Abstract
Mycobacterium avium subsp. paratuberculosis, the agent of Johne's disease, infects ruminant hosts by translocation through the intestinal mucosa. A number of studies have suggested that M. avium subsp. paratuberculosis interacts with M cells in the Peyer's patches of the small intestine. The invasion of the intestinal mucosa by M. avium subsp. paratuberculosis and Mycobacterium avium subsp. hominissuis, a pathogen known to interact with intestinal cells, was compared. M. avium subsp. paratuberculosis was capable of invading the mucosa, but it was significantly less efficient at dissemination than M. avium subsp. hominissuis. B-cell knockout (KO) mice, which lack Peyer's patches, were used to demonstrate that M. avium subsp. paratuberculosis enters the intestinal mucosa through enterocytes in the absence of M cells. In addition, the results indicated that M. avium subsp. paratuberculosis had equal abilities to cross the mucosa in both Peyer's patch and non-Peyer's patch segments of normal mice. M. avium subsp. paratuberculosis was also shown to interact with epithelial cells by an α5β1 integrin-independent pathway. Upon translocation, dendritic cells ingest M. avium subsp. paratuberculosis, but this process does not lead to efficient dissemination of the infection. In summary, M. avium subsp. paratuberculosis interacts with the intestinal mucosa by crossing both Peyer's patches and non-Peyer's patch areas but does not translocate or disseminate efficiently.
Mycobacterium avium subsp. paratuberculosis is the agent of Johne's disease, a debilitating condition of cattle and other ruminants that is associated with severe diarrhea and wasting (10, 32). Johne's disease causes significant economic loss, with a severe impact on the dairy industry (5, 37). Some studies have implicated M. avium subsp. paratuberculosis as one of the potential etiologic agents or opportunistic pathogens of Crohn's disease patients (5, 37).
M. avium subsp. paratuberculosis infects young calves and is usually transmitted by contaminated stools or milk (10, 29). In the first steps of pathogenesis, the bacterium crosses the intestinal mucosa of the infected host. Previous work has suggested that M. avium subsp. paratuberculosis crosses the intestinal mucosa by entering M cells in the Peyer's patches of calves (15). More recently, a goat kid animal model suggested that the ports of entry in the intestinal mucosa are not limited to M cells (24). In addition, work in vitro showed that M. avium subsp. paratuberculosis can invade bovine kidney epithelial (17) and murine intestinal epithelial (20) cells efficiently. Mycobacterium avium subsp. hominissuis, a subspecies related to M. avium subsp. paratuberculosis (26), invades the intestinal mucosa by interacting primarily with enterocytes (4, 21). AIDS patients acquire M. avium subsp. hominissuis infections mainly through the gastrointestinal tract (6), and studies of macaques have demonstrated that this microorganism is within enterocytes (L. E. Bermudez et al., unpublished observations). It should be noted that in earlier literature, this subspecies is referred to simply as Mycobacterium avium or Mycobacterium avium subsp. avium (5, 12, 31); the new taxonomic description (M. avium subsp. hominissuis) for microorganisms isolated from humans and swine is relatively recent (14) and was not immediately captured or accepted by the scientific community at large (31). Nonetheless, in spite of the close genetic relationship, M. avium subsp. hominissuis is likely the only truly environmental mycobacterium (33), often acquired by humans and pigs, but rarely isolated from birds or cattle (33). In contrast, M. avium subsp. paratuberculosis is the predominant, or possibly the only, mycobacterium isolated from ruminants with Johne's disease and associated with cases of Crohn's disease in humans, but rarely isolated from AIDS patients. Thus, these subspecies represent microorganisms associated with unique disease entities and epidemiological distributions.
The mouse model of M. avium subsp. paratuberculosis may not entirely reflect the disease in cattle, sheep, and goats; nevertheless, it has value for studying aspects of M. avium subsp. paratuberculosis pathogenesis in small laboratory animals (11, 16). M. avium subsp. paratuberculosis has been observed multiplying in the intestinal mucosa of athymic nude gnotobiotic mice (9), indicating that the bacterium can replicate efficiently in the intestinal mucosa in the absence of a competent immune system. The availability of recombinant mouse strains offers the opportunity to address the roles of M cells and enterocytes in the translocation of M. avium subsp. paratuberculosis through the intestinal wall. To study this process, we compared M. avium subsp. paratuberculosis dissemination in wild-type mice and B-cell knockout (KO) mice, which lack Peyer's patches and M cells. Our results indicate that M. avium subsp. paratuberculosis crosses the intestinal mucosa by invading both M cells and enterocytes and that it can translocate, although with less efficiency than M. avium subsp. hominissuis, across mucosal epithelial cells. Bacteria also infected dendritic cells after crossing the epithelial barrier.
MATERIALS AND METHODS
Bacteria.
M. avium subsp. paratuberculosis strain K-10 is a bovine isolate capable of causing disease in both cattle and mice (18). M. avium subsp. paratuberculosis was grown on Middlebrook 7H10 agar plates supplemented with 2 mg/liter of mycobactin J and oleic acid, albumin, dextrose, and catalase (OADC). M. avium subsp. hominissuis strain 101 is capable of infecting immunocompetent and immunosuppressed mice (2). M. avium subsp. hominissuis was cultured on 7H10 agar supplemented with OADC. Bacteria grown for 14 days (M. avium subsp. hominissuis) or 20 days (M. avium subsp. paratuberculosis) were used in the experiments. Staphylococcus aureus SA101 is a clinical isolate obtained from the purulent material of a cutaneous abscess. For the experiments, this bacterium was cultured on Mueller-Hilton agar plates for 48 h.
Mice.
Eight- to 10-week-old female pathogen-free C57BL/6J black mice, weighing 25 g, were obtained from the Jackson Laboratory (Bar Harbor, ME) and were used after 2 weeks of quarantine. C57BL/6J B-cell-deficient mice (immunoglobulin H6 negative, 8 to 10 weeks old, weighing 25 g) were purchased from the Jackson Laboratory and were used after 2 weeks of quarantine. These animals have previously been shown to lack Peyer's patches and M cells (8). In some experiments, 10- to 12-week-old mice were used. All experiments were performed according to the guidelines of the institutional animal care and use committee.
