Abstract
We have developed and validated a high-performance liquid chromatography method coupled with a mass detector to quantify itraconazole, voriconazole, and posaconazole using quinoxaline as the internal standard. The method involves protein precipitation with acetonitrile. Mean accuracy (percent deviation from the true value) and precision (relative standard deviation percentage) were less than 15%. Mean recovery was more than 80% for all drugs quantified. The lower limit of quantification was 0.031 μg/ml for itraconazole and posaconazole and 0.039 μg/ml for voriconazole. The calibration range tested was from 0.031 to 8 μg/ml for itraconazole and posaconazole and from 0.039 to 10 μg/ml for voriconazole.
The incidence of mycoses has continued to increase over the past 2 decades, especially in immunocompromised patients. Notwithstanding the fact that in the last decades new antifungal agents have been approved, there is still a therapeutic need for azole compounds, such as itraconazole (ITC), posaconazole (PSC), and voriconazole (VRC), which inhibit 14a-demethylase, a key enzyme in the ergosterol biosynthesis of yeasts and molds (40).
Antifungal prophylaxis, empirical therapy, and treatment of established fungal infections in the hematology patient population may be associated with significant toxicity or drug interactions, leading to subtherapeutic antifungal drug concentrations and poorer clinical outcomes (47). For example, a relationship between plasma concentrations and antifungal efficacy was shown for ITC (19), and the ratio between the area under the concentration-time curve (AUC) and MIC was identified to be predictive for the treatment efficacy of voriconazole and posaconazole, as well (1, 2). Antifungal therapeutic drug monitoring (TDM) could be an important tool in clinical practice if compliance is poor, the therapeutic window is narrow, or drug interactions and toxicity are common adverse effects. Therefore, quantification of drug in plasma samples is an important issue in clinical practice to improve efficacy and to decrease toxicity.
Many methods to individually quantify ITZ (6, 7, 9, 17, 18, 26, 31-34, 37, 45, 46), PSC (8, 35, 39), and VRC (11, 20, 24, 25, 28, 29, 36, 38) in human plasma have been published. Only one method described the quantification of the three triazoles plus fluconazole, ITC metabolite, and ketokonazole in human plasma using a solid-phase extraction procedure.
The aim of this study was to develop and validate a high-performance liquid chromatography-mass spectrometry (HPLC-MS) method useful in routine TDM for quantitation of ITC, PSC, and VRC in human plasma using a protein precipitation extraction procedure and direct injection in an HPLC system.
MATERIALS AND METHODS
Chemicals.
ITC and dimethyl diquinoxaline (QX), used as the internal standard (IS), were purchased from Sigma Aldrich (St. Louis, MO), and PSC and VRC were purchased from Sequoia Research (Pangbourne, United Kingdom). Acetonitrile (HPLC grade) and methanol (HPLC grade) were purchased from J.T. Baker (Deventer, Holland). Formic acid was from Riedel-de Haen (Seelze, Germany). HPLC-grade water was produced by a Milli-DI system by Millipore (Billerica, MA).
Stock solutions and calibration standards.
Stock solutions of ITC, PSC, and VRC were prepared in methanol at a concentration of 1 mg/ml, while a stock solution of QX was prepared in methanol and HPLC-grade water (50:50 [vol/vol]). A working solution of IS was prepared daily at a concentration of 12.50 μg/ml in methanol and HPLC-grade water (50:50 [vol/vol]). All stock solutions were stored at −20°C for no longer than 3 months, based on stability data shown in previously published works (24, 35, 37, 45). The extraction solution consisted of 100% acetonitrile. Stock solutions of ITC, PSC, and VRC were used to prepare plasma calibration samples (standards [STDs]) and quality controls (QCs). The highest level of calibration curve (STD 9) and QCs were prepared, adding an aliquot of each stock solution to obtain final concentrations of 8 μg/ml for ITC and PSC and 10 μg/ml for VRC. Calibration standards were prepared daily by serial dilution of STD 9 to obtain 9 different samples plus blank plasma.
