Abstract
Hybrid antimicrobials containing an antibacterial linked to a multidrug resistance (MDR) pump inhibitor make up a promising new class of agents for countering efflux-mediated bacterial drug resistance. This study explores the effects of varying the relative orientation of the antibacterial and efflux pump inhibitor components in three isomeric hybrids (SS14, SS14-M, and SS14-P) which link the antibacterial alkaloid and known substrate for the NorA MDR pump berberine to different positions on INF55 (5-nitro-2-phenylindole), an inhibitor of NorA. The MICs for all three hybrids against wild-type, NorA-knockout, and NorA-overexpressing Staphylococcus aureus cells were found to be similar (9.4 to 40.2 μM), indicating that these compounds are not effectively effluxed by NorA. The three hybrids were also found to have similar curing effects in a Caenorhabditis elegans live infection model. Each hybrid was shown to accumulate in S. aureus cells to a greater extent than either berberine or berberine in the presence of INF55, and the uptake kinetics of SS14 were found to differ from those of SS14-M and SS14-P. The effects on the uptake and efflux of the NorA substrate ethidium bromide into S. aureus cells in the presence or absence of the hybrids were used to confirm MDR inhibition by the hybrids. MDR-inhibitory activity was confirmed for SS14-M and SS14-P but not for SS14. Molecular dynamics simulations revealed that SS14 prefers to adopt a conformation that is not prevalent in either SS14-M or SS14-P, which may explain why some properties of SS14 diverge from those of its two isomers. In summary, subtle repositioning of the pump-blocking INF55 moiety in berberine-INF55 hybrids was found to have a minimal effect on their antibacterial activities but to significantly alter their effects on MDR pumps.
A key stimulus for current antibacterial research is the emergence of multidrug-resistant (MDR) pathogenic bacteria (10, 27, 30). Clinically relevant bacterial drug resistance is often mediated through MDR efflux pumps in both Gram-positive and Gram-negative bacteria (19, 28). MDR pumps compromise the activity of structurally diverse antibiotics by reducing their intracellular concentrations to subtoxic levels. In response to this, many classes of small-molecule MDR inhibitors are being pursued for use as potentiators in combination with antibiotics (16, 20, 21). A major disadvantage of the combination approach is the challenge of matching the pharmacokinetics and physicochemical properties of two structurally unrelated molecules. One promising alternative strategy is to covalently link the pump inhibitor and the antibacterial agent together into a single noncleavable hybrid molecule (2, 3, 9, 11, 31). Such hybrids carry the potential advantage of simultaneously delivering equimolar quantities of the two agents to bacterial cells (5, 6) and avoiding the complications arising from coadministration.
We previously reported on a prototype hybrid, SS14 (2), containing the antibacterial alkaloid berberine with a substitution at its 13 position consisting of a noncleavable 2′-CH2 linkage to 5-nitro-2-phenyindole (INF55), a known inhibitor of the NorA MDR pump (22). Berberine was chosen for inclusion in the hybrid because it is a NorA substrate (12) and because it is potentially an excellent antimicrobial capable of accumulating in bacterial cells in the absence of MDR activity (33). Further, the antibacterial activity of berberine is thought to arise from its effects on cell membranes and through its interactions with DNA (1). Since these mechanisms are not target specific, it is unlikely that bacterial resistance to berberine could develop through target modification.
In designing SS14, we reasoned that the berberine moiety could elicit its antimicrobial effects while the INF55 component concurrently inhibited MDR pumps to reduce efflux of the hybrid. SS14 was shown to accumulate in the important human pathogen Staphylococcus aureus (2) and to be a more potent antibacterial than either berberine alone (15) or berberine in combination with INF55 (32). In this report, several properties of two new hybrids, SS14-M and SS14-P, the 3′- and 4′-substituted regioisomers of SS14, respectively, are assessed alongside those of SS14 in order to explore the effects of varying the relative orientation of the putative antibacterial and pump-blocking moieties in these compounds.
MATERIALS AND METHODS
Chemistry.
The synthesis of SS14 has been described previously (2). The synthesis of SS14-M and SS14-P is to be reported elsewhere (7).
Bacterial strains.
The following bacterial strains were used: S. aureus 8325-4 (wild-type), K1758 (ΔnorA) (29), K2378 (ΔnorA pK374::norA; pK374::norA is a plasmid that results in overexpression of norA from S. aureus SA1199) (17), Enterococcus faecalis MMH594 (14), and Escherichia coli HB101.
In vitro antibacterial activity.
Cells (105 ml−1) were inoculated into broth and dispensed at 50 μl well−1 in 384-well microtiter plates. MICs were determined in triplicate by serial 2-fold dilution of the test compounds. The MIC was defined as the concentration of the agent that completely inhibited cell growth during 18 h of incubation at 37°C. Growth was assayed with a microtiter plate reader (Spectramax PLUS384; Molecular Devices) by monitoring the absorption at 600 nm.
Caenorhabditis elegans live infection model.