Infection of mice.
The first set of experiments compared the infection of C57BL/6J mice by M. avium subsp. paratuberculosis versus M. avium subsp. hominissuis. Bacteria were cultured on solid medium as described above, with the inoculum prepared at 2.4 × 107 CFU/ml (M. avium subsp. hominissuis 101) or 3.8 × 107 CFU/ml (M. avium subsp. paratuberculosis K-10). Mice were given 0.1 ml of the bacterial suspension per os and were followed for 1, 2, 4, 8, and 16 weeks postinfection (wpi). Ten mice were used for each time point in each of two sets of experiments. At each time point, mice were killed, and the spleens, livers, and terminal ilea were harvested, homogenized, serially diluted, and plated onto Middlebrook 7H10 agar plates supplemented with OADC, mycobactin J, and PACT (polymyxin B at 5 μg/ml, amphotericin B at 4.5 μg/ml, carbenicillin at 22 μg/ml, and trimethoprim at 2 μg/ml).
Intestinal loop.
The intestinal-loop assay was carried out basically as described previously for M. avium subsp. hominissuis (21). Briefly, both C57BL/6 black mice and C57BL/6J B-cell-deficient mice (immunoglobulin H6 negative), 8 to 10 weeks old, weighing 25 g, were anesthetized by intraperitoneal administration of phenobarbital. Mice were maintained under profound anesthesia during the entire procedure. Following anesthesia, the abdominal cavity was carefully opened, and a segment of the small intestine that was approximately 3 cm long, proximal to the ileocecal area, was identified. A suture line was tied at both ends of the segment of intestine, tightly enough to close the intestinal lumen while not interfering with the blood flow. A suspension containing approximately 1 × 107 M. avium subsp. hominissuis or 2.5 × 107 M. avium subsp. paratuberculosis organisms in Hanks' balanced salt solution (HBSS) was injected into the proximal position of the isolated intestinal segment. Mice were kept alive for 1 and 3 h, after which they were killed, and the intestinal segment was removed, opened longitudinally, and rinsed with HBSS to remove unbound or weakly bound bacteria. The removed intestinal segment was placed in 5 ml of 7H9 broth with 30% glycerol and was homogenized. The suspension was then serially diluted in 7H9 broth before being plated onto 7H10 agar, supplemented with mycobactin J and PACT to inhibit intestinal biota, for quantification of viable organisms associated with the intestinal mucosa-submucosa. Plates were cultured for several days at 37°C, and the concentration of bacteria (expressed as CFU per gram of tissue) was calculated as (average CFU per plate × dilution factor × 5 ml)/(intestinal-segment weight).
Differential uptake by Peyer's patch and non-Peyer's patch segments.
To determine whether M. avium subsp. paratuberculosis enters the intestinal mucosa preferentially by M cells or enterocytes, we performed an in vivo assay in which 10-week-old C57BL/6J mice were given M. avium subsp. paratuberculosis (3 × 108 bacteria) orally. At 4 h and 24 h, the mice (5 mice per time point per experimental group) were sacrificed. Their abdomens were opened, and for each mouse, 5 segments (length, 1 cm) comprising regions with Peyer's patches and 5 segments without Peyer's patches were obtained, opened longitudinally, washed, homogenized, and plated onto 7H10 agar with mycobactin J.
Histopathology.
The intestinal ilea from four wild-type mice and four B-cell KO mice were obtained, fixed with 10% neutral buffered formalin, and stained with hematoxylin-eosin or an acid-fast stain as previously described (13).
Electron microscopy.
Mice were infected with M. avium subsp. paratuberculosis, and 1 or 2 days after infection, the animals were harvested; the intestinal segment was cut and opened longitudinally, briefly washed in HBSS, and immersed in 4% paraformaldehyde and 2% glutaraldehyde for 12 h at 4°C. Then the material was placed in HBSS and stained with a 1% aqueous solution of osmium tetroxide for 1 h at 4°C, as previously described (17). Samples were dehydrated in ethanol at room temperature, embedded in resin, and polymerized at 52°C. Photographs were taken using a transmission electron microscope (EM) at a magnification of ×10,000.
Translocation assay.
Translocation assays were performed as previously described (17), using the Transwell 2-chamber culture system (Costar; Corning, NY) containing a 0.33-cm2 porous membrane (pore size, 3.0 μm). Monolayers were established on the top of the membrane by seeding it with 1 × 106 Madin-Darby bovine kidney (MDBK) cells in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS). The culture medium was changed every 2 days, and the integrity of the monolayer was monitored by the following methods: (i) microscopic observation, (ii) measurement of the transwell resistance using a Millipore (Bedford, MA) transwell device as reported elsewhere (17), and (iii) the trypan blue (0.25%) permeability assay (optical density at 580 nm), as described previously (3). Trypan blue (0.25%) was added to the monolayer, and 3 h later, the supernatant of the bottom chamber was obtained for a spectrophotometer reading. The control included the medium alone (baseline). The top chamber was infected either with 5 × 105 bacteria or with 2 × 105 bacteria that had previously been exposed to whole cow milk, as described previously (17). After 2, 6, or 24 h of infection, 500 μl of filtrate was collected from the bottom chamber, and the system was replenished with fresh culture medium. The translocation ability was calculated as the cumulative percentage of the initial inoculum that was recovered in the bottom chamber at each time point.
Bacterial uptake assays.
Bacteria (105) were added to MDBK cells in medium with 10% FBS for 1 h. Extracellular bacteria were removed, and monolayers were washed three times with HBSS. Monolayers were then lysed and the number of intracellular bacteria quantified.
Bovine dendritic cells.
