Abstract
In vivo development of daptomycin resistance (DAPr) among Staphylococcus aureus strains, especially methicillin-resistant S. aureus (MRSA) strains, in conjunction with clinical treatment failures, has emerged as a major problem. This has raised the question of DAP-based combination regimens to enhance efficacy against such strains. We studied five recent DAP-susceptible (DAPs)/DAPr clinical MRSA strain pairs obtained from patients who failed DAP monotherapy regimens, as well as one DAPs/DAPr MRSA strain pair in which the resistant strain was generated by in vitro passage in DAP. Of note, we identified a DAP-oxacillin (OX) “seesaw” phenomenon in vitro in which development of DAPr was accompanied by a concomitant fall in OX resistance, as demonstrated by 3- to 4-fold decreases in the OX MIC, a susceptibility shift by population analyses, and enhanced early killing by OX in time-kill assays. In addition, the combination of DAP and OX exerted modest improvement in in vitro bactericidal effects. Using an experimental model of infective endocarditis and two DAPs/DAPr strain pairs, we demonstrated that (i) OX monotherapy was ineffective at clearing DAPr strains from any target tissue in this model (heart valve, kidneys, or spleen) and (ii) DAP-OX combination therapy was highly effective in DAPr strain clearances from these organs. The mechanism(s) of the seesaw effect remains to be defined but does not appear to involve excision of the staphylococcal cassette chromosome mec (SCCmec) that carries mecA.
Daptomycin (DAP) is a cyclic lipopeptide antibiotic active against a wide range of Gram-positive organisms, including methicillin-resistant Staphylococcus aureus (MRSA), vancomycin (VAN)-intermediate S. aureus (VISA), and vancomycin-resistant S. aureus (VRSA) strains (39, 54). However, an increasing number of reports has described the in vivo loss of DAP susceptibility in association with DAP clinical treatment failures in S. aureus infections (2, 17, 47). Clearly, innovative approaches to this problem need to be expeditiously applied to enhance/retain DAP efficacy, including dose escalations and combination therapy strategies.
It has been previously reported that in some clinical VISA and VRSA strains, as well as in vitro-selected VISA strains, as the VAN MIC was observed to rise, the concomitant MIC to semisynthetic antistaphylococcal penicillins (e.g., oxacillin [OX] or nafcillin) progressively fell. This phenomenon was termed the “seesaw effect” (42-44). This loss of methicillin resistance has most often been due to excision of the staphylococcal cassette chromosome mec element (SCCmec) that carries mecA, the gene encoding penicillin-binding protein 2a (PBP2a), which is responsible for the MRSA phenotype (11, 12, 37). Although uncommon in vivo (3), the seesaw effect was recently observed in a clinical VISA isolate with a retained mecA gene obtained during VAN therapy; of interest, after discontinuation of VAN therapy, the methicillin resistance phenotype was restored (32). Importantly, the combination of VAN and nafcillin has been shown to exert synergistic killing activities in vitro and in an experimental model of infective endocarditis (IE) (8) with both VISA and VRSA strains (14).
We have found a similar seesaw relationship in S. aureus strains that progressively acquire DAPr during DAP exposure, and their respective OX MICs declined in parallel (29). In the current study, we investigated (i) the extent of this seesaw effect in vitro by utilizing five clinical DAPs/DAPr strain pairs and (ii) whether combination regimens of DAP and OX would enhance both the in vitro and in vivo efficacies of these single agents against DAPr strains.
Note that although the currently accepted term for reduced in vitro susceptibility to daptomycin is “nonsusceptible,” we use the terms “daptomycin resistant” (DAPr) and “daptomycin susceptible” (DAPs) in this paper for a more facile presentation.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains used in this study are listed in Table 1. A series of isogenic DAPs/DAPr MRSA strain pairs were used in this study, and represent (i) recent clinical isolates, (ii) in vitro-selected DAPr strain sets, and (iii) a range of the most common pulsotypes causing clinical infections in the United States (USA100, USA300, USA400, and USA500). Two of the clinically derived strain pairs and the one in vitro-derived strain pair have been previously reported (15, 29, 31). The remaining three clinically derived strain pairs were randomly selected from the Cubist Pharmaceuticals Registry collection of such bloodstream isolates (courtesy of Aileen Rubio, Cubist Pharmaceuticals). Molecular typing by pulsed-field gel electrophoresis using the SmaI restriction enzyme (data not shown) and SCCmec typing (Table 1) confirmed that each DAPr strain was clonal to the respective parental isolate.
TABLE 1.
Strains used in this study
| Strain (pulsotype)a | SCCmec type | Description | MIC (μg/ml) |
Reference(s) | |
|---|---|---|---|---|---|
| DAP | OX | ||||
| CB1118 (USA400) | IV | Selected in vitro by serial passage in DAP | 0.5 | 24 | 15, 29 |
| CB2205 (USA400) | IV | Selected in vitro by serial passage in DAP | 8 | 6 | |
| MRSA 11/11 (USA300)* | IV | Clinical endocarditis isolate | 0.38 | 32 | 31 |
| REF2145 (USA300)* | IV | Clinical endocarditis isolate | 4 | 6 | |
| CB1482 (USA500) | IV | Clinical bloodstream isolate | 0.5 | 64 | 15 |
| CB184 (USA500) | IV | Clinical bloodstream isolate | 2 | 16 | |
| BMC1001 (USA500) | IV | Clinical bloodstream isolate | 0.5 | >256 | This study |
| BMC1002 (USA500) | IV | Clinical bloodstream isolate | 2 | 128 | |
| CB5053 (USA100)* | II | Clinical bloodstream isolate | 0.5 | 64 | This study |
| CB5054 (USA100)* | II | Clinical bloodstream isolate | 2 | 24 | |
| CB5035 (USA100) | II | Clinical bloodstream isolate | 0.38 | 192 | This study |
| CB5036 (USA100) | II | Clinical bloodstream isolate | 2 | 96 | |
*, strain used in the in vivo endocarditis studies.