The nematode assay was performed essentially as described previously (2) with some modifications. Eggs from gravid adult glp-4(bn2) sek-1(km4) worms (4, 35) were isolated using the hypochlorite method and hatched at 15°C for 2 days. The resulting L1-stage worms (n = 4,000) were grown on 90-mm plates of SK-NS agar (modified enhanced NGM [0.35% {wt/vol} peptone, 0.3% {wt/vol} Nacl, 5 μg/ml cholesterol, 1 mM CaCl2, 1mM MgSO4, 25 mM KH2PO4, 2% agar] agar medium with 62.5 U/ml nystatin and 100 μg/ml streptomycin) containing lawns of E. coli HB101. The worms were grown at 25°C for 52 h to produce sterile, young adults. A culture of E. faecalis MMH594 was grown in brain heart infusion (BHI) broth (BD Difco) with 80 μg/ml kanamycin at 37°C for 6 h, and 100 μl of the culture was spread onto each 90-mm plate of BHI agar with 80 μg/ml kanamycin. Lawns of E. faecalis were grown at 25°C for 16 h and transferred to 15°C for 8 h. The sterile adult worms were resuspended in M9 buffer and washed twice with M9 buffer in a 1:10 ratio to remove the E. coli cells. Approximately 8,000 worms were pipetted onto each lawn of E. faecalis and incubated for 15 h at 15°C. Upon infection, the worms were resuspended in M9 buffer and used as described below. Plates (384 wells; Corning 3712) were filled with 55 μl medium. Using the Union Biometrica Copas worm sorter according to the manufacturer's protocols, 15 of the infected worms were transferred to each well in a total volume of approximately 15 μl. The total volume per well was 70 μl with (final concentrations) 20% BHI, 60% M9 buffer, 80 μg/ml kanamycin, 62.5 U/ml nystatin, 1% dimethyl sulfoxide (DMSO), and 19% sheath solution (Union Biometrica). The plates were vortexed for 5 s, centrifuged at 1,000 × g for 10 s, and sealed with gas-permeable membranes (BreathEasy; Diversified). The plates were placed in a single layer on the top shelf of a 26.3°C incubator and incubated without agitation for 5 days at 85% relative humidity. Using a Molecular Devices Discovery-1 microscope with a ×2 objective, bright-field transmitted light images showing the entire well were captured as 16-bit TIFF images. These images were converted into 8-bit JPEG images using the CellProfiler program (www.cellprofiler.org), and the resulting images were scored manually for worm survival by analyzing worm body positioning. Eight wells prepared with four independent compound dilutions were analyzed for each treatment.
Bacterial cell uptake experiments.
Bacterial cell uptake experiments were performed essentially as described previously (31) with some minor modifications. Briefly, S aureus cultures were grown at 37°C until the optical density (OD; at 600 nm) reached 1.5. The cells were pelleted by centrifugation, washed twice with phosphate-buffered saline (PBS), and resuspended in PBS containing 10 mM dextrose to obtain an OD of ∼0.8. The cells were then incubated for 1 h at 37°C (with aeration) before they were washed twice with PBS (containing 10 mM dextrose) and further diluted to an OD of ∼0.3 in PBS. The assay was performed in 96-well flat-bottom white microtiter plates in a final volume of 200 μl. Compounds were added at a concentration of 3 μM each. For the ethidium bromide uptake experiments, the hybrid compounds or INF55 was added first. Fluorescence was measured using a SpectraMax Gemini XS fluorimeter (Molecular Devices). Uptake experiments with berberine, INF55, and the SS14 hybrids were performed at excitation and emission wavelengths of 355 and 517 nm, respectively. Experiments with ethidium bromide were performed at least in triplicate at excitation and emission wavelengths of 530 and 600 nm, respectively. The background fluorescence for all compounds in the absence of cells was subtracted from the raw data.
Ethidium bromide efflux experiments.
The efflux assay was performed as described previously with some modifications (31). NorA-overexpressing (K2378 NorA++) S. aureus cells were grown at 37°C to an OD of ∼0.9, pelleted, washed twice with PBS, and then resuspended in PBS to an OD of ∼0.8. The cells were then loaded with 3 μM ethidium bromide and 30 μg/ml of reserpine and incubated at 37°C for 20 min. After the cells were washed twice with ice-cold PBS, they were added to a chilled 96-well flat-bottom black microtiter plate containing ice-cold PBS-10 mM dextrose at an OD of 0.3 in a total volume of 200 μl. Hybrids were added before the cells to give final concentrations of 3 μM. As a negative control (i.e., no ethidium efflux), PBS (without dextrose) containing 30 μg/ml of reserpine, a known efflux pump inhibitor (26), was added instead of the SS14 hybrids. Fluorescence was measured with a SpectraMax Gemini XS apparatus at excitation and emission wavelengths of 530 and 600 nm, respectively.
Molecular modeling.