Bovine dendritic cells were obtained by differentiation of bovine monocytes by treatment with human recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF; 10 μg/ml) and interleukin-4 (IL-4; 10 μg/ml) (Genzyme, Cambridge, MA). Mononuclear phagocytes from healthy cows at the Oregon State University (OSU) dairy farm, which are negative for Johne's disease, were isolated by using Percoll density gradient. Monocytes were then resuspended in RPMI 1640 supplemented with 10% FBS. The monocytes obtained were seeded at 2 × 105 per well and were treated with recombinant cytokines. Monocytes were matured for 7 days (35, 36) and were used when the morphology was indicative of dendritic cells. Dendritic cells were then maintained in RPMI 1640 supplemented with 10% FBS. To infect the dendritic cells (approximately 105 cells), we used 106 bacteria for 1 h. Afterward, monolayers were washed three times, and the lysate was plated onto 7H10 agar with mycobactin J. In some experiments, polarized monolayers of MDBK cells in a Transwell system were infected with 5 × 106 M. avium subsp. paratuberculosis bacteria for 2 h. Twenty-four hours after the infection, the translocated bacteria in the supernatant in the lower chamber were quantified. The cell culture supernatant was obtained and transferred to a 24-well plate with adherent dendritic cells. Then 1 × 105 dendritic cells were incubated with 1 × 105 translocated M. avium subsp. paratuberculosis bacteria for 1 h. As controls, bacteria in 7H9 medium supplemented with mycobactin J were used. Monolayers were repeatedly washed and lysed, and the internalized bacteria were quantified.
Statistical analysis.
The comparisons among experimental groups and the control were evaluated for statistical significance by using Student's t test or the Mann-Whitney test.
RESULTS
Infection of C57BL/6J with M. avium subsp. hominissuis or M. avium subsp. paratuberculosis.
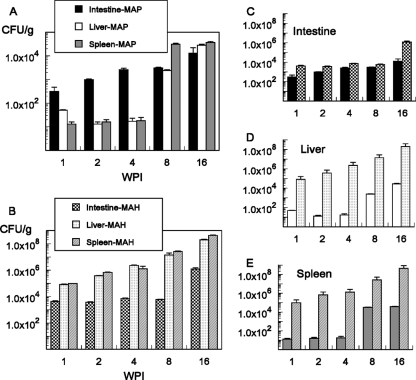
To evaluate whether M. avium subsp. paratuberculosis invades the intestinal mucosae of mice, and if it does so differently from M. avium subsp. hominissuis, mice were infected orally, and the numbers of bacteria in the terminal ileum, liver, and spleen were monitored over time. In C57BL/6 black mice, the intestinal bacillary counts for M. avium subsp. paratuberculosis increased monotonically during the 16-week period. In contrast, M. avium subsp. paratuberculosis did not grow to significant numbers in the liver or spleen at 4 wpi, but moderate dissemination to the liver and the spleen was observed at 8 and 16 wpi (Fig. 1 A). Intestines remained the organ with the greatest microbial burden during this period. Infection with M. avium subsp. hominissuis followed a different course, with bacillary counts increasing monotonically in the liver and the spleen, while intestinal counts remained approximately constant, increasing only at 16 wpi (Fig. 1B). Comparison on a per-organ basis led to the observation that M. avium subsp. paratuberculosis and M. avium subsp. hominissuis reached statistically significantly different levels in the intestines of infected animals, but the difference was less than 100-fold for all cases (Fig. 1C). Dramatic differences, with extremely high counts for M. avium subsp. hominissuis-infected animals, were observed in the liver (Fig. 1D) and the spleen (Fig. 1E). In all cases, M. avium subsp. hominissuis burdens 500-fold greater than M. avium subsp. paratuberculosis burdens were observed. In summary, M. avium subsp. paratuberculosis is less efficient at dissemination in the host than M. avium subsp. hominissuis. These results further confirmed that the mouse can be used as a model for investigating the interaction between M. avium subsp. paratuberculosis and the intestinal mucosa.
FIG. 1.
Microbial burdens in organs of infected C57BL/6 black mice. (A and B) Mice were infected as described in Materials and Methods, and microbial burdens in animals infected with M. avium subsp. paratuberculosis (MAP) (A) or M. avium subsp. hominissuis (MAH) (B) were determined as a function of time. Note that different scales are used for the y axes in panels A and B. (C, D, and E) The same data are plotted by using the same y axis scale for all graphs, highlighting the comparison of organ burdens between the two M. avium subspecies. Mice were infected with 2.4 × 106 M. avium subsp. hominissuis organisms or with 3.8 × 106 M. avium subsp. paratuberculosis organisms. Each bar represents the mean data collected from 20 mice. Error bars, standard errors of the means. P < 0.05 for the comparison between M. avium subsp. hominissuis and M. avium subsp. paratuberculosis at the same time point for each of the organs.
Intestinal loop.
To determine whether M. avium subsp. paratuberculosis needs Peyer's patches in order to cross the intestinal mucosa, we used an intestinal-loop model with C57BL/6 wild-type and B-cell knockout mice, which lack Peyer's patches. As seen in Table 1, both M. avium subsp. paratuberculosis and M. avium subsp. hominissuis (used as a control) (21) are able to enter the intestinal mucosa equally well in the presence or absence of Peyer's patches. That finding, though, does not necessarily indicate that M. avium subsp. paratuberculosis invades the intestinal mucosa through the Peyer's patches and Peyer's patch-deficient segments in the wild-type mouse.
TABLE 1.
Efficacy of invasion of the intestinal mucosae of C57BL/6 mice and B-cell-deficient mice (lacking Peyer's patches) by M. avium subsp. paratuberculosis using an intestinal-loop modela
| Bacterium | % of inoculum inside the mucosab |
|||
|---|---|---|---|---|
| C57BL/6J mice |
B-cell-deficient mice |
|||
| 1 h | 3 h | 1 h | 3 h | |
| M. avium subsp. paratuberculosis | 5.1 ± 0.3 | 36 ± 10 | 4.3 ± 0.5 | 30 ± 6 |
| M. avium subsp. hominissuis | 7.6 ± 0.4 | 54 ± 9 | 6.8 ± 0.3 | 52 ± 4 |
Bacteria (1.7 × 107 M. avium subsp. hominissuis organisms or 2.5 × 107 M. avium subsp. paratuberculosis organisms) were injected into the intestinal loop.
The experiment was repeated twice, with a total of 10 mice per time point. P was >0.05 for comparison of the percentages of invasion of C57BL/6J versus B-cell-deficient mice.
Preferential site of invasion.
