Abstract
Macrolide resistance is a major concern in the treatment of Streptococcus pneumoniae. Inducible macrolide resistance in this pneumococcus is mediated by the efflux pump MefE/Mel. We show here that the human antimicrobial peptide LL-37 induces the mefE promoter and confers resistance to erythromycin and LL-37. Such induction may impact the efficacy of host defenses and of macrolide-based treatment of pneumococcal disease.
Macrolides are in widespread use and are recommended as the first line of treatment for community-acquired pneumonia (CAP), except in areas compromised by high rates of macrolide resistance (25). Macrolide resistance is therefore a major concern in the treatment of Streptococcus pneumoniae, a major causative agent of CAP, sinusitis, otitis media, and meningitis. In S. pneumoniae, macrolide resistance is conferred primarily by the methylase ErmB (MIC ≥ 64 μg/ml) or the macrolide efflux pump Mef/Mel (1, 20, 30). Mef/Mel-mediated efflux has been shown to be specific for 14- and 15-membered macrolides (2, 32). Mef is encoded by two variants, either mefE or mefA, with mefE being more prevalent in the United States (2). MefE/Mel is encoded on the mobile genetic element mega (8) and has been shown to be inducible by 14- and 15-membered macrolides (1, 2, 32). MefE/Mel-mediated MICs for erythromycin range from 1 to 32 μg/ml and increase by 4-fold upon macrolide induction, on average (37). The clinical implications of low-level macrolide resistance (1 to 8 μg/ml) are controversial (3, 14, 17, 26). Recent studies provide evidence that infection with low-level macrolide-resistant pneumococci constitutes a risk factor for treatment failure (5, 4, 13, 22, 23). About 35% of all pneumococcal isolates from community-acquired respiratory tract infections in the Unites States are macrolide resistant, and Mef/Mel is present in more than 50% of the resistant isolates (15).
Cationic antimicrobial peptides (CAMPs) are small cationic, amphiphilic peptides. The major mammalian CAMPs are cathelicidins and defensins, which are constitutively expressed in macrophages and neutrophils and inducibly produced by epithelial cells and at mucosal surfaces. They play an important role in host defense against bacterial infections and are a major component of the innate immune response (11, 19). Sublethal concentrations of CAMPs are known to alter the levels of bacterial gene expression (9). In this respect, we determined that the sole human cathelicidin, LL-37, could alter mefE and mel expression and that this alters pneumococcal susceptibility to macrolides and LL-37.
LL-37 induces the mefE promoter.
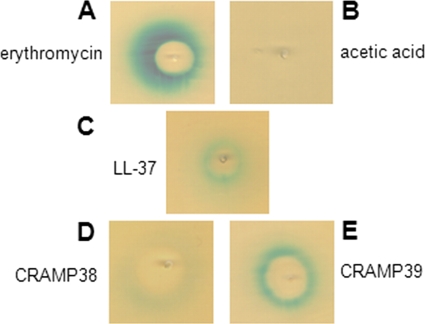
In a screen for inducers of the MefE/Mel efflux pump, several cationic antimicrobial peptides were tested in a plate diffusion assay with a mefE promoter-lacZ reporter strain. The reporter strain was constructed by introducing a 1.1-kb fragment containing the mefE promoter (PmefE) region of S. pneumoniae strain GA17457 (bp 1 to 1,178 based on the published, identical mega nucleotide sequence of strain GA3488; GenBank accession number AF274302 [8]) as an XbaI/EcoRI fragment upstream of the lacZ gene in vector pPP2 (10). The resulting plasmid was used to transform S. pneumoniae strain GA17457, leading to the integration of PmefE-lacZ in the bga locus in reporter strain XZ7042. GA17457 is a clinical isolate (serotype 19A), which has a mega element as its sole erythromycin resistance determinant. For plate diffusion assays, XZ7042 was streaked onto indicator plates (Todd-Hewitt [TH] medium supplemented with 1.5% agarose, 0.5% yeast extract, 300 U/ml catalase, and 0.004% 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside [X-Gal]), and a test compound was applied to the center of the plates. After 36 h at 37°C with 5% CO2, the plates were evaluated for a zone of growth inhibition and an adjacent zone of induction. Under inducing conditions, as shown for erythromycin (Fig. 1A), the zone of induction appears as a blue halo around the inhibition zone.
