Abstract
In the inflamed retina, CD4+ T cells can cause retinal damage when they are not properly regulated. Since tissue expression of major histocompatibility complex (MHC) class II and costimulatory molecules is a key mechanism for regulating effector T cells, we tested the hypothesis that upregulation of these proteins in the retina contributes to the regulation of CD4 T cells. Here we report that in retinas infected with the protozoan parasite Toxoplasma gondii, MHC class II is upregulated on infiltrating leukocytes as well as on resident retinal cells, including photoreceptors. Flow cytometric analysis indicated that B7 costimulatory family members (CD80, CD86, ICOS-L, and programmed death ligand 2 [PD-L2]) were not expressed on class II+ cells. In contrast, PD-L1 (also named B7-H1 or CD274) was expressed on the majority of both hematopoietic and resident retinal MHC class II-expressing cells. Retinal cells from Toxoplasma-infected animals were able to suppress T-cell activation in a PD-L1-dependent manner. Finally, we demonstrate that the expression of MHC class II and PD-L1 was critically dependent on gamma interferon (IFN-γ) expression. These data suggest that retinal MHC class II and PD-L1 expression is a novel mechanism by which the retina protects itself from CD4 T-cell-mediated immune damage in ocular toxoplasmosis and other types of retinal immune responses.
Toxoplasma gondii is an obligate intracellular protozoan parasite that infects humans and animals either congenitally or postnatally (38, 42). There are no vaccines to prevent this infection, and currently prescribed therapies are poorly tolerated and have severe side effects. In addition, the parasite responds to these drugs as well as to the host's immune response by transforming into quiescent, asymptomatic cysts in tissues such as the eye, brain, and muscle (54). These cysts occasionally reactivate, and the released parasites will cause disease unless a properly regulated immune response is mounted. The retina is a common site for cyst reactivation and causes a disease, called ocular toxoplasmosis, that is one of the most common infections of the posterior retina (21).
The ability of a host to control a reactivated Toxoplasma infection is dependent largely on CD8+ T cells and, to a lesser extent, on CD4+ T cells (13, 43, 52). However, CD4+ T cells must be tightly regulated, because these cells cause immune-mediated tissue destruction in the eye and other tissues (23, 32, 36). Because damaged retinal cells cannot be repaired or regenerated, it is imperative to understand how CD4+ T cells are regulated in the retina in ocular toxoplasmosis. As central regulators of CD4+ T cells, major histocompatibility complex (MHC) class II-expressing cells are likely to be critical in controlling CD4-based T-cell responses.
MHC class II expression and function in the retina have been the source of some debate. This is because retinal MHC class II expression is low as a result of the high expression of immunosuppressive cytokines and the blood-ocular barrier (49). Regardless, retinal microglia, endothelial cells, retinal pigment epithelial (RPE) cells, and other, less well characterized cells can express MHC class II under certain conditions (18, 25, 57). But the identity and function of MHC class II-expressing cells in Toxoplasma-infected retinas remain unknown.
At sites of inflammation, CD4+ T cells are regulated by two distinct signals from their target cells. First, they must come in contact with a cell expressing peptide-loaded MHC class II. Second, costimulatory molecules dictate the response of the engaged T cells. One major group of costimulatory molecules is the B7 protein family, which includes CD80, CD86, and ICOS-L (16). These proteins, which bind CD28 (CD80 and CD86) or ICOS (ICOS-L), are important for resistance to Toxoplasma (51, 56). Two additional B7 family members, programmed death ligand 1 (PD-L1) and PD-L2, bind the T-cell surface protein PD-1 and downregulate activated T cells (16). While PD-L1 and PD-L2 are functionally redundant, they have distinct expression patterns; PD-L2 is expressed primarily by cells derived from hematopoietic cells, and PD-L1 is expressed on hematopoietic and nonhematopoietic cells. In mice and humans, PD-L1 but not PD-L2 is expressed in various regions of the eye, including the cornea, ciliary body, iris, and retina (22, 48, 58). Ocular PD-L1 expression is important for the prevention of corneal allograft rejection, suggesting that it is a key player in maintaining immune privilege in the anterior chamber of the eye (22, 48). However, the importance of PD-L1 in the posterior chamber in inflammatory events such as Toxoplasma infections is unknown.
Here we report that CD4 recall responses to Toxoplasma antigen are blocked by retinal cells isolated from intravitreally infected mice. Flow cytometric and immunohistochemical analyses demonstrate that both infiltrating leukocytes and resident retinal cells expressed MHC class II and that MHC class II expression was dependent on gamma interferon (IFN-γ). Surprisingly, an overwhelming majority of the MHC class II-expressing cells did not express positively acting costimulatory molecules but rather expressed PD-L1. Finally, the suppressive effect of parasite-infected retinal cells on T cells was PD-L1 dependent.
MATERIALS AND METHODS
Parasites.
The RH and cpsII pyrimidine auxotrophic Toxoplasma strains were grown in human foreskin fibroblasts as previously described (7, 11). To prepare parasites for injections, infected monolayers were scraped and parasites were mechanically released from their host cells by being passed three times through a 27-gauge syringe needle. The parasite suspension was filtered through a 3-μm-pore-size polycarbonate filter to separate parasites from host cells. Parasites were extensively washed in phenol red-free Dulbecco's modified Eagle medium (DMEM) and were then resuspended in phenol red-free DMEM. Soluble tachyzoite antigen (STAg) was prepared in the presence of a protease inhibitor cocktail (Calbiochem) as previously described (27), except that the lysate was centrifuged at 16,000 × g for 20 min at 4°C.
Mice and intravitreal infections.
Injections were performed as described previously (7). Needles with a tip size of no more than 50 μm were loaded with a parasite suspension using a pneumatic pump delivery system. Six- to 12-week-old C57BL/6 or IFN-γ−/− mice (purchased from Jackson Labs, Bar Harbor, ME) were anesthetized with ketamine-xylazine, and then the needle was inserted immediately behind the limbus-parallel conjunctival vessels. After the needle was inserted, 0.5 μl of the parasite suspension or phenol red-free DMEM (as a mock control) was injected. Mice were intraperitoneally vaccinated with 104 irradiated cpsII parasites. All protocols adhered to University of Oklahoma Health Sciences Center's IACUC and to ARVO's Statement on the Use of Animals in Ophthalmic and Vision Research.
Flow cytometry.
