Abstract
Malaria continues to be a major threat to global health. Artemisinin combination therapy (ACT) is the recommended treatment for clinical malaria; however, recent reports of parasite resistance to artemisinin in certain areas where malaria is endemic have stressed the need for developing more efficacious ACT. We report that cysteamine (Cys), the aminothiol used to treat nephropathic cystinosis in humans, strongly potentiates the efficacy of artemisinin against the Plasmodium parasite in vivo. Using a mouse model of infection with Plasmodium chabaudi AS, we observe that Cys dosing used to treat cystinosis in humans can strongly potentiate (by 3- to 4-fold) the antimalarial properties of the artemisinin derivatives artesunate and dihydroartemisinin. Addition of Cys to suboptimal doses of artemisinin delays the appearance of blood parasitemia, strongly reduces the extent of parasite replication, and significantly improves survival in a model of lethal P. chabaudi infection. Cys, the natural product of the enzyme pantetheinase, has a history of safe use for the clinical management of cystinosis. Our findings suggest that Cys could be included in novel ACTs to improve efficacy against Plasmodium parasite replication, including artemisinin-resistant isolates. Future work will include clinical evaluation of novel Cys-containing ACTs and elucidation of the mechanism underlying the potentiation effect of Cys.
Malaria still represents a huge global health burden, with 500 to 600 million clinical cases resulting in 1 to 2 million deaths annually (www.who.int). The impact is particularly devastating in resource-poor countries of sub-Saharan Africa and Southeast Asia, where malaria is endemic and access to appropriate antimalarial drugs can be limited. Furthermore, as a result of widespread use and misuse of antimalarial drugs, the Plasmodium parasite has developed resistance to commonly used drugs, such as chloroquine, mefloquine, and sulfadoxine-pyrimethamine (23). Artemisinin is a sesquiterpene lactone endoperoxide (extracted from Artemisia annua) with potent antimalarial activity, and artemisinin combination therapy (ACT) is the strategy recommended by the World Health Organization for clinical care of malaria (www.who.int). However, recent reports of delayed parasite clearance times following standard ACT treatment in patients from the Pailin province of Cambodia have suggested emergence of resistance to artemisinin in the Plasmodium parasite (12). These alarming reports have highlighted the urgency for the development of novel and more effective chemotherapeutic strategies, including modification of current ACT composition.
We have used a mouse model of blood-stage infection with P. chabaudi AS to identify novel genetic factors affecting host response to malaria. We mapped two major loci, Char4 (chromosome 3) and Char9 (chromosome 10), that control differential responses of innately susceptible A/J (high parasitemia, high mortality) and uniquely resistant AcB55 (low parasitemia, high rate of survival) mice to P. chabaudi infection. Char4-associated resistance was found to result from a loss-of-function mutation (I90N) in the erythrocyte enzyme pyruvate kinase (Pklr) (28). Subsequently, we observed that erythrocytes heterozygous or homozygous for defective variants in human PKLR are resistant to P. falciparum infection ex vivo (reduced parasite replication and increased phagocytosis), validating our initial observations with the mouse (5). On the other hand, we established that Char9-associated susceptibility is caused by a loss of activity in the pantetheinase enzyme encoded by the Vnn1/Vnn3 genes (27). Pantetheinase is an amidohydrolase that hydrolyzes pantetheine (product of coenzyme A degradation) to pantothenic acid (also called pantothenate or vitamin B5) and the small aminothiol cysteamine (Cys) (NH2-CH2-CH2-SH2) (13). Strikingly, exogenous administration of cysteamine to pantetheinase-deficient malaria-susceptible A/J mice reversed the phenotype by reducing blood parasitemia levels and increasing survival time (26, 27).
Cysteamine, in the form of cysteamine bitartrate (Cystagon), is currently used for the clinical treatment of nephropathic cystinosis in humans (21). Cystinosis is a lysosomal storage disease caused by mutations in the lysosomal cystine transporter cystinosin, which result in lysosomal accumulation of cystine and concomitant cytopathic effects (3). Cysteamine depletes cells of cystine in vitro and in vivo and dramatically improves the prognosis for children with cystinosis. Using a mouse model of P. chabaudi infection, we have investigated the potential antimalarial activity of cysteamine at pharmacological doses that mimic those currently used in patients suffering from cystinosis. We have also tested the potential for cysteamine to enhance the antimalarial activity of known drugs, including artemisinin derivatives. Our results show that cysteamine can strongly potentiate and synergize with artemisinin derivatives to reduce blood parasitemia and rescue lethality in P. chabaudi-infected mice.
MATERIALS AND METHODS
Mice.
A/J and C57BL/6 (B6) mice were purchased from Jackson Laboratories (Bar Harbor, ME) and were housed at McGill University. All animals were handled and cared for according to the guidelines of the Canadian Council on Animal Care. A lactate dehydrogenase (LDH) virus-free isolate of P. chabaudi AS was maintained by weekly passage in A/J mice. Mice were infected intravenously (i.v.) into the tail vein with 105, 106, or 107 parasitized red blood cells (pRBC) suspended in pyrogen-free saline. Following infection, the percentage of pRBC was determined daily with thin blood smears stained with Dif-Quik (Dade Behring, Newark, DE), as described previously (19).
Pharmacokinetic (PK) studies of cysteamine hydrochloride in vivo.
Cysteamine was detected in plasma by high-performance liquid chromatography (HPLC) analysis with UV detection (10). Briefly, blood was collected in EDTA/heparin-containing tubes, and plasma was obtained by centrifugation. Plasma thiols were reduced by treatment with Tris(2-carboxyethyl)phosphine (0.05 M final concentration, 20 min at 20°C), and proteins were precipitated with trichloroacetic acid (TCA; 10% final concentration). Free thiols from the protein-free supernatant were derivatized using SBD-F (7-benzo-2-oxa-1,3-diazole-4-sulfonic acid), used at a final concentration of 0.2 mg/ml (1 h at 60°C) in 0.05 M borate buffer (pH 9.5). The mixture was then analyzed by HPLC: the mobile phase consisted of an aqueous solvent (0.1 M acetic acid, 0.1 sodium acetate, pH 4.3) running on a Supelco LC-8 column, and elution of plasma analytes was carried out with a 0 to 10% acetonitrile gradient. SBD-F derivatized analytes were detected by reading fluorescence at 515 nm (excitation at 385 nm). Cysteamine elution peaks were quantified (surface area), and plasma concentrations were calculated using a set of internal cysteamine standards processed at the same time. Area under the concentration-time curve (expressed as minutes per micromolar) from time zero to the last measurable concentration (AUC0-last) was calculated using the trapezoid approximation method.