FIG. 1.
Induction of PmefE-lacZ by CAMPs. Plate diffusion assay shown on X-Gal indicator plates with erythromycin (A; positive control, 10 μg), acetic acid (B; negative control, 15 μl), human cathelicidin LL-37 (C; 75 μg), and mouse cathelicidins CRAMP38 (D; 75 μg) and CRAMP39 (E; 75 μg) tested against PmefE-lacZ reporter strain XZ7042.
We tested several CAMPs of different origins, including polymyxin B (bacterial, 750 μg), cathepsin G-derived peptide (CG117-136; human, 75 μg), protegrin 1 (porcine, 75 μg), and tachyplesin 1 (horseshoe crab, 75 μg), and a mixture of α-defensins, containing the human neutrophil peptides HNP1-3 (25 μg) and the cathelicidins LL-37 (human, 75 μg) and CRAMP38 and CRAMP39 (both murine, 75 μg each). CRAMP38 and CRAMP39 are two alternatively processed forms of the LL-37 mouse homolog CRAMP (7). All peptides were prepared synthetically, except for HNP1-3 (Hycult Biotechnology, Uden, Netherlands), and were dissolved in 0.01% acetic acid. Acetic acid did not inhibit growth or induce PmefE-lacZ in XZ7042 (Fig. 1B). All tested peptides showed zones of growth inhibition in XZ7042 (data not shown), but only LL-37, CRAMP38, and CRAMP39 induced PmefE-lacZ (Fig. 1C to E). These results demonstrated that LL-37 induced PmefE-lacZ and that this induction was specific for the cathelicidins.
LL-37 induction of PmefE confers resistance to LL-37 and erythromycin.
In order to learn if induction of PmefE by LL-37 has biological significance, we tested whether induction of PmefE-lacZ with LL-37 confers resistance to LL-37 or erythromycin using microdilution assays optimized for MIC determination of CAMPs (28). Cultures were grown to an optical density at 600 nm (OD600) of 0.4, split, and grown with or without addition of subinhibitory concentrations of LL-37 or erythromycin for 1 h. The subinhibitory concentrations used (Table 1) did not appear to affect growth of the bacteria, as assessed by determination of the CFU, but were sufficiently high to induce PmefE-lacZ in the reporter strain (data not shown). A final concentration of 2 × 105 bacteria/ml adjusted in 5-fold-diluted TH medium supplemented with 0.5% yeast extract was added to serial dilutions of LL-37 or erythromycin. After 1 h at 37°C, samples were plated on TSA II blood agar plates (BD), and after 16 h at 37°C with 5% CO2, the MICs and the minimal bactericidal concentrations (MBCs) were determined.
TABLE 1.
MICs and MBCs of wild-type, mega, and mefE-mel mutant strains for LL-37 and erythromycine
| Strain | Genotype | MIC (MBC)a |
Etest valued | |||||
|---|---|---|---|---|---|---|---|---|
| LL-37 |
Em |
|||||||
| Uninduced | Induced with LL-37b | Induced with Emc | Uninduced | Induced with LL-37b | Induced with Emc | |||
| GA17457 | Wild type, parent | 500 (1,000) | 2,000 (>2,000) | 2,000 (>2,000) | 2 (16) | 8 (32) | 8 (>64) | 12 |
| XZ8006 | mega::aad9 | 125 (250) | 125 (250) | N/A | 0.5 (4) | 0.5 (4) | N/A | 0.125 |
| XZ8009 | mefE-mel::aph3 | 125 (250) | 64 (250) | N/A | 0.5 (4) | 0.125 (2) | N/A | 0.125 |
| XZ7042 | bgaA::PmefE-lacZ | 500 (1,000) | 2,000 (>2,000) | 2,000 (>2,000) | 1 (8) | 16 (32) | 4 (>64) | 12 |
| XZ8004 | mega::aad9 bgaA::PmefE-lacZ | 250 (500) | 125 (250) | N/A | 0.25 (4) | 0.5 (4) | N/A | 0.125 |
MICs and MBCs (μg/ml) determined by microdilution assays adapted to CAMPs (28). Em, erythromycin; N/A, not applicable because the strain was erythromycin sensitive.
