Abstract
The development of arachidonic acid (ARA) for treatment of schistosomiasis is an entirely novel approach based on a breakthrough discovery in schistosome biology revealing that activation of parasite tegument-bound neutral sphingomyelinase (nSMase) by unsaturated fatty acids, such as ARA, induces exposure of parasite surface membrane antigens to antibody binding and eventual attrition of developing schistosomula and adult worms. Here, we demonstrate that 5 mM ARA leads to irreversible killing of ex vivo 1-, 3-, 4-, 5-, and 6-week-old Schistosoma mansoni and 9-, 10-, and 12-week-old Schistosoma haematobium worms within 3 to 4 h, depending on the parasite age, even when the worms were maintained in up to 50% fetal calf serum. ARA-mediated worm attrition was prevented by nSMase inhibitors, such as CaCl2 and GW4869. Scanning and transmission electron microscopy revealed that ARA-mediated worm killing was associated with spine destruction, membrane blebbing, and disorganization of the apical membrane structure. ARA-mediated S. mansoni and S. haematobium worm attrition was reproduced in vivo in a series of 6 independent experiments using BALB/c or C57BL/6 mice, indicating that ARA in a pure form (Sigma) or included in infant formula (Nestle) consistently led to 40 to 80% decrease in the total worm burden. Arachidonic acid is already marketed for human use in the United States and Canada for proper development of newborns and muscle growth of athletes; thus, ARA has potential as a safe and cost-effective addition to antischistosomal therapy.
Schistosomiasis is considered the second most important parasitic infection, after malaria, in terms of its public health and economic impacts, affecting 207 million people in the developing world, with 779 million, mostly children, at risk of infection (27). Schistosomiasis is caused by the platyhelminth worms of the genus Schistosoma, with 3 species (Schistosoma mansoni, S. haematobium, and S. japonicum) accounting for the majority of human infections. Cercariae penetrate the unbroken skin of humans or animals, where they change into schistosomula. Once in the blood capillaries, the schistosomula are carried passively by the blood flow until they reach the lungs. Depending on the species, schistosomula stay inside the pulmonary capillaries for 3 to 16 days and then make their way to the liver via the splanchnic vasculature. Maturity is reached between 28 and 35 or 70 and 90 days postinfection for S. mansoni and S. haematobium, respectively. Eggs deposited daily in massive numbers must traverse the walls of the blood venules to enter the lumen of the intestine or bladder and then be excreted with the feces or urine. The morbidity associated with schistosomiasis results from the immunologic reactions to egg-derived antigens, as well as the mechanical and toxic irritation caused by eggs trapped in the walls of blood vessels (11).
Praziquantel (PZQ), an isoquinoline-parazine derivative, immediately proved much superior to any other schistosomicidal drug and quickly became the drug of choice in most areas of endemicity. However, evidence of emerging drug resistance and low efficacy of PZQ have been reported in Egypt and Senegal (1). Moreover, the possibility that PZQ binds to host actin cannot be entirely precluded (9, 22, 28). Accordingly, it is imperative to develop new effective drugs for treatment and prevention of the infection (4).
The antimalarial drug artemether, a methoxy derivative of artemisinin, has been shown to be active against the juvenile stages of S. mansoni (34) and S. haematobium (35) in experimentally infected animals, while it is less effective on adult worms. However, a recent study has shown that a single oral dose of 400 mg artemether/kg of bodyweight to mice infected with approximately 80 cercariae of S. mansoni 21 (prepatent) or 49 (patent period) days earlier led to 71 to 81% reduction in the total worm burden (17). Mefloquine, another antimalarial drug, was also found to have significant antischistosome activity in vivo, as a single dose (200 or 400 mg/kg) administered orally to mice infected with adult S. mansoni resulted in high worm burden reductions of 72.3% to 100% (16, 17). It has been shown that artemether interacts with hemin to exert a toxic effect on schistosomes, while mefloquine is believed to inhibit hemozoin formation (17). However, there are objections regarding possible interference with the primary use of artemether and mefloquine as antimalarials (31). Oxadiazoles have been selected based on their inhibitory activities against theS. mansoni redox protein thioredoxin-glutathione reductase. An oxadiazole 2-oxide was found to be highly active against all intramammalian life cycle stages of S. mansoni. However, the curative regimen involved 5-day doses given daily over 5 days intraperitoneally (24).