Cysteamine, chloroquine, artesunate, and dihydroartemisinin administration in vivo.
Cysteamine hydrochloride (Sigma, Burlington, Ontario, Canada) was prepared in phosphate-buffered saline (PBS). Artesunate and dihydroartemisinin (DHA) were generous gifts of Dafra Pharmaceuticals; artesunate and DHA were prepared in 5% sodium bicarbonate and diluted in water to appropriate concentrations. All solutions were prepared fresh daily and filter sterilized, and injections were performed intraperitoneally (i.p.) or subcutaneously (s.c.) for 4 days or according to the treatment regimen. Mice were weighed prior to treatment to determine appropriate doses, and injection volumes ranged from 100 to 400 μl per mouse. In the case of animals treated with two drugs, artemisinin derivatives were administered first (due to the short half-life of cysteamine), and cysteamine was administered 5 to 10 min later on alternate sides. Untreated control animals were injected with PBS alone.
Statistical tests.
Groups with normally distributed data points were compared using parametric unpaired t tests, while groups with non-Gaussian distributions were compared using nonparametric Mann-Whitney tests. Survival differences were analyzed using the log-rank test. Synergistic effects were defined as the percent inhibition of the combination therapy that was >10% greater than the sum of the percent inhibition of the individual mice. Standard error of percent inhibition was calculated from the parasitemia level of individual mice compared to the mean parasitemia level of the control group.
RESULTS
Characteristics of cysteamine activity against Plasmodium chabaudi infection in vivo.
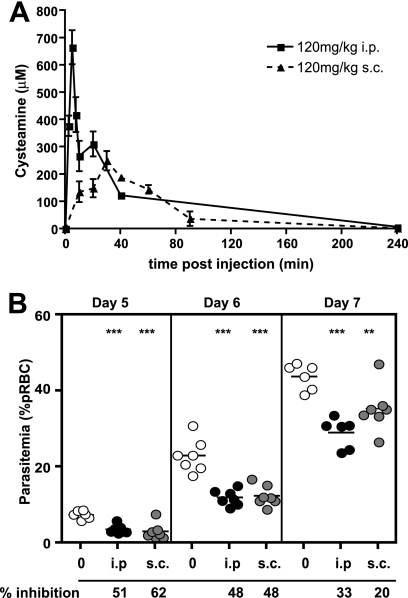
To gain more insight into the antimalarial effect of cysteamine (Cys) in vivo, we compared the pharmacokinetic characteristics (plasma level) of Cys administered through the s.c. and i.p. routes. We measured peak plasma concentration (Cmax) and total bioavailability (AUC) after administration of a single dose of 120 mg/kg of body weight of Cys hydrochloride (Fig. 1 A). The Cmax was higher (665 μM) and was reached more rapidly (time to Cmax [Tmax] of <5 min) following i.p. injection than by the s.c. route, for which a Cmax of 250 μM was attained with a Tmax of 30 min. On the other hand, total Cys bioavailability levels (AUC0-last) were comparable for both routes (24,282 versus 15,277 min·μM for i.p. and s.c. routes, respectively). To determine which pharmacokinetic parameter (AUC versus Cmax) is important for efficacy against Plasmodium, we compared the i.p. and s.c. routes of injection in a continuous treatment regimen, starting 1 day prior to infection (105 P. chabaudi pRBC, i.v.) and continuing daily for 11 days. Parasitemia was monitored on thin blood smears at days 5, 6, and 7 following infection (Fig. 1B). Treatment of infected animals with 120 mg/kg Cys administered either s.c. or i.p. caused a highly significant (P < 0.01) 50% reduction in parasitemia at days 5 and 6, compared to that for saline-injected controls. These results suggest that total Cys exposure (AUC0-last) is the critical pharmacokinetic parameter for the antimalarial effect of Cys.
FIG. 1.
Effect of cysteamine on replication of Plasmodium chabaudi in vivo. (A) The plasma levels of cysteamine-free base (measured by HPLC) following either intraperitoneal (i.p.) or subcutaneous (s.c.) injections (120 mg/kg) were measured in 3 mice and used to calculate Cmax and AUC pharmacokinetic parameters (see text). Error bars indicate standard deviation from the mean. (B) A/J female mice were infected with P. chabaudi (105 pRBC i.v.) and treated daily (either s.c. or i.p.) with cysteamine (120 mg/kg) starting at day −1 to day 10. Blood parasitemia was monitored on days 5, 6, and 7 and is plotted. The % inhibition of parasite replication was calculated by comparison to the blood parasitemia measured in PBS-treated controls and is indicated below the graphs. Each dot represents a mouse. Levels of statistical significance are represented by asterisks; ***, P < 0.01; **, P < 0.05 (compared to PBS control group).
Cysteamine dosing used in the treatment of cystinosis reduces parasitemia during P. chabaudi infection in vivo.