QCs were prepared at the concentrations of 5 μg/ml, 3 μg/ml, 1.5 μg/ml, and 0.1 μg/ml for ITC and PSC and 6 μg/ml, 4 μg/ml, 1.5 μg/ml, 0.12 μg/ml, and 0.3 μg/ml for VRC. STD 9 and QCs were stocked at −20°C for no longer than 3 months; under these conditions, samples have been shown to be stable, as reported in previously published works (24, 35, 37, 45).
Plasma from healthy donors was kindly supplied by the Blood Bank of Maria Vittoria Hospital (Turin, Italy). Plasma from patients taking ITC (n = 32; 50 to 200 mg twice daily), PSC (n = 14; 100 to 300 mg twice daily), and VRC (n = 42; 50 to 300 mg twice daily) was obtained from Regina Margherita Hospital (Turin, Italy) after written informed consensus was received, in TDM routine activity.
Sample preparation.
Two hundred μl of plasma samples were pipetted into a polytetrafluoroethylene tube, and 50 μl of IS working solution was added to each tube. Samples were extracted by protein precipitation using 200 μl of acetonitrile. Each sample was vortexed for at least 15 s and then centrifuged at 12,000 rpm for 10 min at 4°C. One hundred μl of supernatant was transferred to a glass vial and diluted with 100 μl of water. Fifty μl of sample was then injected into the HPLC-MS system. All extraction procedures were performed at room temperature. The HPLC-MS system used was a Waters system (Milford, MA), with a binary pump (model 1525), in-line degasser AF, 717-plus autosampler, and Micromass ZQ mass detector. The LC-MS Empower 2 Pro software program (version year 2005; Waters) was used.
Chromatographic conditions.
Chromatographic separation was performed at 35°C, using a column oven, on a C18 Atlantis T-3 5-μm (150 mm by 4.6 mm, inside diameter [i.d.]) column (Waters, Milford, MA), protected by a Security Guard with a C18 (4.0 mm by 3.0 mm, i.d.) precolumn (Phenomenex; CA). The mobile phase composed initially of 50:50 water with formic acid (0.05%)/acetonitrile with formic acid (0.05%) was then ramped to 20:80 within 6.5 min. The flow rate was set at 1 ml/min.
Detector settings were as follows: electrospray ionization (ESI), positive-polarity ionization; capillary voltage, 3.5 kV; source temperature, 110°C; desolvation temperature, 350°C; nitrogen desolvation flow, 400 liters/h; nitrogen cone flow, 50 liters/h. The ion m/z values monitored were each transition: 705.6 → 353.2 for ITC, 700.8 → 351.0 for PSC, 349.3 → 350.3 for VRC, and 312.4 + H+→ 313.4 for QX. Cone voltage was 25 V for ITC, PSC, and VRC; for QX, it was set at 50 V.
This work was carried out in a UNI EN ISO 9001:2000 certified laboratory (certificate no. IT-64386 for “Design, development and application of determination methods for anti-infective drugs”; www.tdm-torino.org).
RESULTS
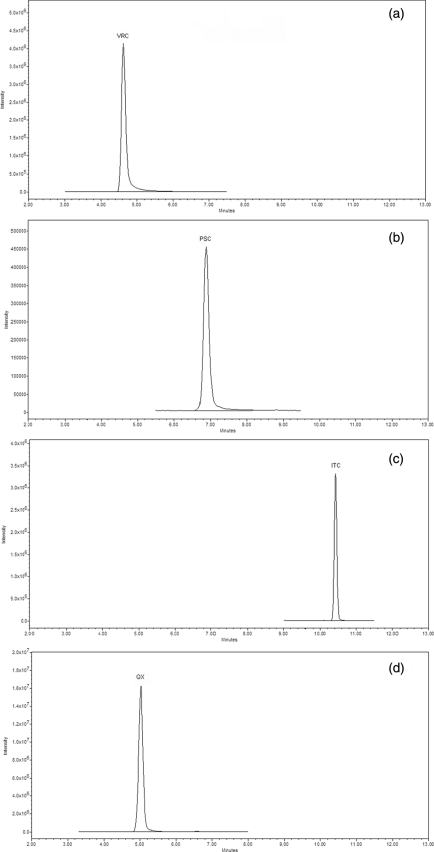
Retention times were 4.73 (±0.20), 7.23 (±0.20), 10.38 (±0.20), and 5.10 (±0.20) min for VRC, PSC, ITC, and QX, respectively. Chromatograms of plasma samples are shown in Fig. 1. Assay validation was done according to Food and Drug Administration (FDA) guidelines published in 2001 (10). Selectivity tests, performed analyzing six different blank plasma samples, showed an absence of interfering drugs coadministered to the patients. Drugs tested were amikacin, caspofungin, vancomycin, piperacillin tazobactam, omeprazole, teicoplanin, linezolid, levofloxacin, and meropenem. Accuracy and precision were assayed using eight determinations for each QC concentration on different days. Accuracy was calculated by determining the deviation in percentage of the mean from the true value. Intra- and interday precision were calculated by determining the relative standard deviation (% RSD) at each QC concentration. Results of validation are shown in Table 1. In accordance with FDA guidelines, accuracy, expressed as percent deviation from the nominal value, and precision, expressed as percent RSD, did not exceed 15%.
