Abstract
Candida infections frequently involve drug-resistant biofilm growth on device surfaces. Glucan synthase gene FKS1 has been linked to triazole resistance in Candida biofilms. We tested the impact of FKS1 modulation on susceptibility to additional antifungal classes. Reduction of FKS1 expression rendered biofilms more susceptible to amphotericin B, anidulafungin, and flucytosine. Increased resistance to anidulafungin and amphotericin B was observed for biofilms overexpressing FKS1. These findings suggest that Candida biofilm glucan sequestration is a multidrug resistance mechanism.
In hospital settings, Candida spp. often cause disease by adhering to the surface of a medical device and adapting to a biofilm lifestyle (7, 10). Biofilms consist of cells attached to a surface and embedded in a protective matrix produced by the organisms (5). C. albicans biofilm cells are phenotypically distinct, and their ability to survive exposure to high antifungal concentrations presents a serious therapeutic dilemma (1, 2, 11, 14, 19-21). Biofilm cells exhibit up to 1,000-fold-increased resistance relative to free-floating, or planktonic, cells (3, 9, 12, 18).
Glucan synthesis by Fks1p has been implicated in C. albicans biofilm resistance to the azole drug fluconazole (17). FKS1 disruption was found to reduce manufacture and deposition of β-1,3-glucan in the biofilm matrix, resulting in susceptibility to fluconazole. The matrix glucan was shown to sequester the triazole, preventing it from reaching its target. The mechanism is biofilm specific and has been studied only for the triazoles.
The purpose of this study was to determine the role of FKS1 in C. albicans biofilm resistance to other available antifungal drug classes. We chose to study three strains with differing expressions of FKS1 and concomitant variations in matrix glucan. The strains included a heterozygous deletion mutant (FKS1/fks1Δ), an FKS1 overexpression mutant (TDH3-FKS1) with one FKS1 allele under the control of TDH3 promoter and one allele intact, and a reference strain (4, 17). Finally, because FKS1 is essential in C. albicans, a conditional TET-FKS1 mutant was also included (22). The TET-FKS1 strain has one allele deleted and one allele under the control of a tetracycline- or doxycycline-repressible promoter. An echinocandin (anidulafungin), flucytosine, and amphotericin B deoxycholate were selected for their different mechanisms of action.
For biofilm antifungal susceptibility testing, C. albicans biofilms were grown in 96-well polystyrene plates as previously described (16, 20). Wells were inoculated with 106 cells/ml in RPMI medium-MOPS (morpholinepropanesulfonic acid). After an adherence period (6 or 24 h), biofilms were washed with phosphate-buffered saline (PBS). Fresh media and antifungals were applied, and plates were incubated for an additional 24 h at 37°C. The concentration ranges included those above and below the planktonic MIC values and included 0.001 to 0.125 μg/ml anidulafungin, 0.03 to 8 μg/ml flucytosine, and 0.008 to 2 μg/ml amphotericin B deoxycholate (13). After 24 h of incubation at 30°C, an XTT [2,3-bis(2-methoxy-4-nitro-5-sulfo-phenyl)-2H-tetrazolium-5-carboxanilide] reduction assay was performed and endpoints were determined spectrophotometrically at 492 nm as a measure of cell metabolic activity (16, 20).
For the FKS1/fks1Δ strain, the TDH3-FKS1 strain, and the reference strain, we measured the impact of antifungal wells compared to the no-drug control wells. The impact of doxycycline repression of FKS1 on antifungal susceptibility during biofilm formation was similarly examined using the TET-FKS1 strain with a doxycycline concentration range of 1 to 240 ng/ml in a 96-well checkerboard format. After adherence, biofilms were incubated in the presence of the doxycycline and antifungal in combination for 24 h prior to the XTT assay. For planktonic studies, MICs were determined two times in duplicate and measured visually using CLSI endpoints (15).