Mice were sacrificed by CO2 asphyxiation, and their eyes were immediately harvested, placed in phosphate-buffered saline (PBS), and mechanically disrupted with a stomacher (Tekmar, Cincinnati, OH). The cell suspension was then passed through a 40-μm-pore-size mesh to remove the lens and eye cup. Cells were incubated first in Fc block (BD Biosciences, San Jose, CA) and then with the following antibodies (or with appropriate isotype controls): (i) fluorescein isothiocyanate (FITC)-conjugated antibodies against Ly6C (clone AL-21; BD Biosciences), CD80 (clone 16-10A1; eBiosciences), CD86 (clone RMMP-2; Invitrogen), Siglec-H (clone eBIO440C; eBioscience), CD45 (clone 30-F11; Invitrogen), CD11c (clone N418; Invitrogen) (conjugated with Alexa Fluor 488), or I-Ab (clone AF6-120.1; BD Biosciences); (ii) phycoerythrin (PE)-conjugated antibodies against Ly6G (clone 1A8; BD Biosciences), PD-L1 (clone M1H5; eBiosciences), PDCA (clone eBio927; eBiosciences), ICOS-L (clone HK5.3; eBiosciences), or B220 (clone RA3-6B2; eBiosciences); (iii) a Tri-Color-conjugated antibody against CD8 (clone CT-CD8α; Invitrogen); (iv) a peridinin chlorophyll protein (PerCP)-Cy5.5-conjugated antibody against CD11b (clone M1/70; BD Biosciences); and (iv) allophycocyanin-conjugated antibodies against I-Ab (clone M5/114.15.2; eBiosciences) or CD45 (clone 30-F11; BD Biosciences). Antibody dilutions were optimized using total splenocytes from uninfected mice. Single-color controls were used for compensation and gating.
Immunocytochemistry.
Mock- and parasite-infected eyes were harvested and fixed for 30 min at room temperature in 3% paraformaldehyde in PBS. Eyes were then placed in increasing concentrations of sucrose (10 to 30%) in PBS at room temperature, followed by overnight incubation at 4°C in PBS containing 30% sucrose. The cryoprotected eyes were then embedded in Tissue-Tek OCT compound (Sakura Fintek, Torrance, CA) and were rapidly frozen in liquid nitrogen. Then 15-μm-thick sections were prepared with a cryostat. Sections were placed on glass slides, incubated briefly in ice-cold methanol, and then stored at −20°C until use. Sections were quenched with 20 mM glycine in PBS, blocked with 10% horse serum, and then incubated overnight at 4°C with antibodies against MHC class II (I-Ab) or mouse IgG as a control, or against SAG1 or rabbit IgG as a control. The slides were washed and then incubated with Alexa Fluor 594-conjugated anti-mouse IgG to detect anti-I-Ab or with an Alexa Fluor 488-conjugated anti-rabbit antibody to detect anti-SAG1. Sections were imaged with an Olympus FluoView FV500 confocal laser scanning microscope (Olympus, Center Valley, PA). Laser power, pinhole settings, photomultiplier tube settings, and intensity thresholds were kept constant for each antibody and its isotype control.
T-cell proliferation assay.
Splenic T cells from cpsII-immunized mice were enriched by negative selection using an AutoMACS cell sorter and antibodies against MHC class II+, CD11c+, NK1.1+, and B220+ cells conjugated to magnetic microbeads (Miltenyi Biotec, Auburn, CA). Antigen-presenting cells (APCs) used in the assay were enriched CD11c+ dendritic cells (DCs) prepared by digesting spleens from naïve mice with 5 mg/ml collagenase IV (Worthington Biochemical, Lakewood, NJ) and 0.1 mg/ml DNase (Sigma Chemical, St. Louis, MO) in Hanks balanced salt solution (HBSS) for 30 min at 37°C. The single-cell suspension was treated with ACK lysis buffer (Cambrex, East Rutherford, NJ) to lyse the red blood cells. The splenocytes were then incubated with magnetic-bead-conjugated antibodies against TCR, CD3, and NK1.1 (Miltenyi Biotech) and were passed through an AutoMACS cell sorter to negatively select for the CD11c+ DCs. The purity of enriched DCs and T cells was always >85% as determined by flow cytometry. Enriched APCs were loaded with STAg protein (1.0 mg/ml) in serum-free RPMI medium by incubation at 37°C for 1 h. T-cell proliferation was measured in each well of a 96-well plate by plating 105 purified T cells with 5 × 104 STAg-loaded APCs (or 200 μg of STAg alone as a negative control). In addition, retinal cells prepared from mice 6 days after they were either mock infected or intravitreally infected with 104 RH parasites were added to the T cells. Plates were incubated for 66 h, and then 1 μCi of [3H]thymidine was added to each well for 6 h. Cells were harvested on glass fiber filters (PhD Cell Harvester; Cambridge Technology, Inc., Cambridge, MA) and counted by liquid scintillation.
IFN-γ cytokine measurement.
Mock-infected and parasite-infected eyes were harvested from euthanized mice. The eyes were then enucleated, and posterior segments were dissected away from the rest of the eye. The posterior segments were then placed in lysis buffer and homogenized with a hand-held homogenizer. Singleplex mouse IFN-γ kits were purchased from Bio-Rad (Hercules, CA) and were used according to the manufacturer's instructions in conjunction with Bio-Plex instrumentation (Bio-Rad). IFN-γ concentrations in tissue supernatants were measured in duplicate and determined by interpolation from standard curves using Bio-Plex software.
RESULTS
Three distinct populations of cells express MHC class II in Toxoplasma-infected eyes.
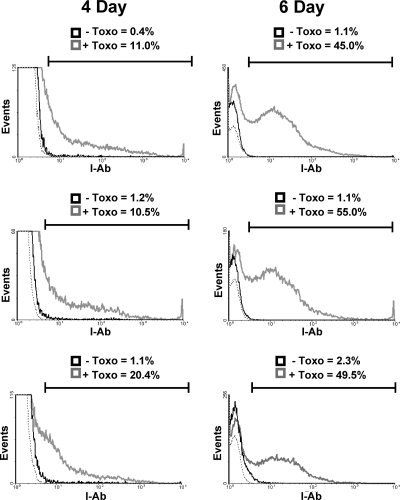
Dysregulated CD4+ T cells can cause retinal tissue damage in a variety of instances, including Toxoplasma infections and experimental autoimmune uveitis. Since CD4+ T-cell binding to peptide-loaded MHC class II on the target cell surface is a key mechanism for the regulation of effector CD4+ T-cell activity, MHC class II expression was examined in the retinas of Toxoplasma-infected mice. Thus, mice were either mock infected or intravitreally infected with 104 tachyzoites, and 4 or 6 days later, eyes were isolated and processed for flow cytometry to detect MHC class II. While ∼1% of cells in mock-infected eyes were MHC class II+ (and these were class IIlo), more than 10% of the cells were MHC class II+ 4 days postinfection (Fig. 1, left panels). By 6 days postinfection, the numbers of MHC class II+ cells in parasite-infected eyes increased to >46%, while the numbers of class II+ cells in the mock-infected samples remained constant (Fig. 1, right panels). Similar time-dependent increases in staining were observed when eyes were analyzed 6 and 8 days after they were intravitreally injected with 102 parasites (not shown).
FIG. 1.
MHC class II expression in Toxoplasma-infected eyes. Mice were either mock infected (solid black lines) or intravitreally injected (gray lines) with 104 parasites. Four and 6 days later, eyes were harvested and analyzed by flow cytometry for MHC class II expression. The gate includes all MHC class II+ events as determined by isotype staining controls (dashed line). The y axes of the 4-day histograms were decreased to highlight class II+ cells. Shown are three representative histograms from independent experiments.