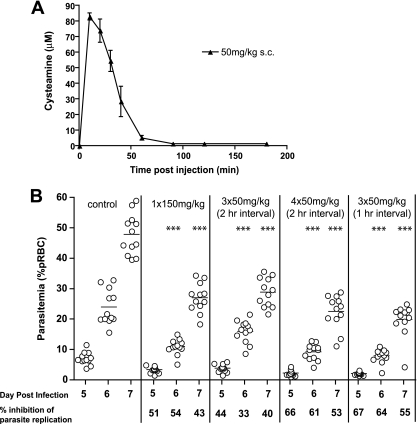
We next determined whether Cys at dosing equivalent to that used in the clinical treatment of nephropathic cystinosis in humans has an effect on the course and severity of P. chabaudi infection in mice. In cystinosis patients, Cys is given orally as Cys bitartrate (Cystagon). The PK profile of an oral dose of 1,475 mg of Cys bitartrate (500-mg cysteamine base) includes a peak plasma concentration of 39 μM (Cmax), with a concomitant AUC0-last of 3,613 min·μM (17). Results in Fig. 2 A show that a single s.c. injection of 50 mg/kg Cys hydrochloride in mice has a PK profile comparable to that of one oral dose of Cystagon in humans, including a Cmax of ∼80 μM and an AUC of 2,845 min·μM. We next evaluated the efficacy of different regimens of 50 mg/kg Cys s.c. (number of injections, interval between injections) on replication of P. chabaudi in vivo. P. chabaudi-infected mice were treated daily, starting at day −1 and continuing to day 10, with Cys at 1 × 150 mg/kg, 3 × 50 mg/kg given at 2-h intervals, 4 × 50 mg/kg given at 2-h intervals, or 3 × 50 mg/kg given at 1-h intervals, and blood parasitemia was monitored at days 5, 6, and 7 (Fig. 2B). Significant reduction (40 to 67%) of blood parasitemia was seen for all treatment regimens, with the strongest effect achieved with 3 × 50 mg/kg given at 1-h intervals. All 50-mg/kg repeated dosing regimens (s.c.) showed inhibitory effects on parasitemia that were similar to that produced by a single s.c. injection of 150 mg/kg Cys, in agreement with data from Fig. 1 showing that the AUC is the critical parameter for efficacy. These results suggest that multiple Cys treatments at doses similar to those used in humans for cystinosis can significantly reduce blood-stage replication of Plasmodium parasites in mice. However, the inhibitory effect of Cys on Plasmodium replication is moderate and inferior to that of known antimalarial drugs.
FIG. 2.
Effect of cysteamine dosing used for treatment of cystinosis on replication of Plasmodium chabaudi in vivo. (A) The plasma levels of cysteamine-free base (measured by HPLC) following subcutaneous (s.c.) injection (50 mg/kg) were measured in 3 mice and used to calculate Cmax and AUC pharmacokinetic parameters (see text). Error bars indicate standard deviation from the mean. (B) A/J female mice were infected with P. chabaudi (105 pRBC i.v.) and treated daily with cysteamine (s.c.) from day −1 to day 10, with the indicated dosing: 1 × 150 mg/kg, 3 × 50 mg/kg, or 4 × 50 mg/kg, given at 1 or 2 h intervals. Blood parasitemia was monitored on days 5, 6, and 7 and is plotted. The % inhibition of parasite replication was calculated by comparison to the blood parasitemia measured in PBS-treated controls and is indicated below the graphs. Each dot represents a mouse. Levels of statistical significance are represented by asterisks; ***, P < 0.01; **, P < 0.05 (compared to PBS control group).
Cysteamine and artemisinin derivatives show synergistic effects against Plasmodium in vivo.
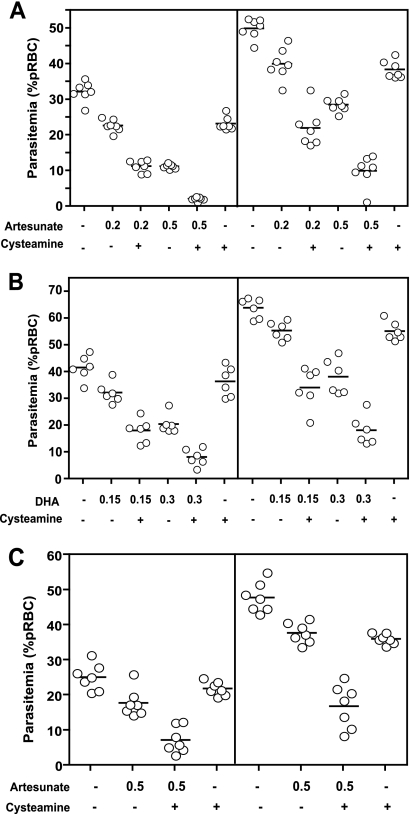
We tested the effect of Cys on the potency and efficacy of the antimalarial artemisinin derivatives. In these studies, artemisinin derivatives were given at suboptimal concentrations to distinguish between the lack of an effect and additive or synergistic effects of Cys addition. Synergy (35) is defined as a total antimalarial activity (reduction in blood parasitemia compared to untreated controls in a 4-day test) of the two compounds administered together being greater than the sum of the independent activities of the two compounds given alone. We tested combinations of Cys and either artesunate (ART) or dihydroartemisinin (DHA), the bioactive form of artemisinin, in a standard 4-day in vivo test, as described previously (18). Pantetheinase-deficient A/J mice were infected with P. chabaudi (107 pRBC, i.v.) and treated with Cys (170 mg/kg) and/or suboptimal doses of ART (0.2 or 0.5 mg/kg) (Fig. 3 A) or DHA (0.15 or 0.3 mg/kg) (Fig. 3B) from day 0 to day 3, and parasitemia was monitored on days 4 and 5. Suboptimal doses of the artemisinin derivatives alone resulted in parasitemia inhibition ranging from 20 to 30%, while higher doses of these drugs could inhibit parasitemia 40 to 60%, compared to controls (Fig. 3A and B; Table 1). However, addition of Cys to either ART or DHA resulted in stronger inhibition of parasitemia than the additive effect of the two compounds, indicating a synergistic effect (Table 1, asterisks). Synergy was noticed for all concentrations of ART and DHA tested. Mice receiving both Cys and ART/DHA also showed fewer symptoms of disease (ruffled fur, lethargy) than did mice receiving either PBS or only one compound. To assess whether the synergistic effect between Cys and ART was restricted to A/J mice deficient in pantetheinase, we repeated the experiment in pantetheinase-sufficient and malaria-resistant C57BL/6 mice (Fig. 3C). Potentiation of the antimalarial activity of ART (0.5 mg/kg) by Cys was also clearly evident in these C57BL/6 mice at both days 4 and 5 postinfection, with combined treatment causing a 65 to 71% reduction in parasitemia compared to that for PBS controls, greater than either compound tested alone (13 to 29%) (Table 1).
FIG. 3.
