, D. Artis, J. J. Cebra, and G. D. Wu. 2005. Activation of RegIIIβ/γ and interferon-γ expression in the intestinal tract of SCID mice: an innate response to bacterial colonisation of the gut. Gut 54:623-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim, B., N. Feng, C. F. Narvaez, X. S. He, S. K. Eo, C. W. Lim, and H. B. Greenberg. 2008. The influence of CD4+ CD25+ Foxp3+ regulatory T cells on the immune response to rotavirus infection. Vaccine 26:5601-5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kitamura, D., J. Roes, R. Kuhn, and K. Rajewsky. 1991. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin μ chain gene. Nature 350:423-426. [DOI] [PubMed] [Google Scholar]
- 41.Kollmann, T. R., J. Crabtree, A. Rein-Weston, D. Blimkie, F. Thommai, X. Y. Wang, P. M. Lavoie, J. Furlong, E. S. Fortuno III, A. M. Hajjar, N. R. Hawkins, S. G. Self, and C. B. Wilson. 2009. Neonatal innate TLR-mediated responses are distinct from those of adults. J. Immunol. 183:7150-7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kollmann, T. R., B. Reikie, D. Blimkie, S. S. Way, A. M. Hajjar, K. Arispe, A. Shaulov, and C. B. Wilson. 2007. Induction of protective immunity to Listeria monocytogenes in neonates. J. Immunol. 178:3695-3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Korn, T., M. Mitsdoerffer, A. L. Croxford, A. Awasthi, V. A. Dardalhon, G. Galileos, P. Vollmar, G. L. Stritesky, M. H. Kaplan, A. Waisman, V. K. Kuchroo, and M. Oukka. 2008. IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells. Proc. Natl. Acad. Sci. U. S. A. 105:18460-18465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kovarik, J., M. Gaillard, X. Martinez, P. Bozzotti, P. H. Lambert, T. F. Wild, and C. A. Siegrist. 2001. Induction of adult-like antibody, Th1, and CTL responses to measles hemagglutinin by early life murine immunization with an attenuated vaccinia-derived NYVAC(K1L) viral vector. Virology 285:12-20. [DOI] [PubMed] [Google Scholar]
- 45.Leav, B. A., M. Yoshida, K. Rogers, S. Cohen, N. Godiwala, R. S. Blumberg, and H. Ward. 2005. An early intestinal mucosal source of gamma interferon is associated with resistance to and control of Cryptosporidium parvum infection in mice. Infect. Immun. 73:8425-8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee, H. H., C. M. Hoeman, J. C. Hardaway, F. B. Guloglu, J. S. Ellis, R. Jain, R. Divekar, D. M. Tartar, C. L. Haymaker, and H. Zaghouani. 2008. Delayed maturation of an IL-12-producing dendritic cell subset explains the early Th2 bias in neonatal immunity. J. Exp. Med. 205:2269-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Levy, O., E. E. Suter, R. L. Miller, and M. R. Wessels. 2006. Unique efficacy of Toll-like receptor 8 agonists in activating human neonatal antigen-presenting cells. Blood 108:1284-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li, L., H. H. Lee, J. J. Bell, R. K. Gregg, J. S. Ellis, A. Gessner, and H. Zaghouani. 2004. IL-4 utilizes an alternative receptor to drive apoptosis of Th1 cells and skews neonatal immunity toward Th2. Immunity 20:429-440. [DOI] [PubMed] [Google Scholar]
- 49.Liang, S. C., X.-Y. Tan, D. P. Luxenberg, R. Karim, K. Dunussi-Joannopoulos, M. Collins, and L. A. Fouser. 2006. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 203:2271-2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Loeffler, D. I., K. Smolen, L. Aplin, B. Cai, and T. R. Kollmann. 2009. Fine-tuning the safety and immunogenicity of Listeria monocytogenes-based neonatal vaccine platforms. Vaccine 27:919-927. [DOI] [PubMed] [Google Scholar]
