Abstract
Polymyxins are cationic lipopeptides (five cationic charges) and the last resort for the treatment of serious Gram-negative infections caused by multiresistant strains. NAB741 has a cyclic peptide portion identical to that of polymyxin B but carries in the linear peptide portion a threonyl-d-serinyl residue (no cationic charges) instead of the diaminobutyryl-threonyl-diaminobutyryl residue (two cationic charges). At the N terminus of the peptide, NAB741 carries an acetyl group instead of a mixture of methyl octanoyl and methyl heptanoyl residues. NAB741 sensitized Escherichia coli, Klebsiella pneumoniae, Enterobacter cloacae, and Acinetobacter baumannii to antibiotics against which the intact outer membrane is an effective permeability barrier. When tested by using Etest strips on plates containing increasing concentrations of NAB741, the fractional inhibition concentration index (FICI) of the combination of NAB741 with rifampin ranged from ≤0.111 to 0.158 and that with clarithromycin from ≤0.094 to 0.292. When tested by the checkerboard method, the corresponding FICI values against E. coli ATCC 25922 were ≤0.141 to ≤0.155 with rifampin and 0.094 with clarithromycin. In addition, at 4 μg/ml, NAB741 decreased the MICs of azithromycin, mupirocin, fusidic acid, and vancomycin for E. coli strains and E. cloacae by factors ranging from 8 to 200. A sister peptide, NAB752, carrying a threonyl-aminobutyryl residue as the linear peptide portion, was inactive. Furthermore, NAB741 sensitized E. coli to the bactericidal activity of fresh guinea pig serum. The renal clearance of NAB741 was approximately 400-fold, 16-fold, and 8-fold higher than those measured for colistin, NAB7061, and NAB739, respectively.
The emergence of progressively more and more multiresistant strains of Gram-negative bacteria is a major public health concern (2, 3, 4, 10, 14, 18). To date, major therapeutic challenges have been caused by such strains of Pseudomonas aeruginosa, Acinetobacter baumannii, and Klebsiella pneumoniae, but an even worse threat is the current development and spread of the carbapenemase-producing multiresistant strains of Escherichia coli, an opportunist capable of causing common infections in otherwise healthy patients outside hospitals. Emerging novel plasmid-encoded resistance determinants, originally found in other Gram-negative species, have already been found in strains of E. coli worldwide (2, 3, 4, 10, 14, 18). Besides KPC and several other carbapenemases (16), they include the qnr-mediated fluoroquinolone resistance determinant (11, 17), as well as the 16S rRNA methylases that confer panresistance to aminoglycosides (8, 9).
The options to treat infections caused by multiresistant Gram-negative bacteria are becoming very scarce. Tigecycline is active against most strains of Enterobacteriaceae, but the low blood levels and other pharmacokinetic properties might limit its use in bacteremic pyelonephritis (2, 4, 7). There are no novel classes of agents in clinical development for the treatment of infections by Gram-negative bacteria, and the pipeline will remain dry in the next 10 years (2, 3, 14, 18).
Still another approach is to develop agents that permeabilize the outer membrane (OM) of Gram-negative bacteria to other antibacterial agents (23). Polymyxins (polymyxin B and colistin) are cyclic lipodecapeptides, each carrying five free amino groups and a net charge of +5 under physiological conditions. They are bactericidal antibiotics that permeabilize the OM. Very importantly, and in contrast to most of the other cationic peptides, their action is restricted to Gram-negative bacteria (23). Gram-positive bacteria, eukaryotic microbes, and mammalian cells are typically resistant to polymyxins. Furthermore, the OM-permeabilizing action of polymyxins is stereospecific (20). Today, polymyxins are increasingly the last resort for the treatment of serious infections by Gram-negative bacteria caused by the very resistant strains. In spite of their notable specificity, the use of polymyxins is shadowed by their occasional toxicity, especially nephrotoxicity (2, 3).
We have previously described novel polymyxin derivatives that carry only three positive charges (24, 25). NAB739 has a cyclic peptide portion identical to that of polymyxin B, but in the linear portion of the peptide, it carries a threonyl-d-serinyl residue (no cationic charge) instead of a diaminobutyryl-threonyl-diaminobutyryl residue (two cationic charges). The MIC90 of NAB739 for E. coli is identical to that of polymyxin B (1 μg/ml) (25). NAB7061 (linear portion of the peptide, threonyl-aminobutyryl [Abu]) lacks direct antibacterial activity but sensitizes the target bacteria to hydrophobic antibiotics (25, 26).
In this study, we describe further modifications, including NAB741. It has cyclic and linear peptide portions identical to those of NAB739 but differs from NAB739, NAB7061, and polymyxin B by carrying only an acetyl residue in the N terminus of the peptide instead of a significantly longer hydrophobic residue, i.e., octanoyl in NAB739 and NAB7061 and 6-methyloctanoyl or 6-methylheptanoyl in polymyxin B. We show that NAB741 has preserved the OM-permeabilizing activity of polymyxins. However, as also shown here, differences in the N-terminal moiety and the linear peptide portion affect the renal clearance of the compounds.
MATERIALS AND METHODS
Peptide synthesis.
