Abstract
Extracellular ATP can be released by many cell types under conditions of cellular stress and signals through activation of purinergic receptors. Bladder uroepithelial cells grown in vitro have previously been shown to release ATP in response to stretch. In the present study, we investigated ATP release from uroepithelial cells infected with bacteria and the effect of ATP on the host cell proinflammatory interleukin 8 (IL-8) response. The human kidney epithelial cell line A498 and the human uroepithelial cell line UROtsa were grown in culture and stimulated by the uropathogenic Escherichia coli (UPEC) IA2 strain or the stable ATP analogue ATP-γ-S. ATP and IL-8 levels were measured in cell culture medium with a luciferin-luciferase assay and enzyme-linked immunosorbent assay (ELISA), respectively. The results showed that UPEC infection of uroepithelial cells for 1 h significantly increased (P < 0.01) the extracellular ATP levels. ATP-γ-S (10 and 100 μM) stimulated release of IL-8 from UROtsa and A498 cells after 6 and 24 h. Experiments with different purinoceptor agonists suggested that P2Y receptors, and not P2X receptors, were responsible for the ATP-γ-S-induced IL-8 release. The potency profile further suggested involvement of P2Y1, P2Y2, and/or P2Y11 receptors, and reverse transcription-PCR (RT-PCR) studies confirmed that the cells expressed these receptors. The amount of IL-8 released increased 12-fold in UPEC-infected cells, and apyrase, an enzyme that degrades ATP, reduced this increase by approximately 50%. The present study suggests that enhanced ATP release and P2Y receptor activation during urinary tract infection may represent a novel, non-TLR4-mediated mechanism for production of proinflammatory IL-8 in human urinary tract epithelial cells.
Extracellular ATP is released from a variety of cells not only as a consequence of cell injury or cell death but also via nonlytic mechanisms (25). ATP has attracted increasing attention as a danger signal released during the initial phase of infection and inflammation in order to trigger innate immunity (18). In a healthy bladder, release of ATP from uroepithelial cells by stretch and distension mediates the sensation of bladder fullness by activation of P2X3 receptors on suburothelial sensory nerves (5). Pathophysiological conditions cause enhanced bladder release of ATP as shown in patients diagnosed with interstitial cystitis (29, 30, 31). Although several studies have shown that pathology often results in augmented ATP release from the urothelium, there have not been any studies of ATP release from urothelium infected with bacteria. Patients with bacteriuria have increased urinary levels of ATP (21), but it is not known whether the bacteria, host, or both produce ATP during infection. In their function as extracellular mediators, nucleotides activate P2 nucleotide receptors that include the ionotrophic P2X receptors (P2X1 to P2X7) and G-protein-coupled P2Y receptors (P2Y1, P2Y2, P2Y4, P2Y6, and P2Y11 to P2Y14) (33). In the urinary tract, extracellular ATP may communicate through P2X and P2Y receptors with different cells, such as uroepithelial cells, inflammatory cells, nerves, and myofibroblasts (5). Evidence that P2 receptors, in particular P2Y receptors, may regulate host immunity and modulate phagocytosis, chemotaxis, and cytokine production is now accumulating (14, 16). The P2Y receptor subtypes P2Y1, P2Y2, and P2Y4 are expressed throughout the cat bladder urothelium (7), but P2Y receptor expression has not been examined in the human urothelium.
Innate immunity is the first line of defense in the urinary tract and triggered by uropathogenic bacteria through activation of Toll-like receptors (TLRs) located on the uroepithelial cell surface (32). Lipopolysaccharide (LPS) is an established ligand for Toll-like receptor 4 (TLR4), but associated coeffector proteins, such as CD14 and MD2, are needed for LPS-induced stimulation of TLR4 (4, 27). TLR4 and CD14 expression by the human urinary tract epithelium remains controversial (3, 23, 24), and in vitro studies show that the TLR4 receptor can be activated in a LPS-independent manner by P-fimbriated uropathogenic Escherichia coli (UPEC) (12). Once activated, the uroepithelial cells play a central role in the host proinflammatory response through production of chemotactic substances, such as interleukin 6 (IL-6) and IL-8. Several lines of evidence demonstrate that IL-8 is essential for neutrophil transmigration across the infected urothelium and that neutrophils are important for clearance of bacterial infections in the urinary tract (32). The role of ATP in the host response has not previously been addressed in the urinary tract. In this study, we examined whether extracellular ATP may use P2 receptors to trigger the innate mucosal response. The human renal epithelial cell line A498 that has been extensively used as a model for host response signaling in urinary tract mucosa (12, 15) and the normal human uroepithelial cell line UROtsa (22) were used as host cells in this study.
MATERIALS AND METHODS
Urinary tract epithelial cells and bacteria.
The human kidney epithelial cell line A498 (HTB-44 [kidney carcinoma]) was obtained from the American Tissue Culture Collection (ATCC) (Manassas, VA) and the human uroepithelial cell line UROtsa was kindly provided by Scott Garrett (University of North Dakota). The UROtsa cell line is a normal, immortalized cell culture model of human urothelium that has proven to be a good model for the bladder epithelium (22). The cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine, 1 mM sodium pyruvate, 1 mM nonessential amino acids, 100 U/ml penicillin, and 100 μg/ml streptomycin (all from Sigma Aldrich, St. Louis, MO) at 37°C and in a humidified atmosphere with 5% CO2 and subcultured when confluent.