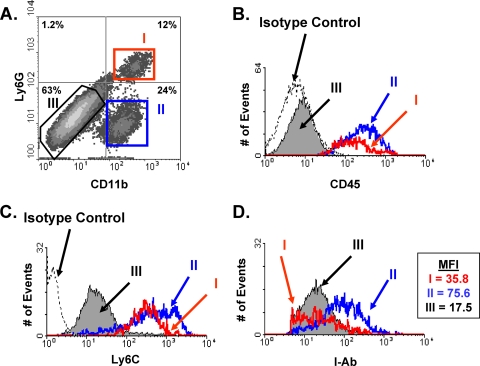
Hematoxylin-and-eosin (H&E)-stained sections from parasite-infected eyes revealed that large numbers of leukocytes with nuclear morphologies typical of monocytes and neutrophils were present in the retinas and, to a lesser extent, in the vitreous of Toxoplasma-infected eyes (7). To determine whether the infiltrating leukocytes were MHC class II+, intravitreally infected eyes were harvested, processed for flow cytometry, and stained with antibodies against MHC class II, CD11b, Ly6G, and CD45. This cocktail of antibodies was used because it can discriminate between neutrophils (CD45+ CD11b+ Ly6G+) and inflammatory monocytes (CD45+ CD11b+ Ly6G−) (15) as well as between retinal microglia (CD45lo) and resident neuroretinal cells (CD45−). Three distinct populations of MHC class II-expressing cells were found in Toxoplasma-infected retinas: populations I (CD45+ CD11b+ Ly6G+), II (CD45+ CD11b+ Ly6G−), and III (CD45− CD11b− Ly6G−) (Fig. 2A and B). In addition, populations I and II stained strongly with anti-Ly6C, suggesting that the Ly6G− cells in population II are inflammatory monocytes (Fig. 2C). These data indicated that populations I, II, and III consisted of neutrophils, monocytes, and resident retinal cells, respectively.
FIG. 2.
Three distinct populations of MHC class II-expressing cells in Toxoplasma-infected eyes. Mice were intravitreally injected with 104 parasites, and 6 days later, eyes were harvested and analyzed by using flow cytometry. (A) FACS plot of CD11b and Ly6G expression on MHC class II+-gated cells. The distinct populations are highlighted as I (CD11b+ Ly6G+), II (CD11b+ Ly6G−), and III (CD11b− Ly6G−). (B through D) Histograms of CD45 (B), Ly6C (C), and MHC class II (D) expression levels in each of the 3 populations. The dashed lines in panels B and C represent isotype control staining levels. MFI, mean fluorescence intensity of each population. Representative results from three independent experiments are shown.
The histograms in Fig. 1 indicate that MHC class II expression levels were widespread in Toxoplasma-infected retinas. We therefore tested whether all cells expressed heterogenous amounts of MHC class II or whether each population expressed class II at specific levels. The data indicated that neutrophils (population I) and resident retinal cells (population III) expressed low levels of MHC class II, while monocytes (population II) expressed it at much higher levels (Fig. 2D).
Dendritic cell marker staining of MHC class II+ cells in Toxoplasma-infected retinas.
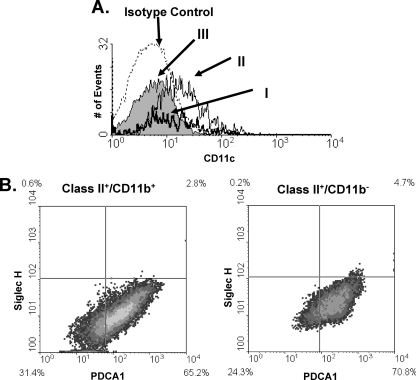
DCs are a large group of APCs that, in general, can be placed into 1 of 3 major classes: conventional DCs (cDCs); inflammatory, blood-derived DCs (iDCs); and plasmacytoid DCs (pDCs). Because CD11c is expressed at high levels on cDCs and iDCs and at low levels on pDCs, we assessed CD11c expression on class II+ cells 6 days after intravitreal injection of mice with 104 parasites. We found that CD11c was expressed on cells in population II at levels comparable to those of splenic CD11c+ DCs (not shown). CD11c expression was, however, undetectable on cells in populations I and III (Fig. 3A). In addition, none of the class II+ cells stained with anti-CD8α or with the dendritic cell marker 33D1 (not shown). These data, combined with the fact that population II cells were Ly6C+, suggested that in parasite-infected retinas, blood-derived monocytes differentiate into iDCs. In addition, the CD45− class II+ cells do not appear to be derived from the 33D1-expressing resident retinal dendritic cells recently identified by Xu and coworkers (57).
FIG. 3.
Dendritic cell staining in Toxoplasma-infected eyes. Mice were intravitreally injected with 104 parasites. Six days later, eyes were harvested and analyzed using flow cytometry. (A) Histogram of CD11c expression on the three populations of MHC class II-expressing cells from Fig. 2A. (B) FACS plots of PDCA1 and Siglec-H staining of class II+ CD11b+ and class II+ CD11b− cells.
To determine whether pDCs were present in Toxoplasma-infected retinas, PDCA1 expression on MHC class II+ cells was analyzed. We used a PE-conjugated anti-PDCA1 antibody, which precluded us from distinguishing the 3 populations of cells as shown in Fig. 2. However, we defined the class II+ CD11b+ cells as infiltrating leukocytes and class II+ CD11b− cells as resident retinal cells, since >95% of CD11b+ cells were CD45+ (not shown). Surprisingly, more than 65% of cells from either group were PDCA1+ (Fig. 3B and C). Since IFN-γ is upregulated in parasite-infected retinas (see Fig. 7A) and regulates PDCA1 expression (2), we examined the expression of a second pDC marker, Siglec-H (1). Fewer than 6% of PDCA1+ cells in either the CD11b+ or the CD11b− population were also Siglec-H+, strongly suggesting that the class II+ cells were not pDCs. Collectively, these data indicate that iDCs are the major class of dendritic cells in Toxoplasma-infected retinas and that the majority of class II+ cells in infected eyes are not dendritic cells.
FIG. 7.
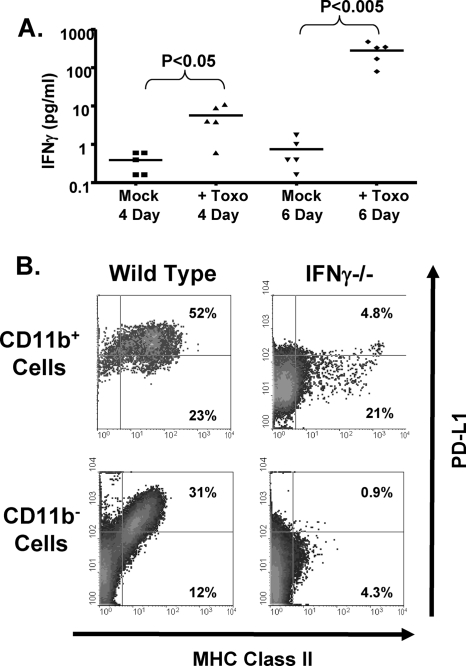
IFN-γ is important for MHC class II and PD-L1 expression in Toxoplasma-infected eyes. (A) Mice were intravitreally injected with 104 parasites (Toxo). After 4 and 6 days, eyes were harvested and enucleated, and IFN-γ levels were measured in lysates prepared from posterior segments. P values were determined using an unpaired, nonparametric Student t test. (B) Wild-type and IFN-γKO mice were intravitreally injected with 104 parasites. Six days later, eyes were harvested and processed for flow cytometry. Shown are representative FACS plots of MHC class II and PD-L1 expression from three independent experiments.
