Abstract
PA-824 is a novel nitroimidazo-oxazine being evaluated for its potential to improve tuberculosis (TB) therapy. This randomized study evaluated safety, tolerability, pharmacokinetics, and extended early bactericidal activity of PA-824 in drug-sensitive, sputum smear-positive, adult pulmonary tuberculosis patients. Fifteen patients per cohort received 1 of 4 doses of oral PA-824: 200, 600, 1,000, or 1,200 mg per day for 14 days. Eight subjects received once daily standard antituberculosis treatment as positive control. The primary efficacy endpoint was the mean rate of change in log CFU of Mycobacterium tuberculosis in sputum incubated on agar plates from serial overnight sputum collections, expressed as log10 CFU/day/ml (± standard deviation [SD]). The drug demonstrated increases that were dose linear but less than dose proportional in serum concentrations in doses from 200 to 1,000 mg daily. Dosing of 1,200 mg gave no additional exposure compared to 1,000 mg daily. The mean daily CFU fall under standard treatment was 0.148 (±0.055), consistent with that found in previous studies. The mean daily fall under PA-824 was 0.098 (±0.072) and was equivalent for all four dosages. PA-824 appeared safe and well tolerated; the incidence of adverse events potentially related to PA-824 appeared dose related. We conclude that PA-824 demonstrated bactericidal activity over the dose range of 200 to 1,200 mg daily over 14 days. Because maximum efficacy was unexpectedly achieved at the lowest dosage tested, the activity of lower dosages should now be explored.
Over four decades ago, the introduction of rifampin catalyzed a series of clinical trials leading to current recommendations for tuberculosis (TB) treatment, with the combination of isoniazid (INH), rifampin (RIF), and pyrazinamide (PZA), supported by ethambutol (EMB) or streptomycin (SM) (17, 27). However, this regimen must be taken consistently for 6 months, preferably under supervision, to ensure sterilization of tuberculosis lesions and to prevent development of drug resistance. This relatively long treatment duration, coupled with increasing financial and administrative difficulties experienced by health care systems and the burgeoning HIV/AIDS epidemic, threatens the success of tuberculosis control programs worldwide, particularly in high-incidence areas such as sub-Saharan Africa and parts of Asia. Treatment default is all too frequent (14), and the situation is further exacerbated by the increasing proportion of new cases of tuberculosis resistant to one or more first-line agents (7, 28). New drugs are urgently needed to meet these challenges. They are needed to assist in the management of drug resistance, but also, at least as importantly, to assist in shortening treatment regimens to make tuberculosis treatment easier to supervise and reduce the therapeutic burden that tuberculosis management places on both patients and health services. In addition, current regimens cause difficulties for tuberculosis patients coinfected with HIV due to the deleterious interaction of existing first-line antituberculosis drugs and antiretroviral agents.
PA-824 is a small nitroimidazo-oxazine molecule that has shown potential both in vitro and in vivo of being able to assist both in shortening the treatment regimen for tuberculosis and contributing to the treatment of drug-resistant tuberculosis patients (19, 20). Studies using anaerobic culture models have also found activity against nonreplicating Mycobacterium tuberculosis (10, 16, 24, 25). In vitro studies found the MIC of PA-824 (≤0.015 to 0.25 μg/ml) to be comparable to that of INH (0.03 to 0.06 μg/ml) (16). Studies in healthy volunteers indicated that PA-824 was well tolerated and bioavailable after oral doses without significant drug-related side effects, changes in vital signs, or abnormal laboratory values (8, 9). PA-824 has been shown to cause isolated and reversible increases in serum creatinine levels, which appear to be due to inhibition of creatinine secretion and are considered clinically benign (8, 9). The dosage levels of 200 mg, 600 mg, 1,000 mg, and 1,200 mg used in this study were selected on the basis of preclinical toxicology and efficacy data and clinical pharmacokinetic properties of PA-824 observed in healthy adult volunteers and were chosen to assist identification of the lowest efficacious dosage and the highest dosage safely inducing maximal bactericidal efficacy.
Early bactericidal activity (EBA) refers to an agent's ability to kill mycobacteria originating within pulmonary cavities during the first weeks of treatment. Determination of EBA in sputum smear-positive pulmonary tuberculosis patients by quantification of viable CFU of M. tuberculosis in an overnight sputum collection allows the comparison of different drugs with one another with regard to clinical bactericidal activity and early assessment of safety in a small number of intensively studied patients. The evaluation of a dosage-related response can guide the selection of a dosage to take forward to later clinical studies (5, 6, 12, 13). Initial studies using this technique were conducted over the first two days of treatment, as it was during this period that the most significant differences between agents were seen (12). Further experience has shown that significant advantages may accrue from extending the study period; for example, the important sterilizing agent PZA showed bactericidal activity only after four days of treatment (12), as did the novel diarylquinoline currently in development, TMC207 (4, 23).