FIG. 1.
Chromatograms for plasma from patients taking VRC at 7 mg/kg of body weight twice daily (a), PSC at 6 mg/kg twice daily (b), ITC at 3 mg/kg once daily (c), or QX (d), referring to plasma from a patient taking ITC at 3 mg/kg once daily. Ctrough (plasma concentrations evaluated just before taking drugs) values were 2.18, 0.92, and 1.46 μg/ml for VRC, PSC, and ITC, respectively. ITC, m/z 705.6 → 353.2;, PSC, m/z 700.8 → 351.0; VRC, m/z 349.3 → 350.3; QX, m/z 312.4 → 313.4.
TABLE 1.
Intra- and interday accuracy and precision
| Drug | Nominal valuea (μg/ml) | Accuracy (% deviation) | Precision (% RSD) |
|
|---|---|---|---|---|
| Intraday | Interday | |||
| ITC | 0.10 | 6.23 | 9.12 | 6.34 |
| 1.50 | 2.30 | 4.30 | 8.70 | |
| 3.00 | 0.14 | 5.72 | 12.01 | |
| 5.00 | 4.60 | 7.86 | 12.64 | |
| PSC | 0.10 | 8.08 | 4.89 | 8.70 |
| 1.50 | 1.30 | 3.70 | 7.10 | |
| 3.00 | 3.83 | 4.20 | 6.61 | |
| 5.00 | 4.99 | 5.77 | 6.96 | |
| VRC | 0.12 | 1.50 | 5.81 | 13.30 |
| 1.50 | 1.70 | 3.60 | 9.10 | |
| 4.00 | 5.78 | 4.09 | 8.09 | |
| 6.00 | 9.55 | 5.90 | 6.09 | |
Plasmatic concentrations of quality controls.
Recovery was calculated by comparing peak heights of extracted plasma samples (QCs) with those of unextracted quality controls. Mean recovery was 114.50%, 85.50%, 97.20%, and 95.60% for ITC, PSC, VRC, and QX, respectively.
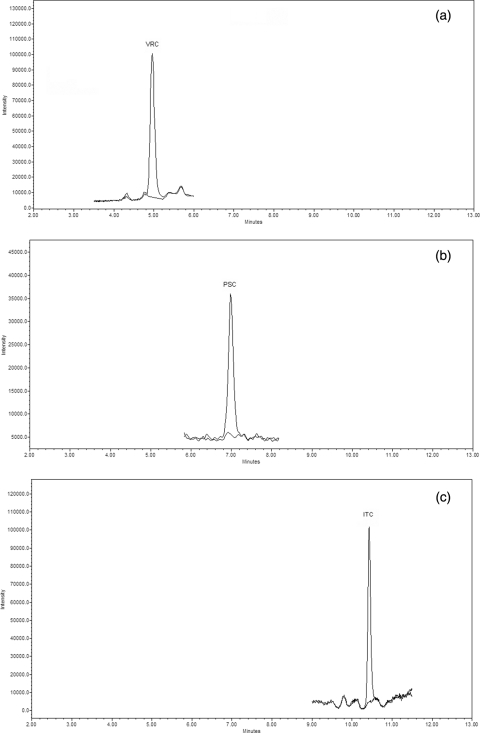
The lower limit of quantification (LLOQ), as suggested by FDA guidelines (10), was considered the lowest standard on the calibration curve. Therefore, the LLOQ for ITC and PSC was 0.031 μg/ml, and that for VRC was 0.039 μg/ml (Fig. 2). The matrix effect was investigated as described by Taylor et al. (42), assaying 6 different blank plasma samples. Peak heights of analytes spiked in a solution of water-acetonitrile (50:50 [vol/vol]) at three different concentrations were compared with peaks obtained in a solution of postextraction blank plasma. The percent deviation of the peak area at the three concentrations for all analytes is comparable, and it never exceeded −15.0%, showing an absence of the “matrix effect.”
