Abstract
Lamivudine (LMV)-adefovir pivoxil (ADV) combination therapy suppresses the replication of LMV-resistant hepatitis B virus (HBV), although its efficacy in suppressing HBV varies among patients. This study analyzed the clinical, virological, and pharmaceutical factors that influence the effect of the combination therapy. Patients negative for hepatitis B virus e antigen (HBeAg) and with low HBV DNA titers immediately prior to the combination therapy effectively cleared serum HBV DNA (P = 0.0348 and P = 0.0310, respectively). The maximum concentration of ADV in serum (ADV Cmax) was higher in patients who showed HBV DNA clearance (P = 0.0392), and the cumulative clearance rates of HBV DNA were significantly higher in patients with ADV Cmax equal to or greater than 24 ng/ml (P = 0.0284). HBeAg negativity and lower HBV DNA at the start of the combination therapy and higher ADV Cmax were found to be independent factors for serum HBV DNA clearance. Serum creatinine increased significantly during the combination therapy, and the ADV Cmax was higher in patients with low creatinine clearance rates. In conclusion, higher serum concentrations of ADV are associated with a good response to therapy based on clearance of HBV DNA in serum. However, care should be taken to prevent worsening of renal function due to high ADV serum concentrations.
Hepatitis B virus (HBV) infection is a serious global health problem. The risk of chronic HBV infection in immunocompetent adults is generally less than 5% but increases significantly in young children and immunocompromised adults (15, 36). Chronically infected individuals often develop chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma, and more than 500,000 people die every year from advanced liver diseases (6). Complete elimination of the virus is difficult, and patients are generally treated with interferon and nucleoside/nucleotide analogues, which suppress viral replication and prevent the progression of liver disease by combating inflammation (11, 23, 33). However, the emergence of drug-resistant viral mutants and hepatitis flare-up (breakthrough hepatitis) is a serious concern for such suppressive therapies (7, 9, 20, 32).
Lamivudine (LMV) is the first approved nucleoside analogue that terminates viral DNA synthesis by inhibiting chain elongation (31). Serum HBV DNA levels decrease soon after commencement of LMV therapy. However, long-term therapy frequently results in the emergence of drug-resistant HBV mutants (8, 24). In one study, the rate of LMV resistance increased from 24% in patients treated for 1 year to 70% after 4 years of treatment (21). LMV resistance is usually associated with amino acid substitutions in the YMDD motif of the viral reverse transcriptase (RT) (rtM204V/I/S) (4, 5, 19, 26). Additional substitutions, rtL180M and rtV173L, then further enhance the mutated transcriptase activity (1, 12, 26). The emergence of resistant mutants also often results in viral breakthrough and subsequent breakthrough hepatitis (21). The nucleotide analogue adefovir dipivoxil (ADV) potently suppresses the replication of both wild-type and LMV-resistant HBV both in vitro and in vivo (17, 25, 27, 37). LMV-ADV combination therapy is therefore recommended as a standard therapy for breakthrough hepatitis in Japan. Although both the combination therapy and ADV monotherapy are reported to be efficacious in patients with LMV-resistant HBV (28), the combination therapy carries a lower risk of emerging LMV-ADV double-resistant mutants (13, 14, 18). Recently, mutations conferring resistance to both LMV and ADV (through a combination of rtA181T/V and rtI233V or rtA181T/V and rtN236T) have been reported (2, 30), although the incidence of these mutations remains lower than the incidence associated with monotherapy.
We recently observed that some patients on LMV-ADV combination therapy who developed LMV resistance showed a poor response to long-term combination therapy. Decrease of serum HBV DNA levels in these patients leveled off, and HBV DNA levels sometimes remained higher than 4 log copies/ml.
The present study investigated those factors that affect the virological response to LMV-ADV combination therapy. We considered the nucleotide and amino acid sequences of HBV reverse transcriptase virological factors and the LMV/ADV concentrations pharmacological factors and then correlated the results with the clinical data of the patients.
MATERIALS AND METHODS
Patients.
Between July 2003 and May 2009, 59 consecutive patients with chronic hepatitis or cirrhosis due to LMV-resistant HBV infection were treated with LMV-ADV combination therapy at Hiroshima University Hospital. Of these, 53 patients who received the combination therapy for more than 48 weeks were analyzed in this study. Patients began to receive the combination therapy based on the following criteria: (i) increase in serum HBV DNA levels of ≥1 log copy/ml in comparison with the nadir level during LMV monotherapy with or without breakthrough hepatitis, (ii) detection of mutations in the HBV RT domain related to LMV resistance by direct sequence analysis before the combination therapy, and (iii) serum creatinine levels of <1.5 mg/dl. The study protocol conformed to the 1975 Declaration of Helsinki and was approved by the Hiroshima University Hospital ethics committee. Written informed consent was obtained from each patient. Patients coinfected with hepatitis C virus or human immunodeficiency virus were excluded from the study. In addition, patients were not administered drugs that affected serum concentrations of LMV and ADV.