The uropathogenic Escherichia coli (UPEC) IA2 strain was originally isolated from a patient with acute pyelonephritis, and it expresses both P and type 1 fimbriae. The IA2 strain was cultured on tryptic soy agar plates (TSA) (Becton, Dickinson and Company, Sparks, MD) at 37°C.
ATP assay.
ATP levels in cell culture medium from uninfected cells or cells infected with E. coli IA2 were analyzed with the ATP bioluminescence assay kit (Sigma), and luminescence was measured on a Fluostar Optima instrument (BMG Labtechnologies, Germany). UROtsa cells were grown in 24-well plates, and when the cells were confluent, the medium was replaced with serum-free medium (without penicillin and streptomycin) containing 108 CFU/ml of UPEC strain IA2 and incubated for 1 or 2 h. Uninfected cells were used as a control. To evaluate ATP levels generated by UPEC per se, experiments were performed in the absence of UROtsa cells but in the presence of 108 CFU/ml of UPEC strain IA2. In some experiments, UROtsa cells were exposed to 108 CFU/ml of strain IA2 for 1 h, the bacteria were removed, and the medium was replaced with fresh medium. Samples were collected after 1, 2, and 4 h. Collected cell culture medium was filtered in Spin-X centrifugation tubes (0.22-μm filter) (Sigma) at 14,000 × g for 1 min. Samples were stored at −70°C until analysis.
Measurement of IL-8 by ELISA.
The cells were grown in 24-well plates in medium with 2% FBS and stimulated with ATP-γ-S (1 to 100 μM; Roche Diagnostics GmbH, Germany) for 6 and 24 h or stimulated with UTP (100 μM; Sigma), α,β-methylene ATP (α,β-MeATP) (100 μM; Sigma), or MRS2365 (N-metano-carba-2-methylthio-ADP) (0.1 and 1 μM; Tocris Bioscience, Bristol, United Kingdom) for 6 h. Cells incubated with medium alone were used as nonstimulated controls. For bacterial infection, confluent monolayers of A498 cells were incubated with medium containing 108 CFU/ml of UPEC strain IA2 for 6 h. Bacterial multiplication was limited by incubating the cells with gentamicin (50 μg/ml) for at least 12 h prior to infection. Gentamicin was excluded during infections. In some experiments, A498 cells were treated with apyrase (0.6 and 2 U/ml; Sigma) alone or during infection with IA2. The medium was collected, centrifuged at 5,000 × g for 5 min, and analyzed for IL-8 content with the BD OptEIA human IL-8 enzyme-linked immunosorbent assay (ELISA) kit II (BD Biosciences Pharmingen, San Diego, CA). IL-8 was determined by measuring the optical density at 450 nm in a Labsystem Multiscan Plus fluorescence spectrophotometer.
Reverse transcription-PCR (RT-PCR).
Total RNA from uninfected or UPEC-infected cells (108 CFU/ml) was isolated using RNeasy minikit (Qiagen Inc., Valencia, CA) according to the manufacturer's instructions. Total RNA from A498 and UROtsa cells (1 μg) was converted to cDNA using oligo(dT)16 primers (1 μM) (Applied Biosystems, Foster City, CA) and the Omniscript RT kit (Qiagen Inc.). RNA was controlled for genomic DNA contamination. PCR (25-μl reaction mixture volume) was performed using Ready-To-Go PCR beads (Amersham Biosciences, Buckinghamshire, United Kingdom). Two microliters of the RT reaction mixture was mixed with primers (0.5 μM) for P2Y1, P2Y2, and P2Y11 receptors or for glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and PCR was performed in a thermal cycler (Mastercycler gradient; Eppendorf). The primers were as follows: for P2Y1, the forward primer was 5′-CTT GGT GCT GAT TCT GGG CTG-3′ and the reverse primer was 5′-GCT CGG GAG AGT CTC CTT CTG-3′; for P2Y2, the forward primer was 5′-CCG CTC GCT GGA CCT CAG CTG-3′ and the reverse primer was 5′-CTC ACT GCT GCC CAA CAC ATC-3′; for P2Y11, the forward primer was 5′-GAG GCC TGC ATC AAG TGT CTG-3′ and the reverse primer was 5′-ACG TTG AGC ACC CGC ATG ATG-3′; and for GAPDH, the forward primer was 5′-ATT CCA TGG CAC CGT CAA GGC T-3′ and the reverse primer was 5′-TCA GGT CCA CCA CTG ACA CGT T-3′. PCR was run with an initial denaturation step of 10 min at 95°C, followed by 35 cycles, with 1 cycle consisting of 45 s at 94°C, 45 s at 60°C, and 45 s at 72°C, followed by a final extension step of 10 min at 72°C. To evaluate UPEC-induced changes in P2Y receptor expression, the cycle numbers were lower (28 to 30 cycles) to ensure submaximal expression at the linear part of the amplification curve. PCR products were analyzed by agarose gel (2%) electrophoresis and visualized by ethidium bromide staining.