Polymyxin derivatives were synthesized by conventional solid-phase chemistry as described in detail previously (25). Briefly, the α-amino function was protected by fluorenylmethoxycarbonyl (Fmoc), and the γ-amino group of the diaminobutyryl (Dab) residue involved in cyclization of the peptide was protected by t-butoxycarbonyl (tBoc). The functional group of asparagine was protected by tritylation. All the other amino acids with functional side chain groups were protected by benzyloxycarbonyl (Z). The amino acids were purchased already protected from a standard supplier. Acylation was performed for 30 min by using a 4-fold molar excess of each amino acid or the fatty acid. Acetylation was performed by using acetic anhydride-diisopropylethylamine-dimethylformamide (1:1:18 by volume).
The cyclization mixture (25) was added to the peptide (dissolved in dimethylformamide) and allowed to react for 2 h. The cyclized, protected peptide was precipitated by the addition of cold diethyl ether and washed with water. The remaining side chain protection groups (Z) were removed by catalytic dehydrogenation as described previously (25). The peptides were purified by reversed-phase chromatography using conventional gradients of acetonitrile-water-trifluoroacetic acid; the eluate fractions corresponding to the peptides were lyophilized. The peptide purity, estimated by reversed-phase high-performance liquid chromatography (HPLC), was more than 95%. Gram-scale synthesis of NAB741 (acetate salt; purity, 96.2%, as estimated by HPLC) was performed by Bachem AG (Bubendorf, Switzerland).
Bacterial strains.
E. coli IH3080 (K1:O18:H7) is a virulent encapsulated strain used in our other recent studies (25, 26, 31). The ATCC strains included E. coli ATCC 25922, K. pneumoniae ATCC 13883, E. cloacae ATCC 23335, A. baumannii ATCC 19606, P. aeruginosa ATCC 27853, Staphylococcus aureus ATCC 25923, and Candida albicans ATCC 28366. The E. coli strains also included blood isolates from Sweden (Culture Collection, University of Gothenburg (CCUG), Sweden; CCUG strains, n = 7) and from Finland (Helsinki University Hospital, Finland; F strains, n = 5). Furthermore, blood isolates of K. pneumoniae (three F strains, one CCUG strain), E. cloacae (three F strains, one CCUG strain), and A. baumannii (two F strains) were included.
Antibacterial assays.
The simple agar well diffusion method, described previously by us (25), was used as a screening test. It detects both the direct antibacterial activities of the compounds and their abilities to sensitize a set of target bacteria to the hydrophobic model antibiotic (rifampin). The test employed LB agar plates (LB Agar Lennox; Difco, BD, Sparks, MD) and LB plates containing increasing concentrations (0.1 μg/ml, 0.3 μg/ml, and 1.0 μg/ml) of rifampin (Sigma-Aldrich, St. Louis, MO). The plates were inoculated with bacterial suspensions in 0.9% NaCl (0.5 McFarland standard) according to the CLSI standard M2-A9 (8) to enable an even confluent growth. Four quantities (0.4 μg, 1 μg, 4 μg, and 10 μg) of each peptide dissolved in 0.9% NaCl were tested. Controls included polymyxin B sulfate (P0972; Sigma-Aldrich) and polymyxin B nonapeptide (PMBN) (Sigma-Aldrich), as well as the NAB compounds NAB739 and NAB7061 (25). After an 18-h incubation at 37°C, the diameters of the growth inhibition zones were measured and the corresponding surface areas (in mm2) were calculated.
The MIC of NAB741 was measured in microtiter plates using cation-adjusted Mueller-Hinton II broth (Difco 212322) and an inoculum size of 5 × 105 CFU/ml, according to CLSI standard M7-A7 (6).
The synergism of NAB741 with other antibiotics was studied as described previously (25) using E-strips (Biodisk Ltd., Solna, Sweden) on plates containing Mueller-Hinton agar (product no. LabO39; LabM Ltd., Bury, Lancashire, United Kingdom) and increasing concentrations of NAB741 (acetate salt, 1 μg/ml to 16 μg/ml in 2-fold increments), as well as on Mueller-Hinton agar without NAB741. The inoculum was according to the manufacturer's instructions and corresponded to that in CLSI standard M2-A9 (5).
The synergism with rifampin (Sigma-Aldrich) and with clarithromycin (Sigma-Aldrich) was also studied in microtiter plates as previously described (25), using the checkerboard method, cation-adjusted Mueller-Hinton II broth (Difco 212322), and an inoculum size of 5 × 105 CFU/ml, according to CLSI standard M7-A7 (6).
Synergism with fresh normal serum.
The ability of NAB741 to sensitize the encapsulated, smooth strain E. coli IH3080 to the bactericidal action of normal guinea pig serum (GPS) was studied by the method previously employed for the study of polymyxin B nonapeptide (27). E. coli was grown in LB broth (Lennox; Difco, BD) at 37°C into early logarithmic growth phase, washed with phosphate-buffered saline (PBS) (8.0 g of NaCl, 0.2 g of KCl, 1.44 g of Na2HPO4·2H2O, and 0.2 g of KH2PO4 per liter; pH 7.4), and resuspended in PBS to approximately 109 cells/ml. Then, 10% GPS in PBS was inoculated with approximately 500 CFU of bacteria per ml and pipetted in 0.2-ml aliquots into the wells of the microtiter plates. The wells already contained increasing amounts of NAB741 (acetate salt) in 0.020 ml normal saline. After incubation of the plate at 37°C for 2 h, the wells were emptied onto LB plates. The plates were incubated overnight at 37°C, and the colonies were counted. Controls included a treatment in 10% heat-inactivated GPS (inactivation was by incubating GPS at 56°C for 30 min), as well as a treatment in the absence of GPS.