The 53 patients were divided into two groups according to virological response: virological responders (VR) and non-VR. Since cessation of the combination therapy in LMV-resistant chronic hepatitis B patients is likely to lead to severe acute exacerbation, response to the therapy was assessed under extended combination therapy. VR were defined by sustained negative serum HBV DNA (<2.6 log copies/ml by the Amplicor HBV Monitor test [Roche Diagnostics, Basel, Switzerland]) for at least 12 weeks, while non-VR showed sustained positive HBV DNA tests until the final observation. In cases of cessation of the combination therapy, the point of discontinuation was defined as the final observation point. Table 1 details the clinical and virological features of the two groups.
TABLE 1.
Baseline characteristics of 53 patients who received LMV-ADV combination therapy
| Characteristica | Value |
||
|---|---|---|---|
| VR (n = 39) | Non-VR (n = 14) | P value | |
| Sex (male/female) | 31/8 | 8/6 | NSc |
| CH/LC | 24/15 | 8/6 | NS |
| HBV genotype C | 39 | 14 | |
| At start of LMV monotherapy | |||
| Age (yr) | 54b (31-70) | 52b (27-66) | NS |
| HBV DNA (log copies/ml) | 6.7b (2.6-8.5) | 6.7 (3.9-8.4) | NS |
| HBeAg (+/−) | 14/25 | 10/4 | 0.0236 |
| Duration of LMV monotherapy (wk) | 96b (0-166) | 69b (0-213) | NS |
| At start of LMV plus ADV combination therapy | |||
| Age (yr) | 56b (32-73) | 54b (27-69) | NS |
| BMI (kg/cm2) | 22.3b (15.6-27.3) | 22.2b (18.6-26.2) | NS |
| Breakthrough hepatitis (+/−) | 25/14 | 8/6 | NS |
| HBV DNA (log copies/ml) | 5.6b (2.6-8.7) | 7.2b (4.4-8.0) | 0.0310 |
| HBeAg (+/−) | 15/24 | 10/4 | 0.0348 |
| ALT (IU/liter) | 44b (12-654) | 39b (18-310) | NS |
| Cr (mg/dl) | 0.74b (0.49-1.28) | 0.73b (0.45-1.05) | NS |
| CLCR (ml/min/1.73 m2) | 114.3b (56.7-163.1) | 101.4b (74.9-180.7) | NS |
| Duration of combination therapy (wk) | 186b (68-311) | 168b (58-276) | NS |
CH, chronic hepatitis; LC, liver cirrhosis; ALT, alanine transaminase; Cr, creatinine; +, positive; −, negative.
Median value.
NS, not significant.
The patients were administered daily oral doses of 10 mg ADV and 100 mg LMV. Sera were collected from the patients every month during the combination therapy and stored at −80°C until they were used. Serum HBV DNA, liver function, complete blood count, and serum creatinine were measured every month.
Sequence analysis of the HBV polymerase RT domain.
HBV DNA was extracted from 100 μl of stored serum samples using the Smitest R&D (Genome Science Laboratories, Tokyo, Japan) and dissolved in 20 μl of sterile water. The extracted DNA was then amplified by nested PCR using 1 μl of DNA as a template for the first PCR. PCR was performed in 25 μl of reaction mixture containing 2.5 mM MgCl2, 0.4 mM each deoxynucleoside triphosphate (dNTP), 20 pmol of each primer, and 1.25 units of LA Taq (Takara Bio Inc., Shiga, Japan) with the buffer supplied by the manufacturer. The first PCR products were diluted 10-fold, and 1 μl was used as a template for the second PCR. The primers used in this study were S2F (nucleotides [nt] 3189 to 3215; 5′-CAGGGATCCTCAGGCCATGCAGTGGAAC-3′) and X2R1 (nt 1606 to 1625; 5′-GTTCACGGTGGTCTCCATGC-3′) for the first PCR, and B2 (nt 65 to 84; 5′-GGCTCMAGTTCMGGAACAGT-3′) (where M is A or C) and X2R1 for the second PCR. The PCR protocol was as follows: initial denaturation at 94°C for 2 min and 35 cycles of denaturation at 94°C for 1 min, annealing at 58°C for 1 min, extension at 72°C for 2 min, and final extension at 72°C for 7 min. After amplification, the final PCR products were gel purified with the QIAquick gel extraction kit (Qiagen, Hilden, Germany) and sequenced using the dye terminator cycle sequencing kit (Applied Biosystems, Foster City, CA). Sequence analysis was performed on an ABI Prism 3100 Avant Genetic Analyzer (Applied Biosystems).
Measurement of serum concentrations of LMV and ADV.
Serum concentrations of LMV and ADV were measured at the last time of observation in 39 of 53 patients who received the combination therapy. Blood sampling for trough values of LMV and ADV was performed at least 24 h after the drugs were taken. Subsequent blood sampling was performed 1 and 2 h after both of the drugs were taken for concentration measurement by liquid chromatography-tandem mass spectrometry (LC-MS-MS) analysis, using an LC-20A system (Shimadzu, Japan) and a Chromolith Performance RP-18e high-performance liquid chromatography (HPLC) column (Waters) for chromatography and an API4000 system (MDS Sciex, Canada) for mass detection and analysis. The instrument was operated in electrospray-positive-ionization mode, and the signal was detected by multiple-reaction monitoring. We defined the highest concentration for the three time points as the maximum concentration of LMV (LMV Cmax) or ADV (ADV Cmax) in serum. The AUC0-2 (the area under the drug concentration-time curve at 0 to 2 h) of LMV and ADV was calculated by the trapezoidal rule.