Here, we propose a novel oral treatment for schistosomiasis mansoni and schistosomiasis haematobium based on arachidonic acid (ARA) and show its in vitro and in vivo activities against S. mansoni and S. haematobium juvenile and adult stages. ARA is a powerful activator of the parasite tegument-associated, magnesium-dependent neutral sphingomyelinase (nSmase) (http://www.genedb.org/gene/Smp_162880). Subsequent sphingomyelin hydrolysis elicits an increase in membrane permeability, bending, and aggregation and dramatic perturbations in lipid content and rigidity, allowing the free movement of antigenic molecules in the plane of the surface membranes and avid binding of specific antibodies (8, 29, 30).
ARA, a 20-carbon fatty acid with 4 double bonds, the first of which is located at the sixth carbon atom from the methyl (omega) end [20:4(ω-6)], is present in the phospholipids (especially phosphatidylethanolamine, phosphatidylcholine, and phosphatidylinositides) of body cell membranes and is abundant in the brain and muscles. It is a precursor in the production of eicosanoids, the prostaglandins, thromboxanes, prostacycline, and the leukotrienes through enzymes, including cyclooxygenase, lipoxygenase, and peroxidase (2). Proper development of the brain, retina, and other body tissues depends upon provision of ARA either directly in the diet (lean meat, egg yolks, and some fish oils) or through synthesis from linoleic acid (36). It is notable that ingestion of ω-3 essential fatty acids, namely, eicosapentaenoic acid and docosahexaenoic acid (DHA), entirely protects against any inflammatory effect ARA might elicit by displacement, competitive inhibition, and direct counteraction (25). ARA and DHA proved entirely safe and were recently incorporated into infant formulas (14, 18).
MATERIALS AND METHODS
Drugs and reagents.
ARA was obtained from Sigma Chemical Co. (St. Louis, MO). Nestle Good Start Supreme DHA and ARA infant formula contains 320 mg/100 g DHA and 640 mg/100 g ARA and was purchased from Diapers (Montclair, NJ).
Animals and parasites.
Male Syrian hamsters and female 6- to 8-week-old BALB/c and C57BL/6 mice were raised and maintained throughout experimentation at the Schistosome Biological Materials Supply Program, Theodore Bilharz Research Institute (SBSP/TBRI), Giza, Egypt. All animal experiments were performed following the recommendations of the current edition of the Guide for the Care and Use of Laboratory Animals, Institute of Laboratory Animal Resources, National Research Council.
Cercariae of Egyptian strains of S. mansoni and S. haematobium were obtained from SBSP/TBRI and used for infection immediately after being shed from Biomphalaria alexandrina, and Bulinus truncatus snails, respectively.
Lung stage schistosomula were recovered from Syrian hamsters 6 days after percutaneous infection with 2,000 cercariae of S. mansoni or S. haematobium/hamster. Lung pieces were incubated in RPMI 1640 medium supplemented with 200 U/ml penicillin, 200 μg/ml streptomycin, 25 mM HEPES (RPMI medium), 5 U/ml heparin, and 10% fetal calf serum (all from BioWhittaker Europe, Verviers, Belgium) for 4 h at 37°C in a humidified atmosphere containing 5.0% CO2, to allow the schistosomula to emerge. The larval suspension was then poured through a sterile Nitex 132-mesh nylon screen; treated for 10 min at 4°C with sterile 0.16 M ammonium chloride-0.017 M Tris buffer, pH 8.0, to lyse the erythrocytes; and isolated from contaminant lung cells by centrifugation over 40% Percoll (Pharmacia, Uppsala, Sweden) in RPMI medium as described previously (29, 30). All larvae were viable, transparent, elongated, and highly motile.
Adult worms were recovered from Syrian hamsters at the time intervals specified in the text after percutaneous infection with 400 ± 25 cercariae of S. mansoni or S. haematobium as described previously (26). Infection and hepatic perfusion were performed under anesthesia at SBSP/TBRI. Adult worms were transported from SBSP/TBRI to the laboratory in ice-cold RPMI medium and washed 10 times, taking great care for parasite tegument integrity, and male (M) and female (F) worms were placed in wells of sterile, flat-bottom, 24-well plates (Corning, NY). Only viable, contractile worms showing total tegument integrity, as assessed by ×40 light microscopy (Olympus Inverted Microscope Model IX70; Olympus, Tokyo, Japan) were included in the different investigations.