Cytotoxicity assay on V79 cells.
Cytotoxicity assays were performed using the Chinese hamster lung fibroblast cell line V79 (European Collection of Cell Cultures, Salisbury, United Kingdom) as described previously (25). Briefly, the cell culture (2 to 3 days old; more than 50% confluent) was trypsinized, and a cell suspension in fresh medium was used to seed petri dishes with approximately 200 cells each. After incubation for 24 h, the medium was replaced with fresh medium containing NAB741 (acetate salt, 0.25 μg/ml to 128 μg/ml, in 2-fold increments). Negative and positive controls consisted of plates with no NAB741 and with phenol (500 μg/ml), respectively. Each treatment included three replicate dishes. After the 24-h treatment period, the cultures were washed three times, covered with fresh medium, and left to form colonies for 3 days, and the colonies were counted.
Pharmacokinetic studies in rats.
All experiments were approved by the Faculty of Pharmacy and Pharmaceutical Sciences Animal Ethics Committee, Monash University (Victoria, Australia). They were performed based upon the methods described by Li et al. (13) and in our previous study on novel polymyxin derivatives (1). Each rat (n = 4; Sprague-Dawley; male; body weight, 245 to 285 g) was anesthetized using isoflurane, and a polyethylene cannula was inserted into the jugular vein. The rats were placed into a metabolic cage and allowed to recover from the procedure overnight. The test compound (NAB741, acetate salt; 1 mg/kg of body weight) was administered as a bolus (in 200 μl sterile 0.9% saline) through the cannula, followed by washing with 0.8 ml of saline. Nine blood samples (0, 10, 20, 30, 60, 90, 120, 180, and 240 min), each 200 μl, were manually collected through the cannula. When the samples were collected, the first 100 μl of blood was withdrawn and kept in the syringe. After the actual sample was collected with another syringe, the content of the first syringe was returned to the rat, together with 400 μl of heparinized saline. Theblood samples were centrifuged to obtain plasma. Urine samples were collected at 0- to 4-h, 4- to 6-h, and 6- to 24-h intervals. The plasma and urine samples were stored at −80°C.
The samples were analyzed using liquid chromatography and mass spectrometry with an electrospray ionization interface (LC/electrospray ionization MS). To a 100-μl sample of plasma or urine, 10 μl of internal standard (NAB739, acetate salt [80 μg/ml) and 200 μl (for plasma samples) or 100 μl (for urine samples) of acetonitrile were added, and the mixture was vortex mixed for 1 min and centrifuged at 10,000 × g for 10 min. The chromatography employed the HPLC C18 column (50 by 2 mm), 0.1% formic acid as solvent A, 0.1% acetonitrile as solvent B, a flow rate of 0.2 ml/min, and the following gradient: 5% to 30% B in 6 min, 30% to 90% B in 0.5 min, 90% B held for 2.5 min, and 90% to 5% B in 1 min. The eluent between 5.90 and 7.00 min, and 9.00 and 10.10 min was directed to the MS system using a switching valve. The positive protonated molecular ions of NAB741 at m/z 496.7 and 331.4 and of NAB739 at m/z 538.8 and 359.6 were monitored. NAB741 and NAB739 were eluted at 6.65 ± 0.05 min and at 9.45 ± 0.05 min, respectively. Calibration curves were constructed using blank rat plasma or urine with 10 concentrations of NAB741 ranging from 0.010 mg/liter to 10.0 mg/liter. The limit of quantitation (LOQ) was 0.010 mg/liter for both plasma and urine assays. Good linearity of calibration curves was achieved (r2 = 0.998 for the plasma assay and 0.999 for the urine assay). The accuracy and reproducibility for the plasma assay were within 18% at 0.090 mg/liter and within 6% at 0.90 mg/liter or 9.00 mg/liter. The accuracy and reproducibility for the urine assay were within 9% at 0.35 mg/liter, 7.00 mg/liter, or 35.0 mg/liter.
Noncompartmental pharmacokinetic analysis of NAB741 in plasma was performed using WinNonlin software (version 4.0; Mountain View, CA), with the model of NA201 (intravenous [i.v.] bolus input for plasma data).
RESULTS AND DISCUSSION
Structure-function relationships of the polymyxin derivatives.
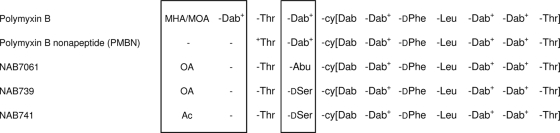
PMBN carries five positive charges (three in the cyclic peptide portion and two in the linear peptide portion) but is “tailless,” i.e., it lacks the fatty acid tail of natural polymyxins. On the other hand, NAB7061 and NAB739 lack the positive charges in the linear peptide portion but possess the fatty acid tail (octanoyl residue) (25). In the present study, we developed hybrid derivatives (such as NAB741) that not only lack the fatty acid tail, but also lack both of the positive charges in the linear peptide portion. However, the free alpha amino group in their N termini is decorated with an acetyl group to eliminate the positive charge. Figure 1 shows the structures of polymyxin B, PMBN, NAB7061, NAB739, and NAB741.
FIG. 1.
Structures of polymyxin B, polymyxin B nonapeptide, NAB7061, NAB739, and NAB741. Locations where the compounds are not identical are boxed. MHA/MOA, the mixture of methyl octanoyl and methyl heptanoyl; OA, octanoyl; Ac, acetyl; cy, the cyclic portion, indicated with brackets. The positive charge of the free α and γ-amino groups is also shown.