- 51.Ma, Y., and A. C. Ross. 2005. The anti-tetanus immune response of neonatal mice is augmented by retinoic acid combined with polyriboinosinic:polyribocytidylic acid. Proc. Natl. Acad. Sci. U. S. A. 102:13556-13561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Macpherson, A. J., and K. Smith. 2006. Mesenteric lymph nodes at the center of immune anatomy. J. Exp. Med. 203:497-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marchant, A., T. Goetghebuer, M. O. Ota, I. Wolfe, S. J. Ceesay, D. De Groote, T. Corrah, S. Bennett, J. Wheeler, K. Huygen, P. Aaby, K. P. McAdam, and M. J. Newport. 1999. Newborns develop a Th1-type immune response to Mycobacterium bovis bacillus Calmette-Guerin vaccination. J. Immunol. 163:2249-2255. [PubMed] [Google Scholar]
- 54.Marin-Gallen, S., F. J. Perez-Cano, M. Castell, C. Castellote, and A. Franch. 2008. Intestinal intraepithelial NK and NKT cell ontogeny in Lewis rats. Dev. Comp. Immunol. 32:1405-1408. [DOI] [PubMed] [Google Scholar]
- 55.Martinez, X., C. Brandt, F. Saddallah, C. Tougne, C. Barrios, F. Wild, G. Dougan, P. H. Lambert, and C. A. Siegrist. 1997. DNA immunization circumvents deficient induction of T helper type 1 and cytotoxic T lymphocyte responses in neonates and during early life. Proc. Natl. Acad. Sci. U. S. A. 94:8726-8731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Min, B., R. McHugh, G. D. Sempowski, C. Mackall, G. Foucras, and W. E. Paul. 2003. Neonates support lymphopenia-induced proliferation. Immunity 18:131-140. [DOI] [PubMed] [Google Scholar]
- 57.Pihlgren, M., C. Tougne, P. Bozzotti, A. Fulurija, M. A. Duchosal, P. H. Lambert, and C. A. Siegrist. 2003. Unresponsiveness to lymphoid-mediated signals at the neonatal follicular dendritic cell precursor level contributes to delayed germinal center induction and limitations of neonatal antibody responses to T-dependent antigens. J. Immunol. 170:2824-2832. [DOI] [PubMed] [Google Scholar]
- 58.Reference deleted.
- 59.Ramirez, K., A. V. E. Capozzo, S. A. Lloyd, M. B. Sztein, J. P. Nataro, and M. F. Pasetti. 2009. Mucosally delivered Salmonella typhi expressing the Yersinia pestis F1 antigen elicits mucosal and systemic immunity early in life and primes the neonatal immune system for a vigorous anamnestic response to parenteral F1 boost. J. Immunol. 182:1211-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ramirez, K., Y. Ditamo, L. Rodriguez, W. L. Picking, M. L. van Roosmalen, K. Leenhouts, and M. F. Pasetti. 2010. Neonatal mucosal immunization with a non-living, non-genetically modified Lactococcus lactis vaccine carrier induces systemic and local Th1-type immunity and protects against lethal bacterial infection. Mucosal Immunol. 3:159-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rhee, S. J., W. A. Walker, and B. J. Cherayil. 2005. Developmentally regulated intestinal expression of IFN-γ and its target genes and the age-specific response to enteric Salmonella infection. J. Immunol. 175:1127-1136. [DOI] [PubMed] [Google Scholar]
- 62.Sarzotti, M., T. A. Dean, M. P. Remington, C. D. Ly, P. A. Furth, and D. S. Robbins. 1997. Induction of cytotoxic T cell responses in newborn mice by DNA immunization. Vaccine 15:795-797. [DOI] [PubMed] [Google Scholar]
- 63.Schulz, S. M., G. Kohler, C. Holscher, Y. Iwakura, and G. Alber. 2008. IL-17A is produced by Th17, γδ T cells and other CD4− lymphocytes during infection with Salmonella enterica serovar Enteritidis and has a mild effect in bacterial clearance. Int. Immunol. 20:1129-1138. [DOI] [PubMed] [Google Scholar]