Statistical analysis.
Data are shown as means ± standard errors of the means (SEMs) and n indicates the number of independent experiments. Student's unpaired t test or analysis of variance (ANOVA), followed by Dunnett's post hoc test, was used with statistical significance considered at P < 0.05.
RESULTS
Extracellular ATP levels in medium from uroepithelial cells and UPEC.
To determine whether UPEC infection causes accumulation of extracellular ATP, we compared ATP levels in cell culture medium from uninfected UROtsa cells with the levels in cells infected with UPEC strain IA2 (108 CFU/ml). The ATP concentration in medium alone was below the detection level (Fig. 1). After 1 h of infection, the ATP concentration in the cell culture medium increased from 457 ± 146 to 1,720 ± 348 pM (n = 6) (P < 0.01) and after 2 h from 646 ± 304 to 2,320 ± 522 pM (n = 6) (P < 0.05). However, since UPEC per se also accumulates ATP (465 ± 160 pM after 1 h and 1,695 ± 120 pM after 2 h), the ATP released by infected UROtsa cells was calculated by subtracting the bacterial ATP levels from the levels measured in infected cells (Fig. 1A and B, black bars). These data showed that extracellular ATP levels increased significantly in the medium of UPEC-infected UROtsa cells after 1 h of infection (P < 0.01), but not after 2 h of infection. Notably, ATP levels found in medium from UPEC alone increased 3.6-fold during the 1- and 2-h measuring period, while ATP levels in medium from the uroepithelial cells alone increased marginally (1.4-fold). The results showed that UPEC-infected UROtsa cells release more ATP than uninfected cells and that UPEC bacteria per se release ATP.
FIG. 1.
(A and B) Measurement of ATP levels in cell culture medium from uninfected and E. coli IA2-infected UROtsa cells after 1 h (A) or 2 h (B). The ATP levels in medium alone and in wells with only cells or only bacteria (IA2 [108 CFU/ml]) are also given. The black bars show estimated ATP levels generated by the infected cells and are calculated by subtracting the ATP levels generated by IA2 itself from the measured ATP levels in the coincubation experiments (cells plus IA2 [108 CFU/ml]). Data are presented as means ± SEMs (n = 6). (C) UROtsa cells were exposed to IA2 (108 CFU/ml) for 1 h, the bacteria were removed, and the medium was replaced with fresh medium. ATP levels were measured 1, 2, and 4 h after the medium was replaced with fresh medium. Uninfected cells were used as a control (Ctrl). Data are presented as means plus SEMs (error bars) (n = 4 or 5). Values that are statistically significantly different are indicated by brackets and asterisks as follows: *, P < 0.05; **, P < 0.01.
We next investigated whether UPEC-infected cells continue to release ATP after the bacteria have been removed. In these experiments, UROtsa cells were exposed to E. coli IA2 for 1 h, the bacteria were removed, and the medium was replaced with fresh medium. The ATP levels increased gradually in the cell culture medium of UPEC-infected cells (Fig. 1C). In uninfected cells, the ATP levels increased 1 h after the medium was replaced with fresh medium, followed by a slow decrease in ATP levels for the next 3 h (Fig. 1C). Trypan blue staining confirmed that cell viability was not affected by bacterial infection during the 4-hour period.
ATP-γ-S stimulates IL-8 release.
Having demonstrated that UPEC infection increases extracellular ATP levels, we evaluated whether ATP is able to modulate the host proinflammatory IL-8 response. The stable ATP analogue ATP-γ-S (1, 10, and 100 μM) was used to stimulate host cells for 6 and 24 h. ATP-γ-S caused a concentration-dependent increase in IL-8 release in UROtsa cells, and the release increased approximately 2-fold after 6 and 24 (P < 0.01) hours of stimulation (Fig. 2A). The IL-8 response of the renal epithelial cell line A498 when exposed to UPEC and LPS has been well characterized (12, 15), but the IL-8 response of A498 cells to extracellular ATP has not been studied previously. The ATP-γ-S-induced IL-8 response was pronounced in A498 cells with an approximately 4-fold increase after 6 (P < 0.001) and 24 (P < 0.01) hours of stimulation (Fig. 2B). These data show that extracellular ATP is able to trigger the innate host response, as represented by increased IL-8 release.
FIG. 2.
Production of IL-8 after stimulation of cells with ATP-γ-S (1, 10, and 100 μM) for 6 or 24 h in UROtsa (A) and A498 (B) cells. The amount of IL-8 produced by ATP-γ-S is expressed as picograms per milliliter, and data are presented as means plus SEMs (error bars) (n = 3 or 4). Values that are statistically significantly different from the values for nonstimulated (NS) cells are indicated by brackets and asterisks as follows: *, P < 0.05; **, P < 0.01; and ***, P < 0.001.
Agonist potency profile on IL-8 release.