It has also been suggested that activity during the period of 2 to 14 days after treatment is started might represent the sterilizing capacity of an agent (13). Recently, evidence has been presented that the time to positivity (TTP) of M. tuberculosis in automated liquid culture systems reflects the metabolic activity of inoculated viable M. tuberculosis in sputum. TTP might represent an alternative method to colony counting for estimating the activity of viable M. tuberculosis in sputum, which might be valuable for exploring the bactericidal activities of new antituberculosis agents (22).
This paper reports a proof-of-concept study that evaluated the bactericidal activity of PA-824 given as monotherapy over a range of dosages during the first 14 days of treatment of treatment-naïve patients with smear-positive pulmonary tuberculosis. Safety, tolerability, and pharmacokinetics (PK) were also evaluated.
MATERIALS AND METHODS
Patients and procedures.
Treatment-naïve, sputum smear-positive patients identified at outpatient clinics in Cape Town, South Africa, were screened for eligibility. Consenting subjects who met all entry criteria were hospitalized for the entire duration of study drug intake at one of two study centers (Task Applied Science, Karl Bremer Hospital, and Centre for Tuberculosis Research Innovation, UCT Lung Institute). Patients were randomized centrally to receive monotherapy with PA-824 dosages of 200 mg, 600 mg, 1,000 mg, or 1,200 mg orally once daily for 14 consecutive days in double-blind fashion. A fifth cohort was randomized to unblinded standard antituberculosis treatment as positive control (INH, RIF, PZA, EMB). All drug administration was supervised. Male or female smear-positive patients (at least a score of 1+ on the WHO-International Union Against Tuberculosis and Lung Disease [IUATLD] scale [11]; age, 18 to 64 years; body weight, 40 kg to 90 kg, inclusive) were eligible if they were free of serious underlying medical conditions that might jeopardize the patient's safety during the study or could render the study endpoints difficult to interpret. Individuals with HIV infection under antiretroviral treatment or with a CD4 cell count of ≤300 × 106/liter were excluded, as were those with bacilli resistant to RIF (GenoType MTBDRplus; Hain Lifesciences, Nehren, Germany). Recent exposure to INH was excluded by urine testing (BBL Taxo INH test strips; Becton Dickinson, Johannesburg, South Africa). Sputum was collected overnight (16 h) for two nights before drug intake, daily from days 1 to 4, and then every other day until day 14. Safety assessments included daily history, vital signs, physical examination, and monitoring of adverse events. Full blood count, coagulation studies, serum chemistry, urinalysis, and 12-lead echocardiograms (ECGs) were performed on days 1, 7, and 14 of drug intake as well as 2 weeks after the last dose.
Microbiology and pharmacokinetics.
All microbiology was done centrally (Department of Medical Biochemistry, Faculty of Health Sciences, University of Stellenbosch, Cape Town, South Africa). Samples were stored and transported to the laboratory under refrigerated conditions. CFU counting was performed as previously described (5, 6). Briefly, sputum was homogenized, diluted, digested (Sputasol; Oxoid, Cambridge, United Kingdom), incubated on selective 7H10 agar plates (Becton Dickinson), and CFU counted after 3 to 4 weeks of incubation. For measurement of time to positivity (TTP), we used a standardized liquid culture system (BACTEC mycobacteria growth indicator tube [MGIT] 960; Becton Dickinson). Briefly, homogenized sputum was decontaminated (MycoPrep; Becton Dickinson), centrifuged, and resuspended, and 0.5 ml of the resulting 2 ml was used for incubation in duplicate. We determined susceptibility to SM, INH, RIF, EMB (Sire kit for MGIT; Becton Dickinson) and the MIC of PA-824 (agar proportion method with concentrations from 0.1 μg/ml to 3.2 μg/ml). Identification of M. tuberculosis was performed with a molecular method (26).
We collected a complete PK profile and serial 12-lead ECGs on days 1, 8, and 14, trough samples daily on days 1 through 14, and additional samples at 24 h, 30 h, and 2 weeks after the last dose in patients receiving PA-824. PA-824 concentrations were determined with a validated high-performance liquid chromatography method. The PK profile was assessed from participants' individual plasma concentrations by applying a noncompartmental approach. We used the SAS System for Windows (v8.2) for statistical calculations and WinNonlin (v5.0.1; Professional) for nonlinear adjustments for determining the elimination half-life.
Statistics.