Hybrids were constructed using the DS Modeling 1.6 program (Accelrys, San Diego, CA, 2006), and atom types and partial charges were assigned using the Accelrys CHARMm force field (23). The berberine quaternary nitrogen of each hybrid was assigned a formal +1 charge. A simulated annealing protocol was ported from the superseded program Insight II (version 97.0; MSI Inc. [now Accelrys], San Diego, CA, 1997) into the DS Modeling 1.6 program and subjected to an in-house rewrite of the annealing scripts for use with CHARMm. Each hybrid was subjected to stepwise minimization and relaxation using the CHARMm molecular dynamics module of the DS Modeling 1.6 program (version 29b1) program (8). The in vacuo simulation used default nonbonding parameters (cutoff, 13.50; cutoff on, 8.00; cutoff off, 12.00). The structures were minimized using steepest descents for 3,000 steps (gradient tolerance, 0.1) with all nonhydrogens explicitly fixed and then minimized with the adopted-basis-set Newton-Raphson (ABNR) algorithm for 30,000 steps (gradient tolerance, 0.01) with no constraints. The system was annealed for 5,000 cycles by heating to 800 K in 20 K increments (2,000 steps), cooled to 0 K in 10 K increments (4,000 steps), and then maintained at this temperature for 6,000 steps.
RESULTS AND DISCUSSION
In vitro antibacterial activity.
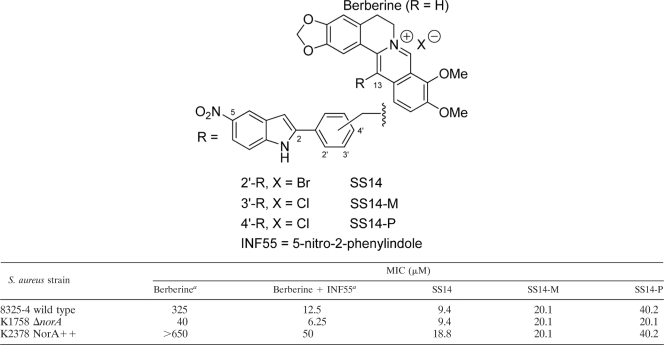
Berberine is a mild antibacterial that is effluxed from cells by MDR pumps, including NorA. Deletion of norA renders bacterial cells more sensitive to berberine, and overexpression of NorA increases bacterial resistance to the drug. Coadministration of the NorA inhibitor INF55 potentiates the antibacterial activity of berberine. Conjugating berberine to INF55 in the form of hybrid SS14 creates an antimicrobial more potent (2) than the combination of the two agents, as does conjugation in the form of a related hybrid bearing a methylene ether (—CH2O—) linkage (31). We previously characterized these effects in wild-type (8325-4), NorA-knockout (K1758 ΔnorA), and NorA-overexpressing (K2378 NorA++) S. aureus cells (2, 31); and a selection of these data are included in Table 1 to facilitate comparison with the MICs of SS14-M and SS14-P. It is of note that the MIC for berberine against the NorA-knockout strain decreases from 40 μM when it is administered alone to 6.25 μM when it is administered with INF55. This NorA-independent effect of INF55 suggests that INF55 inhibits berberine-effluxing MDR pumps, in addition to NorA.
TABLE 1.
MICs for SS14, SS14-M, and SS14-P against three S. aureus strainsa
Values were retrieved from a previous report (2). INF55 at 525 μM shows no activity against these strains.
SS14, SS14-M, and SS14-P were found to have similar activities against the three S. aureus strains (Table 1), although SS14 showed a slightly higher potency than its isomers against the wild-type and NorA-knockout strains. In contrast to the MICs for berberine and berberine in the presence of INF55, the MICs for the individual hybrids were found to be independent of NorA activity and remained essentially unchanged (<2-fold difference) for all three S. aureus strains. We conclude that SS14, SS14-M, and SS14-P are comparable antibacterials that are not efficiently effluxed by NorA.
Antibacterial activity in a live infection model.
A simple live infection model uses the nematode Caenorhabditis elegans as a host for the bacterial pathogen Enterococcus faecalis (2, 24). In this model, the gastrointestinal tract of nematodes is infected by feeding them on lawns of E. faecalis, which leads to their death via a multifactorial process over the course of 5 days. We previously showed that infected nematodes can be cured with antibiotics that are active against E. faecalis (e.g., vancomycin) and with SS14 (2). The worm-curing effects of SS14 have been studied here in parallel with those of SS14-M and SS14-P in order to compare their activities in this live infection model.
Figure 1 shows that mock (DMSO)-treated worms display a median survival time (time to 50% lethality [LT50]) of just under 48 h. Treatment with all three hybrids produced a significant curative effect (P < 0.0001 for treatments compared to mock treatment), extending the LT50 values out beyond 110 h. No significant difference between the effects of the three hybrids on worm survival was observed.
FIG. 1.