MHC class II expression is promiscuous throughout all layers of Toxoplasma-infected retinas.
Resident retinal cells, including microglia, endothelial cells, Müller cells, and retinal pigment epithelial cells, can express MHC class II in vitro and in some cases in vivo. In parasite-infected retinas, the CD45−/lo MHC class II+ cells were CD11b−, suggesting that they were not retinal microglia, which are CD11b+ myeloid-derived cells (18, 25). To determine whether MHC class II+ CD45− cells were endothelial cells, expression of the CD31 endothelial cell marker was assessed by flow cytometry. The data indicated that class II+ cells were CD31−, indicating that in Toxoplasma-infected retinas, endothelial cells were not MHC class II+ (not shown).
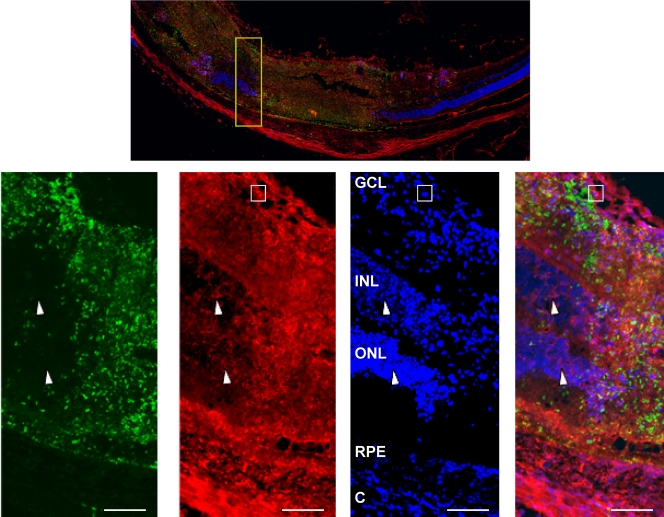
Due to a lack of established flow cytometry markers for other types of retinal cells, we used immunohistochemistry to identify the CD45− MHC class II+ cells. Thus, frozen sections from eyes harvested 6 days after they were intravitreally injected with 104 parasites were stained with antibodies against MHC class II and the Toxoplasma tachyzoite surface protein SAG1, as well as with 4′,6-diamidino-2-phenylindole (DAPI) to identify the retinal layers (Fig. 4). SAG1 staining, which was localized primarily to necrotic regions of the retina, indicated the presence of large numbers of parasites in parasite-infected retinas. MHC class II staining was not detectable in mock-infected retinas (not shown), which was consistent with our flow cytometry data as well as with other reports that retinas normally express very few class II+ cells (4). In contrast, there was widespread, strong MHC class II expression in all regions of the infected retina that were SAG1+ (Fig. 4). High class II expression was noted in cells containing bean-shaped nuclei, suggesting that these were the class IIhi inflammatory monocytes (Fig. 4, boxed area). We also noted class II staining, which was greater than that in isotype control-stained retinas (not shown), in regions with little SAG1 staining (Fig. 4, arrowheads). These included both the outer nuclear layer (ONL), which contains photoreceptors, and the inner nuclear layer (INL), which contains several neurons, such as bipolar and horizontal cells. Thus, MHC class II expression in Toxoplasma-infected retinas is not restricted to cells derived from hematopoietic cells but is also present on multiple types of resident retinal cells.
FIG. 4.
MHC class II is expressed on resident retinal cells in Toxoplasma-infected retinas. Mice were intravitreally injected with 104 parasites. Six days later, eyes were harvested, fixed, and processed for immunocytochemistry. Sections were stained to detect either DAPI (blue), MHC class II (red), or SAG1 (green). (Top) Low-magnification (×40) image of the posterior retina from an infected eye. (Bottom) High-magnification (×400) images of the area highlighted in the yellow box in the top image. Note the SAG1 staining and the intense class II staining in necrotic regions of the retina. Arrowheads point to class II+ nuclear cells in inner and outer nuclear layers. The boxes highlight a crescent-shaped, class IIhi monocyte. Bars, 100 μm. Abbreviations: C, choroid; RPE, retinal pigmented epithelium; ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer.
Costimulatory-molecule expression on MHC class II+ cells in parasite-infected retinas.
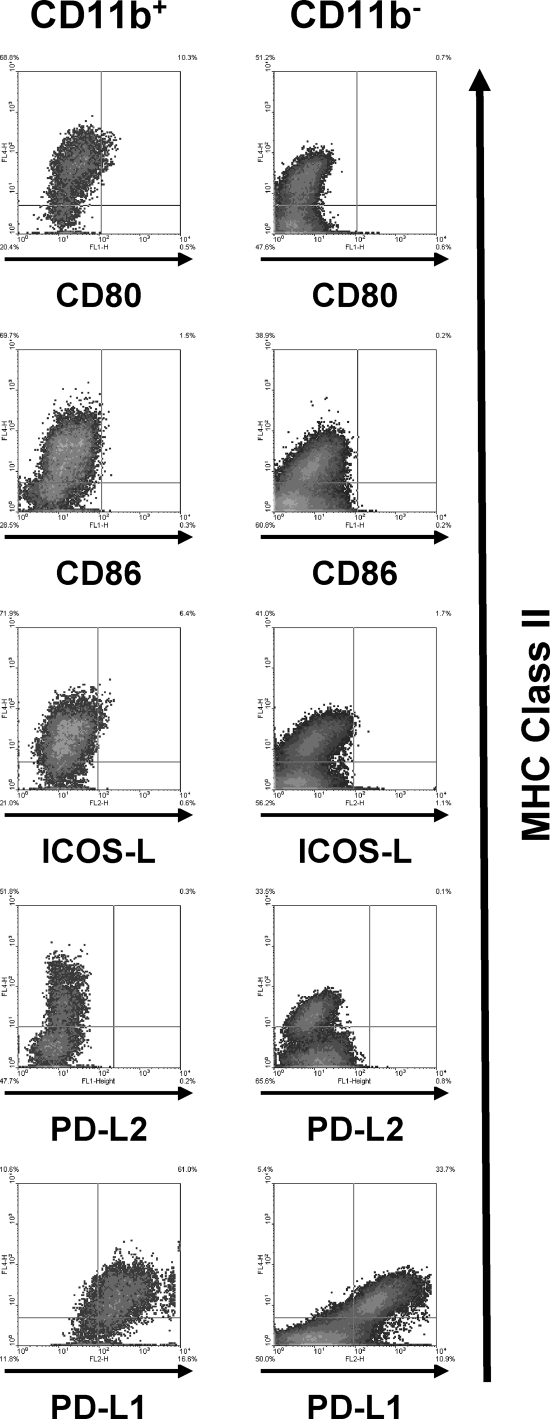
The outcome of an interaction between a T cell and its target cell is dependent on costimulatory molecules, which may either stimulate or inhibit a T cell. We therefore examined the expression of members of the B7 family of costimulatory molecules. Single-cell suspensions prepared from retinas infected with 104 parasites were stained with antibodies against positive-acting (CD80, CD86, and ICOS-L) and negative-acting (PD-L1 and PD-L2) coinhibitory members. Expression of CD86 or PD-L2 could not be detected on class II+ cells, and only a small number of CD11b+ class II+ cells expressed CD80 or ICOS-L (Fig. 5). In contrast, PD-L1 was expressed at high levels on >80% of both CD11b+ class II+ cells and CD11b− class II+ cells. Similar results were obtained using retinal cells analyzed 4 days after infection (data not shown). These data indicate that the majority of class II+ cells express PD-L1 but not other B7 family members.