FIG. 2.
Overlapping chromatograms of LLOQ and blank plasma. VRC, 0.039 μg/ml (a); PSC, 0.031 μg/ml (b); ITC, 0.031 μg/ml (c).
Drug and IS stability in stock solutions and in plasma samples was not investigated because many previous works showed ITC (37, 45), PSC (35), and VRC stability data (24) for stock solutions and long-term stability. For autosampler stability, we observed that the three drugs were stable for at least 24 h. Based on previously published data, we stocked ITC, PSC, and VRC stock solutions at 4°C for 3 months and plasma samples at −20°C for at least 3 months, avoided freezing and thawing samples more than three times, and carried on the extraction process at room temperature in no more than 3 h; the total time of analysis in the HPLC system was no more than 24 h, based on autosampler stability tests.
Calibration curves were created by plotting the area ratios of drugs relative to the IS against the various drug concentrations in the spiked plasma standards. A quadratic forced-through-zero calibration curve was used for all drugs. Regression coefficients were higher than 0.998 for the three drugs quantified.
The mean Ctrough values (drug concentration measured before antimicrobial administration) and lowest and upper Ctrough values observed for plasma patients are listed in Table 2.
TABLE 2.
Mean Ctrough valuea and upper and lower Ctrough values
| Drug | PK parameterb | Concn (μg/ml) |
|---|---|---|
| ITC | Mean Ctrough | 0.25 |
| Upper Ctrough | 1.18 | |
| Lower Ctrough | 0.04 | |
| PSC | Mean Ctrough | 1.14 |
| Upper Ctrough | 3.20 | |
| Lower Ctrough | 0.04 | |
| VRC | Mean Ctrough | 2.86 |
| Upper Ctrough | 8.32 | |
| Lower Ctrough | 0.09 |
Drug concentration measured before antimicrobial administration.
PK, pharmacokinetic.
DISCUSSION
Antifungal TDM is an important tool in clinical practice, especially for patients with hematological disorders, to avoid toxicity and to improve the outcome (47). Drug interactions are often related to subtherapeutic concentrations caused by the specific metabolic pathway of triazoles. In particular, ITC is both an inhibitor and a substrate of cytochrome P450 3A4 (CYP3A4) (47) and of the P-glycoprotein transporter system (23, 32), and PSC is an inhibitor of CYP3A4 and is metabolized by glucuronization (40, 47). VRC is a substrate and inhibitor of CYP2C19, CYP2C8/9, and CYP3A4 (23, 47). Considering ITC, drug interactions with substrates of this enzyme have been reported (30).
A recent study found that interindividual plasmatic concentrations of VRC are generally high (28) both in children and in adults and that diverse manifestations of toxicity can possibly be attributed to high VRC concentrations (5, 41). In particular, a recent study showed that the response to therapy was more frequent in patients with VRC levels greater than 1 μg/ml and that levels greater than 5.5 μg/ml are associated with an increasing probability of showing toxicity (27).