An empirical sample size, similar to that of previous EBA studies, of 15 patients per PA-824 group was chosen (6). All confidence coefficients were 0.95, and significance test levels were 0.05 (two sided). The mean of a maximum of four CFU counts at each time point was calculated. The primary efficacy endpoint was the EBA over 14 days [EBA(0-14)] calculated for each individual with the formula EBA(0-14) = [mean log10 CFU(day 0) − log10 CFU(day 14)]/14, averaged per treatment group. Secondary efficacy endpoints were EBA(0-2) and EBA(2-14), calculated in analogous fashion, and the mycobacterial metabolic activity was measured by change in TTP calculated in the same manner as that for EBA. EBA and TTP were also described with linear, bilinear, or nonlinear regression over time, depending on which method best fitted the data.
Ethics.
The study was approved by the appropriate regulatory agencies and local and central ethics review committees and was conducted in compliance with ICH Good Clinical Practice Guidelines. All participants gave written informed consent.
RESULTS
Study population.
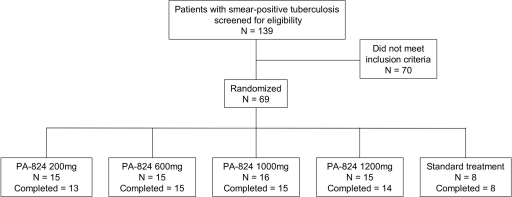
Out of 139 patients screened, 69 met all entry criteria. Sixty-five patients completed the drug intake phase. The dispositions of the patients are summarized in Fig. 1, and demographic, anthropometric, and diagnostic features appear in Table 1. There were no significant differences between the PA-824 groups. Ten out of 69 patients tested seropositive for HIV (14.5%).
FIG. 1.
Patient disposition. Four participants did not complete the 14-day period of drug intake. Two patients randomized to PA-824 200 mg and one patient randomized to PA-824 1,200 mg were withdrawn due to adverse events not related to study medication (hemoptysis, n = 1; Wolff-Parkinson-White syndrome, n = 1; urinary tract infection, n = 1). One patient randomized to 1,000 mg PA-824 withdrew consent.
TABLE 1.
Demographic, anthropometric, and diagnostic features of patients completing the period of drug intakea
| Characteristic | PA-824 group |
Standard treatment groupb | Total | |||
|---|---|---|---|---|---|---|
| 200 mg | 600 mg | 1,000 mg | 1,200 mg | |||
| No. of patients | 13 | 15 | 15 | 14 | 8 | 65 |
| No. of male patients | 7 | 9 | 8 | 8 | 4 | 36 |
| Age (yr) | 30.8 (8.8) | 30.6 (9.7) | 31.4 (9.1) | 31.1 (10.6) | 21.9 (3.4) | 29.8 (9.3) |
| Weight (kg) | 53.2 (6.3) | 55.9 (10.5) | 52.3 (10.1) | 54.1 (8.5) | 48.0 (8.7) | 53.2 (9.1) |
| BMI (kg/m2) | 19.3 (2.8) | 20.1 (3.9) | 19.1 (2.7) | 19.5 (3.2) | 17.5 (1.8) | 19.3 (3.1) |
| Baseline CFU count (log10/ml sputum) | 6.592 (1.100) | 6.335 (0.759) | 6.309 (0.935) | 6.057 (1.097) | 6.152 (0.792) | 6.159 (0.917) |
Values are means (±SD) unless indicated otherwise. BMI, body mass index.
For a definition of standard treatment, see the text.
Bactericidal efficacy.
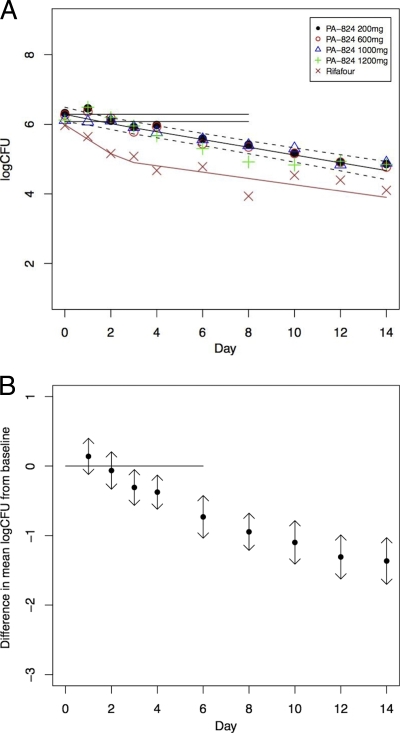
The bactericidal activity of PA-824 evaluated by CFU counting is summarized in Table 2 (see Table S1 in the supplemental material), and the activity demonstrated by TTP is shown in Table 3 (see Table S2 in the supplemental material). At all dosage levels, PA-824 displayed clear bactericidal activity, reducing the mycobacterial load in sputum over days 2 to 14 at a rate comparable to that of a combination of all of the current first-line antituberculosis agents (12, 13). The log10 CFU time trend was best modeled by bilinear regression. For the four PA-824 dosage groups, the departure from a straight line was not significant. For PA-824, the EBA(0-14), EBA(0-2), and EBA(2-14) were similar at all dosages evaluated. Figure 2 A illustrates the mean CFU per day with a fitted bilinear regression line for all treatment groups. Figure 2B illustrates the mean difference of each CFU measurement from baseline for all PA-824 dosage groups combined and indicates that total bactericidal activity becomes significantly different from zero between days 2 and 3. The bactericidal activity of standard treatment over 14 days was biphasic, consistent with and similar in magnitude to that reported previously for the same drug combination. This result validates the underlying mycobacteriological study methodology (2, 12).