FIG. 5.
B7 family member expression on MHC class II+ cells. Mice were intravitreally injected with 104 parasites. Six days later, eyes were harvested and stained with anti-MHC class II and the indicated antibodies. Representative FACS plots from three independent experiments are shown.
Toxoplasma-infected retinal cells suppress T-cell activation via a PD-L1-dependent mechanism.
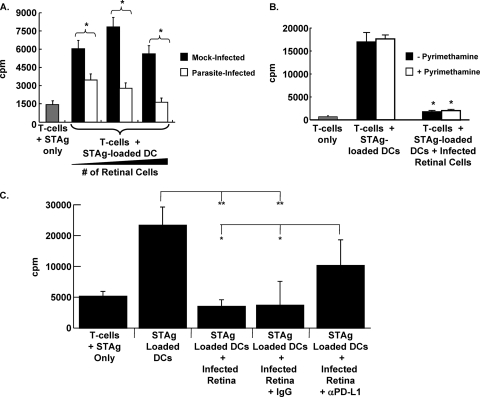
To examine the functional significance of MHC class II and PD-L1 expression in parasite-infected retinas, we first tested what effect the addition of cells from Toxoplasma-infected retinas had on the recall response of T cells to Toxoplasma antigen-loaded dendritic cells by measuring [3H]thymidine incorporation. Thus, purified splenic T cells from cpsII-vaccinated mice were stimulated for 72 h with STAg-loaded CD11c+ dendritic cells together with retinal cells from mock-infected or intravitreally infected mice. T-cell proliferation was increased when cells were cultured with STAg-loaded APCs and retinal cells from mock-infected mice (Fig. 6A, filled bars). In contrast, a significant, dose-dependent reduction in T-cell proliferation was observed when the T cells were exposed to increasing numbers of retinal cells from intravitreally infected mice (Fig. 6A, open bars). This effect on T-cell proliferation was not a consequence of Toxoplasma replication within T cells and lysis of T cells, since the addition of 1 μM pyrimethamine, a significant inhibitor of parasite replication, had no significant affect on the ability of parasite-infected retinal cells to suppress T-cell responses (Fig. 6B).
FIG. 6.
T-cell suppression by retinal cells from Toxoplasma-infected retinas is PD-L1 dependent. (A) Splenic T cells isolated from cpsII-vaccinated mice were incubated with STAg alone (shaded bar) or STAg-loaded splenic APCs in the presence of increasing numbers (1 × 105, 5 × 104, or 1 × 104) of retinal cells from either mock-infected (filled bars) or parasite-infected (open bars) mice. Shown are the averages for quadruplicate samples repeated two independent times. *, P < 0.005 by a two-tailed, unpaired Student t test. (B) Splenic T cells from vaccinated mice were incubated with STAg alone (shaded bar) or with STAg-loaded DCs in the absence or presence of parasite-infected retinal cells. Pyrimethamine (1 μM) was included as indicated. Shown are results of a representative experiment repeated three times in quadruplicate. *, P < 0.005 by Student's t test. (C) Splenic T cells from vaccinated mice were incubated with the indicated cells (5 × 104 parasite-infected retinal cells were used where indicated) and antibodies. Shown are results of a representative experiment carried out in quadruplicate. Asterisks indicate significance by Student's t test (**, P < 0.01; *, P < 0.05).
We next tested whether the inhibition of T-cell recall responses by parasite-infected retinal cells was PD-L1 dependent. Thus, the T-cell recall assay was repeated, but anti-PD-L1 or an isotype control antibody was added to the T-cell cultures incubated with retinal cells from parasite-infected mice. The data indicated that the isotype control antibody had no significant effect on T-cell inhibition by infected retinal cells (Fig. 6C). In contrast, the anti-PD-L1 antibody significantly abrogated the ability of the infected retinal cells to suppress T-cell proliferation. These data indicate that PD-L1 is functionally expressed in Toxoplasma-infected eyes.
IFN-γ is required for MHC class II and PD-L1 expression in Toxoplasma-infected eyes.
We next assessed whether IFN-γ was required for MHC class II and PD-L1 expression in parasite-infected eyes, since IFN-γ is a critical regulator of these proteins (8, 28, 29, 35, 41). IFN-γ protein levels were measured in the eyes of mice 4 and 6 days after the mice were mock-infected or intravitreally infected with 104 parasites. IFN-γ was upregulated approximately 20-fold 4 days postinfection and 500-fold 6 days postinfection (Fig. 7A).
We next compared MHC class II expression 6 days after wild-type and IFN-γ knockout (IFN-γKO) mice were intravitreally infected. We found that >85% of CD11b+ cells were class II+ in wild-type mice, while only 26% of these cells were class II+ in IFN-γKO mice. In CD11b− cells, class II expression was also reduced from approximately 43% in wild-type mice to 6% in IFN-γKO mice (Fig. 7B). Similarly, PD-L1 expression in both CD11b+ and CD11b− cells was also critically dependent on IFN-γ. However, a distinct population of cells that strongly expressed both MHC class II and PD-L1 was refractory to the loss of IFN-γ, suggesting that other signals regulate these cells. These data indicate that MHC class II expression and PD-L1 expression in Toxoplasma-infected eyes are largely dependent on IFN-γ.
DISCUSSION
Immune privilege is important for the retina, because most damaged neural retinal cells cannot be repaired or replaced. The retina maintains its immune-privileged state, in part, by a paucity of endogenous MHC class II expression in the retina (49, 50). Recently, however, cells that do express MHC class II in inflamed retinas have been identified, and the majority of these cells are infiltrating leukocytes, RPE cells, or resident microglia (17, 18, 25, 33, 34, 44, 57). In Toxoplasma-infected eyes, MHC class II was expressed on a much more heterogenous population of resident retinal cells, including retinal neurons such as photoreceptors. It is not clear why other models of inflammation have yet to detect MHC class II expression on these retinal cells. One reason could be that one of the major retinal inflammation models is experimental autoimmune uveoretinitis, which is stimulated by inducing autoimmune responses to a photoreceptor antigen (6). Thus, MHC class II expression on these cells would be difficult to detect. Our work, therefore, further highlights the need to study retinal inflammation with both autoimmune and infection models.