The peptide parts of the acetylated compounds NAB741 and NAB752 are identical to those of NAB739 and NAB7061, respectively. Table 1 compares the antibacterial activities of all four of these with those of polymyxin B and PMBN in a screening assay used in our previous study (25). The activity of NAB741 against E. coli IH3080 (the smooth, encapsulated O18:K1 strain) and A. baumannii ATCC 19606 closely resembled that of NAB7061 and PMBN. Accordingly, NAB741 remarkably increased the activity of rifampin.
TABLE 1.
Antibacterial activities of NAB741, its structural relatives, and four reference compounds against E. coli, A. baumannii, and P. aeruginosa
| Compound name | Structurea |
Activityb against: |
||||||
|---|---|---|---|---|---|---|---|---|
| R | Peptide sequence in linear portion |
E. coli IH3080 |
A. baumannii ATCC 19606 |
P. aeruginosa ATCC 27853 |
||||
| Direct | With rifampin | Direct | With rifampin | Direct | With rifampin | |||
| NAB741 | Ac | Ts [0] | 12 ± 16 (3) | 230 ± 71 (3) | 9 ± 10 (3) | 223 ± 23 (3) | 4 ± 2 (3) | 20 ± 14 (3) |
| NAB752 | Ac | TZ [0] | 3 ± 0 (2) | 8 ± 7 (2) | 3 ± 0 (2) | 9 ± 1 (2) | 3 ± 0 (2) | 3 ± 0 (2) |
| NAB747 | Me | Ts [1] | 3 | 130 | 3 | 79 | 7 | 38 |
| NAB745 | Ac | Tn [0] | 3 | 110 | 3 | 154 | 3 | 3 |
| Polymyxin B | MO(H)A | XTX [2] | 129 ± 15 (6) | 133 ± 15 (5) | 185 ± 14 (3) | 202 ± 38 (3) | 151 ± 13 (4) | 166 ± 13 (4) |
| PMBN | TX [2] | 3 ± 0 (8) | 143 ± 34 (8) | 3 ± 0 (3) | 155 ± 31 (3) | 144 ± 22 (6) | 169 ± 48 (6) | |
| NAB7061 | OA | TZ [0] | 32 ± 18 (4) | 206 ± 38 (4) | 3 ± 0 (2) | 252 ± 71 (2) | 2 ± 31 (3) | 55 ± 22 (3) |
| NAB739 | OA | Ts [0] | 170 ± 22 (8) | 212 ± 33 (8) | 161 ± 25 (7) | 395 ± 121 (7) | 81 ± 17 (7) | 87 ± 26 (7) |
The cyclic heptapeptide portion of each compound is identical to that present in polymyxin B. R denotes the moiety linked to the alpha amino group of the N terminus. The one-letter codes for amino acid residues are as follows: N, Asn; T, threonine; X, diaminobutyric acid; Z, aminobutyric acid. Lowercase underlined letters represent residues that are in the d configuration. Ac, acetyl; Me, methyl; MO(H)A, the mixture of 6-methyl octanoic and 6-methyl heptanoic acid; OA, octanoic acid. The number of free amino groups in the linear portion is shown in brackets.
Growth inhibition (in square millimeters) around a well that contained 10 μg of the peptide on an LB plate without rifampin (direct activity) or with rifampin (1 μg/ml). Inhibition areas ≥100 square millimeters are in boldface. The values are means ±SD from experiments, the number of which is given in parentheses. Values without SD are from single experiments.
NAB752 was inactive, even though it differs from NAB741 only by having in the linear portion acetyl-Thr-Abu instead of acetyl-Thr-dSer. This was not surprising, since we have previously shown that among polymyxin derivatives carrying only three positive charges, the presence of two hydroxyl groups in the linear portion of the peptide is advantageous for antibacterial activity (25).
Two related derivatives, NAB745 (acetyl-Thr-dAsn) and NAB747 (methyl-Thr-dSer), were only partially active (Table 1).
None of the novel tailless compounds had any notable activity against P. aeruginosa ATCC 27853. This is in sharp contrast to PMBN, which displayed antipseudomonal activity even in the absence of rifampin (Table 1), as noted previously (21, 28).
MICs of NAB741.
The lack of any potent direct antibacterial activity was verified by measuring the MICs of NAB741 for E. coli IH3080, E. coli ATCC 25922, K. pneumoniae ATCC 13883, E. cloacae ATCC 23355, and A. baumannii ATCC 19606. For each strain, the MIC was ≥32 μg/ml. Furthermore, the MICs for the CCUG and F strains of E. coli (n = 12), K. pneumoniae (n = 4), E. cloacae (n = 4), and A. baumannii (n = 2) were ≥32 μg/ml, with the exception of the MIC for E. coli F19, which was 16 μg/ml. Resistant strains (MIC ≥ 32 μg/ml) also included P. aeruginosa ATCC 27853, as well as the polymyxin-resistant organisms S. aureus ATCC 25923 and C. albicans ATCC 28366.
Ability of NAB741 to sensitize E. coli, K. pneumoniae, E. cloacae, and A. baumannii to antibacterial agents.