TABLE 2.
Early bactericidal activity for days 0 to 14, 0 to 2, and 2 to 14 measured by fall in CFU on solid mediuma
| Dosagec | PA-824 group |
Standard treatment groupb |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 200 mg |
600 mg |
1,000 mg |
1,200 mg |
All groups |
||||||||
| Mean log10 CFU/ml | n | Mean log10 CFU/ml | n | Mean log10 CFU/ml | n | Mean log10 CFU/ml | n | Mean log10 CFU/ml | n | Mean log10 CFU/ml | n | |
| EBA(0-14) | 0.106 (0.049) | 12 | 0.107 (0.053) | 14 | 0.091 (0.083) | 15 | 0.088 (0.084) | 11 | 0.098 (0.072) | 52 | 0.148 (0.055) | 7 |
| EBA(0-2) | 0.109 (0.487) | 15 | 0.096 (0.226) | 13 | 0.025 (0.340) | 15 | −0.035 (0.420) | 15 | 0.047 (0.373) | 58 | 0.403 (0.290) | 8 |
| EBA(2-14) | 0.106 (0.063) | 12 | 0.113 (0.079) | 12 | 0.095 (0.062) | 14 | 0.113 (0.099) | 11 | 0.106 (0.077) | 49 | 0.112 (0.050) | 7 |
Values are mean log10 CFU/ml sputum/day (±SD). n, number of participants with results; EBA, early bactericidal activity.
For a definition of standard treatment, see the text.
Note that EBA(0-14) can deviate from the arithmetic sum of EBA(0-2) and EBA(2-14) due to missing data points.
TABLE 3.
Estimation of bactericidal activity by prolongation of TTP in liquid culture for days 0 to 14, 0 to 2, and 2 to 14a
| Dosagec | PA-824 group |
Standard treatment groupb |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 200 mg |
600 mg |
1,000 mg |
1,200 mg |
All groups |
||||||||
| Mean h/day (±SD) | n | Mean h/day (±SD) | n | Mean h/day (±SD) | n | Mean h/day (±SD) | n | Mean h/day (±SD) | n | Mean h/day (±SD) | n | |
| EBA(0-14) | 3.818 (2.327) | 12 | 4.776 (2.879) | 14 | 4.865 (3.461) | 13 | 4.440 (2.169) | 12 | 4.494 (2.724) | 51 | 9.741 (5.249) | 8 |
| EBA(0-2) | 1.115 (15.256) | 13 | 5.788 (12.173) | 13 | 2.795 (9.230) | 11 | 1.400 (7.659) | 15 | 2.721 (11.225) | 52 | 24.125 (12.794) | 8 |
| EBA(2-14) | 3.833 (2.954) | 11 | 5.09 (2.768) | 13 | 4.069 (1.916) | 12 | 4.868 (3.224) | 12 | 4.491 (2.718) | 48 | 7.344 (4.660) | 8 |
n, number of participants with results.
For a definition of standard treatment, see the text.
Note that EBA(0-14) can deviate from the arithmetic sum of EBA(0-2) and EBA(2-14) due to missing data points.
FIG. 2.
(A) CFU counts of all treatments over time. Shown is a fitted bilinear regression line for all PA-824 treatment groups, with 95% confidence intervals represented by dotted lines, illustrating the daily decline in CFU from baseline as mean log CFU/day. The horizontal lines are the mean baseline count (top) with lower 95% confidence interval (bottom). Single data points represent mean values of individual treatment groups. Rifafour is standard, four-drug treatment. (B) Change in CFU counts of all PA-824 treatments combined over time. Mean change from baseline in CFU is measured as log10 CFU/day for all PA-824 treatment groups together with 95% confidence intervals. The horizontal line is the mean baseline count. A significant decrease is found from day 3 onwards, indicated by confidence intervals no longer including baseline.