Statistical analysis of clinical data.
The background characteristics and serum concentrations were compared using the chi-square test and the Mann-Whitney U test. The cumulative probability of undetectable HBV DNA was analyzed by the Kaplan-Meier method, and differences between the curves were tested by the log rank test. P values of less than 0.05 were considered statistically significant.
RESULTS
Effects of LMV-ADV combination therapy.
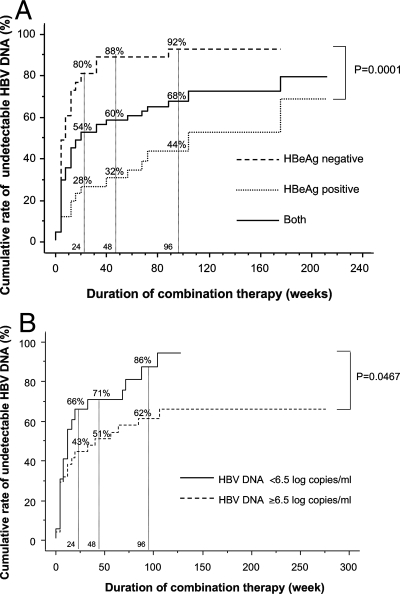
Table 1 details the clinical characteristics of 39 VR and 14 non-VR subjects. Serum HBV DNA in 39 VR decreased to continuously undetectable levels, while serum HBV DNA in 14 non-VR decreased to plateau levels but never became undetectable by the final observation. A larger proportion of VR than non-VR were HBeAg negative prior to the start of LMV monotherapy. Similarly, a larger proportion of VR than non-VR patients were HBeAg negative and had lower serum HBV DNA immediately prior to the combination therapy. The cumulative clearance rates of HBV DNA were significantly higher in HBeAg-negative patients and in those with lower HBV DNA levels (<6.5 log copies/ml) just before the combination therapy than in patients positive for HBeAg or with HBV DNA levels equal to or greater than 6.5 log copies/ml (Fig. 1A and B). Out of 25 patients who were HBeAg positive immediately prior to combination therapy, none had seroconverted to anti-HBe after completing the combination therapy, and none of the total 53 showed viral breakthrough or breakthrough hepatitis during the combination therapy.
FIG. 1.
Cumulative HBV DNA clearance rates in patients treated with lamivudine plus adefovir. Patients were assessed for HBeAg status (A) and HBV DNA levels (B).
Genotyping of LMV- and ADV-resistant mutants.
The nucleotide and amino acid sequences were determined for the RT domain in 47 of the 53 patients by the direct-sequencing method at the time just before HBV DNA clearance or at the nadir of HBV DNA levels after initiation of the combination therapy. Negative amplification of HBV DNA because of low HBV DNA values precluded such analysis in the remaining 6 patients. As shown in Table 2, the amino acid substitutions rtS85A and A181T, previously reported to confer ADV resistance (16, 40), were detected in 2 patients and 1 patient, respectively. The 2 patients with an rtS85A mutation also had YMDD motif mutations (Table 2), and their HBV DNA levels decreased gradually to undetectable levels at 62 and 177 weeks after the beginning of combination therapy, respectively. In contrast, HBV levels in the patient with a unique rtA181T mutation did not decrease to undetectable levels following 58 weeks of combination therapy until the patient was successfully treated with entecavir (ETV) monotherapy (reference 39 and data not shown).
TABLE 2.
Amino acid sequence substitutions in the HBV RT domain
| Substitutiona | No. of patients with substitutiona |
|
|---|---|---|
| VR (n = 33) | Non-VR (n = 14) | |
| rtM204 M | ||
| Alone | 7 | 3 |
| +rtA181T | 0 | 1 |
| rtM204V/I | ||
| Alone | 19 | 10 |
| +rtV214A/E | 2 | 0 |
| +rtQ215H | 2 | 0 |
| +rtV84I | 1 | 0 |
| +rtS85A | 1 | 0 |
| +rtS85A+ rtV214E | 1 | 0 |
Two known ADV-resistant amino acid substitutions (A181T and S85A) are underlined.
Virological response to the combination therapy according to serum concentrations of LMV and ADV.
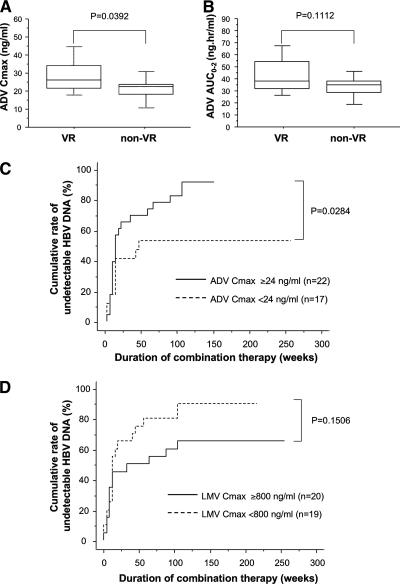
To further explore the poor response of non-VR to therapy, drug concentration analysis was then undertaken in 29 VR and 10 non-VR, and the Cmax and AUC0-2 values of LMV and ADV were compared. ADV Cmax was significantly higher in VR than in non-VR (Fig. 2A), although the difference for ADV AUC0-2 was not statistically significant (Fig. 2B). The median values of ADV Cmax and ADV AUC0-2 were 24 ng/ml and 37 ng·h/ml, respectively. The cumulative HBV DNA clearance rates were significantly higher in patients with high ADV Cmax values (≥24 ng/ml) (Fig. 2C), and most of these patients belonged to the VR group (Table 3). However, the Cmax and AUC0-2 of LMV were not significantly different between the VR and non-VR groups, and there was no difference in HBV clearance rates between patients with high or low Cmax or AUC0-2 of LMV (Fig. 2D and data not shown). The AUC0-2 and Cmax levels of both LMV and ADV did not correlate with the body mass index (BMI) (data not shown).