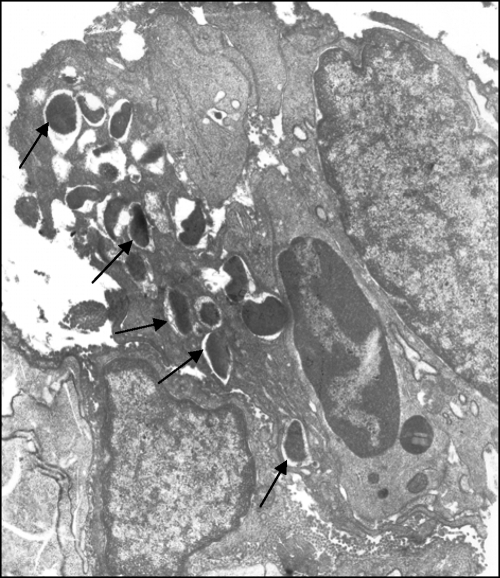
To determine the site of entry of M. avium subsp. paratuberculosis into the intestinal tract, mice were infected orally, and three segments (1 cm each) containing Peyer's patches and three segments containing non-Peyer's patch regions were homogenized and plated. As shown in Table 2, M. avium subsp. paratuberculosis enters equally well into the mucosal area of Peyer's patches and into surrounding areas that do not contain M cells. Moreover, M. avium subsp. paratuberculosis bacilli were visualized in association with enterocytes by transmission electron microscopy (Fig. 2).
TABLE 2.
Preferential site of M. avium subsp. paratuberculosis invasion of the intestinal mucosae of C57BL/6J mice
| Time | Concn of bacteria (CFU/g of intestinal tissue)a |
|
|---|---|---|
| Peyer's patch segments | Non-Peyer's patch segments | |
| 4 h | 3.6 ± 0.4 | 4.1 ± 0.8 |
| 24 h | 8 ± 2 | 9.3 ± 1.7 |
Number of colonies in 1 cm of terminal ileum. Each value is the average for five mice (three segments per mouse). P > 0.05 for all comparisons.
FIG. 2.
Transmission electron micrograph of a sample from a mouse orally infected with M. avium subsp. paratuberculosis. The intestine was harvested at 2 days postinfection. M. avium subsp. paratuberculosis can be observed within enterocytes (arrows). Magnification, ×10,000.
To determine whether the enterocyte route was important for infection, we carried out an in vivo experiment in which wild-type C57BL/6J mice were infected orally with M. avium subsp. paratuberculosis, and 4 h and 24 h later, the number of bacteria in the intestinal wall was determined. As shown in Table 2, comparison between the number of bacteria in Peyer's patch and non-Peyer's patch areas identified a slight preference for the bacteria to enter the non-Peyer's patch segments, though the differences were not statistically significant (P > 0.05). Histopathological observations indicated that wild-type mice infected with M. avium subsp. paratuberculosis displayed approximately the same number of bacteria in the submucosa as that in corresponding tissues from B-cell KO mice (data not shown).
Interactions with a polarized monolayer.
M. avium subsp. paratuberculosis incubated with polarized MDBK epithelial cells crossed the monolayer from the apical to the basolateral surface. As shown in Table 3, significant crossing occurs after 2 h. Crossing of the monolayer by M. avium subsp. paratuberculosis did not alter the transmembrane resistance. When bacteria were incubated in the presence of whole milk, a significantly greater number of organisms translocated MDBK cells at early time points (Table 3).
TABLE 3.
Translocation of M. avium subsp. paratuberculosis across an epithelial cell monolayer
| Condition | 2 h |
6 h |
24 h |
|||
|---|---|---|---|---|---|---|
| % of inoculum that translocated | Transmembrane resistance (Ω)a | % of inoculum that translocated | Transmembrane resistance (Ω) | % of inoculum that translocated | Transmembrane resistance (Ω) | |
| No exposure to milk (5 × 105 bacteria) | 0.2 ± 0.05 | 320 ± 10 | 0.4 ± 0.04c | 310 ± 30 | 0.6 ± 0.05 | 330 ± 24 |
| Preincubation with milk (2 × 105 bacteria)b | 0.4 ± 0.06 | 320 ± 26 | 0.7 ± 0.04c,d | 328 ± 24 | 1.5 ± 0.06c,d | 325 ± 39 |
Before the addition of bacteria, the transmembrane resistance was 320 Ω.
M. avium subsp. paratuberculosis was incubated in the presence of whole milk for 2 h, washed, and used in the experiments.
P < 0.05 for comparison with the previous time point.
P < 0.05 for comparison with M. avium subsp. paratuberculosis incubated without milk.
Role of α5β1 integrin in M. avium subsp. paratuberculosis uptake by MDBK cells.
Past work had suggested the possible role of fibronectin in the translocation of the bacterium through the intestinal tract. To investigate if M. avium subsp. paratuberculosis invades MDBK cells using the fibronectin receptor, we treated MDBK monolayers with a mouse anti-α5β1 IgG antibody or with an irrelevant anti-Escherichia coli lipopolysaccharide (LPS) IgG2a antibody at 30 μg/ml. Because we did not know whether the anti-human integrin would bind to the bovine antigen, we used a Staphylococcus aureus clinical isolate as a control. Incubation of S. aureus in the presence of the anti-α5β1 antibody led to an 85% ± 5% reduction in uptake in 1 h. In contrast, the entry of M. avium subsp. paratuberculosis was reduced by 37.3%. In the initial inoculum, most of the M. avium subsp. paratuberculosis, which was internalized by MDBK cells, entered by a different pathway than the fibronectin receptor. As shown in Table 4, the control antibody results support the absence of nonspecific effects.
TABLE 4.
Impact of the β1 integrin receptor on the entry of M. avium subsp. paratuberculosis into MDBK cells
| Experimental group |
S. aureus |
M. avium subsp. paratuberculosis |
||
|---|---|---|---|---|
| No. of intracellular bacteriaa | % Δb | No. of intracellular bacteriaa | % Δ | |
| Control (no antibody) | (3.4 ± 0.5) × 104 | (2.6 ± 0.4) × 103 | ||
| Anti-α5β1 | (5.1 ± 0.3) × 103 | 85c | (9.7 ± 0.2) × 102 | 37.3d |
| Anti-LPS | (3.2 ± 0.4) × 104 | −5d | (3.0 ± 0.3) × 103 | 15d |
The inoculum was 1.8 × 105 organisms for S. aureus and 1.1 × 105 organisms for M. avium subsp. paratuberculosis.