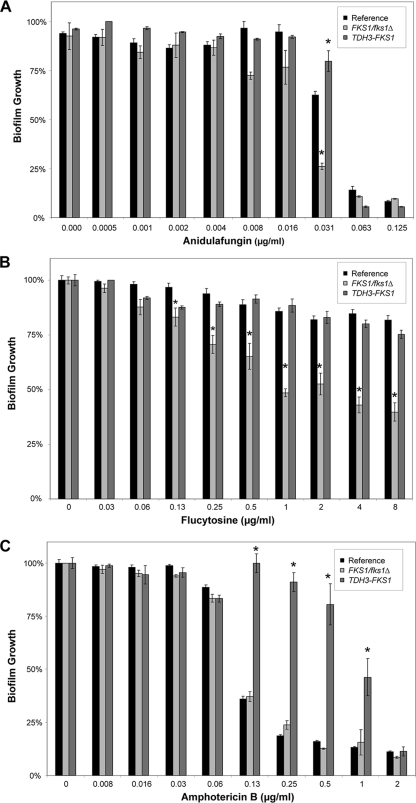
Anidulafungin was the most effective against parent C. albicans biofilms, while flucytosine had minimal or no activity at the highest concentration tested (Fig. 1). The biofilm formed by the FKS1/fks1Δ heterozygote was more susceptible to flucytosine and anidulafungin, with drug impact at 2- to 8-fold-lower concentrations. Heterozygous FKS1 disruption did not impact amphotericin B activity in this assay design. To determine if a difference for amphotericin B might be due to the phase of growth, a later phase of biofilm growth (24 h) was tested. By this method, FKS1/fks1Δ biofilms were more susceptible to amphotericin B than reference strain biofilms were, but the difference was less than that observed for the other antifungal drug classes (not shown). For example, treatment with amphotericin B at 0.25 μg/ml decreased FKS1/fks1Δ biofilms by 80%, compared to 60% for the reference strain (P < 0.05; Student's t test).
FIG. 1.
Impact of FKS1 modulation on antifungal susceptibility in C. albicans biofilms. FKS1-modulated biofilms were grown in 96-well plates for 6 h and treated with serial dilutions of anidulafungin (A), flucytosine (B), or amphotericin B deoxycholate (C) for an additional 24 h. Endpoints were assessed using an XTT assay, and data are shown as percentages of biofilm growth relative to growth of untreated controls. Assays were performed in triplicate, and each error bar represents one standard error. Statistical significance was determined by analysis of variance with pairwise comparisons using the Holm-Sidak method. *, P < 0.05.
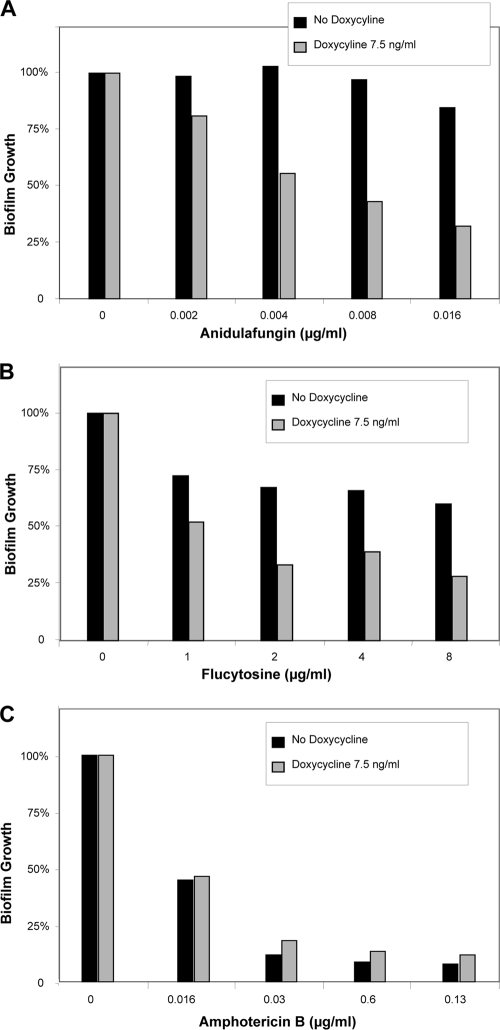
The TET-FKS1 strain recapitulated the phenotypes for susceptibility to echinocandin and flucytosine, with 4- to 8-fold-lower drug concentrations effective for the condition with doxycycline repression of FKS1 (Fig. 2). Interestingly, modulation of FKS1 by doxycycline or heterozygous disruption did not render biofilms more susceptible to amphotericin B. The explanation for this difference is not clear. Doxycycline did not impact C. albicans reference strain growth or drug susceptibility at the concentrations used in these experiments (data not shown).