FIG. 2.
Serum concentrations of ADV and effects of combination therapy. (A and B) Effects of the combination therapy based on ADV Cmax (A) and AUC0-2 (B) determinations. In these box-and-whisker plots, the lines within the boxes represent median values; the upper and lower lines of the boxes represent the 25th and 75th percentiles, respectively; and the upper and lower bars outside the boxes represent the 90th and 10th percentiles, respectively. (C and D) Cumulative clearance rates of HBV DNA by ADV Cmax (C) and LMV Cmax (D).
TABLE 3.
Serum concentration of ADV and efficacy of LMV-ADV combination therapy
| Parameter and value | No. (%) with value |
P value | |
|---|---|---|---|
| VR | Non-VR | ||
| AUC0-2 (ng·h/ml) | |||
| ≥37 | 15 (52) | 3 (30) | 0.2071 |
| <37 | 14 (48) | 7 (70) | |
| Total | 29 | 10 | |
| Cmax (ng/ml) | |||
| ≥24 | 20 (69) | 2 (20) | 0.0097 |
| <24 | 9 (31) | 8 (80) | |
| Total | 29 | 10 | |
Analysis of independent predictive factors for VR.
To analyze predictive factors for achieving VR, multivariate analysis was conducted. When factors appearing in Tables 1, 2, and 3 were analyzed simultaneously, higher ADV Cmax and HBeAg negativity and lower HBV DNA at the start of the combination therapy were found to be independent factors for VR (Table 4). ADV Cmax, in particular, was a strong determinant factor for VR (odds ratio, 16.818; 95% confidence interval [CI], 2.833 to 99.836).
TABLE 4.
Multivariate analysis of factors associated with HBV DNA clearance in LMV-ADV combination therapy
| Factora | Category | P value | Odds ratio | 95% CI |
|---|---|---|---|---|
| HBeAg | 1 (positive) | 0.0170 | 1 | 1.475-25.129 |
| 2 (negative) | 7.194 | |||
| HBV DNA (log copies/ml) | 1 (≥6.5) | 0.0485 | 1 | 1.178-22.367 |
| 2 (<6.5) | 4.185 | |||
| ADV Cmax (ng/ml) | 1 (<24) | 0.0019 | 1 | 2.833-99.836 |
| 2 (≥24) | 16.818 |
At the start of LMV-ADV combination therapy. Factors: gender, age, background liver status, HBeAg, HBV DNA, ALT, Cr, RT mutation, ADV Cmax, and ADV AUC0-2.
Renal function and serum concentrations of the drugs.
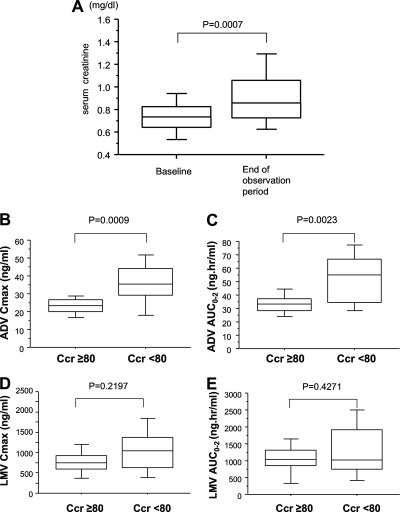
LMV and ADV are excreted from the kidney. Serum creatinine levels increased in 17 (32.1%) of 53 patients during the combination therapy, while the median serum creatinine levels increased significantly from 0.74 mg/dl at baseline to 0.86 mg/dl at the end of the observation period in 53 patients treated with LMV and ADV (Fig. 3A). The dose of ADV was reduced in 6 (11.3%) of the 53 patients to 5 mg/day or 10 mg every 2 days, and ADV administration was stopped in 3 (5.7%) patients due to elevated serum creatinine levels (≥1.5 mg/dl). The HBV DNA titers of 6 patients who reduced the dose of ADV never had a flare-up after the reduction. Five of the 6 patients belonged to the VR and one to the non-VR group. Serum creatinine levels returned to pretherapy values in all patients who reduced or stopped treatment with ADV. Next, we investigated whether the drug concentration was related to renal function. The Cmax and AUC0-2 values of LMV and ADV were compared between patients whose creatinine clearance rates (CLCR) were normal and those whose rates were low. As shown in Fig. 3B and C, both Cmax and AUC0-2 of ADV were significantly higher in patients with CLCR of <80 ml/min/1.73 m2. In contrast, there was no relationship found between CLCR and Cmax/AUC0-2 of LMV (Fig. 3D and E).
FIG. 3.