All S. aureus strains were grown in either tryptic soy broth (TSB; Difco Laboratories, Detroit, MI) or Mueller-Hinton broth (MHB; Difco Laboratories).
Antimicrobial agents and MIC testing.
DAP and OX were purchased from Cubist Pharmaceuticals (Lexington, MA) and Sigma Chemical Co. (St. Louis, MO), respectively. The MICs of DAP and OX were determined by standard micro-Etest according to the manufacturer's recommended protocols. A minimum of two independent experimental runs was performed. The Clinical and Laboratory Standards Institute (CLSI) has established MIC guidelines for DAP susceptibility (≤1 μg/ml) only and not for DAP resistance (9). Thus, S. aureus strains with MICs of ≥2 μg/ml are termed DAPr in this report.
Population analysis.
Population analysis of the strain sets was performed with both DAP (0 to 32 μg/ml) and OX (0 to 512 μg/ml) as described before (30, 57). A minimum of two independent experimental runs was performed.
In vitro time-kill curves.
Time-kill experiments (0, 2, 4, 6, and 24 h) were performed using MHB with an initial inoculum of 106 CFU/ml in the presence of sublethal to lethal concentrations of OX, based on individual strain Etest MIC data. This relatively high inoculum (106 CFU) was chosen to encompass bacterial counts commonly achieved in all target tissues of animals with experimental IE (52, 53, 55, 56). A minimum of two independent experimental runs was performed.
Synergy kill curves.
Bactericidal synergy assays for DAP and OX were performed using MHB as described before (36). Briefly, we employed an initial inoculum of ∼106 CFU/ml in the presence of one-quarter of the MIC of OX in combination with one-quarter or one-half of the MIC for DAP (relative to each single agent). Synergy was defined as previously described (36). A minimum of two independent experimental runs was performed.
mecA retention and SCCmec typing analyses.
mecA is carried on an SCCmec genetic element that integrates into the chromosome (20, 21). Diversity in SCCmec architecture has led to the delineation of eight evolutionarily distinct types (20). Detection of mecA and SCCmec typing for the study strains were performed by PCR-based assays as described previously (5); SCCmec types were assigned using recent guidelines according to the types of mec class and ccr complex detected (21).
In vivo experimental IE.
A well-characterized catheter-induced rabbit model of aortic infective endocarditis (IE) was used as described previously (52, 53, 55) to assess whether combination therapy regimens of DAP and OX would enhance single-drug efficacy against DAPr strains. In brief, 24 h after aortic catheter placement, we infected equivalent groups of animals intravenously with a 95% infective dose (ID95) inoculum for the respective infecting S. aureus strains as established in pilot studies (∼2 × 105 CFU/animal). The two strain sets selected (MRSA 11/11-REF2145 and CB5053-CB5054) were prioritized based on the fact that (i) they were both clinical bacteremic or IE-causing isolates, (ii) each demonstrated the DAP-OX seesaw effect, and (iii) they were virulent in the IE model based on pilot studies.
At 24 h after induction of IE, animals were randomized into groups with the following treatments: none (untreated controls), DAP alone at 12 mg/kg once daily, OX alone at 200 mg/kg intramuscularly (i.m.) three times a day (t.i.d.), and DAP-OX combination at the doses listed above. The DAP dose regimen used mimics the currently approved regimen for treating serious infections in humans (38); the OX dose was used as in prior efficacy studies of S. aureus IE in the same model (19, 51). After 3 days of treatment, animals in each group were sacrificed and target organs (cardiac vegetations, kidneys, and spleen) quantitatively cultured. The mean log10 CFU/g (± standard deviation [SD]) was calculated for each tissue in each group for statistical comparisons.
No serum antibiotic levels were obtained, as the pharmacokinetics of the above dose regimens in this model have been previously published (7, 19, 51).
Statistical analysis.
To statistically compare S. aureus tissue densities among the various groups, we used the Kruskal-Wallis analysis of variance (ANOVA) test with the Tukey post hoc correction for multiple comparisons. Significance was determined at a P value of <0.05.
RESULTS
DAP and OX MICs.
As shown in Table 1, we observed an in vitro DAP-OX seesaw relationship in all six MRSA strain pairs, in which the OX MICs declined in parallel with the emergence of DAPr. None of the OX MICs fell below the breakpoint for in vitro susceptibility (<4 μg/ml).
Population analyses.
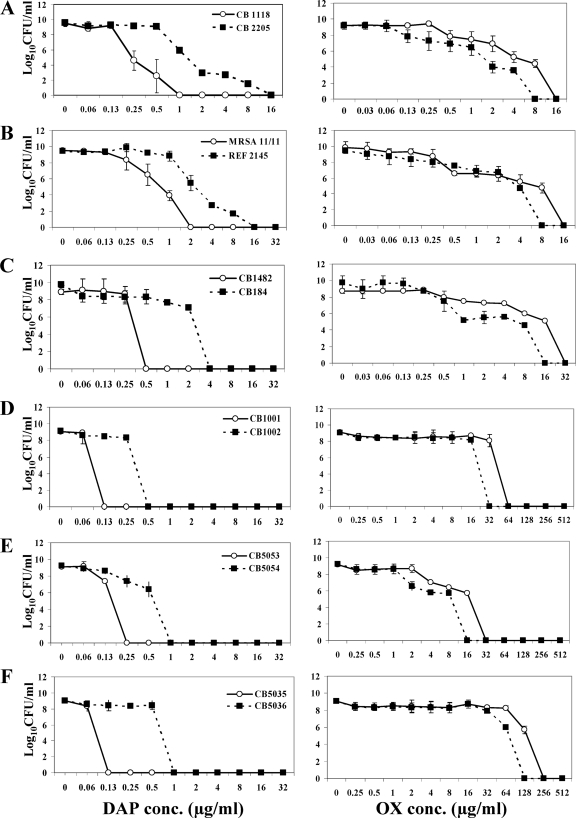
For all six DAPr strains, the DAP-associated population curves were shifted to the right (more resistant), while the OX-associated population curves were uniformly shifted to the left (more susceptible) (Fig. 1A to F).