The synergy of NAB741 with rifampin, as well as with another hydrophobic antibiotic, clarithromycin, was tested using the checkerboard method and cation-adjusted Mueller-Hinton II broth (Table 2). Very strong synergism with both agents was observed, with FICI as low as ≤0.141 to ≤0.155 for rifampin and 0.094 for clarithromycin. At 8 μg/ml, NAB741 decreased the MIC of rifampin by a factor of 32 and that of clarithromycin by factors ranging from ≥64 to ≥128. At 4 μg/ml, the factors ranged from 4 to 8 for rifampin and from ≥32 to ≥64 for clarithromycin. NAB741 alone was not inhibitory (tested up to the NAB741 concentration of 32 μg/ml).
TABLE 2.
MICs of rifampin and clarithromycin for E. coli ATCC 25922 in the presence of increasing concentrations of NAB741a
| NAB741 concn(μg/ml) | MIC of the antibiotic (μg/ml) in the presence of NAB741 |
|
|---|---|---|
| Rifampin | Clarithromycin | |
| 0 | 8 | ≥64 |
| 2 | 4 | 32 |
| 4 | 1-2 | 1-2 |
| 8 | 0.25 | 0.5-1 |
| 16 | 0.063-0.125 | 0.25 |
| 32 | 0.031-0.063 | 0.063 |
Determined by the checkerboard method in cation-adjusted Mueller-Hinton II broth (results of two determinations). The FICI in the presence of NAB741 was ≤0.141 to ≤0.155 with rifampin and 0.094 with clarithromycin.
The MICs of rifampin and clarithromycin were then determined for two strains of E. coli (ATCC 25922 and IH3080), as well as for K. pneumoniae ATCC13883, E. cloacae ATCC 23355, and A. baumannii ATCC 19606, by using Etest strips on Mueller-Hinton agar containing increasing concentrations of NAB741. Under the conditions used, NAB741 displayed no direct growth-inhibitory activity even at the highest concentration tested (16 μg/ml), except that it inhibited the growth of E. cloacae ATCC 23355 at 16 μg/ml.
NAB741 exerted strong synergism with both antibiotics (Table 3). The FICI of the combination of NAB741 and rifampin ranged from ≤0.111 to 0.158, and that of clarithromycin ranged from ≤0.094 to 0.292. At 4 μg/ml, NAB741 sensitized both E. coli strains and E. cloacae to rifampin by factors ranging from 85 to 2,000 and to clarithromycin by factors ranging from 64 to 340. The sensitization factors for K. pneumoniae and A. baumannii were somewhat lower.
TABLE 3.
MICs (μg/ml) of rifampin and clarithromycin for five bacterial strains in the presence of increasing concentrations of NAB741a
| Antibiotic and strain | MIC (μg/ml) of the antibiotic at a NAB741 concn (μg/ml) of: |
Sensitization factorb at 4 μg/ml of NAB741 | FICI | ||
|---|---|---|---|---|---|
| 0 | 2 | 4 | |||
| Rifampin | |||||
| E. coli ATCC 25922 | 4-16 | 0.64 | 0.016 | 250-750 | <0.129-≤0.126 |
| E. coli IH3080 | 4-12 | 0.19 | 0.047 | 85-250 | ≤0.111-≤0.129 |
| K. pneumoniae ATCC 13883 | 12->32 | 1 | 0.5 | 24>64 | ≤0.125-≤0.141 |
| E. cloacae ATCC 23355 | 6->32 | 1.5 | 0.016-0.023 | 260->2,000 | ≤0.126-≤0.129 |
| A. baumannii ATCC 19606 | 1.5-4 | 0.19 | 0.094-0.19 | 16 | ≤0.131-≤0.158 |
| Clarithromycin | |||||
| E. coli ATCC 25922 | 16-48 | 0.5 | 0.094-0.19 | 84-340 | ≤0.094-≤0.128 |
| E. coli IH3080 | 12-16 | 1 | 0.125-0.25 | 64-96 | ≤0.126-≤0.135 |
| K. pneumoniae ATCC 13883 | 12-24 | 4 | 1-2 | 6-24 | ≤0.167-≤0.292 |
| E. cloacae ATCC 23355 | 48-96 | 16 | 0.5-0.75 | 64-96 | ≤0.135-≤0.141 |
| A. baumannii ATCC 19606 | 8-16 | 0.75 | 0.5 | 12-32 | ≤0.156-≤0.208 |
Tested by using E-strips on Mueller-Hinton agar containing increasing concentrations of NAB741. For the growth-inhibitory concentrations of NAB741 alone under these test conditions, see the text. For rifampin and clarithromycin in the absence of NAB741, the data are the results of five to seven and three to four independent determinations, respectively. For determinations of FICI, as well as the MICs, in the presence of 4 μg/ml of NAB741, the data are the results of two independent determinations.
The ratio of the MIC in the absence of NAB741 to that in the presence of 4 μg/ml of NAB741.
Other antibiotics against which the intact OM acts as an effective permeability barrier include azithromycin, mupirocin, fusidic acid, and vancomycin. At a concentration of 4 μg/ml, NAB741 sensitized both E. coli strains and E. cloacae to all these antibiotics by factors ranging from 8 to 200 (Table 4). At this concentration of NAB741, the MIC of vancomycin for E. cloacae was as low as 1.5 μg/ml. For K. pneumoniae and A. baumannii, the highest sensitization factors (from 11 to 32) were to azithromycin (Table 4).
TABLE 4.