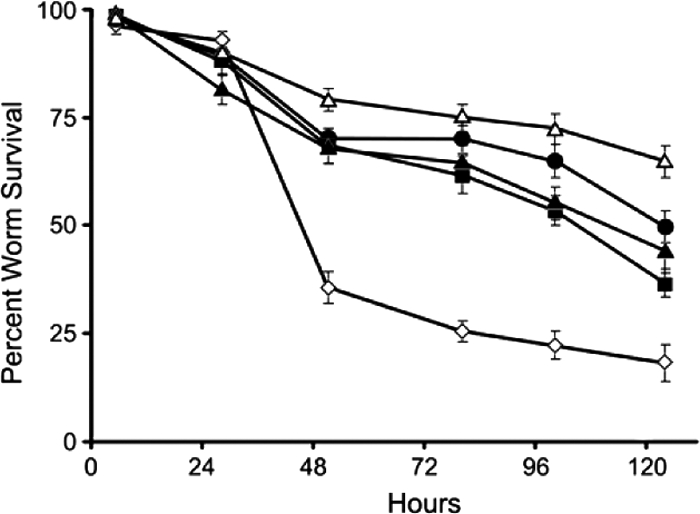
Effects of hybrids SS14 (▴), SS14-M (▪), and SS14-P (•) (at 20 μM) and vancomycin (Δ) (at 8.6 μM) on the survival of C. elegans infected with E. faecalis. ⋄, mock (DMSO) treatment.
Uptake of hybrids in S. aureus cells.
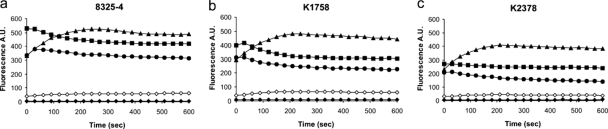
Berberine uptake into microbial cells can be monitored in real time by measuring the fluorescence increases that occur as it binds to cellular DNA (34). This method has been used to show that berberine on its own does not accumulate in S. aureus cells and that its uptake increases in the presence of INF55 (2). Figure 2 shows the data for the uptake of SS14 (31), SS14-M, and SS14-P into wild-type, NorA-knockout, and NorA-overexpressing S. aureus cells. Also shown for comparison are uptake data for berberine and berberine in the presence of INF55.
FIG. 2.
Uptake of berberine (⧫), berberine in the presence of INF55 (⋄), hybrid SS14 (▴), hybrid SS14-M (▪), and hybrid SS14-P (•) into S. aureus cells: (a) wild-type 8325-4 S. aureus cells; (b) NorA-knockout (K1758 ΔnorA) S. aureus cells; (c) NorA-overexpressing (K2378 NorA++) S. aureus cells. Uptake was measured by monitoring the increases in fluorescence at 517 nm (excitation at 355 nm) and is expressed as arbitrary units (A.U.). All compounds were present at a concentration of 3 μM. The curves are representative of at least three independent experiments.
Uptake of the hybrids into all three S. aureus strains was found to be substantially greater than that of either berberine alone or berberine in the presence of INF55. Additionally, uptake of the individual hybrids varied little between wild-type, NorA-knockout, and NorA-overexpressing S. aureus strains, providing further evidence that the hybrids are not efficiently effluxed by NorA. Curiously, the uptake kinetics for SS14 were clearly distinct from those of SS14-M and SS14-P. While uptake of SS14 showed a lag period before it reached and maintained a maximum value, uptake of SS14-M and SS14-P reached their maxima at the start of the experiments, before they declined slightly and eventually leveled off.
Inhibition of MDR pumps by hybrids.
Intuitively, accumulation of hybrids in bacterial cells must arise either because they inhibit the function of efflux pumps or because they are not pump substrates. Differentiating these two effects is theoretically possible by comparing the uptake of berberine into S. aureus cells in the presence or absence of hybrids. If hybrids inhibit pumps, then berberine uptake should increase in their presence. If hybrids are not pump substrates, then there should be no increased berberine uptake in their presence. Previously reported fluorescence-based methods for monitoring berberine uptake into cells (34) cannot be used in these experiments, however, due to the confounding effects of the fluorescence arising from the hybrids interacting with cellular DNA. This problem can be circumvented by using ethidium bromide in place of berberine. Like berberine, ethidium bromide is a hydrophobic cation and a known substrate for MDR pumps, including NorA, NorC, MdeA, SdrM, MepA, and SepA (12, 13, 17, 18, 25, 36, 37). The fluorescence increases observed when berberine interacts with DNA are typically measured at an emission wavelength of 517 nm, after excitation at 355 nm. When ethidium bromide interacts with DNA, increases in fluorescence are measured at the emission wavelength of 600 nm, after excitation at 530 nm. Fluorescence increases at 600 nm can thus be used to monitor the uptake of ethidium bromide into cells in the presence of the berberine hybrids without the confounding effects of hybrid/DNA fluorescence. In this system, increases in ethidium bromide uptake in the presence of hybrids (observed as increases in fluorescence at 600 nm) would confirm that hybrids are MDR inhibitors and do not simply evade the pumps.