Considering the increasing interest in antifungal TDM, we have developed and fully validated a new HPLC-MS method to measure ITC, PSC, and VRC in human plasma. Gordien et al. (13) described an HPLC method coupled with UV detection to simultaneously quantify fluconazole, PSC, VRC, ITC and its metabolite, and ketokonazole; although that method allows quantification of the five azoles and the ITC metabolite in a single assay, plasma samples were purified by a solid-phase extraction procedure, which is more expensive and requires more time than a protein precipitation procedure. They also used linezolid, an antibiotic which may be coadministered in clinical practice, as the internal standard; moreover, the ITC LLOQ was equal to 0.15 μg/ml, a higher level than we observed in our clinical practice. Our method has several advantages which make it highly suitable for routine TDM clinical practice. It requires a small plasma volume, allowing TDM in special populations, such as hematology and pediatric patients. It allows simple and rapid sample preparation, which involves protein precipitation with acetonitrile and direct injection in an HPLC system. The use of QX as the internal standard improves the robustness of the analytical method. The calibration curve covers all drug concentrations found in our clinical practice and in previously published pharmacokinetic studies (3, 14, 21, 44). Moreover, our low LLOQ allows quantification of the lowest drug concentrations found in our clinical practice and in previously published pharmacokinetic studies with respect to other methods (8, 13, 15).
Even if drugs are not coadministered, our chromatographic method allows an analysis to be set up using a single calibration curve, extraction procedure, and HPLC system. Therefore, plasma samples of patients taking ITC, PSC, or VRC could be analyzed by a unique chromatographic method, reducing costs and the time required for analysis, making these methods useful in clinical practice for routine analysis, especially for TDM application, where receiving a report quickly helps clinicians to eventually modify the antifungal dose during therapy. In clinical practice, the impossibility of quantifying the ITC metabolite is only a partial bias, because many previously published studies have shown a relationship between efficacy, toxicity (4, 12, 22, 47), and trough concentrations of ITC. Moreover, no data have clearly reported an association between ITC metabolite concentrations and clinical outcome.
Conclusion.
We developed and validated an HPLC-MS method to determine ITC, PSC, and VRC in human plasma following FDA guidelines (10) and according to UNI EN ISO 9001:2000 certification planning rules, currently used in our certified laboratory (16, 43). This HPLC method was also used to measure the three drugs in plasma samples for the International Interlaboratory Quality Control Program for Therapeutic Drug Monitoring of Antifungal drugs (laboratory no. 26) (16), with mean accuracies, expressed as percent deviation from nominal value, of 6.0, 8, and 7.5% for ITC, PSC, and VRC, respectively. The positive results obtained from this control program show that the method allows correct quantification of the three triazoles in plasma samples in clinical practice, in our routine TDM, and in pharmacokinetic studies.
Footnotes
Published ahead of print on 7 June 2010.
REFERENCES
- 1.Andes, D., K. Marchillo, R. Conklin, G. Krishna, F. Ezzet, A. Cacciapuoti, and D. Loebenberg. 2004. Pharmacodynamics of a new triazole, posaconazole, in a murine model of disseminated candidiasis. Antimicrob. Agents Chemother. 48:137-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andes, D., K. Marchillo, T. Stamstad, and R. Conklin. 2003. In vivo pharmacokinetics and pharmacodynamics of a new triazole, voriconazole, in a murine candidiasis model. Antimicrob. Agents Chemother. 47:3165-3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barone, J. A., J. G. Koh, R. H. Bierman, J. L. Colaizzi, K. A. Swanson, M. C. Gaffar, B. L. Moskovitz, W. Mechlinski, and V. Van de Velde. 1993. Food interaction and steady-state pharmacokinetics of itraconazole capsules in healthy male volunteers. Antimicrob. Agents Chemother. 37:778-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boogaerts, M. A., G. E. Verhoef, P. Zachee, H. Demuynck, L. Verbist, and K. De Beule. 1989. Antifungal prophylaxis with itraconazole in prolonged neutropenia: correlation with plasma levels. Mycoses 32(Suppl. 1):103-108. [DOI] [PubMed] [Google Scholar]
- 5.Boyd, A. E., S. Modi, S. J. Howard, C. B. Moore, B. G. Keevil, and D. W. Denning. 2004. Adverse reactions to voriconazole. Clin. Infect. Dis. 39:1241-1244. [DOI] [PubMed] [Google Scholar]
- 6.Brandsteterova, E., P. Kubalec, A. Rady, and V. Krcmery. 1995. Determination of itraconazole and its metabolite in plasma using SPE-HPLC. Pharmazie 50:597-599. [PubMed] [Google Scholar]
- 7.Breadmore, M. C., A. Prochazkova, R. Theurillat, and W. Thormann. 2003. Determination of itraconazole and hydroxyitraconazole in human serum and plasma by micellar electrokinetic chromatography. J. Chromatogr. A 1014:57-70. [DOI] [PubMed] [Google Scholar]
- 8.Chhun, S., E. Rey, A. Tran, O. Lortholary, G. Pons, and V. Jullien. 2007. Simultaneous quantification of voriconazole and posaconazole in human plasma by high-performance liquid chromatography with ultra-violet detection. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 852:223-228. [DOI] [PubMed] [Google Scholar]
- 9.Darouiche, R. O., A. Setoodeh, and E. J. Anaissie. 1995. Potential use of a simplified method for determination of itraconazole levels in plasma and esophageal tissue by using high-performance liquid chromatography. Antimicrob. Agents Chemother. 39:757-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.FDA. 2001. Guidance for industry: bioanalytical method validation. http://www.fda.gov/cder/guidance/4252fnl.pdf. FDA, Silver Spring, MD.