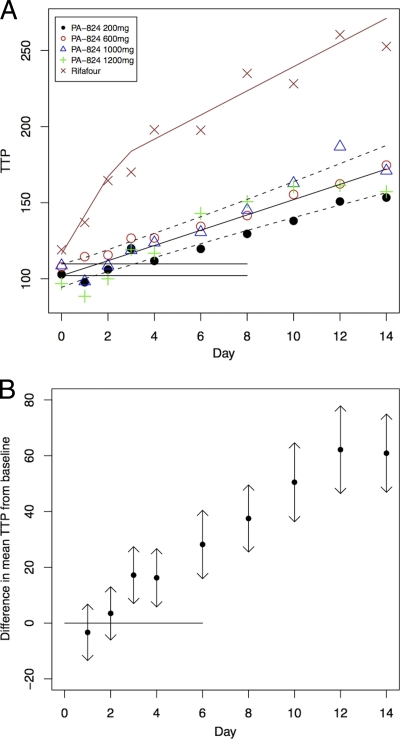
PA-824 activity measured by TTP produced results similar to those determined by sputum CFU quantification. The mean increase in TTP over days 0 through 14 (regression) of the four PA-824 dosage groups did not differ significantly from each other; the overall mean increase in TTP over 14 days (regression) was 4.106 h/day (standard deviation, 4.011). Figure 3A illustrates the mean TTP per day with a fitted bilinear regression line for all treatment groups. Results in Figure 3B, similar to the CFU calculations described above, indicate a statistically significant difference from zero that becomes detectable between days 2 and 3.
FIG. 3.
(A) Time to culture positivity of all treatments over time. Shown is fitted bilinear regression line for all PA-824 treatment groups, with 95% confidence intervals represented by dotted lines, illustrating the daily increase in TTP from baseline as mean hours/day. The horizontal lines are the mean baseline TTP (bottom) with upper 95% confidence interval (top). Single data points represent values of individual treatment groups. Rifafour is standard, four-drug treatment. (B) Change in TTP of all PA-824 treatments combined over time. Mean change from baseline in TTP is measured as hours/day for all PA-824 treatment groups with 95% confidence intervals. The horizontal line is the mean baseline time. A significant increase is found from day 3 onwards (when the confidence intervals no longer include baseline).
All isolated strains tested were susceptible to the investigational product, and M. tuberculosis was speciated as the infecting organism in all patients. The MIC of PA-824 with these strains was <0.1 μg/ml, except for one patient who had a result of 0.4 μg/ml at baseline and <0.1 μg/ml on day 14.
Pharmacokinetics.
PA-824 demonstrated properties consistent with those demonstrated during phase I studies in healthy volunteers. As in phase I single-dose and multiple-dose administrations (8, 9), 1,200 mg PA-824 achieved no substantial increase above that of 1,000 mg in terms of maximum observed plasma concentration (Cmax), area under the plasma concentration-time curve (AUC) from zero to the last measurable concentration, and AUC from zero to infinity. The speed of absorption was moderate with the mean time to reach Cmax ranging across treatment groups from 3.1 to 6.7 h, similar to that observed in healthy volunteers. PA-824 demonstrated a log-linear pattern of elimination. The mean elimination half-life ranged across treatment groups from 17.2 to 24.6 h for the 14-day period. The increase in plasma PA-824 concentrations was less than dose proportional up to 1,000 mg. After assessing trough levels, it appeared that steady state was reached by day 5. Drug clearance appeared fairly constant following single-dose administration across treatment groups, ranging between 6,167 and 10,948 ml/hour. Following multiple-dose administration, clearance appeared fairly constant across treatment groups, although it was somewhat lower on day 14 than after a single dose. This may reflect saturation/inhibition of elimination processes following repeated doses over time and across dose levels. PA-824 has a large volume of distribution (V) following single- and multiple-dose administration. V ranged between 193 and 323 liters following single-dose administration and 93 and 167 liters following multiple-dose administration, indicating that the drug concentrated in tissues. All plasma PA-824 concentrations were below the lower limit of quantification (10 ng/ml) at follow-up on day 29.
Safety.
PA-824 appeared to be safe and well tolerated. Two serious adverse events occurred during the study, both hemoptysis, a common event in pulmonary TB patients. One patient was receiving PA-824 200 mg and the other was receiving standard treatment. Both patients were hospitalized and the events resolved. Thirty-five adverse events were experienced by 26 (38%) of 69 patients; three of these events occurred under standard treatment and the remainder in patients receiving PA-824. The investigator assessed 21 of the 35 adverse events as potentially related to the study medication, of which 15 were mild, five moderate, and one severe. The severe adverse event manifested initially as fever and confusion, which could later be identified as being caused by a urinary tract infection, and was resolved upon treatment. No specific pattern of adverse events was detected in patients treated with PA-824, but a higher incidence of adverse events potentially related to the medication was observed in higher PA-824 dosage groups (PA-824 200 mg, 7%; PA-824 600 mg, 13%; PA-824 1,000 mg, 31%; and PA-824 1,200 mg, 33%). With standard treatment, the incidence of adverse events related to medication was 25%. A dosage-related increase in serum creatinine concentrations was observed as expected based on earlier phase 1 studies; however, no concentrations exceeded the upper limit of reference ranges, and all were normal at follow-up on day 29.