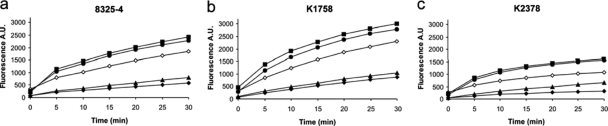
Figure 3 shows that the presence of SS14-M and SS14-P produced substantial increases in ethidium bromide uptake in wild-type, NorA-knockout, and NorA-overexpressing S. aureus cells. The fluorescence increases observed in the presence of these two hybrids were greater in all three S. aureus strains than the increases observed with ethidium bromide alone or ethidium bromide in the presence of INF55. Surprisingly, the fluorescence increases observed with SS14 were only slightly greater than those observed with ethidium bromide alone and lower than those produced by ethidium bromide in the presence of INF55. These results demonstrate that SS14-M and SS14-P inhibit the set of MDR pumps responsible for ethidium bromide efflux in S. aureus, cells while SS14 has only minimal effects on these pumps.
FIG. 3.
Uptake of ethidium bromide (⧫), ethidium bromide in the presence of INF55 (⋄), and ethidium bromide in the presence of hybrids SS14 (▴) (31), SS14-M (▪), and SS14-P (•) into S. aureus cells: (a) wild-type 8325-4 S. aureus cells, (b) NorA-knockout (K1758 ΔnorA) S. aureus cells; (c) NorA overexpressing (K2378 NorA++) S. aureus cells. Uptake was measured by monitoring the increases in fluorescence at 600 nm (excitation at 530 nm) and is expressed as arbitrary units (A.U.). All compounds were present at a concentration of 3 μM. Curves are representative of at least three independent experiments.
Corroborating evidence that hybrids inhibit ethidium bromide-effluxing MDR pumps was sought by monitoring the decreases in fluorescence observed over time as ethidium bromide is effluxed from ethidium bromide-preloaded NorA++ S. aureus cells. Inhibition of MDR pumps by hybrids in this system is evidenced by reduced losses of fluorescence at 600 nm relative to the loss when hybrids are absent. Figure 4 shows that the presence of SS14-M and SS14-P significantly reduced ethidium bromide efflux. INF55 caused a lesser reduction in ethidium bromide efflux than SS14-M and SS14-P, while only a weak effect was observed with SS14. These results correlate with those of the uptake experiments described above and together confirm that SS14-M and SS14-P are MDR inhibitors and true dual-action antibacterials, whereas SS14 has only minor inhibitory effects on MDR pumps.
FIG. 4.
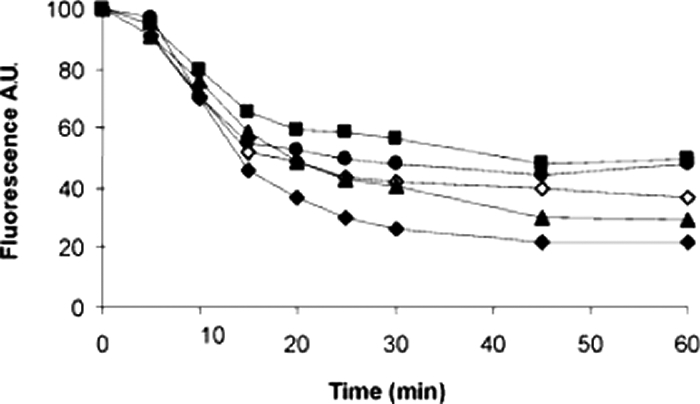
Efflux from NorA-overexpressing (K2378 NorA++) S. aureus cells of ethidium bromide (⧫); ethidium bromide in the presence of INF55 (⋄); and ethidium bromide in the presence of hybrids SS14 (▴) (31), SS14-M (▪), and SS14-P (•). Cells were initially preloaded with ethidium bromide in the presence of the MDR inhibitor reserpine (26) to prevent the loss of ethidium bromide. Hybrids or INF55 in dextrose buffer was added to the cells after they had been washed to remove the reserpine. Efflux was measured by monitoring the decreases in fluorescence at 600 nm (excitation at 530 nm) and is expressed as arbitrary units (A.U.). All compounds were present at a final concentration of 3 μM. The graph is representative of at least three independent experiments. Berberine alone had no effect on ethidium bromide efflux (data not shown).
Conformational analysis of SS14, SS14-M, and SS14-P by molecular dynamics/simulated annealing.
The studies described above demonstrate that SS14, SS14-M, and SS14-P each display very similar antibacterial activity against S. aureus cells and in a C. elegans live infection model. Uptake of SS14 into S. aureus cells and its effects on ethidium bromide uptake and efflux were, however, clearly distinct from those of SS14-M and SS14-P. It was considered that the divergent properties of SS14 might arise from a conformational preference in this hybrid that was not prevalent in its isomers. Molecular dynamics/simulated annealing experiments were thus performed to identify and compare the low-energy conformations of the three isomers.