(A) Comparison of serum creatinine concentrations just before the start of the combination therapy and at the end of the observation period. Renal function and concentrations of LMV and ADV are shown. (B to E) The ADV Cmax (B), LMV Cmax (D), and AUC0-2 of ADV (C) and LMV (E) were compared between patients with high (≥80 ml/min/1.73 m2) and low (<80 ml/min/1.73 m2) CLCR. In these box-and-whisker plots, the lines within the boxes represent median values; the upper and lower lines of the boxes represent the 25th and 75th percentiles, respectively; and the upper and lower bars outside the boxes represent the 90th and 10th percentiles, respectively.
DISCUSSION
The poor response of chronic HBV infection to nucleotide/nucleoside therapy is commonly attributed to amino acid substitutions in the RT domain of HBV polymerase. Several RT amino acid mutations that induce resistance to ADV have been reported, although the incidence is much lower than that reported for LMV. The HBV polymerase RT domain substitutions rtV84, rtS85, rtA181, rtV214, rtQ215, rtI233, rtN236, and rtP237 are associated with ADV resistance (16, 40), and rtA181 and rtQ215 mutations are associated with cross-resistance to LMV and ADV (23, 35). To study the incidence and the effects of amino acid substitutions in the RT domain of HBV polymerase in patients receiving combination therapy, this study initially analyzed serum samples for amino acid sequences in the region. We identified the previously reported A181T and S85A substitutions, as well as substitutions at rt84, rt214, and rt215 that might confer resistance to ADV. However, all these mutations, except for A181T, were found in VR.
These results are consistent with a previous report that most of these mutations confer only limited resistance to ADV therapy (16). In contrast, one of 14 patients who failed to clear HBV DNA in the present study had an apparent ADV resistance mutation. This unique mutation, A181T, which disrupts a stop codon in the HBs gene, is reported to be involved in resistance against both LMV and ADV (39). Therefore, it became apparent in the present series of experiments that only one of 14 patients responded poorly to the combination therapy due to the emergence of a resistant viral clone.
However, none of the remaining 13 patients had amino acid substitutions known to induce resistance to ADV. This is consistent with a recent report by Lampertico et al. (22) citing 11% of patients who failed to clear serum HBV DNA despite 3 years of combination therapy. In addition, none of these patients had a known ADV-resistant strain of HBV. Yatsuji et al (40) also reported 6 of 132 patients with transiently fluctuating HBV DNA levels (from <2.6 to 3.1 log copies/ml) and wild-type genotypes for rtA181 and rtN236.
To further explore the poor response to combination therapy, the concentration of ADV was investigated with respect to the drug's efficacy. Although it is noted that ADV is converted to the diphosphate derivative in hepatocytes by adenylate kinase and inhibits viral DNA polymerase (3, 29), the detailed metabolic pathway remains unclear. According to experimental data from GlaxoSmithKline K.K., when chronic hepatitis B patients were administered oral doses of 10 mg ADV and 100 mg LMV, the ADV Cmax and AUC0-24 were 20.1 ± 3.3 ng/ml and 231.5 ± 33.7 ng·h/ml, respectively (AUC0-2 data not shown). The reported 50% inhibitory concentration (IC50) of ADV is 0.36 to 0.39 μM, equal to 180.5 to 195.6 ng/ml (38, 39) and much higher than the values obtained in this study (ADV Cmax, 5.1 to 54.6 ng/ml). This difference might come from the fact that the concentration of orally administered ADV should be higher in portal blood but lower in the peripheral blood. At any rate, there have been no reports detailing effective serum concentrations of ADV. In this study, the ADV Cmax was higher in VR, and cumulative clearance rates of HBV DNA were higher in patients with higher ADV Cmax values. The reason for the lack of association between the efficacy of the combination therapy and the ADV AUC0-2 remains unclear and might be related to different absorption profiles or metabolic profiles for the drugs or lack of power due to the small number of patients analyzed. However, these results indicate that poor response to the combination therapy arises at least in part from a low serum concentration of ADV. Because 90.9% (20/22) of patients with ADV Cmax values equal to or greater than 24 ng/ml could clear serum HBV DNA, it is expected that non-VR with ADV Cmax values below 24 ng/ml can achieve VR by boosting the serum level of ADV. Therefore, it might be recommended to raise the serum level of ADV to over 24 ng/ml in such cases. Two choices are considered for boosting the serum concentration of ADV: increasing the dose of ADV or using drugs that affect the serum concentration of ADV, such as an inhibitor of organic anion transporters (10, 34).
Meanwhile, renal dysfunction sometimes occurs as a side effect of ADV, and serum creatinine levels actually increased in patients administered the combination therapy; 11.3% of patients had to reduce the dose of ADV, and 5.7% of patients had to discontinue ADV due to elevated serum creatinine levels. Furthermore, the serum concentration of ADV was higher in patients with low CLCR. This finding suggests a possible worsening of renal dysfunction in patients treated with ADV due to the generation of a vicious cause-effect circle (a higher ADV concentration worsens renal function). Although we did not investigate the safety range of ADV concentrations in this study, and the upper limit of the range is not known, it is considered important that adequate and precise doses of ADV should be prescribed to patients, especially those with impaired renal function, instead of simply increasing the serum concentration of ADV. This study suggests that monitoring the serum ADV concentration would be useful to fine tune the appropriate drug dosage.