DISCUSSION
PA-824 appeared safe and well tolerated during 14 days of once-daily dosing in this patient population at dosages of 200 to 1,200 mg/day. The number of adverse events in this study was low and the severity of events mostly mild or moderate, although there was an increase in potentially drug-related events with increasing dosage. The study also showed PA-824 to have a substantial and linear EBA over 14 days comparable to that of the existing first-line TB treatment agents. The extended EBA of PA-824 suggests that this drug may have sterilizing activity in human pulmonary tuberculosis and as such could contribute importantly to the sterilizing and treatment-shortening ability of a multidrug TB treatment regimen.
The EBA of PA-824 was equivalent for all four dosages. This unexpected finding was not predicted by studies in the murine model of tuberculosis (19, 20) and the pharmacokinetic properties of the drug seen in both the current and earlier phase I studies, including sub-dose-proportional increases in serum drug levels across the 200 to 1,000 mg/day dosage groups. The control group treated with the standard regimen showed the expected result, which excluded laboratory methodology as a factor for not detecting a dose-responsive change in EBA. One possible explanation is that the plasma concentrations achieved with all four dosages of PA-824 were above the MIC for virtually the entire dosing period. If the time above MIC, rather than the ratio of AUC or Cmax to MIC, is the pharmacodynamic driver for the EBA of PA-824, it might be expected that all four dosage groups would demonstrate similar efficacies, as all patients who received PA-824 had plasma trough levels above the MIC from day 1 throughout the active treatment period at all dosages tested in this study. The flat dose response observed supports a clear need to further explore the bactericidal activity of PA-824 at lower dosages to define the dosage-responsive range of this drug as measured by EBA, to identify the pharmacodynamic driver(s) within a lower dosage range, and to make an informed choice of dosage for later-stage clinical development and ultimate clinical use.
It should be noted that, as with any drug, there could be considerable advantages to using as low a dosage as possible that achieves maximal efficacy, both in terms of potential tolerability and safety margins and for ultimate drug affordability. The clinical dosage as well as the lowest dosage with definite efficacy, and hence the therapeutic margin of PA-824, both remain to be determined. The good tolerability and efficacy of PA-824 in the dosages used may allow for the selection of a clinical dosage significantly below the highest tolerated dosage while still substantially above the lowest dosage with maximum efficacy, as the latter might well be lower than 200 mg per day—the lowest dose tested in this study.
The present paper is to our knowledge the first to report planned parallel measurements of CFU and TTP in a bactericidal activity study. Whereas TTP measures metabolic activity in liquid medium, CFU counting relies on the visual enumeration of colonies on solid medium and has been the standard in EBA studies over many years. In the present study, a similar impression of drug activity was provided by prolongation of TTP and fall in CFU counts. TTP is a less laborious, potentially more robust measurement using calibrated standardized equipment and fewer steps in preparing sputum for analysis. It has been reported that the yield of growing mycobacteria from sputum is greater when using liquid media (1, 15, 21). It has also been postulated that more actively metabolizing bacteria can grow in both liquid and solid media, whereas more persistent bacilli might be able to grow only in liquid media (3, 18). If so, the bacterial populations growing in liquid medium and on solid medium may not be identical. Due to a lack of significant differences in efficacy between the treatment arms of PA-824, the discriminative power of TTP could not be conclusively evaluated in this study. The significance of changes in TTP in early drug evaluation in comparison to CFU measurements deserves further study.
In conclusion, all dosages of PA-824 evaluated in this study were well tolerated and demonstrated clinically significant extended EBA over 14 days. Since maximum efficacy was achieved at the lowest dosage tested, the extended EBA of lower dosages should be explored.
Supplementary Material
Acknowledgments
This work was supported by the Global Alliance for TB Drug Development, New York, NY.
We thank Ngozi Erondu and Zhenkun Ma for their helpful reading of the manuscript as well as Jane Hutchings and Almarie Uys for their support in organizing the study. We also thank all participating patients, our site staff, the Department of Health, Provincial Administration of the Western Cape, and City Health, City of Cape Town, for permission to recruit patients from their clinics.