Toxoplasma infections result in the development of long-lived tissue cysts in numerous tissues, including immune-privileged sites such as the retina. Studying the activation and regulation of retinal anti-Toxoplasma immune responses after a cyst reactivates has been difficult for several reasons. First, the number of cysts that develop in the retina is highly variable (12, 39). Second, the timing and location of spontaneous cyst reactivation is difficult to predict as well as to detect. Finally, chronically infected mice develop retinal damage only after becoming immune compromised by either genetic or pharmacological intervention. For these reasons, we used the tachyzoite intravitreal infection model to mimic the events that take place after a cyst ruptures and parasites convert to tachyzoites. The utility of this model is highlighted by the observations in this report (functional MHC class II and PD-L1 expression in the retina). Intravitreal infections do, however, have some caveats, as do all infection models. These include the fact that intravitreal injection of tachyzoites bypasses any impact that chronic Toxoplasma tissue cysts and bradyzoites may have on host surveillance and immunity. Nevertheless, the physiological relevance of our findings is supported by recent work demonstrating that PD-L1 mRNA is upregulated in the brains of mice chronically infected with Toxoplasma (20). Moreover, the PD-L1 receptor, PD-1, is expressed on T cells recruited to Toxoplasma-infected brains (55) and retinas (our unpublished results). Another potential caveat is that our experiments were performed primarily by injecting relatively high numbers (104) of parasites. But class II and PD-L1 upregulation also occurred with lower doses of parasites (103), approximating the number that would be released after as few as 1 to 2 cyst bursts (9).
Our data clearly establish IFN-γ as a critical regulator of MHC class II and PD-L1 expression on both infiltrating leukocytes and resident retinal cells. However, IFN-γ does not appear to be sufficient for class II expression, since intravitreal or subretinal IFN-γ injection upregulated MHC class II in the cornea, in the choroid, and on retinal pigment epithelial cells, but not on cells of the inner retina (4, 19). This suggests that Toxoplasma infection induces retinal cells to become responsive to IFN-γ, perhaps by upregulating IFN-γ receptor expression.
Another important question is what cells are the source for IFN-γ in intravitreally infected mice. During reactivated ocular toxoplasmosis, CD8+ and CD4+ T cells are important IFN-γ-secreting cells. However, significant numbers of retinal T cells are not detectable 6 days after the injection of 104 parasites. Thus, a non-T-cell source for IFN-γ is most likely responsible for the early upregulation of MHC class II and PD-L1. Inflammatory monocytes and neutrophils are the two major types of leukocytes in parasite-infected eyes. The monocytes are not predicted to express significant amounts of IFN-γ in parasite-infected retinas, since a failure to recruit these cells to Toxoplasma-infected peritoneal cavities or mucosae did not result in reduced IFN-γ expression (10, 46). Although a failure to recruit neutrophils during an intraperitoneal infection leads to decreased IFN-γ release, this is most likely due to a loss of neutrophil-derived interleukin 12 (IL-12) (3). However, other studies have demonstrated that neutrophils can express and release IFN-γ (26, 30), indicating that in parasite-infected retinas, neutrophil-derived IFN-γ may contribute to MHC class II and PD-L1 expression. Brain microglia (which stain as CD45lo CD11blo cells) are important early sources of IFN-γ after cyst reactivation in the brain (53), and it is therefore possible that retinal microglia respond similarly to Toxoplasma. Finally, a third minor population (<5%) of CD45+ cells that were CD11b− and Ly6G− was present in parasite-infected eyes and could be expressing IFN-γ (not shown). The identity of these cells is unknown, since they did not stain with anti-NK1.1 or anti-B220 antibodies, suggesting that they were not NK cells or B cells, respectively.
The importance of IFN-γ in controlling Toxoplasma replication and in upregulating retinal PD-L1 expression suggests that IFN-γ triggers a negative feedback loop to downregulate recruited effector T cells in an effort to limit immune-mediated pathology. IL-10 is another cytokine demonstrated to downregulate T-cell responses to Toxoplasma in the periphery and the retina (14, 37). However, PD-L1 expression is not dependent on IL-10 (5). Moreover, PD-L1 and IL-10 act in parallel to regulate the immune response during chronic lymphocytic choriomeningitis virus infection (5). Similarly, we propose that in the retina, IL-10 and PD-L1 act in concert to prevent immune-mediated tissue damage. Such a model is supported by the well-documented role of PD-L1 in downregulating T-cell-based responses during viral, bacterial, and parasitic infections (see, e.g., references 5, 24, and 31) as well in various autoimmune models (see, e.g., references 27, 40, 45, and 47). In addition, retinal-cell suppression of T-cell responses was not fully abrogated by the anti-PD-L1 antibody, suggesting the presence of other T-cell-suppressing factors. Our future work will focus on identifying and defining the contributions of these various factors to the immunological responses during ocular toxoplasmosis.
Acknowledgments
We thank John Boothroyd for providing the SAG1 antisera, Paul Kincade for the Siglec-H antibody, and Jerry Niederkorn for critical reading of the manuscript.
This work is supported by grants from the Oklahoma Center for the Advancement of Science and Technology (HR05-138S) and by NIH grants A069986 to I.J.B., AI048097 to A.D.F., EY016459 to J.D.A., AI078993 to M.L.L., and AI41930 to D.J.B.
Editor: J. H. Adams
Footnotes
Published ahead of print on 24 May 2010.
REFERENCES
- 1.Blasius, A. L., M. Cella, J. Maldonado, T. Takai, and M. Colonna. 2006. Siglec-H is an IPC-specific receptor that modulates type I IFN secretion through DAP12. Blood 107:2474-2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blasius, A. L., E. Giurisato, M. Cella, R. D. Schreiber, A. S. Shaw, and M. Colonna. 2006. Bone marrow stromal cell antigen 2 is a specific marker of type I IFN-producing cells in the naive mouse, but a promiscuous cell surface antigen following IFN stimulation. J. Immunol. 177:3260-3265. [DOI] [PubMed] [Google Scholar]
- 3.Bliss, S. K., B. A. Butcher, and E. Y. Denkers. 2000. Rapid recruitment of neutrophils containing prestored IL-12 during microbial infection. J. Immunol. 165:4515-4521. [DOI] [PubMed] [Google Scholar]