FIG. 1.
Population analyses of study strains upon exposure to a range of DAP (left panel) or OX (right panel) concentrations. These data represent the means (± SD) for two separate assays. conc., concentration.
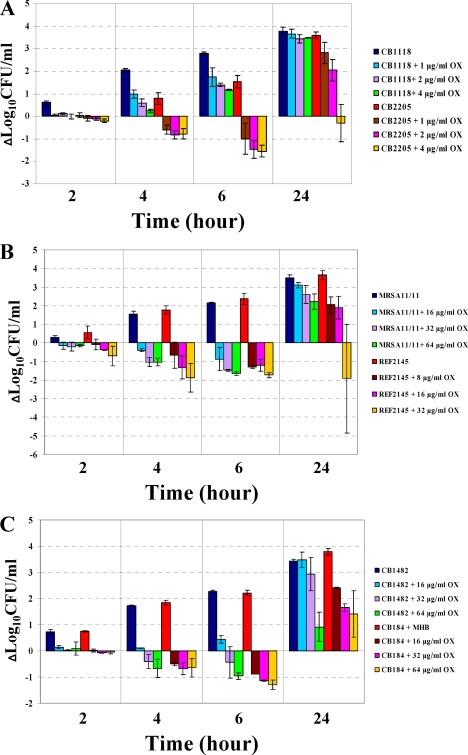
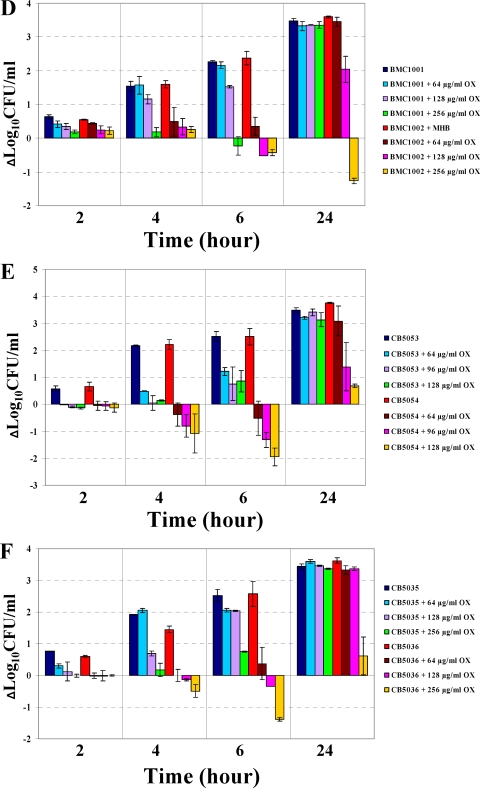
In vitro OX time-kill curves.
As shown in Fig. 2A to F, in all strain pairs, the DAPr strains became more susceptible to the killing effect of OX by 4 or 6 h exposure. The maximal extent of the bactericidal effects at these time points generally ranged from 1 to 2 log10 CFU/ml. Of note, OX prevented regrowth of the DAPr strains CB2205, REF2145, and BMC1002, even after 24 h of incubation at the highest OX concentrations tested (4, 32, and 256 μg/ml, respectively).
FIG. 2.
OX time-kill analyses. Six DAPs and DAPr strain pairs were used for these experiments. (A) CB1118-CB2205; (B) MRSA 11/11-REF2145; (C) CB1482-CB184; (D) BMC1001-BMC1002; (E) CB5053-CB5054; (F) CB5035-CB5036. Time-kill experiments were performed using Mueller-Hinton broth with a 106-CFU/ml inoculum in the presence of 0 to 256 μg/ml OX.
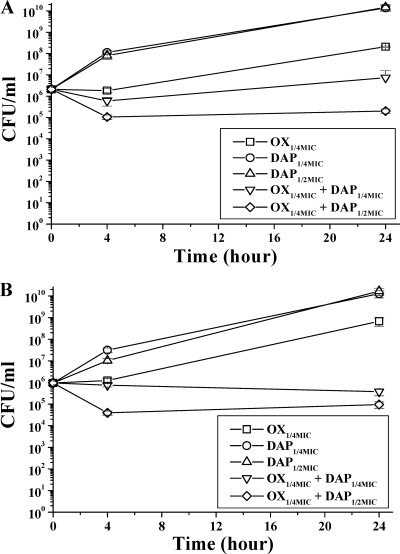
Synergy kill curves.
Two clinical DAPs/DAPr isogenic MRSA strain pairs were selected to determine the potential enhanced in vitro efficacy of DAP-OX combinations (compared to DAP or OX alone). The combination of DAP at one-quarter of the MIC plus OX at one-quarter of the MIC increased the bactericidal activity in the DAPr MRSA strains REF2145 and CB5054 at 24 h (Fig. 3A and B). At one-half of the MIC of DAP plus one-quarter of the MIC of OX, there was a further increase in killing of both DAPr strains relative to that by the single agents.
FIG. 3.
In vitro synergy kill curves for DAPr REF2145 (A) and CB5054 (B) strains. These two strain sets were used in the experimental IE studies detailed in the text. The growth curve for the no-antibiotic growth control for these experiments is not shown but was identical to the growth curve for both strains with one-quarter of the MIC of DAP.
mecA retention and SCCmec typing analyses.
We screened for both the retention of mecA and the SCCmec type present in each pair of isolates. As shown in Table 1, each DAPr isolate retained carriage of mecA like its parental isolate. Isolate pairs with pulsed-field gel types USA300, USA400, and USA500 carried SCCmec type IV (4). In contrast, USA100 isolates carried SCCmec type II. The respective SCCmec types remained the same in all isolate pairs.