- 11.Gage, R., and D. A. Stopher. 1998. A rapid HPLC assay for voriconazole in human plasma. J. Pharm. Biomed. Anal. 17:1449-1453. [DOI] [PubMed] [Google Scholar]
- 12.Glasmacher, A., C. Hahn, C. Leutner, E. Molitor, E. Wardelmann, C. Losem, T. Sauerbruch, G. Marklein, and I. G. Schmidt-Wolf. 1999. Breakthrough invasive fungal infections in neutropenic patients after prophylaxis with itraconazole. Mycoses 42:443-451. [DOI] [PubMed] [Google Scholar]
- 13.Gordien, J. B., A. Pigneux, S. Vigouroux, R. Tabrizi, I. Accoceberry, J. M. Bernadou, A. Rouault, M. C. Saux, and D. Breilh. 2009. Simultaneous determination of five systemic azoles in plasma by high-performance liquid chromatography with ultraviolet detection. J. Pharm. Biomed. Anal. 50:932-938. [DOI] [PubMed] [Google Scholar]
- 14.Hardin, T. C., J. R. Graybill, R. Fetchick, R. Woestenborghs, M. G. Rinaldi, and J. G. Kuhn. 1988. Pharmacokinetics of itraconazole following oral administration to normal volunteers. Antimicrob. Agents Chemother. 32:1310-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khoschsorur, G., F. Fruehwirth, and S. Zelzer. 2005. Isocratic high-performance liquid chromatographic method with ultraviolet detection for simultaneous determination of levels of voriconazole and itraconazole and its hydroxy metabolite in human serum. Antimicrob. Agents Chemother. 49:3569-3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.KKGT. 2009. International Quality Control Program for Antifungal Drugs. KKGT, Den Haag, Netherlands. http://www.kkgt.nl/English%20Version.htm. Accessed 11 November 2009.
- 17.Koks, C. H., R. W. Sparidans, G. Lucassen, K. M. Crommentuyn, and J. H. Beijnen. 2002. Selective high-performance liquid chromatographic assay for itraconazole and hydroxyitraconazole in plasma from human immunodeficiency virus-infected patients. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 767:103-110. [DOI] [PubMed] [Google Scholar]
- 18.Lacroix, C., F. Wojciechowski, and P. Danger. 1995. Simultaneous determination of itraconazole, hydroxy-itraconazole and amphotericin B in human plasma by HPLC with photodiode array detection. Ann. Biol. Clin. (Paris) 53:293-297. [PubMed] [Google Scholar]
- 19.Lampe, D., S. Kreutzberg, and H. J. Prumke. 1994. Therapeutic drug monitoring of itraconazole—a report of experiences. Mycoses 37(Suppl. 2):34-39. (In German.) [PubMed] [Google Scholar]
- 20.Langman, L. J., and F. Boakye-Agyeman. 2007. Measurement of voriconazole in serum and plasma. Clin. Biochem. 40:1378-1385. [DOI] [PubMed] [Google Scholar]
- 21.Lazarus, H. M., J. L. Blumer, S. Yanovich, H. Schlamm, and A. Romero. 2002. Safety and pharmacokinetics of oral voriconazole in patients at risk of fungal infection: a dose escalation study. J. Clin. Pharmacol. 42:395-402. [PubMed] [Google Scholar]
- 22.Lestner, J. M., S. A. Roberts, C. B. Moore, S. J. Howard, D. W. Denning, and W. W. Hope. 2009. Toxicodynamics of itraconazole: implications for therapeutic drug monitoring. Clin. Infect. Dis. 49:928-930. [DOI] [PubMed] [Google Scholar]
- 23.Mathew, B. P., and M. Nath. 2009. Recent approaches to antifungal therapy for invasive mycoses. ChemMedChem 4:310-323. [DOI] [PubMed] [Google Scholar]