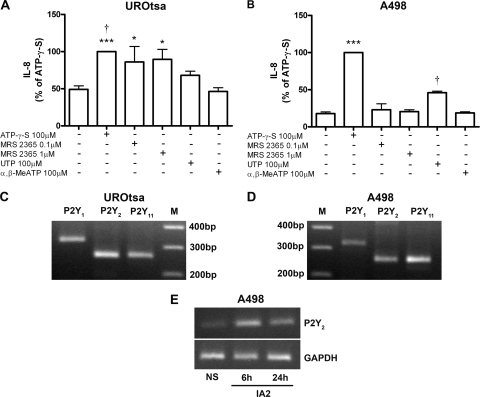
ATP-γ-S mimics the action of ATP, and it is an agonist at P2X, P2Y1, P2Y2, and P2Y11 receptors (17). To identify which purinergic receptor(s) is responsible for activation of the host IL-8 release, the cells were stimulated with different purinoceptor agonists. The P2Y1 agonist MRS2365 (N-metano-carba-2-methylthio-ADP) (0.1 and 1 μM), the P2Y2 and P2Y4 agonist UTP (100 μM), or the P2X agonist α,β-MeATP (100 μM) was applied to the cells for 6 h, and their effect on IL-8 production was compared to that of ATP-γ-S (100 μM). The P2X agonist α,β-MeATP had no effect excluding activation of P2X receptors in the IL-8 response (Fig. 3 A and B). The P2Y1 agonist MRS2365 did not stimulate IL-8 production in A498 cells, but in UROtsa cells, the IL-8 response was comparable to that of ATP-γ-S (Fig. 3A and B). The P2Y2 and P2Y4 agonist UTP stimulated release of IL-8 in both cell lines, although to a lesser extent than that of ATP-γ-S. The statistical comparison between the different agonists are summarized in Fig. 3A and B. Taken together, the potency profile in UROtsa cells was ATP-γ-S = MRS2365 > UTP > α,β-MeATP = control (Fig. 3A), and the potency profile in A498 cells was ATP-γ-S > UTP > MRS2365 = α,β-MeATP = control (Fig. 3B). The agonist profiles we observed suggest involvement of the P2Y1, P2Y2, and/or P2Y11 receptor in UROtsa cells and of the P2Y2 and/or P2Y11 receptor in A498 cells.
FIG. 3.
(A and B) Production of IL-8 after stimulation of UROtsa (A) and A498 (B) cells with ATP-γ-S (100 μM), P2Y1 agonist MRS2365 (0.1 and 1 μM), P2Y2 and P2Y4 agonist UTP (100 μM), or P2X agonist α,β-MeATP (100 μM) for 6 h. The amount of IL-8 produced by ATP-γ-S was set at 100%, and data are presented as means plus SEMs (error bars) (n = 3 or 5 for A498 cells and n = 5 or 7 for UROtsa cells). Statistical significance in panel A is indicated as follows: ***, P < 0.001 versus control or α,β-MeATP; †, P < 0.05 versus UTP; and *, P < 0.05 versus control or α,β-MeATP. Statistical significance in panel B is indicated as follows: ***, P < 0.001 versus control, MRS2365, UTP, or α,β-MeATP; and †, P < 0.001 versus control, MRS2365, or α,β-MeATP. (C and D) RT-PCR demonstrating expression of the P2Y1 (327-bp), P2Y2 (259-bp), and P2Y11 (260-bp) receptor subtypes in UROtsa (C) and A498 (D) cells. Lanes M, molecular size markers (in base pairs). (E) Expression of P2Y2 receptor transcript in A498 cells infected with the UPEC strain IA2. Representative results of three independent experiments are shown for RT-PCR. NS, nonstimulated cells.
Expression of P2Y receptor mRNA.
RT-PCR was performed to confirm that UROtsa and A498 cells express the candidate P2Y receptors identified in the agonist experiments (Fig. 3C and D). Transcripts for P2Y1, P2Y2, and P2Y11 receptors were detected, with no obvious difference between the cell lines except for a slightly lower expression of P2Y1 in A498 cells (Fig. 3D). Thus, in support of the P2Y agonist data, the host cells expressed mRNA for the P2Y1, P2Y2, and P2Y11 receptor subtypes.
To evaluate whether the receptor expression changed in response to infection, cells were infected with UPEC strain IA2 (108 CFU/ml) for 6 to 24 h and the transcript was examined. These data showed an increased expression of the P2Y2 receptor subtype in infected A498 cells compared to uninfected cells (Fig. 3E). The expression of the P2Y1 and P2Y11 receptors did not show any consistent changes in UPEC-infected cells.
Apyrase reduces UPEC-evoked IL-8 release in A498 cells.
To examine whether endogenously produced ATP contributes to UPEC-evoked IL-8 release, we infected host cells with UPEC strain IA2 (108 CFU/ml) in the absence and presence of apyrase. Apyrase is an enzyme that degrades extracellular ATP. These experiments were performed using A498 cells, as the UPEC-evoked IL-8 response in this cell line is distinct and fast, while the UPEC-evoked IL-8 response in UROtsa cells is delayed and lower. Apyrase alone did not affect basal IL-8 release. The amount of IL-8 released increased from 342 ± 102 pg/ml in control cells to 4,057 ± 593 pg/ml (n = 5) (P < 0.001) in IA2-infected cells after 6 h (Fig. 4). The IA2-evoked increase in IL-8 levels were reduced by 44% ± 5.1% (n = 5) (P < 0.05) and 52% ± 4.8% (n = 5) (P < 0.01) in the presence of 0.6 and 2 U/ml of apyrase, respectively (Fig. 4). The bacterial viability was not affected by the apyrase treatment as confirmed by viable counts on tryptic soy agar plates (data not shown). These data suggest that extracellular ATP may contribute to UPEC-evoked IL-8 release.