In vitro studies.
Lung stage larvae and juvenile and adult worms were maintained in wells of sterile 24-well plates in 1.0 ml RPMI medium supplemented with 20% (unless otherwise stated) fetal calf serum (FCS) and 0, 2.5, 5.0, or 10 mM ARA. The pH of the medium was invariably 7.0 to 7.3 in all wells. Worms were monitored for motility, contractility, and viability every hour for a 5-h incubation period at 37°C and 5% CO2, and 24 h after the worms were washed and incubated in drug-free RPMI medium-20% FCS. ARA-treated juvenile and adult worms were washed in FCS-free RPMI medium and processed for scanning and transmission electron microscopy as described previously (33).
nSMase inhibitors were assessed for their effect on ARA in vitro schistosomicidal activity. GW4869 (Calbiochem), a potent, specific, and noncompetitive inhibitor of nSMase (19, 20, 30), was dissolved in dimethyl sulfoxide (DMSO) and used at the preselected concentration of 12.5 μM (30). Calcium chloride (CaCl2), a potent inhibitor of nSMase activity at mM concentrations (15, 23, 29), was used at a final concentration of 2 mM.
In vivo studies.
BALB/c and C57BL/6 mice (20 ± 2 g each) were infected via tail exposure with cercariae of S. mansoni or S. haematobium as described previously (26) and then orally administered pure ARA or Nestle infant formula. A single oral dose of pure ARA (Sigma), dissolved in 100 μl corn oil, was given to test mice on day 7 (500 mg/kg, targeting the lung stage) or day 35 or 70 (1,000 mg/kg, targeting the adult S. mansoni and S. haematobium worms, respectively). Control mice were orally administered 100 μl corn oil. ARA-containing (6.4 mg/g) and ARA-free Nestle infant formula was dissolved in sterile water and offered to the mice instead of drinking water on days 1 to 15 (targeting the lung stage) after infection with S. mansoni or days 25 to 40 or 70 to 85 (targeting the adult worms) following infection with S. mansoni and S. haematobium, respectively. The amount of milk consumed was evaluated daily and was found to be 0.9 ± 0.1 g/mouse, i.e., corresponding to approximately 6 mg ARA/mouse/day, or 300 mg/kg/day.
The total worm burden in control and ARA-treated mice was evaluated on days 42 and 90 for S. mansoni and S. haematobium, respectively.
Statistical analysis.
All values were tested for normality. Student's unpaired t test and the Mann-Whitney test were used to analyze the statistical significance of differences between mean experimental and control values, and a P value of <0.05 was considered significant.
RESULTS
In vitro activities.
Adult S. mansoni (6-week-old) and S. haematobium (12-week-old) worms were dead after incubation for 3 h in RPMI medium supplemented with 20% FCS and 10 mM ARA. Worms exposed to 10 mM ARA in RPMI medium supplemented with 50 or 100% instead of 20% FCS were alive at 3 h, but all died 1 or 2 h later, with S. haematobium appearing slightly more sensitive to ARA schistosomicidal activity than S. mansoni worms (Table 1). Parasite death was irreversible, as judged by examination of worms following washing and incubation overnight in ARA-free RPMI medium supplemented with 20% FCS.
TABLE 1.