In vivo IE model.
The two DAPs and DAPr pairs MRSA 11/11-REF2145 and CB5053-CB5054 had equivalent abilities to induce experimental IE and then disseminate hematogenously to kidneys and spleens. As shown in Table 2, for both DAPr strains, bacterial densities achieved in vegetations were significantly higher than those in kidneys and spleens for untreated control animals at 24 h postinfection (P < 0.05). Following DAP treatment, the bacterial densities of DAPs parental strains MRSA 11/11 and CB5053 were significant decreased in all three target tissues, with each tissue nearly sterilized. Because of the high efficacy of DAP treatment alone against both DAPs parental strains, DAP-OX combinations were not evaluated. In contrast, the respective DAPr strains, REF2145 and CB5054, undergoing DAP monotherapy showed no significant differences in cell densities in any tissues relative to the untreated controls. In addition, although the DAPr strains exhibited decreased OX MICs in vitro relative to the DAPs parental strains, OX treatment alone caused no substantial reduction in bacterial densities in any target tissues. Importantly, the combination of DAP and OX exhibited the best efficacy overall in reducing DAPr cell densities in all three target tissues, better than DAP or OX monotherapy in these animals.
TABLE 2.
MRSA 11/11-REF2145 and CB5053-CB5054 counts in target tissues with DAP and/or OX treatment in the IE model
| Group (no. of animals)a |
S. aureus density (log10 CFU/g tissue) inb: |
||
|---|---|---|---|
| Vegetation | Kidney | Spleen | |
| Control without treatment | |||
| MRSA 11/11 (8) | 7.74 ± 0.79 | 5.51 ± 0.63 | 5.28 ± 0.56 |
| MRSA REF2145 (8) | 8.84 ± 0.56 | 6.29 ± 0.67 | 6.15 ± 0.65 |
| CB5053 (6) | 7.74 ± 0.80 | 5.98 ± 0.98 | 5.84 ± 0.53 |
| CB5054 (6) | 8.76 ± 0.18 | 6.63 ± 0.75 | 5.95 ± 0.65 |
| Treatment | |||
| MRSA 11/11 (12 mg/kg DAP i.v. once daily) (7) | 1.58 ± 0.59 | 0.53 ± 0.28 | 0.60 ± 0.16 |
| MRSA REF2145 (12 mg/kg DAP i.v. once daily) (7) | 7.93 ± 1.12* | 6.74 ± 0.71* | 5.52 ± 1.23* |
| MRSA 11/11 (200 mg/kg OX i.m. t.i.d.) (8) | 5.96 ± 2.23 | 3.99 ± 1.43 | 4.07 ± 1.17 |
| MRSA REF2145 (200 mg/kg OX i.m. t.i.d.) (8) | 7.21 ± 1.39 | 5.09 ± 1.99 | 4.79 ± 1.51 |
| MRSA REF2145 (DAP-OX) (8) | 4.20 ± 1.61** | 2.90 ± 1.50** | 3.12 ± 0.81** |
| CB5053 (12 mg/kg DAP i.v. once daily) (6) | 1.71 ± 0.96 | 0.95 ± 0.60 | 1.07 ± 0.92 |
| CB5054 (12 mg/kg DAP i.v. once daily) (6) | 8.34 ± 0.83* | 6.32 ± 1.31* | 5.78 ± 1.29* |
| CB5053 (200 mg/kg OX i.m. t.i.d.) (7) | 6.18 ± 1.74 | 4.01 ± 1.82 | 3.88 ± 1.19 |
| CB5054 (200 mg/kg OX i.m. t.i.d.) (6) | 8.96 ± 0.44 | 6.35 ± 1.02 | 5.68 ± 0.80 |
| CB5054 (DAP-OX) (10) | 5.70 ± 1.98** | 3.88 ± 1.25** | 3.99 ± 0.95** |
12 mg/kg DAP intravenously (i.v.) once daily is equivalent to a dose of 6 mg/kg once daily in humans (7).
*, P value of <0.001 relative to the value for DAPs MRSA 11/11 or CB5053 with DAP treatment; **, P value of <0.05 relative to the value for REF2145 or CB5054 with OX-only or DAP-only treatment.
DISCUSSION
DAP is a semisynthetic lipopeptide antibiotic approved by the FDA in 2003 for use in a wide variety of S. aureus infections (50). DAP targets the S. aureus cytoplasmic membrane to initiate its bactericidal activity in a calcium-dependent manner, resulting in potassium leakage, inhibition of DNA, RNA, and protein synthesis, and finally cell death (40, 49, 50). Due to its potent staphylocidal activity, DAP has become a clinical mainstay of anti-MRSA therapy, especially in patients with persistent bacteremia (2, 26, 39, 40). However, there have been a number of reports in which initially DAPs S. aureus strains have developed DAPr phenotypes in association with clinical treatment failures (17, 22, 23, 27, 34, 47). Clearly, innovative approaches to this problem, including dose escalations and/or combination therapy strategies, need to be expeditiously applied to enhance/retain DAP efficacy.
It was initially observed that in some in vitro-selected VISA mutants, gradual increases in VAN MICs were accompanied by parallel decreases in the levels of β-lactam resistance (44). This seesaw phenotype was subsequently also observed for some clinical VISA strains (44, 46). It appears that the seesaw phenomenon for VAN and β-lactams can be caused by multiple factors (e.g., mecA-dependent or mecA-independent factors), and its demonstration is reliant on the presence of VAN (37, 41, 45). Interestingly, by both MIC and population analyses, we found a similar seesaw relationship in MRSA strains which progressively acquired DAPr during DAP exposures, i.e., their respective OX MICs declined in parallel to increasing DAP MICs. The DAP-OX seesaw phenotype was observed in all five DAPs/DAPr clinical strain pairs tested, as well as an in vitro-selected DAPs/DAPr pair. Furthermore, all the DAPr strains became more susceptible to early killing by OX in vitro than their respective DAPs parental strains.