- 64.Shimomura, Y., A. Ogawa, M. Kawada, K. Sugimoto, E. Mizoguchi, H.-N. Shi, S. Pillai, A. K. Bhan, and A. Mizoguchi. 2008. A unique B2 B cell subset in the intestine. J. Exp. Med. 205:1343-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Siegrist, C.-A., and R. Aspinall. 2009. B-cell responses to vaccination at the extremes of age. Nat. Rev. Immunol. 9:185-194. [DOI] [PubMed] [Google Scholar]
- 66.Siegrist, C. A. 2001. Neonatal and early life vaccinology. Vaccine 19:3331-3346. [DOI] [PubMed] [Google Scholar]
- 67.Sun, C.-M., L. Fiette, M. Tanguy, C. Leclerc, and R. Lo-Man. 2003. Ontogeny and innate properties of neonatal dendritic cells. Blood 102:585-591. [DOI] [PubMed] [Google Scholar]
- 68.Takatori, H., Y. Kanno, W. T. Watford, C. M. Tato, G. Weiss, I. I. Ivanov, D. R. Littman, and J. J. O'Shea. 2009. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J. Exp. Med. 206:35-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tourais-Esteves, I., N. Bernardet, S. Lacroix-Lamandé, S. Ferret-Bernard, and F. Laurent. 2008. Neonatal goats display a stronger Th1-type cytokine response to TLR ligands than adults. Dev. Comp. Immunol. 32:1231-1241. [DOI] [PubMed] [Google Scholar]
- 70.VanCott, J. L., A. E. Prada, M. M. McNeal, S. C. Stone, M. Basu, B. Huffer, Jr., K. L. Smiley, M. Shao, J. A. Bean, J. D. Clements, A. H. C. Choi, and R. L. Ward. 2006. Mice develop effective but delayed protective immune responses when immunized as neonates either intranasally with nonliving VP6/LT(R192G) or orally with live rhesus rotavirus vaccine candidates. J. Virol. 80:4949-4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vanden Eijnden, S., S. Goriely, D. De Wit, M. Goldman, and F. Willems. 2006. Preferential production of the IL-12(p40)/IL-23(p19) heterodimer by dendritic cells from human newborns. Eur. J. Immunol. 36:21-26. [DOI] [PubMed] [Google Scholar]
- 72.Vekemans, J., A. Amedei, M. O. Ota, M. M. D'Elios, T. Goetghebuer, J. Ismaili, M. J. Newport, G. Del Prete, M. Goldman, K. P. McAdam, and A. Marchant. 2001. Neonatal bacillus Calmette-Guerin vaccination induces adult-like IFN-γ production by CD4+ T lymphocytes. Eur. J. Immunol. 31:1531-1535. [DOI] [PubMed] [Google Scholar]
- 73.Viboud, G. I., and J. B. Bliska. 2005. Yersinia outer proteins: role in modulation of host cell signaling responses and pathogenesis. Annu. Rev. Microbiol. 59:69-89. [DOI] [PubMed] [Google Scholar]
- 74.Walker, W. E., and D. R. Goldstein. 2007. Neonatal B cells suppress innate Toll-like receptor immune responses and modulate alloimmunity. J. Immunol. 179:1700-1710. [DOI] [PubMed] [Google Scholar]
- 75.Williams, M. A., and M. J. Bevan. 2004. Shortening the infectious period does not alter expansion of CD8 T cells but diminishes their capacity to differentiate into memory cells. J. Immunol. 173:6694-6702. [DOI] [PubMed] [Google Scholar]
- 76.Wong, C. Y., G. Mayrhofer, M. W. Heuzenroeder, H. M. Atkinson, D. M. Quinn, and R. L. Flower. 1996. Measurement of virulence of aeromonads using a suckling mouse model of infection. FEMS Immunol. Med. Microbiol. 15:233-241. [DOI] [PubMed] [Google Scholar]
- 77.Zheng, Y., P. A. Valdez, D. M. Danilenko, Y. Hu, S. M. Sa, Q. Gong, A. R. Abbas, Z. Modrusan, N. Ghilardi, F. J. de Sauvage, and W. Ouyang. 2008. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 14:282-289. [DOI] [PubMed] [Google Scholar]
- 78.Zhou, L., J. E. Lopes, M. M. Chong, I. I. Ivanov, R. Min, G. D. Victora, Y. Shen, J. Du, Y. P. Rubtsov, A. Y. Rudensky, S. F. Ziegler, and D. R. Littman. 2008. TGF-β-induced Foxp3 inhibits TH17 cell differentiation by antagonizing RORγΤ function. Nature 453:236-240. [DOI] [PMC free article] [PubMed] [Google Scholar]