Effects of FCS on in vitro schistosomicidal activity of arachidonic acid
| Adult worm | FCS % | ARA concn (mM) | No. of worms investigated | No. of dead worms after incubation for (h): |
|||
|---|---|---|---|---|---|---|---|
| 1 | 3 | 4 | 5a | ||||
| S. mansoni | 20 | 0 | 13 M, 5 F | 0 | 0 | 0 | 0 |
| 10 | 4 M, 5 F | 0 | All | All | All | ||
| 20 | 4 M, 7 F | 0 | All | All | All | ||
| 50 | 0 | 6 M, 6 F | 0 | 0 | 0 | 0 | |
| 10 | 8 M, 9 F | 0 | 0 | 7 M, 9 F | All | ||
| 20 | 7 M, 6 F | 0 | 0 | 4 M, 5 F | All | ||
| 100 | 0 | 4 M, 7 F | 0 | 0 | 0 | 0 | |
| 10 | 7 M, 6 F | 0 | 0 | 0 | All | ||
| 20 | 4 M, 5 F | 0 | 0 | 0 | All | ||
| S. haematobium | 20 | 0 | 3 M, 4 F | 0 | 0 | 0 | 0 |
| 10 | 2 M, 4 F | 0 | All | All | All | ||
| 20 | 2 M, 3 F | 0 | All | All | All | ||
| 50 | 0 | 4 M, 2 F | 0 | 0 | 0 | 0 | |
| 10 | 5 M, 2 F | 0 | 1 M, 2 F | All | All | ||
| 20 | 4 M, 2 F | 0 | All | All | All | ||
| 100 | 0 | 4 M, 5 F | 0 | 0 | 0 | 0 | |
| 10 | 3 M, 2 F | 0 | 2 M, 1 F | All | All | ||
| 20 | 3 M, 3 F | 0 | 1 M, 1 F | 2 M, 2 F | All | ||
All worms were dead after washing and overnight incubation in ARA-free RPMI medium-20% FCS.
A series of 4 independent experiments revealed that adult S. mansoni and S. haematobium worms were irreversibly killed 5 h following incubation in RPMI medium-20% FCS supplemented with 2.5 or 5.0, but not 1.0, mM ARA (Table 2). Juvenile and adult schistosomes appeared highly sensitive to ARA schistosomicidal action, which was obviously more drastic in the juvenile (3-week) and larval (7-day) stages (Table 3).
TABLE 2.
Determination of arachidonic acid in vitro schistosomicidal concentrationsa
| Group | ARA concn (mM) | No. of worms investigated | No. of dead worms after incubation for (h): |
|||
|---|---|---|---|---|---|---|
| 1 | 3 | 4 | 5b | |||
| S. mansoni | 0 | 10 M, 8 F | 0 | 0 | 0 | 0 |
| (6 wk) | 1.0 | 13 M, 8 F | 0 | 0 | 0 | 0 |
| 2.5 | 9 M, 10 F | 0 | 0 | 0 | All | |
| 5.0 | 16 M, 9 F | 0 | 4 F | 8 M, 6 F | All | |
| 10.0 | 13 M, 14 F | 0 | 12 F | All | All | |
| S. haematobium | 0 | 2 M, 4 F | 0 | 0 | 0 | 0 |
| (12 wk) | 1.0 | 2 M, 4 F | 0 | 0 | 0 | 0 |
| 2.5 | 2 M, 3 F | 0 | 0 | 0 | All | |
| 5.0 | 3 M, 3 F | 0 | 2 F | 2 M, 2 F | All | |
| 10.0 | 3 M, 4 F | 0 | 4 F | All | All | |
Representative of 4 independent experiments in which worms were incubated in RPMI medium-20% FCS.
All male and female worms were dead after washing and overnight incubation in ARA-free RPMI medium-20% FCS.
TABLE 3.