Synergistic effect of cysteamine on artemisinin efficacy against replication of Plasmodium chabaudi in vivo. Groups (n = 6) of female A/J (A and B) or C57BL/6 (C) mice were infected with P. chabaudi (107 pRBC, i.v.) and treated for 4 days (days 0, 1, 2, and 3) with indicated doses (in mg/kg) of artesunate (A and C) or dihydroartemisinin (DHA) (B) and/or cysteamine (170 mg/kg, i.p.), and blood parasitemia (expressed as percentage of parasitized erythrocytes) was determined at days 4 (left) and 5 (right) postinfection. In all experiments, control groups were treated with PBS. The presence or absence of cysteamine is indicated by a plus or a minus, respectively, and doses of artemisinin derivatives in mg/kg are indicated below. Each dot represents a mouse and bars indicate the mean of the group.
TABLE 1.
Effect of cysteamine and artemisinin derivative combinations on blood-stage replication of Plasmodium chabaudi in vivo
| Mouse type and drug | Dose (mg/kg) | Cysteamine (170 mg/kg) | Inhibition of parasitemia (% PBS control)a |
|
|---|---|---|---|---|
| Day 4 | Day 5 | |||
| Pantetheinase-deficient A/J | ||||
| Artesunate | 0.2 | − | 30 | 20 |
| Artesunate | 0.2 | + | 65* | 56* |
| Artesunate | 0.5 | − | 65 | 43 |
| Artesunate | 0.5 | + | 93 | 80* |
| DHA | 0.15 | − | 22 | 13 |
| DHA | 0.15 | + | 56* | 46* |
| DHA | 0.3 | − | 50 | 40 |
| DHA | 0.3 | + | 80 | 71* |
| NAb | 0 | + | 28 | 23 |
| Pantetheinase-sufficient C57BL/6 | ||||
| Artesunate | 0.5 | − | 29 | 21 |
| Artesunate | 0.5 | + | 71* | 65* |
| NA | 0 | + | 13 | 25 |
* indicates synergy between the compounds.
NA, no drug administered.
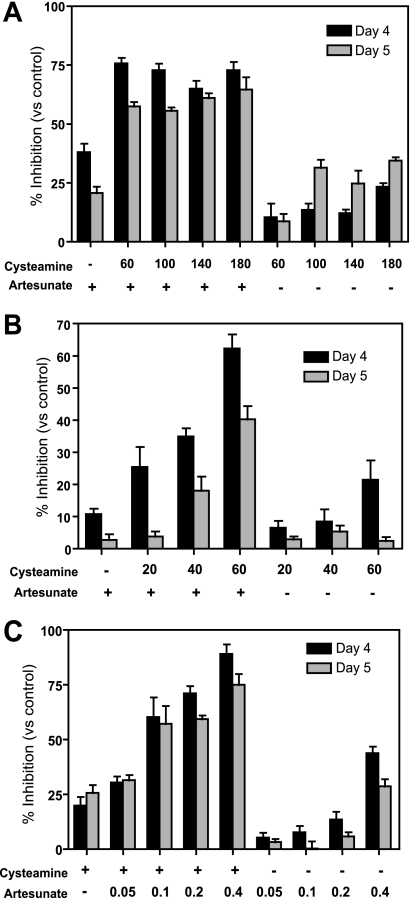
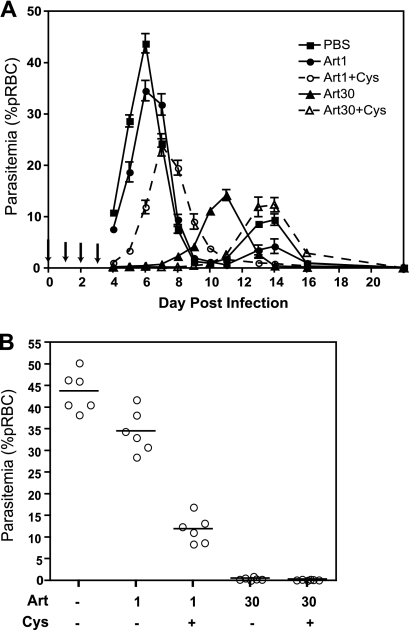
Synergistic inhibition of Plasmodium replication by artesunate and cysteamine is dose dependent.
We subsequently examined if Cys potentiation of ART was dose dependent. Initially, Cys doses of 60, 100, 140, and 180 mg/kg were tested with a suboptimal ART dose of 0.2 mg/kg. The drugs were administered from day 0 to day 3 postinfection, parasitemia was counted at day 4 and day 5, and the percent inhibition was calculated compared to that of PBS-treated controls (Fig. 4 A). At 0.2 mg/kg, ART alone inhibited parasitemia by ∼20% (day 4) and 40% (day 5), while inhibition by Cys alone was partially dose dependent (between 10% and 25%). We detected synergy for all Cys doses tested (varying between 50% and 75% reduction in parasitemia), although without a clear dose-dependent effect in this Cys dosing range. Testing a lower Cys dose range (20, 40, and 60 mg/kg) revealed a clear dose-dependent effect on the synergistic inhibition of parasitemia, with doses as low as 20 to 40 mg/kg showing potentiation of the ART effect (Fig. 4B). We also examined whether Cys could potentiate low doses of artesunate which, given alone, have no significant effect on parasitemia. In this experiment, we administered Cys (170 mg/kg) in combination, or not, with increasing doses of ART (0.05, 0.1, 0.2, and 0.4 mg/kg) in the same 4-day experimental protocol. In these experiments, we detected a striking potentiation (minimum of 3-fold) of low-dose artesunate by Cys, with 60 to 75% inhibition of parasitemia replication for combinations containing low-dose ART at 0.1 and 0.2 mg/kg, compared to <10% for these doses of ART used alone (Fig. 4C).
FIG. 4.
Dose-dependent synergistic effect of cysteamine on artemisinin efficacy against replication of Plasmodium chabaudi in vivo. Groups (n = 6) of female A/J mice were infected with P. chabaudi (107 pRBC, i.v.) and treated for 4 days (days 0, 1, 2, and 3) with increasing doses (indicated) of artesunate (C) and/or cysteamine (A and B) given i.p. Blood parasitemia was determined at days 4 and 5 postinfection, and the inhibitory effects of the different drug treatments on blood-stage P. chabaudi replication were calculated for each animal compared to the mean of PBS-treated controls (expressed as a percentage). The presence or absence of drug is indicated by a plus or minus, respectively, and all doses are in mg/kg. Errors bars represent standard error of the mean.