Subinhibitory concentrations used for induction: GA17457 and XZ7042, 200 μg/ml; XZ8006 and XZ8009, 50 μg; and XZ8004, 100 μg.
Subinhibitory concentration used for induction: 1 μg/ml.
Etest was performed according to the manufacturer's instructions (AB Biodisk, Solna, Sweden).
Shown are the results obtained from one representative experiment, and at least three independent experiments were performed.
Wild-type strain GA17457 and the PmefE-lacZ reporter derivative XZ7042 showed high levels of resistance to LL-37 (Table 1), which were further increased upon growth in subinhibitory concentrations of LL-37 (Table 1). Concomitantly, the MIC for erythromycin increased by 2- to 16-fold. Growth in the presence of erythromycin caused an increase in LL-37 resistance, as well as a 4-fold increase in erythromycin resistance. Taken together, independent of the nature of the inducer, induction increased resistance toward both compounds. In regard to the MBCs, LL-37 induction of resistance was less pronounced than that with erythromycin, consistent with the weaker induction observed with LL-37 in the plate diffusion assay (Fig. 1A and C).
To determine whether the increased resistance to LL-37 and erythromycin relied on the mega element, two mega deletion mutants were constructed. By allelic replacement, the 5.5-kb mega region (bp 1 to 5,532; GenBank accession number AF274302) was replaced in GA17457 and XZ7042 with the spectinomycin resistance cassette (containing the aad9 gene) from pUC-spc (12). The resulting mutants XZ8006 and XZ8004, respectively, were less resistant to LL-37 than their parental strains and were susceptible to erythromycin, confirming that mega was required for resistance (Table 1). In addition, growth in the presence of subinhibitory concentrations of LL-37 did not induce increased resistance to LL-37 or erythromycin (Table 1); hence, inducible resistance in the wild-type strains required the mega element.
We analyzed whether pneumococcal resistance to LL-37 required the MefE/Mel efflux pump encoded on mega. A mefE-mel deletion mutant, XZ8009, was constructed by replacing the region containing the mefE and mel genes (from bp 83 in mefE to bp 3,858 in mel) with the kanamycin resistance cassette (containing the aph3 gene) of pSF191 (33) in GA17457. XZ8009 showed the same levels of resistance to LL-37 as the mega-deletion mutants under all tested conditions (Table 1), demonstrating that the MefE/Mel efflux pump mediated the resistance to LL-37 and erythromycin.
Induction of MefE/Mel-mediated resistance by LL-37 was an unexpected finding, especially after a rather narrow spectrum of 14- and 15-membered macrolides had been described as inducers/substrates of this efflux pump (32, 37). The potential clinical implications of this finding are 2-fold, as follows: (i) failure of macrolide-based treatment of mega-containing strains due to host-mediated induction of MefE/Mel by LL-37, resulting in a higher level of resistance, and (ii) underestimation of macrolide resistance of mega-containing strains, based on in vitro MICs determined under noninducing conditions. To assess the in vivo induction of MefE/Mel by LL-37, further studies are required to determine whether local concentrations of LL-37 in the host are sufficiently high to cause induction. Serum concentrations of LL-37 are reported to be in the μg/ml range and can increase dramatically during acute inflammation; however, the local concentrations may vary greatly (27, 29). Daneman et al. have described that the risk for macrolide treatment failures increased with MICs of ≥1 μg/ml but did not increase further with higher MICs (4). Their finding would be consistent with low-level in vitro MICs, as observed for mega-containing isolates, that increase upon in vivo induction of a resistance determinant, resulting in MICs that exceed the threshold established by existing guidelines (MICs ≥ 16 μg/ml) (25) beyond which macrolides should no longer be used. Taken together, induction of MefE/Mel by LL-37 provides an intriguing, possible explanation for the observed macrolide treatment failures in disease caused by low-level-resistant pneumococci (3, 5, 6, 13, 14, 16, 22, 23).