- 4.Brandt, C., P. Knupfer, G. Boush, R. Gausas, and J. Chandler. 1990. In vivo induction of Ia expression in murine cornea after intravitreal injection of interferon-gamma. Invest. Ophthalmol. Vis. Sci. 31:2248-2253. [PubMed] [Google Scholar]
- 5.Brooks, D. G., S. J. Ha, H. Elsaesser, A. H. Sharpe, G. J. Freeman, and M. B. Oldstone. 2008. IL-10 and PD-L1 operate through distinct pathways to suppress T-cell activity during persistent viral infection. Proc. Natl. Acad. Sci. U. S. A. 105:20428-20433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caspi, R. R., F. G. Roberge, C. C. Chan, B. Wiggert, G. J. Chader, L. A. Rozenszajn, Z. Lando, and R. B. Nussenblatt. 1988. A new model of autoimmune disease. Experimental autoimmune uveoretinitis induced in mice with two different retinal antigens. J. Immunol. 140:1490-1495. [PubMed] [Google Scholar]
- 7.Charles, E., M. C. Callegan, and I. J. Blader. 2007. The SAG1 Toxoplasma surface protein is not required for acute ocular toxoplasmosis in mice. Infect. Immun. 75:2079-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong, H., S. E. Strome, D. R. Salomao, H. Tamura, F. Hirano, D. B. Flies, P. C. Roche, J. Lu, G. Zhu, K. Tamada, V. A. Lennon, E. Celis, and L. Chen. 2002. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat. Med. 8:793-800. [DOI] [PubMed] [Google Scholar]
- 9.Dubey, J. P., D. S. Lindsay, and C. A. Speer. 1998. Structures of Toxoplasma gondii tachyzoites, bradyzoites, and sporozoites and biology and development of tissue cysts. Clin. Microbiol. Rev. 11:267-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunay, I. R., R. A. Damatta, B. Fux, R. Presti, S. Greco, M. Colonna, and L. D. Sibley. 2008. Gr1+ inflammatory monocytes are required for mucosal resistance to the pathogen Toxoplasma gondii. Immunity 29:306-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox, B. A., and D. J. Bzik. 2002. De novo pyrimidine biosynthesis is required for virulence of Toxoplasma gondii. Nature 415:926-929. [DOI] [PubMed] [Google Scholar]
- 12.Gazzinelli, R. T., A. Brezin, Q. Li, R. B. Nussenblatt, and C. C. Chan. 1994. Toxoplasma gondii: acquired ocular toxoplasmosis in the murine model, protective role of TNF-α and IFN-γ. Exp. Parasitol. 78:217-229. [DOI] [PubMed] [Google Scholar]
- 13.Gazzinelli, R. T., F. T. Hakim, S. Hieny, G. M. Shearer, and A. Sher. 1991. Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-γ production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. J. Immunol. 146:286-292. [PubMed] [Google Scholar]
- 14.Gazzinelli, R. T., M. Wysocka, S. Hieny, T. Scharton-Kersten, A. Cheever, R. Kuhn, W. Muller, G. Trinchieri, and A. Sher. 1996. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-γ and TNF-α. J. Immunol. 157:798-805. [PubMed] [Google Scholar]
- 15.Geissmann, F., S. Jung, and D. R. Littman. 2003. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19:71-82. [DOI] [PubMed] [Google Scholar]
- 16.Greenwald, R. J., G. J. Freeman, and A. H. Sharpe. 2005. The B7 family revisited. Annu. Rev. Immunol. 23:515-548. [DOI] [PubMed] [Google Scholar]
- 17.Gregerson, D. S., N. D. Heuss, K. L. Lew, S. W. McPherson, and D. A. Ferrington. 2007. Interaction of retinal pigmented epithelial cells and CD4 T cells leads to T-cell anergy. Invest. Ophthalmol. Vis Sci. 48:4654-4663. [DOI] [PubMed] [Google Scholar]
- 18.Gregerson, D. S., and J. Yang. 2003. CD45-positive cells of the retina and their responsiveness to in vivo and in vitro treatment with IFN-γ or anti-CD40. Invest. Ophthalmol. Vis Sci. 44:3083-3093. [DOI] [PubMed] [Google Scholar]
- 19.Hamel, C. P., B. Detrick, and J. J. Hooks. 1990. Evaluation of Ia expression in rat ocular tissues following inoculation with interferon-gamma. Exp. Eye Res. 50:173-182. [DOI] [PubMed] [Google Scholar]
- 20.Hermes, G., J. W. Ajioka, K. A. Kelly, E. Mui, F. Roberts, K. Kasza, T. Mayr, M. J. Kirisits, R. Wollmann, D. J. Ferguson, C. W. Roberts, J. H. Hwang, T. Trendler, R. P. Kennan, Y. Suzuki, C. Reardon, W. F. Hickey, L. Chen, and R. McLeod. 2008. Neurological and behavioral abnormalities, ventricular dilatation, altered cellular functions, inflammation, and neuronal injury in brains of mice due to common, persistent, parasitic infection. J. Neuroinflamm. 5:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holland, G. N. 2003. Ocular toxoplasmosis: a global reassessment. Part I: epidemiology and course of disease. Am. J. Ophthalmol. 136:973-988. [DOI] [PubMed] [Google Scholar]
- 22.Hori, J., M. Wang, M. Miyashita, K. Tanemoto, H. Takahashi, T. Takemori, K. Okumura, H. Yagita, and M. Azuma. 2006. B7-H1-induced apoptosis as a mechanism of immune privilege of corneal allografts. J. Immunol. 177:5928-5935. [DOI] [PubMed] [Google Scholar]
- 23.Israelski, D. M., F. G. Araujo, F. K. Conley, Y. Suzuki, S. Sharma, and J. S. Remington. 1989. Treatment with anti-L3T4 (CD4) monoclonal antibody reduces the inflammatory response in toxoplasmic encephalitis. J. Immunol. 142:954-958. [PubMed] [Google Scholar]
- 24.Jurado, J. O., I. B. Alvarez, V. Pasquinelli, G. J. Martinez, M. F. Quiroga, E. Abbate, R. M. Musella, H. E. Chuluyan, and V. E. Garcia. 2008. Programmed death (PD)-1:PD-ligand 1/PD-ligand 2 pathway inhibits T cell effector functions during human tuberculosis. J. Immunol. 181:116-125. [DOI] [PubMed] [Google Scholar]
- 25.Kaneko, H., K. M. Nishiguchi, M. Nakamura, S. Kachi, and H. Terasaki. 2008. Characteristics of bone marrow-derived microglia in the normal and injured retina. Invest. Ophthalmol. Vis Sci. 49:4162-4168. [DOI] [PubMed] [Google Scholar]
- 26.Kirby, A. C., U. Yrlid, and M. J. Wick. 2002. The innate immune response differs in primary and secondary Salmonella infection. J. Immunol. 169:4450-4459. [DOI] [PubMed] [Google Scholar]
- 27.Latchman, Y. E., S. C. Liang, Y. Wu, T. Chernova, R. A. Sobel, M. Klemm, V. K. Kuchroo, G. J. Freeman, and A. H. Sharpe. 2004. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc. Natl. Acad. Sci. U. S. A. 101:10691-10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lazar-Molnar, E., A. Gacser, G. J. Freeman, S. C. Almo, S. G. Nathenson, and J. D. Nosanchuk. 2008. The PD-1/PD-L costimulatory pathway critically affects host resistance to the pathogenic fungus Histoplasma capsulatum. Proc. Natl. Acad. Sci. U. S. A. 105:2658-2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee, S. K., S. H. Seo, B. S. Kim, C. D. Kim, J. H. Lee, J. S. Kang, P. J. Maeng, and J. S. Lim. 2005. IFN-γ regulates the expression of B7-H1 in dermal fibroblast cells. J. Dermatol. Sci. 40:95-103. [DOI] [PubMed] [Google Scholar]
- 30.Li, L., L. Huang, S.-S. J. Sung, P. I. Lobo, M. G. Brown, R. K. Gregg, V. H. Engelhard, and M. D. Okusa. 2007. NKT cell activation mediates neutrophil IFN-γ production and renal ischemia-reperfusion injury. J. Immunol. 178:5899-5911. [DOI] [PubMed] [Google Scholar]