FIG. 4.
Production of IL-8 in A498 cells stimulated with UPEC strain IA2 (108 CFU/ml) alone or in combination with apyrase (0.6 and 2 U/ml) for 6 h. The amount of IL-8 produced is expressed as picograms per milliliter, and data are presented as means plus SEMs (error bars) (n = 5). Values that are statistically significantly different from the values for IA2-infected cells are indicated by brackets and asterisks as follows: *, P < 0.05; **, P < 0.01; and ***, P < 0.001. NS, nonstimulated cells.
DISCUSSION
It has been shown previously that bladder uroepithelial cells grown in vitro release ATP in response to stretch and exogenous ATP (29, 30, 31). These previous studies also reported an increased ATP release from uroepithelial cells from patients with interstitial cystitis compared to control patients, suggesting that enhanced extracellular ATP signaling plays a role in the pathophysiology of interstitial cystitis (29). The present study is the first study addressing ATP release from uroepithelial cells during infection with uropathogenic E. coli. We found that both uroepithelial cells and UPEC by themselves produce ATP during in vitro culture conditions. The ATP levels generated by UPEC alone increased 3.6-fold between the 1- and 2-h measuring period, while ATP generated by the host cells increased only marginally. In vitro-cultured intestinal bacteria have previously been shown to generate high concentrations of ATP (2), and more ATP may be expected to accumulate in the absence of a host degradation or reuptake system. UPEC infection of uroepithelial cells for 1 h significantly increased the extracellular ATP levels, while no further increase was seen after 2 h of infection. Thus, coincubation of host uroepithelial cells and UPEC had a synergistic, rather than additive, effect on ATP release, which suggests enhanced extracellular ATP signaling during infectious urinary tract conditions. Consistent with our results, enteropathogenic E. coli infection was found to trigger a large release of ATP from different human cell lines (9, 10).
The accumulation of ATP in the supernatant from UPEC-infected cells may reflect increased release of ATP or decreased hydrolysis due to decreased ectonucleotidase activity. The extent of ATP hydrolysis in the bladder mucosa and isolated uroepithelial cells is normally low (19, 34), most likely due to low expression of the CD39 ectonucleotidase (19). ATP can be released into the extracellular environment as a consequence of cell damage but also through nonlytic mechanism. In our experiments, bacterial infection did not cause cell damage, which was confirmed with trypan blue exclusion. The mechanisms for ATP release in UPEC-infected cells were not investigated, but as shown in the rabbit bladder, vesicular release, connexin hemichannels, and nucleoside transporters are all possible mechanisms for ATP release from uroepithelial cells (34).
We used the hydrolysis-resistant, long-lived ATP analogue ATP-γ-S in our studies to limit involvement of the ATP metabolites ADP, AMP, and adenosine. Thus, the stimulatory effect of ATP-γ-S on IL-8 release is likely to depend on ATP-activating purinergic P2 receptors and not on activation of adenosine (P1) receptors. The fact that the P2X agonist α,β-MeATP (17) was without effect on IL-8 release suggests that the observed effect was not caused by activation of P2X receptors. Indeed, activation of the P2X receptor family, except for the P2X7 receptor, is not generally associated with modulation of cytokine output (14). Since ATP-γ-S is a weak agonist for the P2X7 receptor (15), this receptor is an unlikely candidate for the IL-8-inducing effect of ATP-γ-S. A role for ATP release from damaged or sensitized urothelium followed by activation of P2X receptors on sensory nerves has previously been proposed to trigger the sensation of bladder pain and have a role in bladder pathology (6). Our data provide evidence for a novel mechanism, by which augmented release of ATP may affect the production of proinflammatory cytokines and the host response by activating P2Y receptors.
The purine ATP-γ-S mimics the action of ATP and is an agonist at P2X, P2Y1, P2Y2, and P2Y11 receptors (17). Thus, the effect on IL-8 release observed by ATP-γ-S may be caused by activation of several receptors. To further address which P2Y receptor might be responsible for the IL-8-inducing effect of ATP-γ-S, agonists with known profiles on the different P2Y receptors were tested. The P2Y1 and P2Y11 receptors are selective for adenine nucleotides, whereas all other P2Y receptors can be activated by uracil nucleotides (33). The analogue MRS2365 (N-metano-carba-2-methylthio-ADP) is selective for the P2Y1 receptor, and MRS2365 was found to stimulate IL-8 production in UROtsa cells, but not in A498 cells. ATP-γ-S is also an agonist at the P2Y11 receptor (17), and ATP-γ-S was clearly the most effective stimulus of IL-8 production in A498 cells and it did also stimulate IL-8 in UROtsa cells. Signaling through P2Y11 receptors has previously been implicated in enhancement of proinflammatory IL-8 and IL-6 in, e.g., dermal endothelial cells and bile duct epithelia (26, 36). Also, a P2Y1 receptor agonist increased IL-6 secretion from spleen slices and astrocytes (13, 28).