Footnotes
Published ahead of print on 24 May 2010.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Badak, F. Z., D. L. Kiska, S. Setterquist, C. Hartley, M. A. O'Connell, and R. L. Hopfer. 1996. Comparison of mycobacteria growth indicator tube with BACTEC 460 for detection and recovery of mycobacteria from clinical specimens. J. Clin. Microbiol. 34:2236-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Botha, F. J., F. A. Sirgel, D. P. Parkin, B. W. van de Wal, P. R. Donald, and D. A. Mitchison. 1996. Early bactericidal activity of ethambutol, pyrazinamide and the fixed combination of isoniazid, rifampicin and pyrazinamide (Rifater) in patients with pulmonary tuberculosis. S. Afr. Med. J. 86:155-158. [PubMed] [Google Scholar]
- 3.Dhillon, J., D. B. Lowrie, and D. A. Mitchison. 2004. Mycobacterium tuberculosis from chronic murine infections that grows in liquid but not on solid medium. BMC Infect. Dis. 4:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diacon, A. H., A. Pym, M. Grobusch, R. Patientia, R. Rustomjee, L. Page-Shipp, C. Pistorius, R. Krause, M. Bogoshi, G. Churchyard, A. Venter, J. Allen, J. C. Palomino, T. De Marez, R. P. van Heeswijk, N. Lounis, P. Meyvisch, J. Verbeeck, W. Parys, K. de Beule, K. Andries, and D. F. McNeeley. 2009. The diarylquinoline TMC207 for multidrug-resistant tuberculosis. N. Engl. J. Med. 360:2397-2405. [DOI] [PubMed] [Google Scholar]
- 5.Donald, P. R., and A. H. Diacon. 2008. The early bactericidal activity of anti-tuberculosis drugs: a literature review. Tuberculosis (Edinb.) 88(Suppl. 1):S75-S83. [DOI] [PubMed] [Google Scholar]
- 6.Donald, P. R., F. A. Sirgel, A. Venter, D. P. Parkin, H. I. Seifart, B. W. van de Wal, J. S. Maritz, and P. B. Fourie. 2003. Early bactericidal activity of antituberculosis agents. Expert Rev. Anti Infect. Ther. 1:141-155. [DOI] [PubMed] [Google Scholar]
- 7.Donald, P. R., and P. D. van Helden. 2009. The global burden of tuberculosis—combating drug resistance in difficult times. N. Engl. J. Med. 360:2393-2395. [DOI] [PubMed] [Google Scholar]
- 8.Ginsberg, A. M., M. W. Laurenzi, D. J. Rouse, K. D. Whitney, and M. K. Spigelman. 2009. Assessment of the effects of the nitroimidazo-oxazine PA-824 on renal function in healthy subjects. Antimicrob. Agents Chemother. 53:3726-3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ginsberg, A. M., M. W. Laurenzi, D. J. Rouse, K. D. Whitney, and M. K. Spigelman. 2009. Safety, tolerability, and pharmacokinetics of PA-824 in healthy subjects. Antimicrob. Agents Chemother. 53:3720-3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu, Y., A. R. Coates, and D. A. Mitchison. 2008. Comparison of the sterilising activities of the nitroimidazopyran PA-824 and moxifloxacin against persisting Mycobacterium tuberculosis. Int. J. Tuberc. Lung Dis. 12:69-73. [PubMed] [Google Scholar]
- 11.International Union Against Tuberculosis and Lung Disease. 2000. Technical guide: sputum examination for tuberculosis by direct microscopy in low income countries, 5th ed. International Union Against Tuberculosis and Lung Disease, Paris, France. http://www.uphs.upenn.edu/bugdrug/antibiotic_manual/IUATLD_afb%20microscopy_guide.pdf.