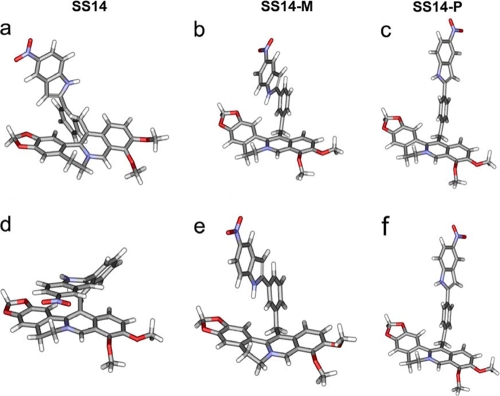
The preferred conformations identified for each isomer fell into two distinct groups: (i) an ensemble of lowest-energy conformations comprising ∼2 to 5% of the total structures and (ii) an ensemble of equilibrium conformations comprising ∼90% of the total structures. The lowest-energy ensemble for SS14 positioned the indole plane at 90° to the plane of the benzene ring of the INF271 moiety, which in turn was oriented orthogonally to the plane of the berberine component (Fig. 5a). Importantly, the majority of the equilibrium conformations of SS14 positioned the planar indole group of the INF55 moiety directly over the positively charged quaternary nitrogen of berberine, apparently being stabilized by a π-stacking or cation-π interaction (Fig. 5d).
FIG. 5.
Representative structures from the lowest-energy conformational ensembles of SS14 (a), SS14-M (b), and SS14-P (c) and the equilibrium conformational ensembles of SS14 (d), SS14-M (e), and SS14-P (f). Structures were calculated using a molecular dynamics/simulated annealing protocol in DS Modeling 1.6 (Accelrys).
The lowest-energy ensemble for SS14-M placed the indole and benzylic groups only slightly out of plane, with this plane being oriented at ∼90° to the berberine component and extended away from it (Fig. 5b). The majority of the equilibrium conformations of SS14-M (Fig. 5e) were very similar to its lowest-energy conformations, differing only in the puckering of the nonaromatic berberine six-membered ring. The lowest-energy (Fig. 5c) and equilibrium conformational (Fig. 5f) ensembles for SS14-P were essentially identical and analogous to the lowest-energy and equilibrium conformations of SS14-M, wherein the INF55 moieties extended away from the berberine unit.
These modeling results indicate that SS14 prefers to adopt a more compact globular conformation that differs markedly from the more extended conformations found in SS14-M and SS14-P. The unique conformation for SS14 identified here may explain why it shows different bacterial cell uptake kinetics and reduced inhibitory effects on MDR pumps relative to those of SS14-M and SS14-P.
In summary, SS14, SS14-M, and SS14-P were shown to be useful probes for exploring the effects of varying the relative positioning of the putative antibacterial and pump-blocking moieties in berberine-INF55 hybrids. The hybrids each showed similar MICs against three S. aureus strains expressing various levels of NorA and identical activities in a live-animal infection model. We conclude that all three compounds are antibacterial agents and are not efficiently effluxed by NorA. All the hybrids accumulated appreciably in S. aureus cells, although SS14 displayed uptake kinetics distinct from those of its two isomers. SS14 also showed only minor effects on the uptake and efflux of ethidium bromide in S. aureus cells, whereas both SS14-M and SS14-P strongly potentiated ethidium bromide uptake and reduced its efflux. We conclude that all three hybrids accumulate in bacterial cells because they are poorly effluxed by MDR pumps but only SS14-M and SS14-P (and not SS14) also function to inhibit MDR pumps and are thus true dual-action antibacterials. Conformational searching using molecular dynamics/simulated annealing showed that SS14-M and SS14-P adopt conformations where the INF55 moiety extends away from the berberine unit, whereas the INF55 moiety in SS14 prefers to fold back over berberine to produce a more compact structure. The different presentation of the INF55 moiety in SS14 is proposed as an explanation for why its properties diverge from those of its isomers. Overall, this study demonstrates that the structural and conformational variations arising from subtle repositioning of the pump-blocking INF55 moiety in berberine-INF55 hybrids have minimal effects on their antibacterial activities but can significantly alter their effects on MDR pumps.
Acknowledgments
We thank the University of Wollongong, NSW, Australia; Harvard Medical School; and Northeastern University, Boston, MA, for supporting this work. The award of an NHMRC C. J. Martin fellowship (fellowship 252922 to Michael J. Kelso) and an American Cancer Society postdoctoral fellowship (fellowship PF-02-130-01-MBC to Terence I. Moy) are gratefully acknowledged. The work was supported by grants R21 AI059483, R01 AI072508, and R01 AI076372 from the NIH and a grant from the Broad Institute of Harvard and MIT, awarded to Frederick M. Ausubel.
A. Conery is acknowledged for helpful discussions and W. K. Smits is acknowledged for critical reading of the manuscript. We kindly thank K. Gornall for providing a sample of SS14 and Glenn Kaatz for providing S. aureus strains.
Footnotes
Published ahead of print on 24 May 2010.