Triphosphate nucleotides, including UTP, ATP, and ATP-γ-S act as full agonists at the P2Y2 receptors (33). The human P2Y4 receptor is also activated by UTP and ATP, with UTP being 50-fold more potent than ATP (20). UTP was not more potent than ATP-γ-S in our study, which suggests that the UTP-induced IL-8 response may involve P2Y2 receptors rather than P2Y4 receptors. In other studies, P2Y2-mediated secretion of IL-6 and IL-8 has been reported in human airway epithelia and dermal endothelial cells (11, 26). Taken together, the agonist profiles do not fit signaling through one receptor alone; therefore, the IL-8 response may involve several P2Y receptor subtypes, most likely P2Y1, P2Y2, and/or P2Y11 subtypes in UROtsa cells and P2Y2 and/or P2Y11 receptors in A498 cells. Additionally, we found basal expression of mRNA for the P2Y receptors P2Y1, P2Y2, and P2Y11 in both UROtsa and A498 cells. The expression of the P2Y2 receptor increased in UPEC-infected A498 cells, but the functional importance of this increase remains to be established. Interestingly, the P2Y14 receptor and its analogue UDP-glucose were recently implicated in triggering innate mucosal immunity in the female reproductive tract (1).
We next examined whether endogenously produced ATP may contribute to UPEC-evoked IL-8 production. Cells infected with UPEC in the presence of the apyrase enzyme, which degrades extracellular ATP, produced significantly less IL-8 than cells infected in the absence of apyrase. This suggests that ATP is produced during UPEC infection and, in turn, activates purinergic receptors to enhance IL-8 secretion. In LPS-stimulated THP-1 cells, the output of IL-8 was also decreased by apyrase (35), suggesting an autocrine type of mechanism for extracellular ATP. Moreover, intestinal bacteria produce large amounts of extracellular ATP that stimulate purinergic receptors in lamina propria cells to induce IL-6 and the differentiation of T lymphocytes (2).
UPEC-evoked IL-8 secretion is known to involve fimbria-mediated TLR4 signaling (12), and our results suggest that ATP may participate in the UPEC-evoked IL-8 response. It is tempting to speculate that P2Y receptors located on the urothelium may sense ATP derived from bacteria and thereby contribute to the first line of defense against bacteria. However, at the mucosal surface, there is a need to distinguish nonpathogenic or asymptomatic strains from pathogenic strains in order to activate an appropriate host response (32). It is not known whether pathogenic bacteria release more ATP than nonpathogenic bacteria do, but measurement of ATP levels can be used to differentiate bacteriuria from abacteriuria (21). Thus, UPEC bacteria alone may produce sufficient ATP for activation of mucosal P2Y receptors, or binding of UPEC to the host cell may be needed to trigger epithelial release of high local concentrations of ATP. ATP is an established endogenous danger signal released by cells undergoing stress or damage (8), and P2Y receptors may behave as danger sensors for ATP released by infected uroepithelial cells to initiate a host response. The fact that ATP-γ-S was able to elicit a strong and sustained IL-8 response provides evidence for a novel, non-TLR4-mediated mechanism underlying production of proinflammatory IL-8 in human urinary tract epithelial cells. However, elucidation of possible cross talk between the TLR4 and P2Y receptor pathways awaits further studies. Besides acting on uroepithelial cells, extracellular ATP seems to be essential for controlling neutrophil migration. IL-8-induced neutrophil chemotaxis was found to require a concurrent activation of neutrophil P2Y receptors (16). Thus, enhanced release of extracellular ATP during a urinary tract infection (UTI) may both trigger uroepithelial IL-8 release and facilitate neutrophil migration by acting on P2Y receptors.
In conclusion, we showed enhanced ATP release from UPEC-infected host epithelial cells and identified P2Y receptors as novel receptors involved in the production of proinflammatory cytokines. Enhanced release of extracellular ATP during a UTI may trigger the early cellular host response and play a role in the recruitment and function of neutrophils.
Acknowledgments
This project was supported by the Swedish Medical Research Council (12601) and the Natural Sciences Faculty at the University of Kalmar.
We are thankful to Anita Koskela for valuable help with the ATP assay.
Editor: B. A. McCormick
Footnotes
Published ahead of print on 1 June 2010.