Acknowledgments
This work was carried out at the Research Center for Molecular Medicine, Faculty of Medicine, Hiroshima University, and the Analysis Center of Life Science, Hiroshima University. We thank Rie Akiyama, Sachi Tanaka, and Miyuki Matsushita for their excellent technical assistance and Yoshiko Nakata and Aya Furukawa for secretarial assistance.
This study was supported in part by a Grant-in-Aid for Scientific Research from the Japanese Ministry of Labor and Health and Welfare.
We have no conflicts of interest.
Footnotes
Published ahead of print on 24 May 2010.
REFERENCES
- 1.Allen, M. I., M. Deslauriers, C. W. Andrews, G. A. Tipples, K. A. Walters, D. L. Tyrrell, N. Brown, and L. D. Condreay. 1998. Identification and characterization of mutations in hepatitis B virus resistant to lamivudine. Lamivudine Clinical Investigation Group. Hepatology 27:1670-1677. [DOI] [PubMed] [Google Scholar]
- 2.Angus, P., R. Vaughan, S. Xiong, H. Yang, W. Delaney, C. Gibbs, C. Brosgart, D. Colledge, R. Edwards, A. Ayres, A. Bartholomeusz, and S. Locarnini. 2003. Resistance to adefovir dipivoxil therapy associated with the selection of a novel mutation in the HBV polymerase. Gastroenterology 125:292-297. [DOI] [PubMed] [Google Scholar]
- 3.Balzarini, J., Z. Hao, P. Herdewijn, D. G. Johns, and E. De Clercq. 1991. Intracellular metabolism and mechanism of anti-retrovirus action of 9-(2-phosphonylmethoxyethyl) adenine, a potent anti-human immunodeficiency virus compound. Proc. Natl. Acad. Sci. U. S. A. 88:1499-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bozdayi, A. M., C. P. Eyigun, A. R. Turkyilmaz, I. Y. Avci, A. Pahsa, and C. Yurdaydin. 2004. A novel pattern (sW195a) in surface gene of HBV DNA due to YSDD (L180M plus M204S) mutation selected during lamivudine therapy and successful treatment with adefovir dipivoxil. J. Clin. Virol. 31:76-77. [DOI] [PubMed] [Google Scholar]
- 5.Bozdayi, A. M., O. Uzunalimoğlu, A. R. Türkyilmaz, N. Aslan, O. Sezgin, T. Sahin, G. Bozdayi, K. Cinar, S. B. Pai, R. Pai, H. Bozkaya, S. Karayaçin, C. Yurdaydin, and R. F. Schinazi. 2003. YSDD: a novel mutation in HBV DNA polymerase confers clinical resistance to lamivudine. J. Viral Hepat. 10:256-265. [DOI] [PubMed] [Google Scholar]
- 6.Bruix, J., and J. M. Llovet. 2003. Hepatitis B virus and hepatocellular carcinoma. J. Hepatol 39(Suppl. 1):S59-S63. [DOI] [PubMed] [Google Scholar]
- 7.Buti, M., R. Jardi, M. Cotrina, F. Rodriguez-Frias, R. Esteban, and J. Guardia. 1998. Transient emergence of hepatitis B variants in a patient with chronic hepatitis B resistant to lamivudine. J. Hepatol. 28:510-513. [DOI] [PubMed] [Google Scholar]
- 8.Chang, T. T., C. L. Lai, R. N. Chien, R. Guan, S. G. Lim, C. M. Lee, K. Y. Ng, G. J. Nicholls, J. C. Dent, and N. W. Leung. 2004. Four years of lamivudine treatment in Chinese patients with chronic hepatitis B. J. Gastroenterol. Hepatol. 19:1276-1282. [DOI] [PubMed] [Google Scholar]
- 9.Chayama, K., Y. Suzuki, M. Kobayashi, M. Kobayashi, A. Tsubota, M. Hashimoto, Y. Miyano, H. Koike, M. Kobayashi, I. Koida, Y. Arase, S. Saitoh, N. Murashima, K. Ikeda, and H. Kumada. 1998. Emergence and takeover of YMDD motif mutant hepatitis B virus during long-term lamivudine therapy and re-takeover by wild type after cessation of therapy. Hepatology 27:1711-1716. [DOI] [PubMed] [Google Scholar]
- 10.Cihlar, T., D. C. Lin, J. B. Pritchard, M. D. Fuller, D. B. Mendel, and D. H. Sweet. 1999. The antiviral nucleotide analogs cidofovir and adefovir are novel substrates for human and rat renal organic anion transporter 1. Mol. Pharmacol. 56:570-580. [DOI] [PubMed] [Google Scholar]
- 11.Conjeevaram, H. S., and A. S. Lok. 2003. Management of chronic hepatitis B. J. Hepatol 38(Suppl. 1):S90-S103. [DOI] [PubMed] [Google Scholar]
- 12.Delaney, W. E., IV, H. Yang, C. E. Westland, K. Das, E. Arnold, C. S. Gibbs, M. D. Miller, and S. Xiong. 2003. The hepatitis B virus polymerase mutation rtV173L is selected during lamivudine therapy and enhances viral replication in vitro. J. Virol. 77:11833-11841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fung, S. K., P. Andreone, S. H. Han, K. Rajender Reddy, A. Regev, E. B. Keeffe, M. Hussain, C. Cursaro, P. Richtmyer, J. A. Marrero, and A. S. Lok. 2005. Adefovir-resistant hepatitis B can be associated with viral rebound and hepatic decompensation. J. Hepatol. 43:937-943. [DOI] [PubMed] [Google Scholar]