In vitro effects of arachidonic acid against adult and juvenile schistosomesa
| Group | ARA concn (mM) | No. of worms investigated | No. of dead worms after incubation for (h): |
|||
|---|---|---|---|---|---|---|
| 1 | 3 | 4 | 5b | |||
| S. mansoni | 0 | 3 M, 5 F | 0 | 0 | 0 | 0 |
| (6 wk) | 2.5 | 4 M, 4 F | 0 | 0 | All | All |
| 5.0 | 5 M, 7 F | 0 | 1 M, 3 F | 5 M, 6 F | All | |
| S. mansoni | 0 | 30 M, 19 F | 0 | 0 | 0 | 0 |
| (5 wk) | 2.5 | 21 M, 10 F | 0 | 0 | 5 M, 9 F | All |
| 5.0 | 19 M, 13 F | 0 | 0 | 10 M, 10 F | All | |
| S. mansoni | 0 | 28 M, 5 F | 0 | 0 | 0 | 0 |
| (4 wk) | 2.5 | 17 M, 6 F | 0 | 0 | 12 M, 6 F | All |
| 5.0 | 15 M, 7 F | 0 | 2 M | 8 M, 5 F | All | |
| S. mansoni | 0 | 47 | 0 | 0 | 0 | 0 |
| (3 wk) | 2.5 | 40 | 0 | 35 | All | All |
| 5.0 | 41 | 0 | All | All | All | |
| S. mansoni | 0 | 45 | 0 | 0 | 0 | 0 |
| (7 days) | 2.5 | 40 | 0 | All | All | All |
| 5.0 | 42 | 0 | All | All | All | |
| S. haematobium | 0 | 9 M, 4 F | 0 | 0 | 0 | 0 |
| (12 wk) | 2.5 | 9 M, 3 F | 0 | 0 | All | 0 |
| 5.0 | 5 M, 3 F | 0 | 3 M | All | 0 | |
| S. haematobium | 0 | 9 M, 8 F | 0 | 0 | 0 | 0 |
| (10 wk) | 2.5 | 14 M, 10 F | 0 | 0 | 12 M, 8 F | All |
| 5.0 | 9 M, 5 F | 0 | 0 | 3 M, 2 F | All | |
| S. haematobium | 0 | 2 M, 2 F | 0 | 0 | 0 | 0 |
| (8 wk) | 2.5 | 3 M, 3 F | 0 | 0 | 0 | All |
| 5.0 | 4 M, 5 F | 0 | 0 | 4 M, 4 F | All | |
| S. haematobium | 0 | 25 | 0 | 0 | 0 | 0 |
| (7 days) | 2.5 | 20 | 0 | All | All | All |
| 5.0 | 28 | 0 | All | All | All | |
Representative of 2 independent experiments in which worms were incubated in RPMI medium-20% FCS.
All male and female worms were dead after washing and overnight incubation in ARA-free RPMI medium-20% FCS.
Inhibitors of nSMase were added to S. mansoni and S. haematobium adult worm cultures supplemented with 0 or 5 mM ARA. The data from 4 independent experiments indicated that 2 mM CaCl2 and 12.5 μM GW4869 entirely protected schistosomes from ARA lethal action (Table 4).
TABLE 4.
Effects of nSMase inhibitors on arachidonic acid in vitro schistosomicidal activitya
| Group | No. of worms investigated | No. of dead worms after incubation for (h): |
|||
|---|---|---|---|---|---|
| 1 | 3 | 4 | 5 | ||
| S. mansoni (6 wk) | |||||
| 0.5% DMSO | 7 M, 4 F | 0 | 0 | 0 | 0 |
| 12.5 μM GW4869/0.5% DMSO | 4 M, 6 F | 0 | 0 | 0 | 0 |
| 5 mM ARA/0.5% DMSO | 5 M, 5 F | 0 | 0 | 3 M, 5 F | All |
| 5 mM ARA/12.5 μM GW4869/0.5% DMSO | 5 M, 5 F | 0 | 0 | 0 | 0 |
| 2 mM CaCl2 | 5 M, 6 F | 0 | 0 | 0 | 0 |
| 5 mM ARA | 4 M, 4 F | 0 | 0 | 2 F | All |
| 5 mM ARA/2 mM CaCl2 | 7 M, 5 F | 0 | 0 | 0 | 0 |
| S. haematobium (12 wk) | |||||
| 0.5% DMSO | 8 M, 7 F | 0 | 0 | 0 | 0 |
| 12.5 μM GW4869/0.5% DMSO | 6 M, 4 F | 0 | 0 | 0 | 0 |
| 5 mM ARA/0.5% DMSO | 7 M, 5 F | 0 | 0 | 4 M, 3 F | All |
| 5 mM ARA/12.5 μM GW4869/0.5% DMSO | 7 M, 4F | 0 | 0 | 0 | 4 M, 1 F |
| 2 mM CaCl2 | 6 M, 4 F | 0 | 0 | 0 | 0 |
| 5 mM ARA | 8 M, 5 F | 0 | 0 | 6 M, 3 F | All |
| 5 mM ARA/2 mM CaCl2 | 12 M, 5 F | 0 | 0 | 0 | 0 |
Representative of 4 independent experiments in which male and female worms were incubated in 1.0 ml RPMI medium-20% FCS.
Electron microscopy investigations.