S. pneumoniae is naturally resistant to high levels of CAMPs, which has been attributed to mechanisms altering the surface charge, thereby decreasing CAMP affinity, and to several other factors that await further characterization (18, 21, 24, 31, 35, 36). Recently, Majchrzykiewicz et al. (24) have shown that incubation with LL-37 altered expression of ∼10% of the genome in S. pneumoniae, including the genes of known and putative regulatory proteins, suggesting the possibility of an indirect induction of Mef/Mel by LL-37. Our data suggest that the MefE/Mel efflux pump can further contribute to cathelicidin resistance and may thereby contribute to increased survival in the human host. Due to the limitations of our in vitro assay system, which required high concentrations of LL-37 at or beyond the soluble concentration of LL-37 (∼2,000 μg/ml), this important observation needs to be further evaluated for biological significance with in vivo experiments. Actual efflux-mediated cathelicidin resistance has been demonstrated for the MtrCDE efflux pump of Neisseria spp. (28, 34). This additional function of MefE/Mel could account for an increasing number of pneumococcal isolates that contain both macrolide resistance determinants ErmB and MefE/Mel (15). Due to the high-resistance levels associated with ErmB, MefE/Mel does not contribute to macrolide resistance, but it may help protect MefE/Mel-containing strains from antimicrobial host defenses.
Acknowledgments
This work was supported by funding from NIH grants AI 070829 (to D.S.S.) and AI 062755 (to W.M.S.) and a VA Merit Award (to D.S.S.). W.M.S. was supported in part by a Senior Research Career Scientist Award from VA Medical Research Service.
Footnotes
Published ahead of print on 24 May 2010.
REFERENCES
- 1.Ambrose, K. D., R. Nisbet, and D. S. Stephens. 2005. Macrolide efflux in Streptococcus pneumoniae is mediated by a dual efflux pump (mel and mef) and is erythromycin inducible. Antimicrob. Agents Chemother. 49:4203-4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daly, M. M., S. Doktor, R. Flamm, and D. Shortridge. 2004. Characterization and prevalence of MefA, MefE, and the associated msr(D) gene in Streptococcus pneumoniae clinical isolates. J. Clin. Microbiol. 42:3570-3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daneman, N., D. E. Low, A. McGeer, K. A. Green, and D. N. Fisman. 2008. At the threshold: defining clinically meaningful resistance thresholds for antibiotic choice in community-acquired pneumonia. Clin. Infect. Dis. 46:1131-1138. [DOI] [PubMed] [Google Scholar]
- 4.Daneman, N., A. McGeer, K. Green, and D. E. Low. 2006. Macrolide resistance in bacteremic pneumococcal disease: implications for patient management. Clin. Infect. Dis. 43:432-438. [DOI] [PubMed] [Google Scholar]
- 5.File, T. M., Jr. 2006. Clinical implications and treatment of multiresistant Streptococcus pneumoniae pneumonia. Clin. Microbiol. Infect. 12(Suppl. 3):31-41. [DOI] [PubMed] [Google Scholar]
- 6.Fogarty, C., R. Goldschmidt, and K. Bush. 2000. Bacteremic pneumonia due to multidrug-resistant pneumococci in 3 patients treated unsuccessfully with azithromycin and successfully with levofloxacin. Clin. Infect. Dis. 31:613-615. [DOI] [PubMed] [Google Scholar]
- 7.Gallo, R. L., K. J. Kim, M. Bernfield, C. A. Kozak, M. Zanetti, L. Merluzzi, and R. Gennaro. 1997. Identification of CRAMP, a cathelin-related antimicrobial peptide expressed in the embryonic and adult mouse. J. Biol. Chem. 272:13088-13093. [DOI] [PubMed] [Google Scholar]
- 8.Gay, K., and D. S. Stephens. 2001. Structure and dissemination of a chromosomal insertion element encoding macrolide efflux in Streptococcus pneumoniae. J. Infect. Dis. 184:56-65. [DOI] [PubMed] [Google Scholar]