- 24.Michael, C., J. Teichert, and R. Preiss. 2008. Determination of voriconazole in human plasma and saliva using high-performance liquid chromatography with fluorescence detection. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 865:74-80. [DOI] [PubMed] [Google Scholar]
- 25.Nakagawa, S., R. Suzuki, R. Yamazaki, Y. Kusuhara, S. Mitsumoto, H. Kobayashi, S. Shimoeda, S. Ohta, and S. Yamato. 2008. Determination of the antifungal agent voriconazole in human plasma using a simple column-switching high-performance liquid chromatography and its application to a pharmacokinetic study. Chem. Pharm. Bull. (Tokyo) 56:328-331. [DOI] [PubMed] [Google Scholar]
- 26.Ohkubo, T., and T. Osanai. 2005. Determination of itraconazole in human plasma by high-performance liquid chromatography with solid-phase extraction. Ann. Clin. Biochem. 42:94-98. [DOI] [PubMed] [Google Scholar]
- 27.Pascual, A., T. Calandra, S. Bolay, T. Buclin, J. Bille, and O. Marchetti. 2008. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin. Infect. Dis. 46:201-211. [DOI] [PubMed] [Google Scholar]
- 28.Pascual, A., V. Nieth, T. Calandra, J. Bille, S. Bolay, L. A. Decosterd, T. Buclin, P. A. Majcherczyk, D. Sanglard, and O. Marchetti. 2007. Variability of voriconazole plasma levels measured by new high-performance liquid chromatography and bioassay methods. Antimicrob. Agents Chemother. 51:137-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pehourcq, F., C. Jarry, and B. Bannwarth. 2004. Direct injection HPLC micro method for the determination of voriconazole in plasma using an internal surface reversed-phase column. Biomed. Chromatogr. 18:719-722. [DOI] [PubMed] [Google Scholar]
- 30.Poirier, J. M., and G. Cheymol. 1998. Optimisation of itraconazole therapy using target drug concentrations. Clin. Pharmacokinet. 35:461-473. [DOI] [PubMed] [Google Scholar]
- 31.Poirier, J. M., and G. Cheymol. 1997. A rapid and specific liquid chromatographic assay for the determination of itraconazole and hydroxyitraconazole in plasma. Ther. Drug Monit. 19:247-248. [DOI] [PubMed] [Google Scholar]
- 32.Redmann, S., and B. G. Charles. 2006. A rapid HPLC method with fluorometric detection for determination of plasma itraconazole and hydroxy-itraconazole concentrations in cystic fibrosis children with allergic bronchopulmonary aspergillosis. Biomed. Chromatogr. 20:343-348. [DOI] [PubMed] [Google Scholar]
- 33.Rhim, S. Y., J. H. Park, Y. S. Park, D. S. Kim, M. H. Lee, L. M. Shaw, and J. S. Kang. 2009. A sensitive validated LC-MS/MS method for quantification of itraconazole in human plasma for pharmacokinetic and bioequivalence study in 24 Korean volunteers. Pharmazie 64:71-75. [PubMed] [Google Scholar]
- 34.Rifai, N., M. Sakamoto, O. Platt, and C. Brugnara. 1995. A high-performance liquid chromatographic assay for the determination of itraconazole concentration using solid-phase extraction and small sample volume. Ther. Drug Monit. 17:522-525. [DOI] [PubMed] [Google Scholar]
- 35.Shen, J. X., G. Krishna, and R. N. Hayes. 2007. A sensitive liquid chromatography and mass spectrometry method for the determination of posaconazole in human plasma. J. Pharm. Biomed. Anal. 43:228-236. [DOI] [PubMed] [Google Scholar]