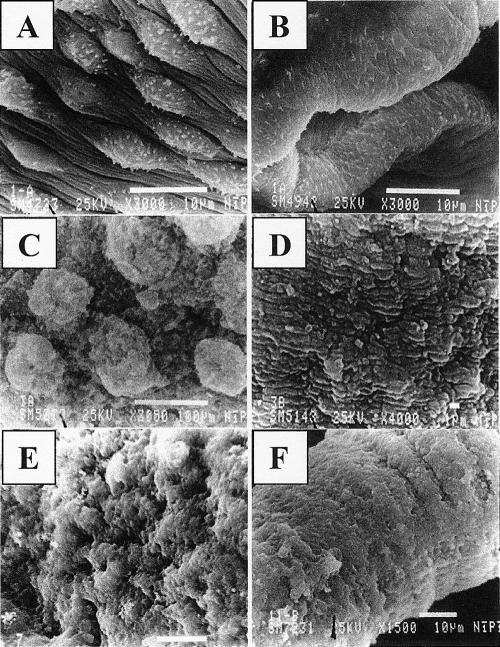
Adult S. mansoni (6-week-old) and S. haematobium (12-week-old) worms incubated for 4 h in medium supplemented with 20% FCS and 0 mM ARA showed intact surface structure and topography (Fig. 1 A and B). Treatment of adult schistosomes with 2.5 mM ARA for 4 h led to a pronounced change in the aspect of the tubercles, which often appeared collapsed, disrupted, and reduced in size and showed spine loss. Additionally, there was evident shrinkage and wrinkling in the areas between tubercles. Exposure of adult male worms to 5.0 mM ARA for 4 h elicited wrinkling and collapse of the tubercles, which looked edematous, short, and blunt. Disruption of the sensory bulbs and swelling and pronounced edema in the intertegumental areas were evident. The most significant morphological degeneration was the conspicuous bubble-like lesions (Fig. 1C). The teguments of female worms exposed to 5.0 mM ARA for 4 h were highly affected, and marked surface swelling and lesions were widespread (Fig. 1D). Dilation and edema of the oral sucker with loss of the lining spines and wrinkling and edema with loss of spines in the ventral sucker were significant (data not shown). Three-week-old S. mansoni worms treated with 2.5 mM ARA for 4 h showed extreme deformation of the tegumental ultrastructure and oral and ventral suckers. Edema, swelling, and severe lesions were widespread (Fig. 1E), supporting the higher sensitivity of 3 week-old juvenile S. mansoni worms to ARA schistosomicidal action. S. haematobium juveniles treated with 2.5 mM ARA for 4 h appeared eroded, deformed, and edematous (Fig. 1F), in complete accord with the high sensitivity of S. haematobium worms to ARA.
FIG. 1.
Scanning electron microscopy investigation of ARA in vitro schistosomicidal effect. S. mansoni (A, C, and E) and S. haematobium (B, D, and F) adult (A to D) or juvenile (E and F) worms incubated for 4 h in RPMI medium supplemented with 20% FCS and 0 (A and B), 5 (C and D), or 2.5 (E and F) mM ARA were examined by scanning electron microscopy.
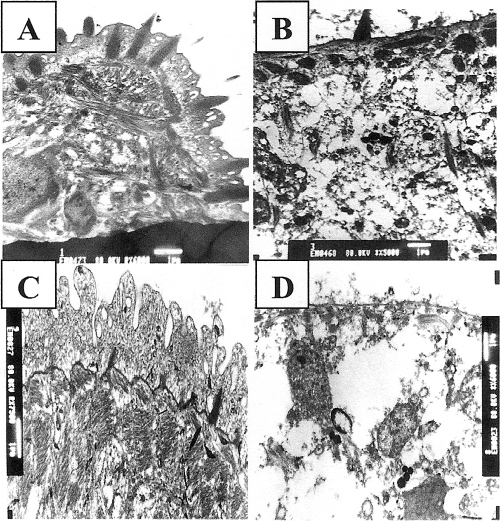
The tegumental ultrastructure of adult S. mansoni and S. haematobium worms incubated for 4 h in RPMI medium supplemented with 20% FCS and 0 mM ARA appeared intact (33) (Fig. 2 A). Treatment of adult worms with 2.5 mM ARA for 4 h elicited changes in the tegument structure that varied in different areas from slight to severe. Thus, some areas presented with a slight degree of basal vacuolation associated with the basal lamina, while other areas showed marked fragmentation and vacuolation, which extended to the muscle layers. Exposure to 5.0 mM ARA led to extensive damage, disorganization, and degeneration of the tegument and the subtegumental musculature (Fig. 2B), thus giving a sound explanation for ARA schistosomicidal action at the cellular level.