Impact of cysteamine and artesunate in combination on the resolution of P. chabaudi infection.
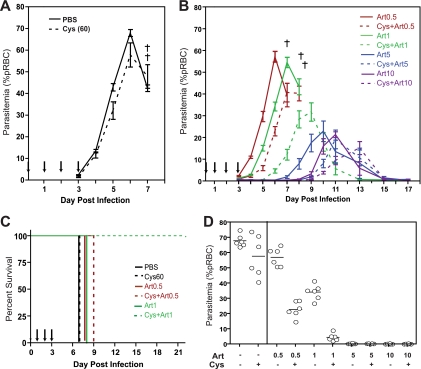
We investigated if low-dose Cys could potentiate standard doses of ART that show therapeutic activity in vivo and concurrently determined possible long-term effects on patent parasitemia, resolution of infection, and survival in a lethal infection model. In this protocol, mice were infected with 106 P. chabaudi pRBC (i.v.) and treated with Cys (60 mg/kg) and/or ART (0.5, 1, 2, 5, and 10 mg/kg) for 4 days (days 0 to 3), while blood parasitemia and survival were followed for 22 days. As expected from this infectious dose, control animals treated with either PBS or Cys alone (60 mg/kg) developed high parasite burdens, which peaked at day 6, and all mice succumbed to the infection by day 7 (Fig. 5 A and C). In animals receiving ART alone, there was a dose-dependent effect on infection, which manifested as a delay in the onset of parasitemia and a reduction of peak parasitemia. Strikingly, the addition of Cys (60 mg/kg) to all ART doses tested had a beneficial effect on infection kinetics, causing both a further delay in onset (by 2 or 3 days) and a reduction of peak levels of parasitemia compared to those for mice receiving only the corresponding dose of ART (Fig. 5B). Notably, the addition of Cys to 0.5 mg/kg or 1 mg/kg of ART caused a strong potentiation of the ART effect, with a further 60 to 70% reduction in parasitemia at day 6 (Fig. 5D). Likewise, although all mice treated with 0.5 mg/kg ART succumbed to the infection early (day 8), mice additionally receiving Cys survived until day 9; moreover, addition of Cys to 1.0 mg/kg ART completely rescued animals from lethality of infection, with 100% survival in this group (Fig. 5B and C).
FIG. 5.
Effect of cysteamine and artesunate combinations on progression and resolution of P. chabaudi infection in vivo. Groups (n = 6) of female A/J mice were infected with P. chabaudi (106 pRBC, i.v.) and treated for 4 days (days 0, 1, 2, and 3) with PBS (A), cysteamine (60 mg/kg, A), or cysteamine (60 mg/kg) combined with increasing doses of artesunate (0.5, 1.0, 5, or 10 mg/kg) (B), all given i.p. Blood parasitemia was measured daily up to day 20 (expressed as percentage of pRBC), and death was recorded (indicated by a cross). Solid and dashed lines represent mice receiving artesunate doses alone or in combination with cysteamine, respectively; artesunate doses are depicted by different color groups, as indicated. Error bars represent standard deviation of the mean, and arrows represent drug treatment days. (C) Kaplan-Meier survival plot for experimental treatment groups for which lethality was observed. Color codes and dashed versus solid lines are as described for panel B. (D) Parasitemia levels at day 6 postinfection for all experimental groups are shown, with each dot representing a mouse. Mean levels are shown as bars.
To investigate whether these effects on parasite burden over the course of infection would also be observed with a pantetheinase-sufficient mouse strain, we performed a similar experiment using female B6 mice. Groups of mice were infected with 106 P. chabaudi pRBC i.v. and treated with PBS, 1 mg/kg or 30 mg/kg of Art, or 1 mg/kg or 30 mg/kg of Art plus 60 mg/kg of Cys for 4 days. We see both a reduction in parasite levels and a delay in the peak when Cys and Art are given in combination, compared to results with Art administered alone, at both high and low doses (Fig. 6 A). As in A/J mice, the effect of Cys addition to 1 mg/kg of Art has a clear effect on early parasite replication at day 6 (Fig. 6B). Although a “curative” dose combination was not achieved with a 4-day treatment regimen, parasite levels remained under 12% pRBC in the 30-mg/kg Art-plus-Cys group, and mice did not display any outward symptoms of disease (lethargy, ruffled fur). B6 mice were able to completely clear parasite burdens and survive the infection, even in the control PBS-treated group, as expected. However, the addition of Cys eliminated the appearance of recrudescent parasitemia around day 14, as seen with the control group (Fig. 6A).
FIG. 6.
Effect of cysteamine and artesunate combinations on progression of P. chabaudi in pantetheinase-sufficient B6 mice. Groups (n = 6) of female B6 mice were infected with P. chabaudi (106 pRBC, i.v.) and treated for 4 days (days 0, 1, 2, and 3) with either PBS or artesunate (1.0 or 30 mg/kg) combined with, or without, cysteamine (60 mg/kg) (A), all given i.p. Blood parasitemia was measured daily up to day 22 (expressed as percentage of pRBC). Solid and dashed lines represent mice receiving artesunate doses alone or in combination with cysteamine, respectively. Error bars represent standard deviation of the mean, and arrows represent drug treatment days. (B) Parasitemia levels at day 6 postinfection for all experimental groups are shown, with each dot representing a mouse. Mean levels are shown as bars.
These results suggest that the synergistic effect of low doses of Cys on artemisinin derivatives not only impacts early parasite burdens but can also significantly improve ultimate outcome to infection.