Recently, Rand and Houck (36) showed consistent synergy in vitro between DAP and OX by time-kill curve analyses. However, all strains were DAPs, and population analysis was not performed in their study. More recently, Credito et al. and others evaluated the synergistic effect of DAP and gentamicin or rifampin also by time-kill analyses and found synergy between DAP and gentamicin against methicillin-susceptible S. aureus (MSSA), MRSA, VISA, and VRSA strains (10, 48). In addition, LaPlante and Rybak (25), using an in vitro pharmacodynamic model which mimics IE, showed enhanced bactericidal activity of combinations of DAP and low-dose gentamicin. However, unlike for these in vitro results, the addition of gentamicin failed to enhance the effectiveness of DAP in the treatment of experimental MRSA-induced IE (28).
These findings and our own in vitro data led us to investigate whether combination therapy regimens of DAP-OX would enhance the in vitro and/or in vivo efficacy over that of each single agent against DAPr strains. In the current study, the combination of DAP and OX was found to increase the early in vitro bactericidal activity relative to that of DAP or OX alone in DAPr strains. Of note, using two clinical DAPs/DAPr isogenic pairs, we showed that the combination of DAP and OX yielded significantly enhanced bacterial clearance from all three target tissues in an experimental IE model relative to monotherapies in the context of only 3 days of treatment. However, despite the exhibition of a notable in vitro seesaw effect of evolving OX susceptibility in each DAPr strain by both MIC and population analyses, OX treatment alone failed to reduce S. aureus cell densities in any target organ. The reason(s) for this in vitro-in vivo disparity may well be multifactorial. For example, a host factor(s) may impact the maintenance of the seesaw effect in vivo. Thus, the increased OX susceptibility phenotype may not be sustainable in vivo. Further, we have previously noted the coemergence of DAPr with resistance to several innate host defense cationic peptides from polymorphonuclear leukocytes (PMNs) and platelets that are important in the pathogenesis of endovascular infections (22, 57). It is conceivable that such microbial resistances may buttress organism survival and override any enhanced bactericidal effects of DAP and OX. In addition, it should be noted that despite the seesaw effect, the two DAPr strains used in the IE model still exhibited in vitro resistance to OX (MIC, >4 μg/ml). Therefore, the OX treatment regimen chosen may not have provided an adequate pharmacokinetic profile to exert an enhanced killing impact as monotherapy. We recognize that the magnitude of the enhanced bactericidal effect of DAP and OX in vitro against the DAPr strains we tested was modest, suggesting that the in vivo synergy detected may have coinvolved key host defense factors.
The mechanism(s) of the seesaw effect are not well understood for either vancomycin-OX or DAP-OX. We ruled out the possibility of mecA excision in the DAPr isolates in the current study. Also, the SCCmec types remained identical within each DAPs/DAPr pair. There are many possibilities that may explain the decreased OX resistance phenotype of the DAPr isolates studied in this investigation. The mechanism of the MRSA phenotype is mainly due to the acquisition of an SCCmec that carries mecA, encoding PBP2a. This enzyme is a transpeptidase with low affinity for β-lactams, allowing it to carry out transpeptidation reactions required for peptidoglycan assembly in the presence of these usually lethal agents (21, 35). However, expression of the MRSA phenotype is multifactorial. In addition to PBP2a, the MRSA phenotype requires the transglycosylase activity supplied by the PBP2 housekeeping gene (35). Additionally, other cell wall biosynthesis enzymes, called factors essential for methicillin resistance (encoded by fem), are required for a mecA-mediated MRSA phenotype (1). In addition, the two-component regulatory system VraSR (24) is essential for the MRSA phenotype in a mecA-independent fashion (6). Finally, the dedicated regulators of mecA gene transcription, MecR1/MecI and BlaI/BlaR1, can also influence the MRSA phenotype via modulation of mecA expression (18). Thus, effective repression of mecA expression by MecI, with poor MecR1-mediated induction, can influence the MRSA phenotype (18). Further, the level of PBP2a produced does not necessarily correlate with the level of β-lactam resistance. For example, strains can express mecA but still exhibit a methicillin-susceptible phenotype (6, 33). SCCmec type IV lacks mecI, whereas SCCmec type II contains mecI and mecR1. However, most SCCmec type II-containing strains have defective mecA repression due to point mutations either in the mecA promoter or in the mecI open reading frame (ORF); the function of mecI and mecR1 is replaced by blaI and blaR1, which has less effective repression and more effective induction of mecA (13, 16). It remains to be determined whether expression profiles of mecA, mecI and mecR1, or blaI and blaR1 differ between our DAPs/DAPr strain pairs. Given the complexity of factors which can ultimately determine the MRSA phenotype, many possibilities need to be explored in order to explain the decreased OX resistance phenotype commonly associated with evolving DAPr. These studies are in progress in our laboratories.
Collectively, these results suggest that combination therapy regimens of DAP and OX has enhanced in vivo efficacy relative to DAP monotherapy in DAPr strains which exhibit the DAP-OX seesaw phenomenon in vitro. This combination antibiotic approach may be relevant to salvaging DAP therapy in patients with evolving increases in DAP MICs during treatment, especially when OX MICs decrease in parallel.
Acknowledgments
This research was supported by grants from the National Institutes of Health (grant AI-39108 to A.S.B. and grant AI-40481 to R.D. and S.B.-V.) and Cubist Pharmaceuticals (to A.S.B.).
We thank Yin Li, Wessam Abdel Hady, and Andres Taboada for excellent technical assistance. We also thank Diana Zychowski for excellent technical assistance in SCCmec typing.
Footnotes
Published ahead of print on 14 June 2010.