Δ, reduction.
P < 0.05 for comparison with the no-antibody control.
P > 0.05 for comparison with the no-antibody control.
Uptake by dendritic cells.
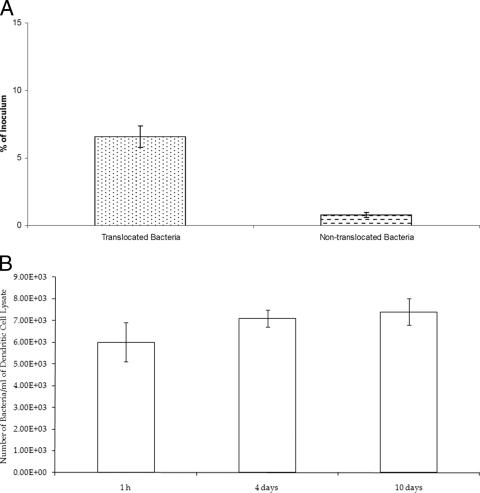
M. avium subsp. paratuberculosis was placed in the presence of dendritic cells, and the percentage of bacteria that were internalized was determined. After 15 min, (3 ± 0.4) × 101 M. avium subsp. paratuberculosis bacteria were observed inside dendritic cells (0.003% of the inoculum), while at 30 min and 1 h, (6 ± 0.6) × 102 (0.01% of the inoculum) and (1.5 ± 0.3) × 104 (1.8% of the inoculum) bacteria were ingested by dendritic cells, respectively (Table 5). Since M. avium subsp. paratuberculosis is likely to encounter dendritic cells upon translocation of the mucosal epithelium, we infected polarized MDBK cells and used the translocated bacteria to infect dendritic cells. As shown in Fig. 3, translocated bacteria were significantly more efficiently internalized by dendritic cells than bacteria grown on plates. Observation of monolayers for as long as 10 days showed a stable number of intracellular bacteria without significant growth.
TABLE 5.
Infection of bovine dendritic cellsa by M. avium subsp. paratuberculosis
| Time | No. of intracellular bacteria | % of inoculum |
|---|---|---|
| Uptake | ||
| 15 min | (3 ± 0.4) × 101 | 0.003 |
| 30 min | (6 ± 0.6) × 102 | 0.01 |
| Intracellular growth | ||
| 1 h | (1.5 ± 0.3) × 104 | |
| 4 days | (3.8 ± 0.3) × 104 | 1.8 |
A total of 1 × 106 monocyte-derived macrophages were incubated with human GM-CSF and IL-4 for maturation into dendritic cells.
FIG. 3.
Uptake and survival of M. avium subsp. paratuberculosis in dendritic cells. (A) Uptake of M. avium subsp. paratuberculosis by bovine dendritic cells following translocation across a polarized monolayer of MDBK cells. Uptake by dendritic cells was allowed for 1 h. Bacteria obtained from 7H9 broth medium supplemented with mycobactin J were used as a control. (B) Number of M. avium subsp. paratuberculosis bacteria in dendritic cells 10 days after infection (no significant differences in growth were observed).
DISCUSSION
In this study, we developed a novel low-dose (ca 2.5 × 107-CFU) oral-infection model of mice in order to compare the pathogenesis of M. avium subsp. hominissuis and M. avium subsp. paratuberculosis. In contrast, other studies using oral infection of mice have used a significantly higher dose (1.0 × 1010 to 1.0 × 1011 CFU). High-dose oral experimental infection with M. avium subsp. paratuberculosis resembles the intraperitoneal infection model, resulting in higher microbial burdens in the livers and spleens of infected animals than in the intestines (11). Our model reflects more closely the natural infection of ruminants, in that there is a higher level of colonization of the intestinal mucosa by M. avium subsp. paratuberculosis early in the infection (up to 4 wpi). However, at a similar infection dose, M. avium subsp. hominissuis is significantly more effective at disseminating in the host, reaching high colonization levels in the liver and spleen. Thus, this model reveals significant differences in the pathogenesis of these subspecies, as reflected in natural infections. In this context, M. avium subsp. paratuberculosis infects calves, and most of the bacteria remain in the intestinal wall, leading to lesions characteristic of Johne's diseases in the adult animal (25). A small percentage of the bacteria disseminate, with some spreading to distant tissues, such as the mammary gland (27) and local lymph nodes (28). M. avium subsp. hominissuis, in contrast, crosses the intestinal mucosa by entering enterocytes (21), where it suppresses chemokine production (22) and, therefore, inflammatory cell migration (13). M. avium subsp. hominissuis disseminates to mesenteric lymph nodes (19) and, in immunosuppressed individuals, to distant sites such as spleen and bone marrow (12).
M. avium subsp. paratuberculosis infects young calves through the gastrointestinal tract (9, 12, 27). It has been hypothesized that this microorganism crosses the intestinal mucosa by the Peyer's patches, reaching the submucosa (12, 19). However, a recent study with goat kids suggested that M. avium subsp. paratuberculosis enters the intestinal mucosa, translocating through enterocytes (20). By using B-cell knockout mice deficient in Peyer's patches, it was observed that M. avium subsp. paratuberculosis can still enter the intestinal wall. M. avium subsp. paratuberculosis can infect the mucosa similarly in C57BL/6J wild-type mice and in B-cell knockout animals, and when given orally, the bacterium can be found both in regions with Peyer's patches and in regions in which there are no Peyer's patches. The histopathological alterations at the initial stage of infection appear to be the same, suggesting that the two subspecies cross the mucosa similarly, but the kinetics of dissemination differ significantly. The molecular basis for this difference between these closely related organisms is unknown. Nonetheless, comparison of the two genomes unveiled the occurrence of genome segments specific for either M. avium subsp. paratuberculosis or M. avium subsp. hominissuis (38). The study of these genomic regions might help to explain these phenotypic differences. Alternatively, differential gene expression of key virulence determinants in these two subspecies may account for these observations.