REFERENCES
- 1.Amin, A. H., T. V. Subbaiah, and K. M. Abbasi. 1969. Berberine sulfate: antimicrobial activity, bioassay and mode of action. Can. J. Microbiol. 15:1067-1076. [DOI] [PubMed] [Google Scholar]
- 2.Ball, A. R., G. Casadei, S. Samosorn, J. B. Bremner, F. M. Ausubel, T. I. Moy, and K. Lewis. 2006. Conjugating berberine to a multidrug resistance pump inhibitor creates an effective antimicrobial. ACS Chem. Biol. 1:594-600. [DOI] [PubMed] [Google Scholar]
- 3.Barbachyn, M. R. 2008. Recent advances in the discovery of hybrid antibacterial agents. Annu. Rep. Med. Chem. 43:281-290. [Google Scholar]
- 4.Beanan, M. J., and S. Strome. 1992. Characterization of a germ-line proliferation mutation in C. elegans. Development 116:755-766. [DOI] [PubMed] [Google Scholar]
- 5.Bremner, J. B. 2007. Some approaches to new antibacterial agents. Pure Appl. Chem. 79:2143-2153. [Google Scholar]
- 6.Bremner, J. B., J. I. Ambrus, and S. Samosorn. 2007. Dual action-based approaches to antibacterial agents. Curr. Med. Chem. 14:1459-1477. [DOI] [PubMed] [Google Scholar]
- 7.Bremner, J. B., and M. J. Kelso. Synthesis of berberine-efflux pump inhibitor hybrid antibacterials. Synth. Commun., in press. [DOI] [PMC free article] [PubMed]
- 8.Brooks, B. R., R. E. Bruccoleri, B. D. Olafson, D. J. States, S. Swaminathan, and M. Karplus. 1983. CHARMM: a program for macromolecular energy minimization and dynamics calculations. J. Comput. Chem. 4:187-217. [Google Scholar]
- 9.Charifson, P. S., A.-L. Grillot, T. H. Grossman, J. D. Parsons, M. Badia, S. Bellon, D. D. Deininger, J. E. Drumm, C. H. Gross, A. LeTiran, Y. Liao, N. Mani, D. P. Nicolau, E. Perola, S. Ronkin, D. Shannon, L. L. Swenson, Q. Tang, P. R. Tessier, S.-K. Tian, M. Trudeau, T. Wang, Y. Wei, H. Zhang, and D. Stamos. 2008. Novel dual-targeting benzimidazole urea inhibitors of DNA gyrase and topoisomerase IV possessing potent antibacterial activity: intelligent design and evolution through the judicious use of structure guided design and structure-activity relationships. J. Med. Chem. 51:5243-5263. [DOI] [PubMed] [Google Scholar]
- 10.Davies, J. 2007. Microbes have the last word. EMBO Rep. 8:616-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.German, N., P. Wei, G. W. Kaatz, and R. J. Kerns. 2008. Synthesis and evaluation of fluoroquinolone derivatives as substrate based inhibitors of bacterial efflux pumps. Eur. J. Med. Chem. 43:2453-2463. [DOI] [PubMed] [Google Scholar]
- 12.Hseih, P. C., S. A. Siegel, B. Rogers, S. Davis, and K. Lewis. 1998. Bacteria lacking a multidrug pump: a sensitive tool for drug discovery. Proc. Natl. Acad. Sci. U. S. A. 95:6602-6606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang, J. Z., P. W. O'Toole, W. Shen, H. Amrine-Madsen, X. H. Jiang, N. Lobo, L. A. Palmer, L. Voelker, F. Fan, M. N. Gwynn, and D. McDevitt. 2004. Novel chromosomally encoded multidrug efflux transporter MdeA in Staphylococcus aureus. Antimicrob. Agents Chemother. 48:909-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huycke, M. M., C. A. Spiegel, and M. S. Gilmore. 1991. Bacteremia caused by hemolytic high-level gentamycin-resistant Enterococcus faecalis. Antimicrob. Agents Chemother. 35:1626-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwasa, K., M. Kamigauchi, M. Ueki, and M. Taniguchi. 1996. Antibacterial activity and structure-activity relationships of berberine analogs. Eur. J. Med. Chem. 31:469-478. [Google Scholar]
- 16.Kaatz, G. W. 2006. Bacterial efflux pump inhibition. Curr. Opin. Invest. Drugs 6:191-198. [PubMed] [Google Scholar]
- 17.Kaatz, G. W., and S. M. Seo. 1997. Mechanisms of fluoroquinolone resistance in genetically related strains of Staphylococcus aureus. Antimicrob. Agents Chemother. 41:2733-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaatz, G. W., F. McAleese, and S. M. Seo. 2005. Multidrug resistance in Staphylococcus aureus due to overexpression of a novel multidrug and toxin extrusion (MATE) transport protein. Antimicrob. Agents Chemother. 49:1857-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li, X.-Z., and H. Nikaido. 2004. Efflux-mediated drug resistance. Drugs 64:159-204. [DOI] [PubMed] [Google Scholar]
- 20.Lomovskaya, O., and K. A. Bostian. 2006. Practical applications and feasibility of efflux pump inhibitors in the clinic—a vision for applied use. Biochem. Pharmacol. 71:910-918. [DOI] [PubMed] [Google Scholar]
- 21.Lomovskaya, O., H. I. Zgurskaya, K. A. Bostian, and K. Lewis. 2008. Multidrug efflux pumps: structure, mechanism, and inhibition, p. 45-69. In R. G. Wax, K. Lewis, A. A. Salyers and H. Taber (ed.), Bacterial resistance to antimicrobials, 2nd ed. CRC Press-Taylor and Francis Group, Boca Raton, FL.