REFERENCES
- 1.Berger-Bachi, B. 1994. Expression of resistance to methicillin. Trends Microbiol. 2:389-393. [DOI] [PubMed] [Google Scholar]
- 2.Boucher, H. W., and G. Sakoulas. 2007. Antimicrobial resistance: perspectives on daptomycin resistance, with emphasis on resistance in Staphylococcus aureus. Clin. Infect. Dis. 45:601-608. [DOI] [PubMed] [Google Scholar]
- 3.Boyle-Vavra, S., R. B. Carey, and R. S. Daum. 2001. Development of vancomycin and lysostaphin resistance in a methicillin-resistant Staphylococcus aureus isolate. J. Antimicrob. Chemother. 48:617-625. [DOI] [PubMed] [Google Scholar]
- 4.Boyle-Vavra, S., and R. S. Daum. 2007. Community-acquired methicillin-resistant Staphylococcus aureus: the role of Panton-Valentine leukocidin. Lab. Invest. 87:3-9. [DOI] [PubMed] [Google Scholar]
- 5.Boyle-Vavra, S., B. Ereshefsky, C.-C. Wang, and R. S. Daum. 2005. Successful multiresistant community-associated methicillin-resistant Staphylococcus aureus lineage from Taipei, Taiwan, that carries either the novel staphylococcal chromosome cassette mec (SCCmec) type VT or SCCmec type IV. J. Clin. Microbiol. 43:4719-4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyle-Vavra, S., S. Yin, and R. S. Daum. 2006. The VraS/VraR two-component regulatory system required for oxacillin resistance in community-acquired methicillin-resistant Staphylococcus aureus. FEMS Microbiol. Lett. 262:163-171. [DOI] [PubMed] [Google Scholar]
- 7.Chambers, H. F., L. Basuino, B. A. Diep, J. Steenbergen, S. Zhang, P. Tattevin, and J. Alder. 2009. Relationship between susceptibility to daptomycin in vitro and activity in vivo in a rabbit model of aortic valve endocarditis. Antimicrob. Agents Chemother. 53:1463-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Climo, M. W., R. L. Patron, and G. L. Archer. 1999. Combinations of vancomycin and β-lactams are synergistic against staphylococci with reduced susceptibilities to vancomycin. Antimicrob. Agents Chemother. 43:1747-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clinical and Laboratory Standards Institute. 2005. Performance standards for antimicrobial susceptibility testing; 15th informational supplement, M100-S15. CLSI, Wayne, PA.
- 10.Credito, K., G. Lin, and P. C. Appelbaum. 2007. Activity of daptomycin alone and in combination with rifampin and gentamicin against Staphylococcus aureus assessed by time-kill methodology. Antimicrob. Agents Chemother. 51:1504-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donnio, P.-Y., L. Louvet, L. Preney, D. Nicolas, J.-L. Avril, and L. Desbordes. 2002. Nine-year surveillance of methicillin-resistant Staphylococcus aureus in a hospital suggests instability of mecA DNA region in an epidemic strain. J. Clin. Microbiol. 40:1048-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donnio, P.-Y., D. C. Oliveira, N. A. Faria, N. Wilhelm, A. Le Coustumier, and H. de Lencastre. 2005. Partial excision of the chromosomal cassette containing the methicillin resistance determinant results in methicillin-susceptible Staphylococcus aureus. J. Clin. Microbiol. 43:4191-4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ender, M., N. McCallum, and B. Berger-Bächi. 2008. Impact of mecA promoter mutations on mecA expression and β-lactam resistance levels. Int. J. Med. Microbiol. 298:607-617. [DOI] [PubMed] [Google Scholar]
- 14.Fox, P. M., R. J. Lampen, K. S. Stumpf, G. L. Archer, and M. W. Climo. 2006. Successful therapy of experimental endocarditis caused by vancomycin-resistant Staphylococcus aureus with a combination of vancomycin and β-lactam antibiotics. Antimicrob. Agents Chemother. 50:2951-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedman, L., J. D. Alder, and J. A. Silverman. 2006. Genetic changes that correlate with reduced susceptibility to daptomycin in Staphylococcus aureus. Antimicrob. Agents Chemother. 50:2137-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldstein, F., J. Perutka, A. Cuirolo, K. Plata, D. Faccone, J. Morris, A. Sournia, M. D. Kitzis, A. Ly, G. Archer, and A. E. Rosato. 2007. Identification and phenotypic characterization of a β-lactam-dependent, methicillin-resistant Staphylococcus aureus strain. Antimicrob. Agents Chemother. 51:2514-2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayden, M. K., K. Rezai, R. A. Hayes, K. Lolans, J. P. Quinn, and R. A. Weinstein. 2005. Development of daptomycin resistance in vivo in methicillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 43:5285-5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hiramatsu, K., K. Asada, E. Suzuki, K. Okonogi, and T. Yokota. 1992. Molecular cloning and nucleotide sequence determination of the regulator region of mecA gene in methicillin-resistant Staphylococcus aureus (MRSA). FEBS Lett. 298:133-136. [DOI] [PubMed] [Google Scholar]