MICs of azithromycin, mupirocin, fusidic acid, and vancomycin in the presence of NAB741 (4 μg/ml)a
| Bacterial strain | MIC (μg/ml) |
|||
|---|---|---|---|---|
| Azithromycin | Mupirocin | Fusidic acid | Vancomycin | |
| E. coli ATCC 25922 | 0.125-0.19 (16-24) | 0.75-1 (64-128) | 1.5 (>170) | 8 (>32) |
| E. coli IH3080 | 0.094-0.38 (8-32) | 1 (24-64) | 2 (>130) | 16 (>16) |
| K. pneumoniae ATCC 13883 | 0.125-0.19 (11-32) | 6 (8) | 48 (>5) | 128 (>2) |
| E. cloacae ATCC 23355 | 0.25 (16) | 0.25-0.5 (50-200) | 2 (>130) | 1.5 (>170) |
| A. baumannii ATCC 19606 | 1.5 (21) | 512-1,024 (<2) | 6 (>43) | 24 (>11) |
Tested by using E-strips on Mueller-Hinton agar. Sensitization factors (the ratio of the MIC in the absence of NAB741 to that in the presence of 4 μg/ml of NAB741) are shown in parentheses. The results are from two (azithromycin and mupirocin) or one (vancomycin or fusidic acid) independent determinations.
Synergism with fresh normal serum.
The combined effects of NAB741 and fresh serum were evaluated by using the smooth, encapsulated E. coli IH3080 as the target. Fresh 10% GPS alone or NAB741 alone (tested up to 4 μg/ml) exerted no bactericidal activity against E. coli IH3080, but a combination of fresh 10% GPS and NAB741 at 1 μg/ml reduced the CFU by 95%. A reduction by more than 99% was observed in 10% GPS at the NAB741 concentration of 4 μg/ml. Heat treatment (at 56°C for 30 min) of GPS completely abolished its synergism with NAB741.
It has long been known that PMBN sensitizes E. coli, K. pneumoniae, and other polymyxin-susceptible strains of Enterobacteriaceae, as well as P. aeruginosa, to serum bactericidal action (12, 15, 27, 29), perhaps by facilitating the insertion of the membrane attack complex (MAC) of serum complement into the hydrophobic membrane milieu (27). The combination of PMBN with human, guinea pig, rabbit, and rat sera is strongly bactericidal, whereas no synergy can be found with mouse serum (27). Also, NAB7061 sensitizes E. coli IH3080 to the bactericidal action of 10% GPS (M. Vaara and T. Vaara, U.S. patent application 20080287345). Whether NAB741 and NAB7061 also enhance the blood clearance of Gram-negative bacteria and sensitize them to complement-mediated killing within neutrophils, as does PMBN (19, 22), remains to be seen. Furthermore, whether they preserve their OM-permeabilizing activities under the acidic conditions prevailing in neutrophil granules, as does PMBN (30), should be studied.
Cytotoxicity.
NAB741 (tested at 0.25 μg/ml to 128 μg/ml in 2-fold increments) did not show cytotoxicity against V79 Chinese hamster lung fibroblasts (treatment time, 24 h).
Pharmacokinetic evaluation of NAB741.
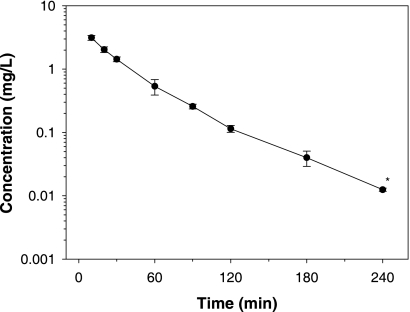
Figure 2 shows the mean (± standard deviation [SD]) concentrations in plasma as a function of time for rats that received NAB741 (acetate salt) intravenously at 1 mg/kg (n = 4). The mean extrapolated area under the concentration-time curve (AUC) represented 0.82 ± 0.50% of the AUC from zero hour to infinity (AUC0-∞). At 10 min, the mean plasma concentration of NAB741 was 3.13 μg/ml (SD, 0.28 μg/ml) (Fig. 2), while the corresponding concentrations of NAB7061 (acetate salt) and NAB739 (acetate salt) were 4.08 μg/ml (SD, 0.84 μg/ml) and 5.01 μg/ml (SD, 0.70 μg/ml), respectively (1).
FIG. 2.
Mean (±SD) plasma concentrations of NAB741 after intravenous administration, in log-linear scale. *, two of the concentration values were below the limit of quantitation (0.010 μl/ml).
In Table 5, the key pharmacokinetic parameters determined for NAB741 are compared to those measured previously for NAB7061 (acetate salt) (1), NAB739 (acetate salt) (1), and colistin (sulfate salt) (13). The volumes of distribution for NAB741 and NAB739 were substantially lower than those observed for NAB7061 and colistin. The half-life of NAB741 was about half that of the other three compounds. The total body clearance of NAB741 was approximately 3-fold, 2-fold, and 50% higher than those for NAB739, NAB7061, and colistin, respectively. The renal clearance of NAB741 was approximately 400-fold, 16-fold, and 8-fold higher than those measured for colistin, NAB7061, and NAB739, respectively. As much as half of the dose of NAB741 was recovered in the urine, while the urinary recovery rates of NAB7061, NAB739, and colistin were 19%, 7%, and almost nil (0.18%), respectively. The mean nonrenal clearances (total body clearance minus the corresponding renal clearance values) for NAB741, NAB7061, NAB739, and colistin were 3.61, 2.10, 3.56, and 5.19 ml/min/kg, respectively.