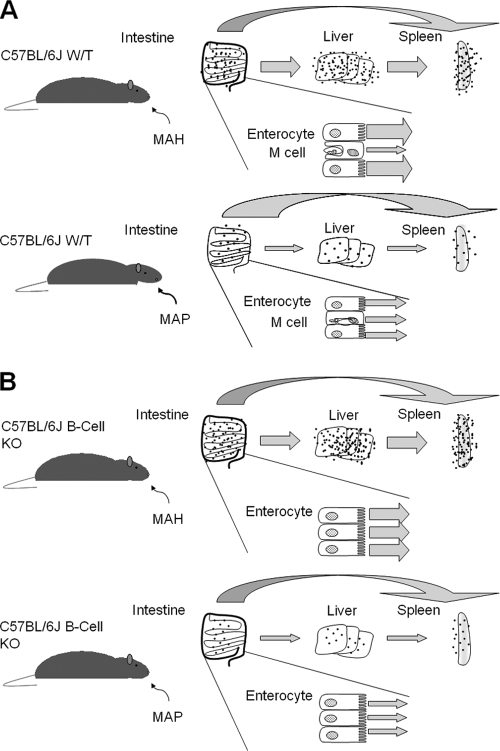
By using a mouse strain lacking Peyer's patches (B-cell KO mice) and comparing the level of infection with that of the wild-type mouse, it became evident that the levels of tissue infection were similar, independently of the existence of Peyer's patches. In the presence of Peyer's patches, the bacterium used the M cells to cross the mucosa, but in the absence of the patches, M. avium subsp. paratuberculosis was capable of interacting with enterocytes (Fig. 2). Additional experiments confirmed that bacteria delivered orally infected both Peyer's patches and non-Peyer's patch regions (Fig. 4 depicts a schematic model). Whether the mechanisms of interaction are the same is currently unknown. Bacterial genes required for the invasion of bovine epithelial cells have been identified (1), but all the evidence so far supports the hypothesis that uptake by M cells follows binding to the β1 integrin receptor (15, 23, 24). Our study confirmed that in vitro, invasion of MDBK cells by M. avium subsp. paratuberculosis does not depend on the β1 integrin receptor as a primary mechanism of uptake.
FIG. 4.
Mouse model of M. avium infection. Shown are predicted outcomes of oral infection of wild-type (C57BL/6J WT) (A) and B-cell-deficient (C57BL/6J B-cell KO) (B) mice with M. avium subsp. hominissuis (MAH) or M. avium subsp. paratuberculosis (MAP). Dots indicate bacillary burdens, and arrow widths are proportional to the numbers of translocated bacilli. In the model depicted, M. avium subsp. hominissuis is translocated mostly via enterocytes in WT mice.
What would be the evolutionary advantage for M. avium subsp. paratuberculosis of crossing the intestinal mucosa by two different pathways? We hypothesize that the function of the M-cell pathway is to recognize and contain the infection, while the enterocyte pathway represents the pathogenic route leading to dissemination. This hypothesis is consistent with the use of the enterocyte entry pathway by M. avium subsp. hominissuis, the intrinsically more virulent subspecies. The other plausible hypothesis is that each mechanism of entry represents a different outcome, both of which are important for the disease. Interaction with the Peyer's patches would result in rapid interaction with phagocytic cells, local spread, and an inflammatory response. In contrast, the infection of enterocytes would lead to a slow inflammatory response, translocation, and dissemination to distant sites, including the mammary glands. In the case of M. avium subsp. hominissuis, infection of enterocytes results in slow translocation followed by faster systemic dissemination (21). Alternatively, cells of the dendritic cell lineage might play a role in the dissemination of M. avium subsp. paratuberculosis. In fact, M. avium subsp. paratuberculosis infection of dendritic cells is inefficient. However, if the bacterium translocates across the epithelial barrier, uptake into dendritic cells increases 3- to 6-fold. This finding suggests that once bacteria cross the intestinal mucosa, they are readily ingested by dendritic cells. Once within dendritic cells, M. avium subsp. paratuberculosis was able to persist for 10 days, despite the lack of growth. Thus, dendritic cells may be responsible for the rapid spread of M. avium subsp. paratuberculosis to mesenteric lymph nodes, as observed by Wu and colleagues (39).
The finding that M. avium subsp. paratuberculosis translocates through enterocytes in our mouse model may also have implications for the potential colonization of the human epithelium. Recently, Golan et al. developed an M. avium subsp. paratuberculosis infection model using SCID mice transplanted with fetal human intestinal xenografts (7). Mice were infected by intraluminal challenge using a relatively low dose (approximately 5 × 107 CFU). The results indicated that goblet cells were predominantly infected compared to enterocytes. However, there are significant differences in the SCID model, including the use of human xenografts, the inoculation route, the complete absence of both B and T cells, and a complex interplay of both mouse and human innate immune cells. These differences may explain the predilection of M. avium subsp. paratuberculosis to invade either enterocytes or goblet cells. In this regard, intracellular pathogens, such as Salmonella spp., have been shown to invade both M cells and enterocytes, and, to a lesser extent, goblet cells, in mouse models, as reviewed by van Asten (34).
Our in vitro studies using polarized monolayers suggest that binding and internalization of M. avium subsp. paratuberculosis do not cause a decrease in transmembrane resistance up to 24 h of infection. In addition, the bacterium translocated through the epithelial monolayer, a finding that is in agreement with recent observations by Wu and colleagues that M. avium subsp. paratuberculosis is found in the mesenteric lymph nodes of cows just 2 h after infection (39). Although surgery and intestinal clumping can be associated with increased permeability and can explain, in part, the results of Wu and colleagues, the observation appears to suggest that M. avium subsp. paratuberculosis crosses the mucosal barrier. However, the percentage of bacteria that translocates is very small. Histopathological observations of cattle infected with M. avium subsp. hominissuis and M. avium subsp. paratuberculosis indicate that while M. avium subsp. hominissuis spreads quickly from the intestinal mucosa, M. avium subsp. paratuberculosis disseminates very slowly (30), which, in fact, was observed in our system.
In conclusion, this study demonstrates that M. avium subsp. paratuberculosis uses both Peyer's patches and enterocytes to cross the intestinal mucosa, a mechanism slightly different from that of M. avium subsp. hominissuis, which enters the mucosa preferentially by enterocytes (21). M. avium subsp. paratuberculosis also differs from M. avium subsp. hominissuis in the efficiency of dissemination. Further studies should focus on the understanding of those differences at the molecular level.