- 36.Simmel, F., J. Soukup, A. Zoerner, J. Radke, and C. Kloft. 2008. Development and validation of an efficient HPLC method for quantification of voriconazole in plasma and microdialysate reflecting an important target site. Anal. Bioanal Chem. 392:479-488. [DOI] [PubMed] [Google Scholar]
- 37.Srivatsan, V., A. K. Dasgupta, P. Kale, R. R. Datla, D. Soni, M. Patel, R. Patel, and C. Mavadhiya. 2004. Simultaneous determination of itraconazole and hydroxyitraconazole in human plasma by high-performance liquid chromatography. J. Chromatogr. A 1031:307-313. [DOI] [PubMed] [Google Scholar]
- 38.Stopher, D. A., and R. Gage. 1997. Determination of a new antifungal agent, voriconazole, by multidimensional high-performance liquid chromatography with direct plasma injection onto a size-exclusion column. J. Chromatogr. B Biomed. Sci. Appl. 691:441-448. [DOI] [PubMed] [Google Scholar]
- 39.Storzinger, D., C. Lichtenstern, S. Swoboda, M. A. Weigand, and T. Hoppe-Tichy. 2008. Posaconazole in intensive care patients I: invasive fungal infections in surgical intensive care and case presentation. Mycoses 51(Suppl. 2):52-57. [DOI] [PubMed] [Google Scholar]
- 40.Storzinger, D., S. Swoboda, C. Lichtenstern, C. Muller, M. A. Weigand, and T. Hoppe-Tichy. 2008. Development and validation of a high-performance liquid chromatography assay for posaconazole utilizing solid-phase extraction. Clin. Chem. Lab. Med. 46:1747-1751. [DOI] [PubMed] [Google Scholar]
- 41.Tan, K., N. Brayshaw, K. Tomaszewski, P. Troke, and N. Wood. 2006. Investigation of the potential relationships between plasma voriconazole concentrations and visual adverse events or liver function test abnormalities. J. Clin. Pharmacol. 46:235-243. [DOI] [PubMed] [Google Scholar]
- 42.Taylor, P. J. 2005. Matrix effects: the Achilles heel of quantitative high-performance liquid chromatography-electrospray-tandem mass spectrometry. Clin. Biochem. 38:328-334. [DOI] [PubMed] [Google Scholar]
- 43.University of Turin. 2009. Therapeutic drug monitoring—Turin. University of Turin, Turin, Italy. http://www.tdm-torino.org/. Accessed 15 November 2009.
- 44.Ullmann, A. J., O. A. Cornely, A. Burchardt, R. Hachem, D. P. Kontoyiannis, K. Topelt, R. Courtney, D. Wexler, G. Krishna, M. Martinho, G. Corcoran, and I. Raad. 2006. Pharmacokinetics, safety, and efficacy of posaconazole in patients with persistent febrile neutropenia or refractory invasive fungal infection. Antimicrob. Agents Chemother. 50:658-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uno, T., M. Shimizu, K. Sugawara, and T. Tateishi. 2006. Sensitive determination of itraconazole and its active metabolite in human plasma by column-switching high-performance liquid chromatography with ultraviolet detection. Ther. Drug Monit. 28:526-531. [DOI] [PubMed] [Google Scholar]
- 46.Wong, J. W., U. R. Nisar, and K. H. Yuen. 2003. Liquid chromatographic method for the determination of plasma itraconazole and its hydroxy metabolite in pharmacokinetic/bioavailability studies. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 798:355-360. [DOI] [PubMed] [Google Scholar]
- 47.Worth, L. J., C. C. Blyth, D. L. Booth, D. C. Kong, D. Marriott, M. Cassumbhoy, J. Ray, M. A. Slavin, and J. R. Wilkes. 2008. Optimizing antifungal drug dosing and monitoring to avoid toxicity and improve outcomes in patients with haematological disorders. Intern. Med. J. 38:521-537. [DOI] [PubMed] [Google Scholar]