FIG. 2.
Impact of doxycycline repression of FKS1 on antifungal susceptibility in C. albicans biofilms. TET-FKS1-modulated biofilms were grown in 96-well plates for 6 h and treated for 24 h with serial dilutions of anidulafungin (A), flucytosine (B), or amphotericin B deoxycholate (C) in combination with doxycycline by using a checkerboard format. Endpoints were assessed using an XTT assay, and data are shown as percentages of biofilm growth relative to growth of untreated controls. Checkerboard assays were performed in duplicate, and results from one assay replicate are shown.
FKS1 overexpression had a similar but lesser impact on biofilm susceptibility to anidulafungin (Fig. 1). Increased resistance to flucytosine was not detectable by these overexpression assays, due to the profound resistance of the reference biofilm at the highest concentrations. The TDH3-FKS1 overexpression biofilm exhibited a marked increased resistance to amphotericin B, supporting a role for glucan in polyene biofilm resistance.
Importantly, modulation of FKS1 did not impact planktonic susceptibility to the various antifungals based on standard CLSI testing and interpretation (Table 1) (15). Because the drug target of anidulafungin is Fks1p, we considered the possibility that genetically modifying expression and regulation of this gene may directly impact susceptibility to the compound (6). For example, echinocandin resistance in planktonic cells has been linked to altered Fks1p kinetics due to point mutations in several hot spots (8). However, the FKS1 heterozygote was similarly susceptible to echinocandin in this planktonic assay, while the strain was more susceptible to echinocandin in the biofilm assay relative to the parent strain, again suggesting a biofilm-specific mode of action (Table 1).
TABLE 1.
Impact of FKS1 modulation on drug susceptibility of planktonic cellsa
| Drug | MIC (μg/ml) for strain type |
||
|---|---|---|---|
| Reference strain | FKS1/fks1Δ | TDH3-FKS1 | |
| Anidulafungin | 0.01 | 0.01 | 0.01 |
| Flucytosine | 0.06 | 0.03 | 0.03 |
| Amphotericin B deoxycholate | 0.03 | 0.03 | 0.03 |
MICs were determined using the CLSI method and endpoints.
FKS1 has been linked to C. albicans resistance through a mechanism specific to biofilms. Investigations using fluconazole and amphotericin B suggest that this process involves antifungal sequestration by the matrix glucan (16, 17, 23). Modulation of FKS1, through either inhibition or overexpression, impacted biofilm susceptibility to all the antifungal agents tested. As observed for FKS1 and fluconazole resistance, this mechanism appears to be biofilm specific, since disruption of FKS1 has no impact on planktonic resistance. Our findings indicate that FKS1 similarly impacts biofilm resistance to other antifungal drug classes, possibly through the same mechanism.
Acknowledgments
We thank C. Douglas, A. Mitchell, and C. Nobile for strains and plasmids.
This work was supported by the National Institutes of Health (grant RO1 AI073289-01).
Footnotes
Published ahead of print on 1 June 2010.