Acknowledgments
We thank Denny Weber for help with the preparation of the manuscript and Bernadette Stang for technical help.
We are also indebted to the Infectious Disease Foundation for the support of this research. L.E.B. was also supported by a grant from JDIP. R.G.B. was supported by funds from the BARD program (IS-3673-05C), the Johne's Disease Integrated Program (JDIP), USDA Cooperative State Service Project NEB 14-141, and the School of Veterinary Medicine and Biomedical Sciences.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 24 May 2010.
REFERENCES
- 1.Alonso-Hearn, M., D. Patel, L. Danelishvili, L. Meunier-Goddik, and L. E. Bermudez. 2008. The Mycobacterium avium subsp. paratuberculosis MAP3464 gene encodes an oxidoreductase involved in invasion of bovine epithelial cells through the activation of host cell Cdc42. Infect. Immun. 76:170-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bermudez, L. E., M. Petrofsky, P. Kolonoski, and L. S. Young. 1992. An animal model of Mycobacterium avium complex disseminated infection after colonization of the intestinal tract. J. Infect. Dis. 165:75-79. [DOI] [PubMed] [Google Scholar]
- 3.Bermudez, L. E., F. J. Sangari, P. Kolonoski, M. Petrofsky, and J. Goodman. 2002. The efficiency of the translocation of Mycobacterium tuberculosis across a bilayer of epithelial and endothelial cells as a model of the alveolar wall is a consequence of transport within mononuclear phagocytes and invasion of alveolar epithelial cells. Infect. Immun. 70:140-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bermudez, L. E., and L. S. Young. 1994. Factors affecting invasion of HT-29 and HEp-2 epithelial cells by organisms of the Mycobacterium avium complex. Infect. Immun. 62:2021-2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chacon, O., L. E. Bermudez, and R. G. Barletta. 2004. Johne's disease, inflammatory bowel disease, and Mycobacterium paratuberculosis. Annu. Rev. Microbiol. 58:329-363. [DOI] [PubMed] [Google Scholar]
- 6.Damsker, B., and E. J. Bottone. 1985. Mycobacterium avium-Mycobacterium intracellulare from the intestinal tracts of patients with the acquired immunodeficiency syndrome: concepts regarding acquisition and pathogenesis. J. Infect. Dis. 151:179-181. [DOI] [PubMed] [Google Scholar]
- 7.Golan, L., A. Livneh-Kol, E. Gonen, S. Yagel, I. Rosenshine, and N. Y. Shpigel. 2009. Mycobacterium avium paratuberculosis invades human small-intestinal goblet cells and elicits inflammation. J. Infect. Dis. 199:350-354. [DOI] [PubMed] [Google Scholar]
- 8.Golovkina, T. V., M. Shlomchik, L. Hannum, and A. Chervonsky. 1999. Organogenic role of B lymphocytes in mucosal immunity. Science 286:1965-1968. [DOI] [PubMed] [Google Scholar]
- 9.Hamilton, H. L., D. M. Follett, L. M. Siegfried, and C. J. Czuprynski. 1989. Intestinal multiplication of Mycobacterium paratuberculosis in athymic nude gnotobiotic mice. Infect. Immun. 57:225-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris, N. B., and R. G. Barletta. 2001. Mycobacterium avium subsp. paratuberculosis in veterinary medicine. Clin. Microbiol. Rev. 14:489-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hines, M. E., II, J. R. Stabel, R. W. Sweeney, F. Griffin, A. M. Talaat, D. Bakker, G. Benedictus, W. C. Davis, G. W. de Lisle, I. A. Gardner, R. A. Juste, V. Kapur, A. Koets, J. McNair, G. Pruitt, and R. H. Whitlock. 2007. Experimental challenge models for Johne's disease: a review and proposed international guidelines. Vet. Microbiol. 122:197-222. [DOI] [PubMed] [Google Scholar]
- 12.Inderlied, C. B., C. A. Kemper, and L. E. Bermudez. 1993. The Mycobacterium avium complex. Clin. Microbiol. Rev. 6:266-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim, S. Y., J. R. Goodman, M. Petrofsky, and L. E. Bermudez. 1998. Mycobacterium avium infection of gut mucosa in mice associated with late inflammatory response and intestinal cell necrosis. J. Med. Microbiol. 47:725-731. [DOI] [PubMed] [Google Scholar]
- 14.Mijs, W., P. de Haas, R. Rossau, T. Van der Laan, L. Rigouts, F. Portaels, and D. van Soolingen. 2002. Molecular evidence to support a proposal to reserve the designation Mycobacterium avium subsp. avium for bird-type isolates and ‘M. avium subsp. hominissuis’ for the human/porcine type of M. avium. Int. J. Syst. Evol. Microbiol. 52:1505-1518. [DOI] [PubMed] [Google Scholar]
- 15.Momotani, E., D. L. Whipple, A. B. Thiermann, and N. F. Cheville. 1988. Role of M cells and macrophages in the entrance of Mycobacterium paratuberculosis into domes of ileal Peyer's patches in calves. Vet. Pathol. 25:131-137. [DOI] [PubMed] [Google Scholar]
- 16.Mutwiri, G. K., D. G. Butler, S. Rosendal, and J. Yager. 1992. Experimental infection of severe combined immunodeficient beige mice with Mycobacterium paratuberculosis of bovine origin. Infect. Immun. 60:4074-4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel, D., L. Danelishvili, Y. Yamazaki, M. Alonso, M. L. Paustian, J. P. Bannantine, L. Meunier-Goddik, and L. E. Bermudez. 2006. The ability of Mycobacterium avium subsp. paratuberculosis to enter bovine epithelial cells is influenced by preexposure to a hyperosmolar environment and intracellular passage in bovine mammary epithelial cells. Infect. Immun. 74:2849-2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paustian, M. L., V. Kapur, and J. P. Bannantine. 2005. Comparative genomic hybridizations reveal genetic regions within the Mycobacterium avium complex that are divergent from Mycobacterium avium subsp. paratuberculosis isolates. J. Bacteriol. 187:2406-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petrofsky, M., and L. E. Bermudez. 2005. CD4+ T cells but not CD8+ or γδ+ lymphocytes are required for host protection against Mycobacterium avium infection and dissemination through the intestinal route. Infect. Immun. 73:2621-2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pott, J., T. Basler, C. U. Duerr, M. Rohde, R. Goethe, and M. W. Hornef. 2009. Internalization-dependent recognition of Mycobacterium avium ssp. paratuberculosis by intestinal epithelial cells. Cell. Microbiol. 11:1802-1815. [DOI] [PubMed] [Google Scholar]