- 9.Gryllos, I., H. J. Tran-Winkler, M. F. Cheng, H. Chung, R. Bolcome III, W. Lu, R. I. Lehrer, and M. R. Wessels. 2008. Induction of group A Streptococcus virulence by a human antimicrobial peptide. Proc. Natl. Acad. Sci. U. S. A. 105:16755-16760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halfmann, A., R. Hakenbeck, and R. Bruckner. 2007. A new integrative reporter plasmid for Streptococcus pneumoniae. FEMS Microbiol. Lett. 268:217-224. [DOI] [PubMed] [Google Scholar]
- 11.Hancock, R. E. 2001. Cationic peptides: effectors in innate immunity and novel antimicrobials. Lancet Infect. Dis. 1:156-164. [DOI] [PubMed] [Google Scholar]
- 12.Husmann, L. K., D. L. Yung, S. K. Hollingshead, and J. R. Scott. 1997. Role of putative virulence factors of Streptococcus pyogenes in mouse models of long-term throat colonization and pneumonia. Infect. Immun. 65:1422-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iannini, P. B., J. A. Paladino, B. Lavin, M. E. Singer, and J. J. Schentag. 2007. A case series of macrolide treatment failures in community acquired pneumonia. J. Chemother. 19:536-545. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs, M. R. 2002. In vivo veritas: in vitro macrolide resistance in systemic Streptococcus pneumoniae infections does result in clinical failure. Clin. Infect. Dis. 35:565-569. [DOI] [PubMed] [Google Scholar]
- 15.Jenkins, S. G., and D. J. Farrell. 2009. Increase in pneumococcus macrolide resistance, United States. Emerg. Infect. Dis. 15:1260-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelley, M. A., D. J. Weber, P. Gilligan, and M. S. Cohen. 2000. Breakthrough pneumococcal bacteremia in patients being treated with azithromycin and clarithromycin. Clin. Infect. Dis. 31:1008-1011. [DOI] [PubMed] [Google Scholar]
- 17.Klugman, K. P., and J. R. Lonks. 2005. Hidden epidemic of macrolide-resistant pneumococci. Emerg. Infect. Dis. 11:802-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kovacs, M., A. Halfmann, I. Fedtke, M. Heintz, A. Peschel, W. Vollmer, R. Hakenbeck, and R. Bruckner. 2006. A functional dlt operon, encoding proteins required for incorporation of d-alanine in teichoic acids in gram-positive bacteria, confers resistance to cationic antimicrobial peptides in Streptococcus pneumoniae. J. Bacteriol. 188:5797-5805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai, Y., and R. L. Gallo. 2009. AMPed up immunity: how antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 30:131-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leclercq, R., and P. Courvalin. 2002. Resistance to macrolides and related antibiotics in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 46:2727-2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee, H. Y., A. Andalibi, P. Webster, S. K. Moon, K. Teufert, S. H. Kang, J. D. Li, M. Nagura, T. Ganz, and D. J. Lim. 2004. Antimicrobial activity of innate immune molecules against Streptococcus pneumoniae, Moraxella catarrhalis and nontypeable Haemophilus influenzae. BMC Infect. Dis. 4:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lonks, J. R. 2004. What is the clinical impact of macrolide resistance? Curr. Infect. Dis. Rep. 6:7-12. [DOI] [PubMed] [Google Scholar]
- 23.Lonks, J. R., J. Garau, L. Gomez, M. Xercavins, A. Ochoa de Echaguen, I. F. Gareen, P. T. Reiss, and A. A. Medeiros. 2002. Failure of macrolide antibiotic treatment in patients with bacteremia due to erythromycin-resistant Streptococcus pneumoniae. Clin. Infect. Dis. 35:556-564. [DOI] [PubMed] [Google Scholar]