- 22.Markham, P. N., E. Westhaus, K. Klyachko, M. E. Johnson, and A. A. Neyfakh. 1999. Multiple novel inhibitors of the NorA multidrug transporter of Staphylococcus aureus. Antimicrob. Agents Chemother. 43:2404-2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Momany, F. A., and R. Rone. 1992. Validation of the general purpose QUANTA 3.2/CHARMm forcefield. J. Comput. Chem. 13:888-900. [Google Scholar]
- 24.Moy, T. I., A. R. Ball, Z. Anklesaria, G. Casadei, K. Lewis, and F. M. Ausubel. 2006. Identification of novel antimicrobials using a live animal infection model. Proc. Natl. Acad. Sci. U. S. A. 103:10414-10419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Narui, K., N. Noguchi, K. Wakasugi, and M. Sasatsu. 2002. Cloning and characterization of a novel chromosomal drug efflux gene in Staphylococcus aureus. Biol. Pharm. Bull. 25:1533-1536. [DOI] [PubMed] [Google Scholar]
- 26.Neyfakh, A. A., C. M. Borsch, and G. W. Kaatz. 1993. Fluoroquinolone resistance protein NorA of Staphylococcus aureus is a multidrug efflux transporter. Antimicrob. Agents Chemother. 37:128-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nordmann, P., T. Naas, N. Fortineau, and L. Poirel. 2007. Superbugs in the coming decade; multidrug resistance and prospects for treatment of Staphylococcus aureus, Enterococcus spp. and Pseudomonas aeruginosa I 2010. Curr. Opin. Microbiol. 10:436-440. [DOI] [PubMed] [Google Scholar]
- 28.Poole, K. 2007. Efflux pumps as antimicrobial resistance mechanisms. Ann. Med. 39:162-176. [DOI] [PubMed] [Google Scholar]
- 29.Price, C. T. D., G. W. Kaatz, and J. E. Gustafson. 2002. The multidrug efflux pump NorA is not required for salicylate-induced reduction in drug accumulation by Staphylococcus aureus. Int. J. Antimicrob. Agents 20:206-213. [DOI] [PubMed] [Google Scholar]
- 30.Rice, L. B. 2006. Unmet medical needs in antibacterial therapy. Biochem. Pharmacol. 71:991-995. [DOI] [PubMed] [Google Scholar]
- 31.Samosorn, S., B. Tanwirat, N. Muhamad, G. Casadei, D. Tomkiewicz, K. Lewis, A. Suksamrarn, T. Prammananan, K. C. Gornall, J. L. Beck, and J. B. Bremner. 2009. Antibacterial activity of berberine-NorA pump inhibitor hybrids with a methylene ether linking group. Bioorg. Med. Chem. 17:3866-3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Samosorn, S., J. B. Bremner, A. Ball, and K. Lewis. 2006. Synthesis of functionalized 2-aryl 5-nitro-1H-indoles and their activity as bacterial NorA effux pump inhibitors. Bioorg. Med. Chem. 14:857-865. [DOI] [PubMed] [Google Scholar]
- 33.Severina, I. I., M. S. Muntyan, K. Lewis, and V. P. Skulachev. 2001. Transfer of cationic antibacterial agents berberine, palmatine and benzalkonium through bimolecular planar phospholipid film and Staphylococcus aureus membrane. IUBMB Life 52:321-324. [DOI] [PubMed] [Google Scholar]
- 34.Stermitz, F. R., P. Lorenz, J. N. Tawara, L. Zenewicz, and K. Lewis. 2000. Synergy in a medicinal plant: antimicrobial action of berberine potentiated by 5′-methoxyhydnocarpin, a multidrug pump inhibitor. Proc. Natl. Acad. Sci. U. S. A. 97:1433-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanaka-Hino, M., A. Sagasti, N. Hisamoto, M. Kawasaki, S. Nakano, J. Ninomiya-Tsuji, C. I. Bargmann, and K. Matsumoto. 2002. SEK-1 MAPKK mediates Ca2+ signaling to determine neuronal asymmetric development in Caenorhabditis elegans. EMBO Rep. 3:56-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Truong-Bolduc, Q. C., J. Strahilevitz, and D. C. Hooper. 2006. NorC, a new efflux pump regulated by MgrA of Staphylococcus aureus. Antimicrob. Agents Chemother. 50:1104-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamada, Y., K. Hideka, S. Shiota, T. Kuroda, and T. Tsuchya. 2006. Gene cloning and characterization of SdrM, a chromosomally-encoded multidrug efflux pump from Staphylococcus aureus. Biol. Pharm. Bull. 29:554-556. [DOI] [PubMed] [Google Scholar]