- 21.Sangari, F. J., J. Goodman, M. Petrofsky, P. Kolonoski, and L. E. Bermudez. 2001. Mycobacterium avium invades the intestinal mucosa primarily by interacting with enterocytes. Infect. Immun. 69:1515-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sangari, F. J., M. Petrofsky, and L. E. Bermudez. 1999. Mycobacterium avium infection of epithelial cells results in inhibition or delay in the release of interleukin-8 and RANTES. Infect. Immun. 67:5069-5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Secott, T. E., T. L. Lin, and C. C. Wu. 2004. Mycobacterium avium subsp. paratuberculosis fibronectin attachment protein facilitates M-cell targeting and invasion through a fibronectin bridge with host integrins. Infect. Immun. 72:3724-3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sigurdardóttir, O. G., A. M. Bakke-McKellep, B. Djonne, and O. Evensen. 2005. Mycobacterium avium subsp. paratuberculosis enters the small intestinal mucosa of goat kids in areas with and without Peyer's patches as demonstrated with the everted sleeve method. Comp. Immunol. Microbiol. Infect. Dis. 28:223-230. [DOI] [PubMed] [Google Scholar]
- 25.Sigurethardóttir, O. G., M. Valheim, and C. M. Press. 2004. Establishment of Mycobacterium avium subsp. paratuberculosis infection in the intestine of ruminants. Adv. Drug Deliv. Rev. 56:819-834. [DOI] [PubMed] [Google Scholar]
- 26.Springer, B., L. Stockman, K. Teschner, G. D. Roberts, and E. C. Bottger. 1996. Two-laboratory collaborative study on identification of mycobacteria: molecular versus phenotypic methods. J. Clin. Microbiol. 34:296-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Streeter, R. N., G. F. Hoffsis, S. Bech-Nielsen, W. P. Shulaw, and D. M. Rings. 1995. Isolation of Mycobacterium paratuberculosis from colostrum and milk of subclinically infected cows. Am. J. Vet. Res. 56:1322-1324. [PubMed] [Google Scholar]
- 28.Sweeney, R. W., R. H. Whitlock, and A. E. Rosenberger. 1992. Mycobacterium paratuberculosis cultured from milk and supramammary lymph nodes of infected asymptomatic cows. J. Clin. Microbiol. 30:166-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor, T. K., C. R. Wilks, and D. S. McQueen. 1981. Isolation of Mycobacterium paratuberculosis from the milk of a cow with Johne's disease. Vet. Rec. 109:532-533. [PubMed] [Google Scholar]
- 30.Thoen, C. O., A. G. Karlson, and E. M. Himes. 1981. Mycobacterial infections in animals. Rev. Infect. Dis. 3:960-972. [DOI] [PubMed] [Google Scholar]
- 31.Thorel, M. F., M. Krichevsky, and V. V. Levy-Frebault. 1990. Numerical taxonomy of mycobactin-dependent mycobacteria, emended description of Mycobacterium avium, and description of Mycobacterium avium subsp. avium subsp. nov., Mycobacterium avium subsp. paratuberculosis subsp. nov., and Mycobacterium avium subsp. silvaticum subsp. nov. Int. J. Syst. Bacteriol. 40:254-260. [DOI] [PubMed] [Google Scholar]
- 32.Tripathi, B. N. 2005. Paratuberculosis (Johne's disease) in cattle. Int. J. Cow Sci. 1:16-26. [Google Scholar]
- 33.Turenne, C. Y., D. M. Collins, D. C. Alexander, and M. A. Behr. 2008. Mycobacterium avium subsp. paratuberculosis and M. avium subsp. avium are independently evolved pathogenic clones of a much broader group of M. avium organisms. J. Bacteriol. 190:2479-2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Asten, A. J., J. F. Koninkx, and J. E. van Dijk. 2005. Salmonella entry: M cells versus absorptive enterocytes. Vet. Microbiol. 108:149-152. [DOI] [PubMed] [Google Scholar]
- 35.Wagner, D., F. J. Sangari, S. Kim, M. Petrofsky, and L. E. Bermudez. 2002. Mycobacterium avium infection of macrophages results in progressive suppression of interleukin-12 production in vitro and in vivo. J. Leukoc. Biol. 71:80-88. [PubMed] [Google Scholar]
- 36.Weiss, D. J., C. D. Souza, O. A. Evanson, M. Sanders, and M. Rutherford. 2008. Bovine monocyte TLR2 receptors differentially regulate the intracellular fate of Mycobacterium avium subsp. paratuberculosis and Mycobacterium avium subsp. avium. J. Leukoc. Biol. 83:48-55. [DOI] [PubMed] [Google Scholar]
- 37.Whittington, R. J., and E. S. Sergeant. 2001. Progress towards understanding the spread, detection and control of Mycobacterium avium subsp. paratuberculosis in animal populations. Aust. Vet. J. 79:267-278. [DOI] [PubMed] [Google Scholar]
- 38.Wu, C. W., J. Glasner, M. Collins, S. Naser, and A. M. Talaat. 2006. Whole-genome plasticity among Mycobacterium avium subspecies: insights from comparative genomic hybridizations. J. Bacteriol. 188:711-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu, C. W., M. Livesey, S. K. Schmoller, E. J. Manning, H. Steinberg, W. C. Davis, M. J. Hamilton, and A. M. Talaat. 2007. Invasion and persistence of Mycobacterium avium subsp. paratuberculosis during early stages of Johne's disease in calves. Infect. Immun. 75:2110-2119. [DOI] [PMC free article] [PubMed] [Google Scholar]