- 14.Fung, S. K., H. B. Chae, R. J. Fontana, H. Conjeevaram, J. Marrero, K. Oberhelman, M. Hussain, and A. S. Lok. 2006. Virologic response and resistance to adefovir in patients with chronic hepatitis B. J. Hepatol. 44:283-290. [DOI] [PubMed] [Google Scholar]
- 15.Ganem, D., and A. M. Prince. 2004. Hepatitis B virus infection—natural history and consequences. N. Engl. J. Med. 350:1118-1129. [DOI] [PubMed] [Google Scholar]
- 16.Ghany, M., and T. J. Liang. 2007. Drug targets and molecular mechanisms of drug resistance in chronic hepatitis B. Gastroenterology 132:1574-1585. [DOI] [PubMed] [Google Scholar]
- 17.Hadziyannis, S. J., N. C. Tassopoulos, E. J. Heathcote, T. T. Chang, G. Kitis, M. Rizzetto, P. Marcellin, S. G. Lim, Z. Goodman, M. S. Wulfsohn, S. Xiong, J. Fry, C. L. Brosgart, and the Adefovir Dipivoxil 438 Study Group. 2003. Adefovir dipivoxil for the treatment of hepatitis B e antigen-negative chronic hepatitis B. N. Engl. J. Med. 348:800-807. [DOI] [PubMed] [Google Scholar]
- 18.Ijaz, S., C. Arnold, S. Dervisevic, J. Mechurova, N. Tatman, R. S. Tedder, and N. V. Naoumov. 2008. Dynamics of lamivudine-resistant hepatitis B virus during adefovir monotherapy versus lamivudine plus adefovir combination therapy. J. Med. Virol. 80:1160-1170. [DOI] [PubMed] [Google Scholar]
- 19.Jardi, R., M. Buti, F. Rodriguez-Frias, M. Cotrina, X. Costa, C. Pascual, R. Esteban, and J. Guardia. 1999. Rapid detection of lamivudine-resistant hepatitis B virus polymerase gene variants. J. Virol. Methods 83:181-187. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi, M., F. Suzuki, N. Akuta, Y. Suzuki, Y. Arase, K. Ikeda, T. Hosaka, H. Sezaki, M. Kobayashi, S. Iwasaki, J. Sato, S. Watahiki, Y. Miyakawa, and H. Kumada. 2006. Response to long-term lamivudine treatment in patients infected with hepatitis B virus genotype A, B, and C. J. Med. Virol. 78:1276-1283. [DOI] [PubMed] [Google Scholar]
- 21.Lai, C. L., J. Dienstag, E. Schiff, N. W. Leung, M. Atkins, C. Hunt, N. Brown, M. Woessner, R. Boehme, and L. Condreay. 2003. Prevalence and clinical correlates of YMDD variants during lamivudine therapy for patients with chronic hepatitis B. Clin. Infect. Dis. 36:687-696. [DOI] [PubMed] [Google Scholar]
- 22.Lampertico, P., M. Viganò, E. Manenti, M. Iavarone, E. Sablon, and M. Colombo. 2007. Low resistance to adefovir combined with lamivudine: a 3-year study of 145 lamivudine-resistant hepatitis B patients. Gastroenterology 133:1445-1451. [DOI] [PubMed] [Google Scholar]
- 23.Lee, Y. S., D. J. Suh, Y. S. Lim, S. W. Jung, K. M. Kim, H. C. Lee, Y. H. Chung, Y. S. Lee, W. Yoo, and S. O. Kim. 2006. Increased risk of adefovir resistance in patients with lamivudine-resistant chronic hepatitis B after 48 weeks of adefovir dipivoxil monotherapy. Hepatology 43:1385-1391. [DOI] [PubMed] [Google Scholar]
- 24.Leung, N. W., C. L. Lai, T. T. Chang, R. Guan, C. M. Lee, K. Y. Ng, S. G. Lim, P. C. Wu, J. C. Dent, S. Edmundson, L. D. Condreay, R. N. Chien, and the Asia Hepatitis Lamivudine Study Group. 2001. Extended lamivudine treatment in patients with chronic hepatitis B enhances hepatitis B e antigen seroconversion rates: results after 3 years of therapy. Hepatology 33:1527-1532. [DOI] [PubMed] [Google Scholar]
- 25.Marcellin, P., T. T. Chang, S. G. Lim, M. J. Tong, W. Sievert, M. L. Shiffman, L. Jeffers, Z. Goodman, M. S. Wulfsohn, S. Xiong, J. Fry, C. L. Brosgart, and the Adefovir Dipivoxil 437 Study Group. 2003. Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N. Engl. J. Med. 348:808-816. [DOI] [PubMed] [Google Scholar]
- 26.Melegari, M., P. P. Scaglioni, and J. R. Wands. 1998. Hepatitis B virus mutants associated with 3TC and famciclovir administration are replication defective. Hepatology 27:628-633. [DOI] [PubMed] [Google Scholar]