- 19.Hirano, L., and A. S. Bayer. 1991. β-Lactam-β-lactamase-inhibitor combinations are active in experimental endocarditis caused by β-lactamase-producing oxacillin-resistant staphylococci. Antimicrob. Agents Chemother. 35:685-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ito, T., K. Hiramatsu, D. C. Oliveira, H. de Lencastre, K. Zhang, H. Westh, F. O'Brien, P. M. Giffard, D. Coleman, F. C. Tenover, S. Boyle-Vavra, R. L. Skov, M. C. Enright, B. Kreiswirth, K. S. Ko, H. Grundmann, F. Laurent, J. E. Sollid, A. M. Kearns, R. Goering, J. F. John, R. Daum, and B. Soderquist. 2009. Classification of staphylococcal cassette chromosome mec (SCCmec): guidelines for reporting novel SCCmec elements. Antimicrob. Agents Chemother. 53:4961-4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ito, T., Y. Katayama, K. Asada, N. Mori, K. Tsutsumimoto, C. Tiensasitorn, and K. Hiramatsu. 2001. Structural comparison of three types of staphylococcal cassette chromosome mec integrated in the chromosome in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 45:1323-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones, T., M. R. Yeaman, G. Sakoulas, S. J. Yang, R. A. Proctor, H. G. Sahl, J. Schrenzel, Y. Q. Xiong, and A. S. Bayer. 2008. Failures in clinical treatment of Staphylococcus aureus infection with daptomycin are associated with alterations in surface charge, membrane phospholipid asymmetry, and drug binding. Antimicrob. Agents Chemother. 52:269-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Julian, K., K. Kosowska-Shick, C. Whitener, M. Roos, H. Labischinski, A. Rubio, L. Parent, L. Ednie, L. Koeth, T. Bogdanovich, and P. C. Appelbaum. 2007. Characterization of a daptomycin-nonsusceptible vancomycin-intermediate Staphylococcus aureus strain in a patient with endocarditis. Antimicrob. Agents Chemother. 51:3445-3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuroda, M., H. Kuroda, T. Oshima, F. Takeuchi, H. Mori, and K. Hiramatsu. 2003. Two-component system VraSR positively modulates the regulation of cell-wall biosynthesis pathway in Staphylococcus aureus. Mol. Microbiol. 49:807-821. [DOI] [PubMed] [Google Scholar]
- 25.LaPlante, K. L., and M. J. Rybak. 2004. Impact of high-inoculum Staphylococcus aureus on the activities of nafcillin, vancomycin, linezolid, and daptomycin, alone and in combination with gentamicin, in an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 48:4665-4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marco, F., C. Garcia de la Maria, Y. Armero, E. Amat, D. Soy, A. Moreno, A. del Rio, M. Almela, C. A. Mestres, J. M. Gatell, M. T. Jimenez de Anta, and J. M. Miro for the Hospital Clinic Experimental Endocarditis Study Group. 2008. Daptomycin is effective in treatment of experimental endocarditis due to methicillin-resistant and glycopeptide-intermediate Staphylococcus aureus. Antimicrob. Agents Chemother. 52:2538-2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mariani, P. G., H. S. Sader, and R. N. Jones. 2006. Development of decreased susceptibility to daptomycin and vancomycin in a Staphylococcus aureus strain during prolonged therapy. J. Antimicrob. Chemother. 58:481-483. [DOI] [PubMed] [Google Scholar]
- 28.Miró, J. M., C. Garcia-de-la-Maria, Y. Armero, D. Soy, A. Moreno, A. del Rio, M. Almela, M. Sarasa, C. A. Mestres, J. M. Gatell, M. T. Jimenez de Anta, F. Marco, and the Hospital Clinic Experimental Endocarditis Study Group. 2009. Addition of gentamicin or rifampin does not enhance the effectiveness of daptomycin in treatment of experimental endocarditis due to methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 53:4172-4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mishra, N. N., S. J. Yang, A. Sawa, A. Rubio, C. C. Nast, M. R. Yeaman, and A. S. Bayer. 2009. Analysis of cell membrane characteristics of in vitro-selected daptomycin-resistant strains of methicillin-resistant Staphylococcus aureus (MRSA). Antimicrob. Agents Chemother. 53:2312-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moore, M. R., F. Perdreau-Remington, and H. F. Chambers. 2003. Vancomycin treatment failure associated with heterogeneous vancomycin-intermediate Staphylococcus aureus in a patient with endocarditis and in the rabbit model of endocarditis. Antimicrob. Agents Chemother. 47:1262-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murthy, M. H., M. E. Olson, R. E. Wickert, P. D. Fey, and Z. Jalali. 2008. Daptomycin non-susceptible methicillin-resistant Staphylococcus aureus U. S. A. 300 isolate. J. Med. Microbiol. 57:1036-1038. [DOI] [PubMed] [Google Scholar]
- 32.Naimi, T. S., D. Anderson, C. O'Boyle, D. J. Boxrud, S. K. Johnson, F. C. Tenover, and R. Lynfield. 2003. Vancomycin-intermediate Staphylococcus aureus with phenotypic susceptibility to methicillin in a patient with recurrent bacteremia. Clin. Infect. Dis. 36:1609-1612. [DOI] [PubMed] [Google Scholar]
- 33.Niemeyer, D. M., M. J. Pucci, J. A. Thanassi, V. K. Sharma, and G. L. Archer. 1996. Role of mecA transcriptional regulation in the phenotypic expression of methicillin resistance in Staphylococcus aureus. J. Bacteriol. 178:5464-5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pillai, S. K., H. S. Gold, G. Sakoulas, C. Wennersten, R. C. Moellering, Jr., and G. M. Eliopoulos. 2007. Daptomycin nonsusceptibility in Staphylococcus aureus with reduced vancomycin susceptibility is independent of alterations in MprF. Antimicrob. Agents Chemother. 51:2223-2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pinho, M. G., H. de Lencastre, and A. Tomasz. 2001. An acquired and a native penicillin-binding protein cooperate in building the cell wall of drug-resistant staphylococci. Proc. Natl. Acad. Sci. U. S. A. 98:10886-10891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rand, K. H., and H. J. Houck. 2004. Synergy of daptomycin with oxacillin and other β-lactams against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 48:2871-2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reipert, A., K. Ehlert, T. Kast, and G. Bierbaum. 2003. Morphological and genetic differences in two isogenic Staphylococcus aureus strains with decreased susceptibilities to vancomycin. Antimicrob. Agents Chemother. 47:568-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rose, W. E., M. J. Rybak, and G. W. Kaatz. 2007. Evaluation of daptomycin treatment of Staphylococcus aureus bacterial endocarditis: an in vitro and in vivo simulation using historical and current dosing strategies. J. Antimicrob. Chemother. 60:334-340. [DOI] [PubMed] [Google Scholar]