REFERENCES
- 1.Al-Fattani, M. A., and L. J. Douglas. 2006. Biofilm matrix of Candida albicans and Candida tropicalis: chemical composition and role in drug resistance. J. Med. Microbiol. 55:999-1008. [DOI] [PubMed] [Google Scholar]
- 2.Baillie, G. S., and L. J. Douglas. 1998. Effect of growth rate on resistance of Candida albicans biofilms to antifungal agents. Antimicrob. Agents Chemother. 42:1900-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chandra, J., D. M. Kuhn, P. K. Mukherjee, L. L. Hoyer, T. McCormick, and M. A. Ghannoum. 2001. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J. Bacteriol. 183:5385-5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis, D., R. B. Wilson, and A. P. Mitchell. 2000. RIM101-dependent and-independent pathways govern pH responses in Candida albicans. Mol. Cell. Biol. 20:971-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donlan, R. M. 2001. Biofilm formation: a clinically relevant microbiological process. Clin. Infect. Dis. 33:1387-1392. [DOI] [PubMed] [Google Scholar]
- 6.Douglas, C. M., J. A. D'Ippolito, G. J. Shei, M. Meinz, J. Onishi, J. A. Marrinan, W. Li, G. K. Abruzzo, A. Flattery, K. Bartizal, A. Mitchell, and M. B. Kurtz. 1997. Identification of the FKS1 gene of Candida albicans as the essential target of 1,3-beta-D-glucan synthase inhibitors. Antimicrob. Agents Chemother. 41:2471-2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Douglas, L. J. 2003. Candida biofilms and their role in infection. Trends Microbiol. 11:30-36. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Effron, G., S. Park, and D. S. Perlin. 2009. Correlating echinocandin MIC and kinetic inhibition of fks1 mutant glucan synthases for Candida albicans: implications for interpretive breakpoints. Antimicrob. Agents Chemother. 53:112-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hawser, S. P., and L. J. Douglas. 1994. Biofilm formation by Candida species on the surface of catheter materials in vitro. Infect. Immun. 62:915-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kojic, E. M., and R. O. Darouiche. 2004. Candida infections of medical devices. Clin. Microbiol. Rev. 17:255-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuhn, D. M., and M. A. Ghannoum. 2004. Candida biofilms: antifungal resistance and emerging therapeutic options. Curr. Opin. Invest. Drugs 5:186-197. [PubMed] [Google Scholar]
- 12.Mah, T. F., B. Pitts, B. Pellock, G. C. Walker, P. S. Stewart, and G. A. O'Toole. 2003. A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature 426:306-310. [DOI] [PubMed] [Google Scholar]
- 13.McDonnell, G. E. 2007. Antisepsis, disinfection, and sterilization: types, action, and resistance. ASM Press, Washington, DC.
- 14.Mukherjee, P. K., J. Chandra, D. M. Kuhn, and M. A. Ghannoum. 2003. Mechanism of fluconazole resistance in Candida albicans biofilms: phase-specific role of efflux pumps and membrane sterols. Infect. Immun. 71:4333-4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.NCCLS/CLSI. 2002. Reference method for broth dilution antifungal susceptibility testing. Document M27-A2, 2nd ed. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 16.Nett, J., L. Lincoln, K. Marchillo, R. Massey, K. Holoyda, B. Hoff, M. VanHandel, and D. Andes. 2007. Putative role of beta-1,3 glucans in Candida albicans biofilm resistance. Antimicrob. Agents Chemother. 51:510-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nett, J. E., H. Sanchez, M. T. Cain, and D. Andes. 2010. Genetic basis of Candida biofilm resistance due to drug sequestering matrix glucan. J. Infect. Dis. 202:171-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Toole, G. A. 2003. To build a biofilm. J. Bacteriol. 185:2687-2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramage, G., S. Bachmann, T. F. Patterson, B. L. Wickes, and J. L. Lopez-Ribot. 2002. Investigation of multidrug efflux pumps in relation to fluconazole resistance in Candida albicans biofilms. J. Antimicrob. Chemother. 49:973-980. [DOI] [PubMed] [Google Scholar]
- 20.Ramage, G., K. Vande Walle, B. L. Wickes, and J. L. Lopez-Ribot. 2001. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob. Agents Chemother. 45:2475-2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramage, G., K. VandeWalle, S. P. Bachmann, B. L. Wickes, and J. L. Lopez-Ribot. 2002. In vitro pharmacodynamic properties of three antifungal agents against preformed Candida albicans biofilms determined by time-kill studies. Antimicrob. Agents Chemother. 46:3634-3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roemer, T., B. Jiang, J. Davison, T. Ketela, K. Veillette, A. Breton, F. Tandia, A. Linteau, S. Sillaots, C. Marta, N. Martel, S. Veronneau, S. Lemieux, S. Kauffman, J. Becker, R. Storms, C. Boone, and H. Bussey. 2003. Large-scale essential gene identification in Candida albicans and applications to antifungal drug discovery. Mol. Microbiol. 50:167-181. [DOI] [PubMed] [Google Scholar]
- 23.Vediyappan, G., T. Rossignol, and C. d'Enfert. 2010. Interaction of Candida albicans biofilms with antifungals: transcriptional response and binding of antifungals to beta-glucans. Antimicrob. Agents Chemother. 54:2096-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]