TABLE 5.
Basic pharmacokinetic parameters of NAB741 compared to those of NAB7061 and NAB739 (1) and of colistin (13)
| Parameter | Value (mean ± SD; n = 4) |
|||
|---|---|---|---|---|
| NAB739 | NAB741 | NAB7061 | Colistin | |
| Half-life (min) | 69.0 ± 21.9 | 32.7 ± 2.41 | 66.2 ± 12.3 | 74.6 ± 13.2 |
| Volume of distribution (ml/kg) | 222 ± 20.5 | 243 ± 24.0 | 339 ± 96 | 496 ± 60 |
| Clearance (ml/min/kg) | 2.63 ± 0.54 | 7.39 ± 0.85 | 3.84 ± 0.75 | 5.22 ± 0.41 |
| Urinary recovery (% of dose) | 19.4 ± 7.38 | 50.9 ± 13.6 | 7.16 ± 3.70 | 0.18 ± 0.14 |
| Renal clearance (ml/min/kg) | 0.53 ± 0.30 | 3.78 ± 1.11 | 0.28 ± 0.16 | 0.010 ± 0.008 |
Conclusions.
We developed a polymyxin B-like peptide antibiotic, NAB741. It carries only an acetyl group in the N terminus of the peptide and a threonyl-d-serinyl residue as the linear portion of the peptide. NAB741 lacks direct antibacterial activity but decreases the MICs of several antibiotics for Gram-negative bacteria, such as E. coli, K. pneumoniae, E. cloacae, and A. baumannii, by factors as high as 100 or more. A sister peptide carrying a threonyl-aminobutyryl residue as the linear portion of the peptide was inactive. This further emphasizes our previous view (25) that the presence of two hydroxyl groups in the linear portion of the peptide is advantageous for the antibacterial activity. The renal clearance of NAB741 was found to be approximately 400-fold higher than that of colistin, indicating that the renal handling of NAB741 is quite different from that of colistin. Whether these differences in the N-terminal moiety and the linear peptide portion have other impacts on the pharmacokinetic and toxicological properties remains to be seen.
Acknowledgments
J.F. thanks Sat Sandhu, who performed all of the mg-scale peptide synthesis chemistry. M.V., O.S., and J.A. thank Sini Virtanen, and N.F.-M. thanks Frank Hansen for excellent technical assistance.
J.L. is an Australian National Health and Medical Research Council R. Douglas Wright Research Fellow. This study was funded by Northern Antibiotics Ltd.
Footnotes
Published ahead of print on 17 May 2010.
REFERENCES
- 1.Ali, F. E., G. Cao, A. Poydal, T. Vaara, R. L. Nation, M. Vaara, and J. Li. 2009. Pharmacokinetics of novel antimicrobial cationic peptides NAB7061 and NAB739 in rats following intravenous administration. J. Antimicrob. Chemother. 64:1067-1070. [DOI] [PubMed] [Google Scholar]
- 2.Arias, C. A., and B. E. Murray. 2009. Antibiotic-resistant bugs in the 21st century—a clinical super-challenge. N. Engl. J. Med. 360:439-443. [DOI] [PubMed] [Google Scholar]
- 3.Boucher, H. W., G. H. Talbot, J. S. Bradley, J. E. Edwards, Jr., D. Gilbert, L. B. Rice, M. Scheld, B. Spellberg, and J. Bartlett. 2009. Bad bugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 48:1-12. [DOI] [PubMed] [Google Scholar]
- 4.Chopra, J., C. Schonfield, M. Everett, A. O'Neill, K. Miller, M. Wilcox, J.-M. Frère, M. Dawson, L. Czaplewski, U. Urleb, and P. Courvalin. 2008. Treatment of health-care-associated infections caused by Gram-negative bacteria: a consensus statement. Lancet Infect. Dis. 8:133-139. [DOI] [PubMed] [Google Scholar]
- 5.Clinical and Laboratory Standards Institute. 2006. M2-A9: Performance standards for antimicrobial disk susceptibility tests. Approved standard, 9th ed. CLSI, Wayne, PA.
- 6.Clinical and Laboratory Standards Institute. 2006. M7-A7. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 7th ed. Approved standard. CLSI, Wayne, PA.