REFERENCES
- 1.Arase, T., H. Uchida, T. Kajitani, M. Ono, K. Tamaki, H. Oda, S. Nishikawa, M. Kagami, T. T. Nagashima, H. Masuda, H. Asada, Y. Yoshimura, and T. Maruyama. 2009. The UDP-glucose receptor P2RY14 triggers innate mucosal immunity in the female reproductive tract by inducing IL-8. J. Immunol. 182:7074-7084. [DOI] [PubMed] [Google Scholar]
- 2.Atarashi, K., J. Nishimura, T. Shima, Y. Umesaki, M. Yamamoto, M. Onoue, H. Yagita, N. Ishii, R. Evans, K. Honda, and K. Takeda. 2008. ATP drives lamina propria T(H)17 cell differentiation. Nature 455:808-812. [DOI] [PubMed] [Google Scholar]
- 3.Bäckhed, F., M. Soderhall, P. Ekman, S. Normark, and A. Richter-Dahlfors. 2001. Induction of innate immune responses by Escherichia coli and purified lipopolysaccharide correlate with organ- and cell-specific expression of Toll-like receptors within the human urinary tract. Cell. Microbiol. 3:153-158. [DOI] [PubMed] [Google Scholar]
- 4.Beutler, B. 2000. Tlr4: central component of the sole mammalian LPS sensor. Curr. Opin. Immunol. 12:20-26. [DOI] [PubMed] [Google Scholar]
- 5.Birder, L. A. 2005. More than just a barrier: urothelium as a drug target for urinary bladder pain. Am. J. Physiol. Renal Physiol. 289:F489-F495. [DOI] [PubMed] [Google Scholar]
- 6.Birder, L. A., and W. C. de Groat. 2007. Mechanisms of disease: involvement of the urothelium in bladder dysfunction. Nat. Clin. Pract. Urol. 4:46-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birder, L. A., H. Z. Ruan, B. Chopra, Z. Xiang, S. Barrick, C. A. Buffington, J. R. Roppolo, A. P. Ford, W. C. de Groat, and G. Burnstock. 2004. Alterations in P2X and P2Y purinergic receptor expression in urinary bladder from normal cats and cats with interstitial cystitis. Am. J. Physiol. Renal Physiol. 287:F1084-F1091. [DOI] [PubMed] [Google Scholar]
- 8.Bours, M. J., E. L. Swennen, F. Di Virgilio, B. N. Cronstein, and P. C. Dagnelie. 2006. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol. Ther. 112:358-404. [DOI] [PubMed] [Google Scholar]
- 9.Crane, J. K., R. A. Olson, H. M. Jones, and M. E. Duffey. 2002. Release of ATP during host cell killing by enteropathogenic E. coli and its role as a secretory mediator. Am. J. Physiol. Gastrointest. Liver Physiol. 283:G74-G86. [DOI] [PubMed] [Google Scholar]
- 10.Crane, J. K., T. M. Naeher, S. S. Choudhari, and E. M. Giroux. 2005. Two pathways for ATP release from host cells in enteropathogenic Escherichia coli infection. Am. J. Physiol. Gastrointest. Liver Physiol. 289:G407-G417. [DOI] [PubMed] [Google Scholar]
- 11.Douillet, C. D., W. P. Robinson III, P. M. Milano, R. C. Boucher, and P. B. Rich. 2006. Nucleotides induce IL-6 release from human airway epithelia via P2Y2 and p38 MAPK-dependent pathways. Am. J. Physiol. Lung Cell. Mol. Physiol. 291:L734-L746. [DOI] [PubMed] [Google Scholar]
- 12.Frendéus, B., C. Wachtler, M. Hedlund, H. Fischer, P. Samuelsson, M. Svensson, and C. Svanborg. 2001. Escherichia coli P fimbriae utilize the Toll-like receptor 4 pathway for cell activation. Mol. Microbiol. 40:37-51. [DOI] [PubMed] [Google Scholar]
- 13.Fujita, T., H. Tozaki-Saitoh, and K. Inoue. 2009. P2Y1 receptor signaling enhances neuroprotection by astrocytes against oxidative stress via IL-6 release in hippocampal cultures. Glia 57:244-257. [DOI] [PubMed] [Google Scholar]
- 14.Gabel, C. A. 2007. P2 purinergic receptor modulation of cytokine production. Purinergic Signal. 3:27-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hedlund, M., M. Svensson, A. Nilsson, R. D. Duan, and C. Svanborg. 1996. Role of the ceramide-signaling pathway in cytokine responses to P-fimbriated Escherichia coli. J. Exp. Med. 183:1037-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kukulski, F., F. Ben Yebdri, J. Lecka, G. Kauffenstein, S. A. Lévesque, M. Martín-Satué, and J. Sévigny. 2009. Extracellular ATP and P2 receptors are required for IL-8 to induce neutrophil migration. Cytokine 46:166-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lambrecht, G. 2000. Agonists and antagonists acting at P2X receptors: selectivity profiles and functional implications. Naunyn Schmiedebergs Arch. Pharmacol. 362:340-350. [DOI] [PubMed] [Google Scholar]
- 18.la Sala, A., D. Ferrari, F. Di Virgilio, M. Idzko, J. Norgauer, and G. Girolomoni. 2003. Alerting and tuning the immune response by extracellular nucleotides. Leukoc. Biol. 73:339-343. [DOI] [PubMed] [Google Scholar]