DISCUSSION
Drug resistance in the Plasmodium parasite has been reported for all major classes of antimalarial drugs and is exacerbating the serious global health problem posed by this infectious disease (36). Widespread resistance to older antimalarial drugs has brought artemisinin derivatives to the forefront of malaria treatment therapies (29, 37). Artemisinin is a sesquiterpene lactone isolated from Artemisia annua, a plant used in traditional Chinese medicine to treat various illnesses. Artemisinin derivatives dihydroartemisinin, oil-soluble arteether, artemether, and water-soluble sodium artesunate rapidly clear parasite burden and resolve clinical symptoms. These drugs have relatively few adverse side effects and have proven effective against multidrug-resistant P. falciparum, making them the standard of care for clinical disease (22, 25). The drawbacks of artemisinin include a short half-life (1), poor solubility, and cost. Monotherapy with artemisinin derivatives is not recommended, in order to avoid development of parasite resistance; in addition, monotherapy results in high levels of parasite recrudescence (8). Artemisinin combination therapies (ACTs) using a partner drug with a different mode of action increase efficacy while limiting the potential for development of artemisinin resistance and are the WHO recommended first-line treatment for uncomplicated malaria (29). ACTs currently in clinical use include artesunate-mefloquine, artesunate-amodiaquine, artesunate-sulfadoxine-pyrimethamine, artesunate-sulfamethoxypyrazine-pyrimethamine, and artemether-lumefantrine (33). Although these combinations have shown robust efficacy, resistance to the partner drug has increased the need for identifying novel partner compounds. Although it is desirable to have a partner drug with a longer half-life than artemisinin to improve efficacy and reduce treatment time (29), exposure to the partner drug (e.g., mefloquine) following elimination of artemisinin may itself select for resistance in the parasite (33). A partner drug with a shorter half-life that still retains efficacy of the ACT (in a 3- to 4-day treatment) may prove beneficial in reducing emergence of resistance to ACT. Recently, the first field reports of resistance to ACT, namely, of P. falciparum isolates from the Thailand-Cambodia border area of Southeast Asia, have appeared in the literature (12, 32). The Pailin province is recognized to be the birthplace of chloroquine- and sulfadoxine-pyrimethamine (SP)-resistant Plasmodium, partly due to low levels of transmission and frequent use of monotherapy. P. falciparum parasites from this region showed lower susceptibility to ART in vivo (higher initial treatment failure rate at day 3 and persistence of parasitemia past treatment) than parasites from neighboring Thailand (12). These troubling reports stress the importance of finding novel and efficacious partners for the artemisinin derivatives.
Cysteamine (Cys) is in clinical use for the treatment of nephropathic cystinosis and is administered orally as cysteamine bitartrate (Cystagon), starting in early childhood (21). Dosing for cystinotic children is ∼50 mg/kg per day divided in 4 doses. Pharmacokinetic analyses of patients have shown Cmax levels of 36 μM with a Tmax of 1.4 h after treatment with this cysteamine bitartrate (6). Continuous use of Cys at these doses over several years shows low toxicity in humans (www.drugs.com/pro). Using an in vivo mouse model of infection with P. chabaudi, we report a moderate but significant effect of Cys used as a single agent on blood-stage parasite replication and on survival from acute infection (26). Here, we show that Cys significantly reduces parasite burden when given i.p. or s.c. and using different treatment regimens. Moreover, Cys doses showing antiparasitic effects present pharmacokinetic profiles similar to those displayed by oral Cys bitartrate doses given for cystinosis in humans (Cmax = 80 μM; AUC = 2,845.1 min·μM). Cys shows efficacy despite a short plasma half-life, a potentially interesting characteristic for a new ACT. Most importantly, Cys shows synergistic effects on the antimalarial activity of the artemisinin derivatives artesunate and dihydroartemisinin. Synergy between the compounds is dose dependent and occurs over a wide dose range, and even low doses (40 to 60 mg/kg) of Cys can potentiate suboptimal doses of artesunate. We have also demonstrated that adding this low dose of Cys to 1 mg/kg of artesunate can result in parasite clearance and survival in 100% of mice that would otherwise have succumbed to the infection; in resistant B6 mice, this manifests as an elimination of recrudescent parasitemia. At this dose, we observed that Cys does not increase the toxicity of artesunate in HeLa cells in vitro (data not shown), suggesting that Cys potentiation of artesunate antimalarial activity occurs in the absence of toxic side effects.
Our results suggest that Cys has many key characteristics of a new partner for artemisinin derivatives in ACT. First, it is well tolerated and has previously been used safely in the clinic for chronic cystinosis (11) and is under clinical evaluation for Huntington's disease (20). Second, it shows good efficacy against P. chabaudi replication in our mouse model in vivo as well as antiparasitic activity against P. falciparum in vitro, when used as a single agent (26). This antiparasitic effect in vivo is achieved at dosing that displays a pharmacokinetic profile similar to that of Cys bitartrate dosing used in humans (6). Third, Cys shows highly significant, dose-dependent synergy when used in combination with artesunate or dihydroartemisinin against P. chabaudi in vivo, with only a 4-day course of treatment. These findings, together with a low cost of synthesis and good stability and bioavailability, point to Cys as an excellent potential addition to current ACTs. Cys potentiation of artemisinin compounds in novel ACT may prove useful to delay the appearance of and/or treat artemisinin-resistant infections.
The potentiation of artemisinin by Cys suggests that both molecules have complementary modes of action. As Cys is able to freely reach the lysosomes of patients suffering from cystinosis, it is conceivable that this small molecule can also penetrate the infected erythrocyte, the malaria parasite, and could enter the digestive vacuole (DV) to act together with artemisinin. As a stand-alone molecule, Cys has been suggested to indirectly modify the cellular pool of glutathione (GSH) and decrease the capacity of the cells to cope with oxidative damages (7). Similarly, this would affect the capacity of the parasite to detoxify heme in the cytosol of the erythrocyte. Cys could also have a direct role in slowing the degradation of hemoglobin in the DV, possibly by forming adducts with the parasite cysteine proteases, falcipains (31).