FIG. 2.
Transmission electron microscopy investigation of ARA in vitro schistosomicidal effect. Adult (A and B) and 3-week-old (C and D) S. mansoni worms incubated for 4 h in RPMI medium supplemented with 20% FCS and 0 (A and C), 5 (B), or 2.5 (D) mM ARA were examined by transmission electron microscopy.
Juvenile S. mansoni and S. haematobium worms' tegument ultrastructure did not differ greatly from that of the adult (Fig. 2A), except for the reduced size of the spines and the lower number of vesicles and dense bodies (Fig. 2C). The response to exposure to 2.5 mM ARA, however, was far more drastic than for adults, with widespread disintegration of the apical membrane layer, disorganization, deformation, and extensive damage to the tegumental syncytium and subtegumental muscle bundles (Fig. 2D).
In vivo studies.
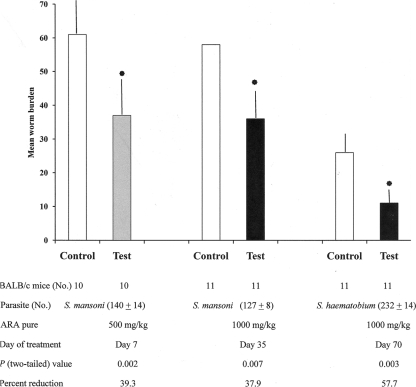
BALB/c and C57BL/6 mice were used to evaluate the in vivo ARA action on survival of S. mansoni and S. haematobium worms. The data shown in Fig. 3 indicate that a single ARA oral dose calculated to elevate the plasma level to approximately 12 mM (targeting the lung stage) or 30 mM (targeting the adult stage) led to a significant (P < 0.05 to < 0.01) reduction of 31.2 to 39.3% in the S. mansoni and 57% in the S. haematobium total worm burden (Fig. 3) and male and female worms (data not shown).
FIG. 3.
Recovery of schistosomes in mice treated with pure ARA. BALB/c mice infected with cercariae of S. mansoni or S. haematobium were treated with a single oral dose of pure ARA, and the worm burden was evaluated 42 and 90 days after infection, respectively. The bars represent mean worm burdens in 10 or 11 mice, and the error bars depict the standard deviation (SD) about the mean. The asterisks indicate significant (P < 0.01) differences.
As with pure ARA, ARA-infant formula oral administration did not lead to detectable change in host survival, activity, appetite, or plasma cholesterol or triglyceride concentrations (data not shown) and was associated with highly significant (P < 0.02 to < 0.001) reduction of 43.0 to 63.6% in the S. mansoni and 81.4% in the S. haematobium total worm burden (Fig. 4).
FIG. 4.
Recovery of schistosomes in mice treated with ARA/infant formula. BALB/c and C57BL/6 mice infected with cercariae of S. mansoni or S. haematobium were treated orally with ARA/infant formula for 15 days at the prepatent or patent period, and the worm burden was evaluated 42 and 90 days after infection, respectively. The bars represent mean worm burdens in 8 or 10 mice, and the error bars depict the SD about the mean. Star, P < 0.05; single asterisk, P < 0.01; and double asterisk, P < 0.001.