- 19.Mohlin, C., S. Säve, M. Nilsson, and K. Persson. 2009. Studies of the extracellular ATP-adenosine pathway in human urinary tract epithelial cells. Pharmacology 84:196-202. [DOI] [PubMed] [Google Scholar]
- 20.Nicholas, R. A., W. C. Watt, E. R. Lazarowski, Q. Li, and K. Harden. 1996. Uridine nucleotide selectivity of three phospholipase C-activating P2 receptors: identification of a UDP-selective, a UTP-selective, and an ATP- and UTP-specific receptor. Mol. Pharmacol. 50:224-249. [PubMed] [Google Scholar]
- 21.Osterberg, E., H. O. Hallander, A. Kallner, A. Lundin, and H. Aberg. 1991. Evaluation of the adenosine triphosphate test in the diagnosis of urinary tract infection. Eur. J. Clin. Microbiol. Infect. Dis. 10:70-73. [DOI] [PubMed] [Google Scholar]
- 22.Rossi, M. R., J. R. Masters, S. Park, J. H. Todd, S. H. Garrett, M. A. Sens, S. Somji, J. Nath, and D. A. Sens. 2001. The immortalized UROtsa cell line as a potential cell culture model of human urothelium. Environ. Health Perspect. 109:801-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scherberich, J. E., and A. Hartinger. 2008. Impact of Toll-like receptor signalling on urinary tract infection. Int. J. Antimicrob. Agents 31(Suppl. 1):S9-S14. [DOI] [PubMed] [Google Scholar]
- 24.Schilling, J. D., S. M. Martin, D. A. Hunstad, K. P. Patel, M. A. Mulvey, S. S. Justice, R. G. Lorenz, and S. J. Hultgren. 2003. CD14- and Toll-like receptor-dependent activation of bladder epithelial cells by lipopolysaccharide and type 1 piliated Escherichia coli. Infect. Immun. 71:1470-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwiebert, E. M., and A. Zsembery. 2003. Extracellular ATP as a signaling molecule for epithelial cells. Biochim. Biophys. Acta 1615:7-32. [DOI] [PubMed] [Google Scholar]
- 26.Seiffert, K., W. Ding, J. A. Wagner, and R. D. Granstein. 2006. ATPgammaS enhances the production of inflammatory mediators by a human dermal endothelial cell line via purinergic receptor signaling. J. Investig. Dermatol. 126:1017-1027. [DOI] [PubMed] [Google Scholar]
- 27.Shimazu, R., S. Akashi, H. Ogata, Y. Nagai, K. Fukudome, K. Miyake, and M. Kimoto. 1999. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J. Exp. Med. 189:1777-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Straub, R. H., G. Pongratz, C. Günzler, A. Michna, S. Baier, F. Kees, W. Falk, and J. Schölmerich. 2002. Immunoregulation of IL-6 secretion by endogenous and exogenous adenosine and by exogenous purinergic agonists in splenic tissue slices. J. Neuroimmunol. 125:73-81. [DOI] [PubMed] [Google Scholar]
- 29.Sun, Y., and T. C. Chai. 2006. Augmented extracellular ATP signaling in bladder urothelial cells from patients with interstitial cystitis. Am. J. Physiol. Cell Physiol. 290:C27-C34. [DOI] [PubMed] [Google Scholar]
- 30.Sun, Y., S. Keay, P. G. De Deyne, and T. C. Chai. 2001. Augmented stretch activated adenosine triphosphate release from bladder uroepithelial cells in patients with interstitial cystitis. J. Urol. 166:1951-1956. [PubMed] [Google Scholar]
- 31.Sun, Y., S. Keay, T. J. Lehrfeld, and T. C. Chai. 2009. Changes in adenosine triphosphate-stimulated ATP release suggest association between cytokine and purinergic signaling in bladder urothelial cells. Urology 74:1163-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Svanborg, C., G. Bergsten, H. Fischer, G. Godaly, M. Gustafsson, D. Karpman, A. C. Lundstedt, B. Ragnarsdottir, M. Svensson, and B. Wullt. 2006. Escherichia coli as a model of host-parasite interaction. Curr. Opin. Microbiol. 9:33-39. [DOI] [PubMed] [Google Scholar]
- 33.von Kügelgen, I., and A. Wetter. 2000. Molecular pharmacology of P2Y receptors. Naunyn Schmiedebergs Arch. Pharmacol. 362:310-323. [DOI] [PubMed] [Google Scholar]
- 34.Wang, E. C., J. M. Lee, W. G. Ruiz, E. M. Balestreire, M. von Bodungen, S. Barrick, D. A. Cockayne, L. A. Birder, and G. Apodaca. 2005. ATP and purinergic receptor-dependent membrane traffic in bladder umbrella cells. J. Clin. Invest. 115:2412-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Warny, M., S. Aboudola, S. C. Robson, J. Sévigny, D. Communi, S. P. Soltoff, and C. P. Kelly. 2001. P2Y(6) nucleotide receptor mediates monocyte interleukin-8 production in response to UDP or lipopolysaccharide. J. Biol. Chem. 276:26051-26056. [DOI] [PubMed] [Google Scholar]
- 36.Yu, J., N. Sheung, E. M. Soliman, C. Spirli, and J. A. Dranoff. 2009. Transcriptional regulation of IL-6 in bile duct epithelia by extracellular ATP. Am. J. Physiol. Gastrointest. Liver Physiol. 296:G563-G571. [DOI] [PMC free article] [PubMed] [Google Scholar]