The exact molecular mechanism of action of artemisinin against the malarial parasite remains unclear. Artemisinin derivatives have been shown to act against other apicomplexan parasites, trematodes, several viral infections, cancer cells, and systemic lupus erythematosus (16, 24, 34). Based on this broad spectrum of efficacy, it is unlikely that ART acts via a single mechanism. Nevertheless, it is generally agreed that cleavage of the peroxide bridge of ART is a key initial activating step for its activity. In Plasmodium, this reactive species causes heme alkylation and accumulation of toxic heme adducts (9). The production of free radicals that alkylate and oxidize proteins and lipids, including the critical iron-sulfur redox centers of the parasite, also contributes to ART toxicity (4, 38). Fe2+ is proposed to play an important role in breaking up the endoperoxide bridge of ART to induce the production of free radicals. Studies with radiolabeled ART derivatives have identified several Plasmodium proteins that are bound by the drug, suggesting multiple possible targets for inhibition. In particular, ART binds to and has been shown to inhibit the activity of the parasite calcium-type ATPase PfATP6 (15, 30). On the other hand, at least two transporters present in the membrane of the parasite DV, namely, PfMDR1 and PfCRT, have been shown to modulate susceptibility to ART (14). Although the mechanistic basis of Cys potentiation of ART activity remains unknown, it is tempting to speculate that Cys may affect one or several aspects of the above-mentioned parameters of the ART antimalarial effect. Cys could affect the pharmacokinetic properties of ART in vivo, possibly increasing its bioavailability. It could also enhance the fragility of the infected erythrocytes to the reactive ART-derived species and/or increase access to ART molecular targets. Finally, Cys may inhibit PfMDR- and PfCRT-mediated efflux of ART from the parasite DV.
We are currently testing the activity of Cys/ART combinations against artemisinin-resistant rodent parasite lines (2) and evaluating the efficacy against mouse models of cerebral malaria caused by infection with the Plasmodium berghei species. Overall, our results suggest that Cys possesses several key characteristics of a partner drug for artemisinin derivatives and should be clinically evaluated in ACT against malaria.
Acknowledgments
We are indebted to Patricia D'Arcy and Susan Gauthier for expert technical assistance in animal studies, to Shannon McLaughlan for diligent determinations of blood parasitemia, and to Mifong Tam for preparing P. chabaudi AS infective inocula.
P.G. is a James McGill professor of biochemistry. This work was supported by a grant from the Canadian Institutes of Health Research to P.G. (CIHR; Team in Global Health Research in Malaria, and Proof of Principle 2 grants).
Footnotes
Published ahead of print on 17 May 2010.
REFERENCES
- 1.Adjuik, M., A. Babiker, P. Garner, P. Olliaro, W. Taylor, and N. White. 2004. Artesunate combinations for treatment of malaria: meta-analysis. Lancet 363:9-17. [DOI] [PubMed] [Google Scholar]
- 2.Afonso, A., P. Hunt, S. Cheesman, A. C. Alves, C. V. Cunha, V. do Rosario, and P. Cravo. 2006. Malaria parasites can develop stable resistance to artemisinin but lack mutations in candidate genes atp6 (encoding the sarcoplasmic and endoplasmic reticulum Ca2+ ATPase), tctp, mdr1, and cg10. Antimicrob. Agents Chemother. 50:480-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anikster, Y., V. Shotelersuk, and W. A. Gahl. 1999. CTNS mutations in patients with cystinosis. Hum. Mutat. 14:454-458. [DOI] [PubMed] [Google Scholar]
- 4.Asawamahasakda, W., I. Ittarat, Y. M. Pu, H. Ziffer, and S. R. Meshnick. 1994. Reaction of antimalarial endoperoxides with specific parasite proteins. Antimicrob. Agents Chemother. 38:1854-1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ayi, K., G. Min-Oo, L. Serghides, M. Crockett, M. Kirby-Allen, I. Quirt, P. Gros, and K. C. Kain. 2008. Pyruvate kinase deficiency and malaria. N. Engl. J. Med. 358:1805-1810. [DOI] [PubMed] [Google Scholar]
- 6.Belldina, E. B., M. Y. Huang, J. A. Schneider, R. C. Brundage, and T. S. Tracy. 2003. Steady-state pharmacokinetics and pharmacodynamics of cysteamine bitartrate in paediatric nephropathic cystinosis patients. Br. J. Clin. Pharmacol. 56:520-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berruyer, C., F. M. Martin, R. Castellano, A. Macone, F. Malergue, S. Garrido-Urbani, V. Millet, J. Imbert, S. Dupre, G. Pitari, P. Naquet, and F. Galland. 2004. Vanin-1−/− mice exhibit a glutathione-mediated tissue resistance to oxidative stress. Mol. Cell. Biol. 24:7214-7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bunnag, D., J. Karbwang, and T. Harinasuta. 1992. Artemether in the treatment of multiple drug resistant falciparum malaria. Southeast Asian J. Trop. Med. Public Health 23:762-767. [PubMed] [Google Scholar]
- 9.Creek, D. J., W. N. Charman, F. C. Chiu, R. J. Prankerd, Y. Dong, J. L. Vennerstrom, and S. A. Charman. 2008. Relationship between antimalarial activity and heme alkylation for spiro- and dispiro-1,2,4-trioxolane antimalarials. Antimicrob. Agents Chemother. 52:1291-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dias, V. C., F. J. Bamforth, M. Tesanovic, M. E. Hyndman, H. G. Parsons, and G. S. Cembrowski. 1998. Evaluation and intermethod comparison of the Bio-Rad high-performance liquid chromatographic method for plasma total homocysteine. Clin. Chem. 44:2199-2201. [PubMed] [Google Scholar]
- 11.Dohil, R., M. Fidler, J. A. Gangoiti, F. Kaskel, J. A. Schneider, and B. A. Barshop. 2010. Twice-daily cysteamine bitartrate therapy for children with cystinosis. J. Pediatr. 156:71-75. [DOI] [PubMed] [Google Scholar]