- 31.Liang, S. C., R. J. Greenwald, Y. E. Latchman, L. Rosas, A. Satoskar, G. J. Freeman, and A. H. Sharpe. 2006. PD-L1 and PD-L2 have distinct roles in regulating host immunity to cutaneous leishmaniasis. Eur. J. Immunol. 36:58-64. [DOI] [PubMed] [Google Scholar]
- 32.Liesenfeld, O., J. Kosek, J. S. Remington, and Y. Suzuki. 1996. Association of CD4+ T cell-dependent, interferon-gamma-mediated necrosis of the small intestine with genetic susceptibility of mice to peroral infection with Toxoplasma gondii. J. Exp. Med. 184:597-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liversidge, J., H. F. Sewell, A. W. Thomson, and J. V. Forrester. 1988. Lymphokine-induced MHC class II antigen expression on cultured retinal pigment epithelial cells and the influence of cyclosporin A. Immunology 63:313-317. [PMC free article] [PubMed] [Google Scholar]
- 34.Liversidge, J. M., H. F. Sewell, and J. V. Forrester. 1988. Human retinal pigment epithelial cells differentially express MHC class II (HLA, DP, DR and DQ) antigens in response to in vitro stimulation with lymphokine or purified IFN-γ. Clin. Exp. Immunol. 73:489-494. [PMC free article] [PubMed] [Google Scholar]
- 35.Loke, P., and J. P. Allison. 2003. PD-L1 and PD-L2 are differentially regulated by Th1 and Th2 cells. Proc. Natl. Acad. Sci. U. S. A. 100:5336-5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu, F., S. Huang, and L. H. Kasper. 2004. CD4+ T cells in the pathogenesis of murine ocular toxoplasmosis. Infect. Immun. 72:4966-4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu, F., S. Huang, and L. H. Kasper. 2003. Interleukin-10 and pathogenesis of murine ocular toxoplasmosis. Infect. Immun. 71:7159-7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luft, B. J., and J. S. Remington. 1992. Toxoplasmic encephalitis in AIDS. Clin. Infect. Dis. 15:211-222. [DOI] [PubMed] [Google Scholar]
- 39.Lyons, R. E., J. P. Anthony, D. J. Ferguson, N. Byrne, J. Alexander, F. Roberts, and C. W. Roberts. 2001. Immunological studies of chronic ocular toxoplasmosis: up-regulation of major histocompatibility complex class I and transforming growth factor beta and a protective role for interleukin-6. Infect. Immun. 69:2589-2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin-Orozco, N., Y. H. Wang, H. Yagita, and C. Dong. 2006. Programmed death (PD) ligand-1/PD-1 interaction is required for CD8+ T cell tolerance to tissue antigens. J. Immunol. 177:8291-8295. [DOI] [PubMed] [Google Scholar]
- 41.Mazanet, M. M., and C. C. Hughes. 2002. B7-H1 is expressed by human endothelial cells and suppresses T cell cytokine synthesis. J. Immunol. 169:3581-3588. [DOI] [PubMed] [Google Scholar]
- 42.Montoya, J. G., and O. Liesenfeld. 2004. Toxoplasmosis. Lancet 363:1965-1976. [DOI] [PubMed] [Google Scholar]
- 43.Parker, S. J., C. W. Roberts, and J. Alexander. 1991. CD8+ T cells are the major lymphocyte subpopulation involved in the protective immune response to Toxoplasma gondii in mice. Clin. Exp. Immunol. 84:207-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Percopo, C. M., J. J. Hooks, T. Shinohara, R. Caspi, and B. Detrick. 1990. Cytokine-mediated activation of a neuronal retinal resident cell provokes antigen presentation. J. Immunol. 145:4101-4107. [PubMed] [Google Scholar]
- 45.Reynoso, E. D., K. G. Elpek, L. Francisco, R. Bronson, A. Bellemare-Pelletier, A. H. Sharpe, G. J. Freeman, and S. J. Turley. 2009. Intestinal tolerance is converted to autoimmune enteritis upon PD-1 ligand blockade. J. Immunol. 182:2102-2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robben, P. M., M. LaRegina, W. A. Kuziel, and L. D. Sibley. 2005. Recruitment of Gr-1+ monocytes is essential for control of acute toxoplasmosis. J. Exp. Med. 201:1761-1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schreiner, B., S. L. Bailey, T. Shin, L. Chen, and S. D. Miller. 2008. PD-1 ligands expressed on myeloid-derived APC in the CNS regulate T-cell responses in EAE. Eur. J. Immunol. 38:2706-2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shen, L., Y. Jin, G. J. Freeman, A. H. Sharpe, and M. R. Dana. 2007. The function of donor versus recipient programmed death-ligand 1 in corneal allograft survival. J. Immunol. 179:3672-3679. [DOI] [PubMed] [Google Scholar]
- 49.Streilein, J. W. 2003. Ocular immune privilege: the eye takes a dim but practical view of immunity and inflammation. J. Leukoc. Biol. 74:179-185. [DOI] [PubMed] [Google Scholar]
- 50.Streilein, J. W., K. Ohta, J. S. Mo, and A. W. Taylor. 2002. Ocular immune privilege and the impact of intraocular inflammation. DNA Cell Biol. 21:453-459. [DOI] [PubMed] [Google Scholar]
- 51.Subauste, C. S., R. de Waal Malefyt, and F. Fuh. 1998. Role of CD80 (B7.1) and CD86 (B7.2) in the immune response to an intracellular pathogen. J. Immunol. 160:1831-1840. [PubMed] [Google Scholar]
- 52.Suzuki, Y., and J. S. Remington. 1988. Dual regulation of resistance against Toxoplasma gondii infection by Lyt-2+ and Lyt-1+, L3T4+ T cells in mice. J. Immunol. 140:3943-3946. [PubMed] [Google Scholar]
- 53.Wang, X., and Y. Suzuki. 2007. Microglia produce IFN-γ independently from T cells during acute toxoplasmosis in the brain. J. Interferon Cytokine Res. 27:599-605. [DOI] [PubMed] [Google Scholar]
- 54.Weiss, L. M., and K. Kim. 2000. The development and biology of bradyzoites of Toxoplasma gondii. Front. Biosci. 5:D391-D405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilson, E. H., T. H. Harris, P. Mrass, B. John, E. D. Tait, G. F. Wu, M. Pepper, E. J. Wherry, F. Dzierzinski, D. Roos, P. G. Haydon, T. M. Laufer, W. Weninger, and C. A. Hunter. 2009. Behavior of parasite-specific effector CD8+ T cells in the brain and visualization of a kinesis-associated system of reticular fibers. Immunity 30:300-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilson, E. H., C. Zaph, M. Mohrs, A. Welcher, J. Siu, D. Artis, and C. A. Hunter. 2006. B7RP-1-ICOS interactions are required for optimal infection-induced expansion of CD4+ Th1 and Th2 responses. J. Immunol. 177:2365-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu, H., R. Dawson, J. V. Forrester, and J. Liversidge. 2007. Identification of novel dendritic cell populations in normal mouse retina. Invest. Ophthalmol. Vis Sci. 48:1701-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang, W., H.-C. Li, P. W. Chen, H. Alizadeh, Y. He, R. N. Hogan, and J. Y. Niederkorn. 2009. PD-L1 expression on human ocular cells and its possible role in regulating immune-mediated ocular inflammation. Invest. Ophthalmol. Vis. Sci. 50:273-280. [DOI] [PubMed] [Google Scholar]