- 27.Perrillo, R., E. Schiff, E. Yoshida, A. Statler, K. Hirsch, T. Wright, K. Gutfreund, P. Lamy, and A. Murray. 2000. Adefovir dipivoxil for the treatment of lamivudine-resistant hepatitis B mutants. Hepatology 32:129-134. [DOI] [PubMed] [Google Scholar]
- 28.Peters, M. G., H. Hann Hw, P. Martin, E. J. Heathcote, P. Buggisch, R. Rubin, M. Bourliere, K. Kowdley, C. Trepo, D. Gray Df, M. Sullivan, K. Kleber, R. Ebrahimi, S. Xiong, and C. L. Brosgart. 2004. Adefovir dipivoxil alone or in combination with lamivudine in patients with lamivudine-resistant chronic hepatitis B. Gastroenterology 126:91-101. [DOI] [PubMed] [Google Scholar]
- 29.Robbins, B. L., J. Greenhaw, M. C. Connelly, and A. Fridland. 1995. Metabolic pathways for activation of the antiviral agent 9-(2-phosphonylmethoxyethyl) adenine in human lymphoid cells. Antimicrob. Agents Chemother. 39:2304-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schildgen, O., H. Sirma, A. Funk, C. Olotu, U. C. Wend, H. Hartmann, M. Helm, J. K. Rockstroh, W. R. Willems, H. Will, and W. H. Gerlich. 2006. Variant of hepatitis B virus with primary resistance to adefovir. N. Engl. J. Med. 354:1807-1812. [DOI] [PubMed] [Google Scholar]
- 31.Severini, A., X. Y. Liu, J. S. Wilson, and D. L. Tyrrell. 1995. Mechanism of inhibition of duck hepatitis B virus polymerase by (-)-beta-L-2′,3′-dideoxy-3′-thiacytidine. Antimicrob. Agents Chemother. 39:1430-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki, F., A. Tsubota, Y. Arase, Y. Suzuki, N. Akuta, T. Hosaka, T. Someya, M. Kobayashi, S. Saitoh, K. Ikeda, M. Kobayashi, M. Matsuda, J. Satoh, K. Takagi, and H. Kumada. 2003. Efficacy of lamivudine therapy and factors associated with emergence of resistance in chronic hepatitis B virus infection in Japan. Intervirology 46:182-189. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki, Y., H. Kumada, K. Ikeda, K. Chayama, Y. Arase, S. Saitoh, A. Tsubota, M. Kobayashi, M. Koike, N. Ogawa, and K. Tanikawa. 1999. Histological changes in liver biopsies after one year of lamivudine treatment in patients with chronic hepatitis B infection. J. Hepatol. 30:743-748. [DOI] [PubMed] [Google Scholar]
- 34.Uwai, Y., H. Ida, Y. Tsuji, T. Katsura, and K. Inui. 2007. Renal transport of adefovir, cidofovir, and tenofovir by SLC22A family members (hOAT1, hOAT3, and hOCT2). Pharm. Res. 24:811-815. [DOI] [PubMed] [Google Scholar]
- 35.Villet, S., C. Pichoud, J. P. Villeneuve, C. Trépo, and F. Zoulim. 2006. Selection of a multiple drug-resistant hepatitis B virus strain in a liver-transplanted patient. Gastroenterology 131:1253-1261. [DOI] [PubMed] [Google Scholar]
- 36.Wright, T. L., and J. Y. Lau. 1993. Clinical aspects of hepatitis B virus infection. Lancet 342:1340-1344. [DOI] [PubMed] [Google Scholar]
- 37.Xiong, X., C. Flores, H. Yang, J. J. Toole, and C. S. Gibbs. 1998. Mutations in hepatitis B DNA polymerase associated with resistance to lamivudine do not confer resistance to adefovir in vitro. Hepatology 28:1669-1673. [DOI] [PubMed] [Google Scholar]
- 38.Yatsuji, H., N. Hiraga, N. Mori, T. Hatakeyama, M. Tsuge, M. Imamura, S. Takahashi, Y. Fujimoto, H. Ochi, H. Abe, T. Maekawa, F. Suzuki, H. Kumada, and K. Chayama. 2007. Successful treatment of an entecavir-resistant hepatitis B virus variant. J. Med. Virol. 79:1811-1817. [DOI] [PubMed] [Google Scholar]
- 39.Yatsuji, H., C. Noguchi, N. Hiraga, N. Mori, M. Tsuge, M. Imamura, S. Takahashi, E. Iwao, Y. Fujimoto, H. Ochi, H. Abe, T. Maekawa, C. Tateno, K. Yoshizato, F. Suzuki, H. Kumada, and K. Chayama. 2006. Emergence of a novel lamivudine-resistant hepatitis B virus variant with a substitution outside the YMDD motif. Antimicrob. Agents Chemother. 50:3867-3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yatsuji, H., F. Suzuki, H. Sezaki, N. Akuta, Y. Suzuki, Y. Kawamura, T. Hosaka, M. Kobayashi, S. Saitoh, Y. Arase, K. Ikeda, S. Watahiki, S. Iwasaki, M. Kobayashi, and H. Kumada. 2008. Low risk of adefovir resistance in lamivudine-resistant chronic hepatitis B patients treated with adefovir plus lamivudine combination therapy: two-year follow-up. J. Hepatol. 48:923-931. [DOI] [PubMed] [Google Scholar]