- 12.Dondorp, A. M., F. Nosten, P. Yi, D. Das, A. P. Phyo, J. Tarning, K. M. Lwin, F. Ariey, W. Hanpithakpong, S. J. Lee, P. Ringwald, K. Silamut, M. Imwong, K. Chotivanich, P. Lim, T. Herdman, S. S. An, S. Yeung, P. Singhasivanon, N. P. Day, N. Lindegardh, D. Socheat, and N. J. White. 2009. Artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 361:455-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dupre, S., M. Graziani, M. Rosei, A. Fabi, and E. Del Grosso. 1970. The enzymatic breakdown of pantethine to pantothenic acid and cystamine. Eur. J. Biochem. 16:571-578. [DOI] [PubMed] [Google Scholar]
- 14.Eastman, R. T., and D. A. Fidock. 2009. Artemisinin-based combination therapies: a vital tool in efforts to eliminate malaria. Nat. Rev. Microbiol. 7:864-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eckstein-Ludwig, U., R. J. Webb, I. D. Van Goethem, J. M. East, A. G. Lee, M. Kimura, P. M. O'Neill, P. G. Bray, S. A. Ward, and S. Krishna. 2003. Artemisinins target the SERCA of Plasmodium falciparum. Nature 424:957-961. [DOI] [PubMed] [Google Scholar]
- 16.Efferth, T., M. R. Romero, D. G. Wolf, T. Stamminger, J. J. Marin, and M. Marschall. 2008. The antiviral activities of artemisinin and artesunate. Clin. Infect. Dis. 47:804-811. [DOI] [PubMed] [Google Scholar]
- 17.Fidler, M. C., B. A. Barshop, J. A. Gangoiti, R. Deutsch, M. Martin, J. A. Schneider, and R. Dohil. 2007. Pharmacokinetics of cysteamine bitartrate following gastrointestinal infusion. Br. J. Clin. Pharmacol. 63:36-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fidock, D. A., P. J. Rosenthal, S. L. Croft, R. Brun, and S. Nwaka. 2004. Antimalarial drug discovery: efficacy models for compound screening. Nat. Rev. Drug Discov. 3:509-520. [DOI] [PubMed] [Google Scholar]
- 19.Fortin, A., L. R. Cardon, M. Tam, E. Skamene, M. M. Stevenson, and P. Gros. 2001. Identification of a new malaria susceptibility locus (Char4) in recombinant congenic strains of mice. Proc. Natl. Acad. Sci. U. S. A. 98:10793-10798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frankish, H. 2006. Drug shows potential for treatment of Huntington's disease. Lancet Neurol. 5:476-477. [DOI] [PubMed] [Google Scholar]
- 21.Gahl, W. A. 2003. Early oral cysteamine therapy for nephropathic cystinosis. Eur. J. Pediatr. 162(Suppl. 1):S38-S41. [DOI] [PubMed] [Google Scholar]
- 22.Greenwood, B. M., D. A. Fidock, D. E. Kyle, S. H. Kappe, P. L. Alonso, F. H. Collins, and P. E. Duffy. 2008. Malaria: progress, perils, and prospects for eradication. J. Clin. Invest. 118:1266-1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hyde, J. E. 2007. Drug-resistant malaria—an insight. FEBS J. 274:4688-4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keiser, J., and J. Utzinger. 2007. Artemisinins and synthetic trioxolanes in the treatment of helminth infections. Curr. Opin. Infect. Dis. 20:605-612. [DOI] [PubMed] [Google Scholar]
- 25.McIntosh, H. M., and P. Olliaro. 2000. Artemisinin derivatives for treating uncomplicated malaria. Cochrane Database Syst. Rev. 2000:CD000256. [DOI] [PMC free article] [PubMed]
- 26.Min-Oo, G., K. Ayi, S. E. Bongfen, M. Tam, I. Radovanovic, S. Gauthier, H. Santiago, A. G. Rothfuchs, E. Roffe, A. Sher, A. Mullick, A. Fortin, M. M. Stevenson, K. C. Kain, and P. Gros. 2010. Cysteamine, the natural metabolite of pantetheinase, shows specific activity against Plasmodium. Exp. Parasitol. 125:315-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Min-Oo, G., A. Fortin, G. Pitari, M. Tam, M. M. Stevenson, and P. Gros. 2007. Complex genetic control of susceptibility to malaria: positional cloning of the Char9 locus. J. Exp. Med. 204:511-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Min-Oo, G., A. Fortin, M. F. Tam, A. Nantel, M. M. Stevenson, and P. Gros. 2003. Pyruvate kinase deficiency in mice protects against malaria. Nat. Genet. 35:357-362. [DOI] [PubMed] [Google Scholar]
- 29.Nosten, F., and N. J. White. 2007. Artemisinin-based combination treatment of falciparum malaria. Am. J. Trop. Med. Hyg. 77:181-192. [PubMed] [Google Scholar]
- 30.Olliaro, P. L., R. K. Haynes, B. Meunier, and Y. Yuthavong. 2001. Possible modes of action of the artemisinin-type compounds. Trends Parasitol. 17:122-126. [DOI] [PubMed] [Google Scholar]
- 31.Rosenthal, P. J. 2004. Cysteine proteases of malaria parasites. Int. J. Parasitol. 34:1489-1499. [DOI] [PubMed] [Google Scholar]
- 32.Samarasekera, U. 2009. Countries race to contain resistance to key antimalarial. Lancet 374:277-280. [DOI] [PubMed] [Google Scholar]
- 33.Sinclair, D., B. Zani, S. Donegan, P. Olliaro, and P. Garner. 2009. Artemisinin-based combination therapy for treating uncomplicated malaria. Cochrane Database Syst. Rev. 2009:CD007483. [DOI] [PMC free article] [PubMed]
- 34.Sissoko, M. S., A. Dabo, H. Traore, M. Diallo, B. Traore, D. Konate, B. Niare, M. Diakite, B. Kamate, A. Traore, A. Bathily, A. Tapily, O. B. Toure, S. Cauwenbergh, H. F. Jansen, and O. K. Doumbo. 2009. Efficacy of artesunate + sulfamethoxypyrazine/pyrimethamine versus praziquantel in the treatment of Schistosoma haematobium in children. PLoS One 4:e6732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tallarida, R. J. 2001. Drug synergism: its detection and applications. J. Pharmacol. Exp. Ther. 298:865-872. [PubMed] [Google Scholar]
- 36.White, N. J. 2004. Antimalarial drug resistance. J. Clin. Invest. 113:1084-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.WHO. 2006. Guidelines for the treatment of malaria. World Health Organization, Geneva, Switzerland.
- 38.Wu, Y. 2002. How might qinghaosu (artemisinin) and related compounds kill the intraerythrocytic malaria parasite? A chemist's view. Acc. Chem. Res. 35:255-259. [DOI] [PubMed] [Google Scholar]