- 12.Jindani, A., V. R. Aber, E. A. Edwards, and D. A. Mitchison. 1980. The early bactericidal activity of drugs in patients with pulmonary tuberculosis. Am. Rev. Respir. Dis. 121:939-949. [DOI] [PubMed] [Google Scholar]
- 13.Jindani, A., C. J. Dore, and D. A. Mitchison. 2003. Bactericidal and sterilizing activities of antituberculosis drugs during the first 14 days. Am. J. Respir. Crit. Care Med. 167:1348-1354. [DOI] [PubMed] [Google Scholar]
- 14.Kruk, M. E., N. R. Schwalbe, and C. A. Aguiar. 2008. Timing of default from tuberculosis treatment: a systematic review. Trop. Med. Int. Health 13:703-712. [DOI] [PubMed] [Google Scholar]
- 15.Lee, J. J., J. Suo, C. B. Lin, J. D. Wang, T. Y. Lin, and Y. C. Tsai. 2003. Comparative evaluation of the BACTEC MGIT 960 system with solid medium for isolation of mycobacteria. Int. J. Tuberc. Lung Dis. 7:569-574. [PubMed] [Google Scholar]
- 16.Lenaerts, A. J., V. Gruppo, K. S. Marietta, C. M. Johnson, D. K. Driscoll, N. M. Tompkins, J. D. Rose, R. C. Reynolds, and I. M. Orme. 2005. Preclinical testing of the nitroimidazopyran PA-824 for activity against Mycobacterium tuberculosis in a series of in vitro and in vivo models. Antimicrob. Agents Chemother. 49:2294-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maggi, N., C. R. Pasqualucci, R. Ballotta, and P. Sensi. 1966. Rifampicin: a new orally active rifamycin. Chemotherapy 11:285-292. [DOI] [PubMed] [Google Scholar]
- 18.Mitchison, D. A., and A. R. Coates. 2004. Predictive in vitro models of the sterilizing activity of anti-tuberculosis drugs. Curr. Pharm. Des. 10:3285-3295. [DOI] [PubMed] [Google Scholar]
- 19.Nuermberger, E., I. Rosenthal, S. Tyagi, K. N. Williams, D. Almeida, C. A. Peloquin, W. R. Bishai, and J. H. Grosset. 2006. Combination chemotherapy with the nitroimidazopyran PA-824 and first-line drugs in a murine model of tuberculosis. Antimicrob. Agents Chemother. 50:2621-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nuermberger, E., S. Tyagi, R. Tasneen, K. N. Williams, D. Almeida, I. Rosenthal, and J. H. Grosset. 2008. Powerful bactericidal and sterilizing activity of a regimen containing PA-824, moxifloxacin, and pyrazinamide in a murine model of tuberculosis. Antimicrob. Agents Chemother. 52:1522-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pfyffer, G. E., H. M. Welscher, P. Kissling, C. Cieslak, M. J. Casal, J. Gutierrez, and S. Rusch-Gerdes. 1997. Comparison of the Mycobacteria Growth Indicator Tube (MGIT) with radiometric and solid culture for recovery of acid-fast bacilli. J. Clin. Microbiol. 35:364-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pheiffer, C., N. M. Carroll, N. Beyers, P. Donald, K. Duncan, P. Uys, and P. van Helden. 2008. Time to detection of Mycobacterium tuberculosis in BACTEC systems as a viable alternative to colony counting. Int. J. Tuberc. Lung Dis. 12:792-798. [PubMed] [Google Scholar]
- 23.Rustomjee, R., A. H. Diacon, J. Allen, A. Venter, C. Reddy, R. F. Patientia, T. C. Mthiyane, T. De Marez, R. van Heeswijk, R. Kerstens, A. Koul, K. De Beule, P. R. Donald, and D. F. McNeeley. 2008. Early bactericidal activity and pharmacokinetics of the diarylquinoline TMC207 in treatment of pulmonary tuberculosis. Antimicrob. Agents Chemother. 52:2831-2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh, R., U. Manjunatha, H. I. Boshoff, Y. H. Ha, P. Niyomrattanakit, R. Ledwidge, C. S. Dowd, I. Y. Lee, P. Kim, L. Zhang, S. Kang, T. H. Keller, J. Jiricek, and C. E. Barry III. 2008. PA-824 kills nonreplicating Mycobacterium tuberculosis by intracellular NO release. Science 322:1392-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stover, C. K., P. Warrener, D. R. Van Devanter, D. R. Sherman, T. M. Arain, M. H. Langhorne, S. W. Anderson, J. A. Towell, Y. Yuan, D. N. McMurray, B. N. Kreiswirth, C. E. Barry, and W. R. Baker. 2000. A small-molecule nitroimidazopyran drug candidate for the treatment of tuberculosis. Nature 405:962-966. [DOI] [PubMed] [Google Scholar]
- 26.Warren, R. M., N. C. Gey van Pittius, M. Barnard, A. Hesseling, E. Engelke, M. de Kock, M. C. Gutierrez, G. K. Chege, T. C. Victor, E. G. Hoal, and P. D. van Helden. 2006. Differentiation of Mycobacterium tuberculosis complex by PCR amplification of genomic regions of difference. Int. J. Tuberc. Lung Dis. 10:818-822. [PubMed] [Google Scholar]
- 27.World Health Organization. 2003. Treatment of tuberculosis. Guidelines for national programmes, 3rd ed. WHO/CDS/TB/2003.313. World Health Organization, Geneva, Switzerland. http://www.who.int/tb/publications/2008/en/index.html.
- 28.World Health Organization. 2008. WHO-IUTALD Global Project on anti-tuberculosis drug resistance surveillance. Anti-tuberculosis drug resistance in the world. Report no. 4. WHO/HTM/TB/2008.394. World Health Organization, Geneva, Switzerland. http://www.who.int/tb/publications/2008/en/index.html.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.