- 39.Sakoulas, G., G. M. Eliopoulos, J. Alder, and C. T. Eliopoulos. 2003. Efficacy of daptomycin in experimental endocarditis due to methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 47:1714-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schriever, C. A., C. Fernandez, K. A. Rodvold, and L. H. Danziger. 2005. Daptomycin: a novel cyclic lipopeptide antimicrobial. Am. J. Health Syst. Pharm. 62:1145-1158. [DOI] [PubMed] [Google Scholar]
- 41.Severin, A., S. W. Wu, K. Tabei, and A. Tomasz. 2004. Penicillin-binding protein 2 is essential for expression of high-level vancomycin resistance and cell wall synthesis in vancomycin-resistant Staphylococcus aureus carrying the enterococcal vanA gene complex. Antimicrob. Agents Chemother. 48:4566-4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sieradzki, K., T. Leski, J. Dick, L. Borio, and A. Tomasz. 2003. Evolution of a vancomycin-intermediate Staphylococcus aureus strain in vivo: multiple changes in the antibiotic resistance phenotypes of a single lineage of methicillin-resistant S. aureus under the impact of antibiotics administered for chemotherapy. J. Clin. Microbiol. 41:1687-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sieradzki, K., B. R. Roberts, W. S. Haber, and A. Tomasz. 1999. The development of vancomycin resistance in a patient with methicillin-resistant Staphylococcus aureus infection. N. Engl. J. Med. 340:517-523. [DOI] [PubMed] [Google Scholar]
- 44.Sieradzki, K., and A. Tomasz. 1999. Gradual alterations in cell wall structure and metabolism in vancomycin-resistant mutants of Staphylococcus aureus. J. Bacteriol. 181:7566-7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sieradzki, K., and A. Tomasz. 1997. Inhibition of cell wall turnover and autolysis by vancomycin in a highly vancomycin-resistant mutant of Staphylococcus aureus. J. Bacteriol. 179:2557-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Silverman, J. A., N. G. Perlmutter, and H. M. Shapiro. 2003. Correlation of daptomycin bactericidal activity and membrane depolarization in Staphylococcus aureus. Antimicrob. Agents Chemother. 47:2538-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Skiest, D. J. 2006. Treatment failure resulting from resistance of Staphylococcus aureus to daptomycin. J. Clin. Microbiol. 44:655-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Snydman, D. R., L. A. McDermott, and S. V. Jacobus. 2005. Evaluation of in vitro interaction of daptomycin with gentamicin or beta-lactam antibiotics against Staphylococcus aureus and enterococci by FIC index and timed-kill curves. J. Chemother. 17:614-621. [DOI] [PubMed] [Google Scholar]
- 49.Steenbergen, J. N., J. Alder, G. M. Thorne, and F. P. Tally. 2005. Daptomycin: a lipopeptide antibiotic for the treatment of serious Gram-positive infections. J. Antimicrob. Chemother. 55:283-288. [DOI] [PubMed] [Google Scholar]
- 50.Tally, F. P., and M. F. DeBruin. 2000. Development of daptomycin for Gram-positive infections. J. Antimicrob. Chemother. 46:523-526. [DOI] [PubMed] [Google Scholar]
- 51.Trotonda, M. P., Y. Q. Xiong, G. Memmi, A. S. Bayer, and A. L. Cheung. 2009. Role of mgrA and sarA in autolysis and resistantce to cell wall-active antibiotics in methicillin-resistant Staphylococcus aureus. J. Infect. Dis. 199:209-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weidenmaier, C., A. Peschel, V. A. J. Kempf, N. Lucindo, M. R. Yeaman, and A. S. Bayer. 2005. DltABCD- and MprF-mediated cell envelope modifications of Staphylococcus aureus confer resistance to platelet microbicidal proteins and contribute to virulence in a rabbit endocarditis model. Infect. Immun. 73:8033-8038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weidenmaier, C., A. Peschel, Y.-Q. Xiong, S. A. Kristian, K. Dietz, M. R. Yeaman, and A. S. Bayer. 2005. Lack of wall teichoic acids in Staphylococcus aureus leads to reduced interactions with endothelial cells and to attenuated virulence in a rabbit model of endocarditis. J. Infect. Dis. 191:1771-1777. [DOI] [PubMed] [Google Scholar]
- 54.Wootton, M., A. P. MacGowan, and T. R. Walsh. 2006. Comparative bactericidal activities of daptomycin and vancomycin against glycopeptide-intermediate Staphylococcus aureus (GISA) and heterogeneous GISA isolates. Antimicrob. Agents Chemother. 50:4195-4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xiong, Y.-Q., L. I. Kupferwasser, P. M. Zack, and A. S. Bayer. 1999. Comparative efficacies of liposomal amikacin (MiKasome) plus oxacillin versus conventional amikacin plus oxacillin in experimental endocarditis induced by Staphylococcus aureus: microbiological and echocardiographic analyses. Antimicrob. Agents Chemother. 43:1737-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xiong, Y. Q., J. V. G. Fowler, M. R. Yeaman, F. Perdreau-Remington, B. N. Kreiswirth, and A. S. Bayer. 2009. Phenotypic and genotypic characteristics of persistent methicillin-resistant Staphylococcus aureus bacteremia in vitro and in an experimental endocarditis model. J. Infect. Dis. 199:201-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang, S. J., B. N. Kreiswirth, G. Sakoulas, M. R. Yeaman, Y. Q. Xiong, A. Sawa, and A. S. Bayer. 2009. Enhanced expression of dltABCD is associated with development of daptomycin nonsusceptibility in a clinical endocarditis isolate of Staphylococcus aureus. J. Infect. Dis. 200:1916-1920. [DOI] [PMC free article] [PubMed] [Google Scholar]