- 7.Curcio, D. 2008. Treatment of recurrent urosepsis with tigecycline: a pharmacological perspective. J. Clin. Microbiol. 46:1892-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doi, Y., J. M. Adams-Haduch, and D. L. Paterson. 2008. Genetic environment of 16S rRNA methylase gene rmtD. Antimicrob. Agents Chemother. 52:2270-2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fritsche, T. R., M. Castanheira, G. H. Miller, R. N. Jones, and E. S. Armstrong. 2008. Detection of methyltransferases conferring high-level resistance to aminoglycosides in Enterobacteriaceae from Europe, North America, and Latin America. Antimicrob. Agents Chemother. 52:1843-1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hawkey, P. M., and A. M. Jones. 2009. The changing epidemiology of resistance. J. Antimicrob. Chemother. 64(Suppl. 1):i3-i10. [DOI] [PubMed] [Google Scholar]
- 11.Kim, H. B., C. H. Park, C. J. Kim, E. C. Kim, G. A. Jacoby, and D. C. Hooper. 2009. Prevalence of plasmid-mediated quinolone resistance determinants over a 9-year period. Antimicrob. Agents Chemother. 53:639-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lam, C., J. Hildebrandt, E. Schutze, and A. F. Wenzel. 1986. Membrane-disorganizing property of polymyxin B nonapeptide. J. Antimicrob. Chemother. 18:9-15. [DOI] [PubMed] [Google Scholar]
- 13.Li, J., R. W. Milne, R. L. Nation, J. D. Turnidge, T. C. Smeaton, and K. Coulthard. 2003. Use of high-performance liquid chromatography to study the pharmacokinetics of colistin sulfate in rats following intravenous administration. Antimicrob. Agents Chemother. 47:1766-1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Livermore, D. 2009. Has the era of untreatable infections arrived? J. Antimicrob. Chemother. 64(Suppl. 1):i29-i36. [DOI] [PubMed] [Google Scholar]
- 15.McCashion, R. N., and W. H. Lynch. 1987. Effects of polymyxin B nonapeptide on Aeromonas salmonicida. Antimicrob. Agents Chemother. 31:1414-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nordmann, P., G. Cuzon, and T. Naas. 2009. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect. Dis. 9:228-236. [DOI] [PubMed] [Google Scholar]
- 17.Pallecchi, L., E. Riccobono, A. Mantella, F. Bartalesi, S. Sennati, H. Gamboa, E. Gotuzzo, A. Bartoloni, and G. M. Rossolini. 2009. High prevalence of qnr genes in commensial enterobacteria from healthy children in Peru and Bolivia. Antimicrob. Agents Chemother. 53:2632-2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Payne, D. J., M. N. Gwynn, D. J. Holmes, and D. L. Pompliano. 2007. Drugs for bad bugs: confronting the challenges of antibacterial discovery. Nat. Rev. 6:29-40. [DOI] [PubMed] [Google Scholar]
- 19.Rose, F., K. U. Heuer, U. Sibelius, S. Hombach-Klonisch, L. Kiss, W. Seeger, and F. Grimminger. 2000. Targeting lipopolysaccharides by the nontoxic polymyxin B nonapeptide sensitizes resistant Escherichia coli to the bactericidal effect of human neutrophils. J. Infect. Dis. 182:191-199. [DOI] [PubMed] [Google Scholar]
- 20.Tsubery, H., I. Ofek, S. Cohen, and M. Fridkin. 2000. The functional association of polymyxin B wih bacterial lipopolysaccharide is stereospecific: studies on polymyxin B nonapeptide. Biochemistry 39:11838-11844. [DOI] [PubMed] [Google Scholar]
- 21.Tsubery, H., I. Ofek, S. Cohen, and M. Fridkin. 2001. N-terminal modifications of polymyxin B nonapeptide and their effect on antibacterial activity. Peptides 22:1675-1681. [DOI] [PubMed] [Google Scholar]
- 22.Tsubery, H., H. Yaakov, S. Cohen, T. Giterman, A. Matiyahou, M. Fridkin, and I. Ofek. 2005. Neopeptide antibiotics that function as opsonins and membrane-permeabilizing agents for Gram-negative bacteria. Antimicrob. Agents Chemother. 49:3122-3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vaara, M. 1992. Agents that increase the permeability of the outer membrane. Microbiol. Rev. 56:395-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vaara, M. 2009. New approaches in peptide antibiotics. Curr. Opin. Pharmacol. 9:571-576. [DOI] [PubMed] [Google Scholar]
- 25.Vaara, M., J. Fox, G. Loidl, O. Siikanen, J. Apajalahti, F. Hansen, N. Frimodt-Møller, J. Nagai, M. Takano, and T. Vaara. 2008. Novel polymyxin derivatives carrying only three positive charges are effective antibacterial agents. Antimicrob. Agents Chemother. 52:3229-3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vaara, M., O. Siikanen, J. Apajalahti, N. Frimodt-Møller, and T. Vaara. 2010. Susceptibility of carbapenemase-producing strains of Klebsiella pneumoniae and Escherichia coli to the direct antibacterial activity of NAB739 and to the synergistic activity of NAB7061 with rifampin and clarithromycin. J. Antimicrob. Chemother. 65:942-945. [DOI] [PubMed] [Google Scholar]
- 27.Vaara, M., P. Viljanen, T. Vaara, and P. H. Mäkelä. 1984. An outer membrane-disorganizing peptide PMBN sensitizes E. coli strains to serum bactericidal action. J. Immunol. 132:2582-2589. [PubMed] [Google Scholar]
- 28.Viljanen, P., and M. Vaara. 1984. Susceptibility of Gram-negative bacteria to polymyxin B nonapeptide. Antimicrob. Agents Chemother. 25:701-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Viljanen, P., H. Käyhty, M. Vaara, and T. Vaara. 1986. Susceptibility of Gram-negative bacteria to the synergistic bactericidal action of serum and polymyxin B nonapeptide. Can. J. Microbiol. 32:66-69. [DOI] [PubMed] [Google Scholar]
- 30.Viljanen, P., P. Koski, and M. Vaara. 1988. Effect of small cationic leukocyte peptides (defensins) on the permeability barrier of the outer membrane. Infect. Immun. 56:2324-2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vingsbo Lundberg, C., T. Vaara, N. Frimodt-Møller, and M. Vaara. 2010. Novel polymyxin derivatives are effective in treating experimental Escherichia coli peritoneal infection in mice. J. Antimicrob. Chemother. 65:981-985. [DOI] [PubMed] [Google Scholar]