- 24.Majchrzykiewicz, J. A., O. P. Kuipers, and J. J. Bijlsma. 2010. Generic and specific adaptative response of Streptococcus pneumoniae to challenge with three distinct antimicrobial peptides: bacitracin, LL-37 and nisin. Antimicrob. Agents Chemother. 54:440-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mandell, L. A., R. G. Wunderink, A. Anzueto, J. G. Bartlett, G. D. Campbell, N. C. Dean, S. F. Dowell, T. M. File, Jr., D. M. Musher, M. S. Niederman, A. Torres, and C. G. Whitney. 2007. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin. Infect. Dis. 44(Suppl. 2):S27-S72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nuermberger, E., and W. R. Bishai. 2004. The clinical significance of macrolide-resistant Streptococcus pneumoniae: it's all relative. Clin. Infect. Dis. 38:99-103. [DOI] [PubMed] [Google Scholar]
- 27.Ong, P. Y., T. Ohtake, C. Brandt, I. Strickland, M. Boguniewicz, T. Ganz, R. L. Gallo, and D. Y. Leung. 2002. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N. Engl. J. Med. 347:1151-1160. [DOI] [PubMed] [Google Scholar]
- 28.Shafer, W. M., X. Qu, A. J. Waring, and R. I. Lehrer. 1998. Modulation of Neisseria gonorrhoeae susceptibility to vertebrate antibacterial peptides due to a member of the resistance/nodulation/division efflux pump family. Proc. Natl. Acad. Sci. U. S. A. 95:1829-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sørensen, O., J. B. Cowland, J. Askaa, and N. Borregaard. 1997. An ELISA for hCAP-18, the cathelicidin present in human neutrophils and plasma. J. Immunol. Methods 206:53-59. [DOI] [PubMed] [Google Scholar]
- 30.Sutcliffe, J., A. Tait-Kamradt, and L. Wondrack. 1996. Streptococcus pneumoniae and Streptococcus pyogenes resistant to macrolides but sensitive to clindamycin: a common resistance pattern mediated by an efflux system. Antimicrob. Agents Chemother. 40:1817-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swiatlo, E., F. R. Champlin, S. C. Holman, W. W. Wilson, and J. M. Watt. 2002. Contribution of choline-binding proteins to cell surface properties of Streptococcus pneumoniae. Infect. Immun. 70:412-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tait-Kamradt, A., J. Clancy, M. Cronan, F. Dib-Hajj, L. Wondrack, W. Yuan, and J. Sutcliffe. 1997. mefE is necessary for the erythromycin-resistant M phenotype in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 41:2251-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tao, L., D. J. LeBlanc, and J. J. Ferretti. 1992. Novel streptococcal-integration shuttle vectors for gene cloning and inactivation. Gene 120:105-110. [DOI] [PubMed] [Google Scholar]
- 34.Tzeng, Y. L., K. D. Ambrose, S. Zughaier, X. Zhou, Y. K. Miller, W. M. Shafer, and D. S. Stephens. 2005. Cationic antimicrobial peptide resistance in Neisseria meningitidis. J. Bacteriol. 187:5387-5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vollmer, W., and A. Tomasz. 2000. The pgdA gene encodes for a peptidoglycan N-acetylglucosamine deacetylase in Streptococcus pneumoniae. J. Biol. Chem. 275:20496-20501. [DOI] [PubMed] [Google Scholar]
- 36.Wartha, F., K. Beiter, B. Albiger, J. Fernebro, A. Zychlinsky, S. Normark, and B. Henriques-Normark. 2007. Capsule and d-alanylated lipoteichoic acids protect Streptococcus pneumoniae against neutrophil extracellular traps. Cell. Microbiol. 9:1162-1171. [DOI] [PubMed] [Google Scholar]
- 37.Wierzbowski, A. K., D. Boyd, M. Mulvey, D. J. Hoban, and G. G. Zhanel. 2005. Expression of the mef(E) gene encoding the macrolide efflux pump protein increases in Streptococcus pneumoniae with increasing resistance to macrolides. Antimicrob. Agents Chemother. 49:4635-4640. [DOI] [PMC free article] [PubMed] [Google Scholar]