DISCUSSION
Exposure of larval, juvenile, and adult stages of S. mansoni and S. haematobium to 5 mM (1.5 mg/ml) ARA led to their irreversible attrition within less than 5 h, indicating that ARA targets all intramammalian stages of the parasite, similarly to mefloquine and oxadiazole 2-oxide (16, 17, 24). It is believed that ARA-mediated killing is essentially due to excessive activation of parasite nSMase, leading to sphingomyelin hydrolysis into ceramide and phosphorylcholine (8). Replacement of sphingomyelin at the external leaflet of the parasite outer lipid bilayer by ceramide induces drastic changes in the membrane charge, fluidity, permeability, and integrity (10). Ceramide was recently reported to be responsible for lipid bilayer scrambling (5, 6). Production of ceramide may be the principal cause of parasite death following excessive nSMase activation, as ceramide is known to act as a second messenger, a key factor in signal transduction pathways associated with cell differentiation, cell cycle arrest, and programmed cell death (apoptosis) (12, 21). Our hypothesis is supported by several pieces of evidence. First, increase of FCS to 50 and 100% in the culture medium delayed ARA lethal action, likely via provision of larger amounts of cholesterol and substances necessary for efficient phospholipid and sphingomyelin synthesis (8). Second, while dead parasites did not show spasmodic body contractions as they did after PZQ treatment, electron microscopy studies revealed entire disruption of the outer lipid bilayers, the strength of which was correlated with the ARA concentration. Third, addition of the nSMase inhibitors CaCl2 (15, 23, 29) and GW4869 (19, 20, 30) entirely protected the parasite from the lethal ARA actions, and nearly all worms remained viable beyond the 5-h exposure period.
In vivo increase of ARA in the mouse circulation to approximately 10 or >10 mM was achieved by a single oral dose of 500 or 1,000 mg/kg pure ARA, respectively. No adverse effects were observed in treated mice regarding appetite, activity, survival, gross pathology, and serum lipid levels. An analysis of results from subchronic adult/rat toxicology studies of several ARA-rich oils from Mortierella alpina also indicated that at doses of these long-chain polyunsaturated fatty acids below 2,000 mg/kg/day, no adverse effects are expected in that species (3, 32). Our oral ARA treatment was associated with significant (P < 0.05 to < 0.01) reduction of 31.2 to 39.3% in the total worm burden and male and female worm counts. The reductions observed are inferior to those observed with oxadiazole 2-oxide, a curative regimen of which involved 5-day doses given intraperitoneally (24). The rather modest ARA-mediated protection could be due to the short life span of the free fatty acid in the circulation (2). In support of this, feeding mice with approximately 300 mg/kg ARA included in infant formula daily for 15 days during the prepatent and patent periods led to highly significant (P < 0.02 to < 0.001) worm burden reduction that reached approximately 60% for S. mansoni and 80% for S. haematobium. No adverse effects were noted in any treated mice, supporting the data showing that administration of 2,000 mg/kg ARA oil for a period of 4 weeks did not induce obvious signs of toxicity in Wistar rats (14).
It is not clear whether in vivo ARA-mediated worm killing is predominantly due to production of ceramide (12, 21) or to exposure of the parasite to the host effector immune cells, antibodies, and toxic molecules and radicals (8, 29, 30). Of note, S. haematobium, which displayed higher in vivo sensitivity to ARA than S. mansoni, was shown to be more sensitive than S. mansoni to in vitro ARA-mediated exposure of otherwise concealed surface membrane antigens (29). Our preliminary unpublished results using immunocompromised mice and hamsters also provided evidence for the latter hypothesis. However, further studies are needed and planned to reach a definite consensus.
The importance of ARA in supporting neurovascular membrane integrity in preterm infants (7) and in the proper function of the immune system (13) is increasingly recognized. Arachidonic acid is already marketed for human use in the United States and Canada for proper development of newborns and muscle growth of athletes. Accordingly, the preclinical and clinical trials and costs will be at a minimum. Based on the above, evaluation of ARA potential for treatment of schistosomiasis could be entirely cost-effective in the near future.
Acknowledgments
Financial support by Arab Foundation for Scientific Research and Technology (ASTF) grant no. BT05205 and Science and Technology Development Fund (STDF) grant no. 144 is gratefully acknowledged. Use of ARA for treatment of schistosomiasis is covered by a patent application published by the United Kingdom Intellectual Property Office on 18 November 2009 under number GB2460056A.
We thank the reviewers for invaluable suggestions and constructive criticisms.
Footnotes
Published ahead of print on 17 May 2010.
REFERENCES
- 1.Botros, S. S., and J. L. Bennett. 2007. Praziquantel resistance. Expert Opin. Drug Discov. 2(Suppl. 1):S35-S40. [DOI] [PubMed] [Google Scholar]
- 2.Brash, A. R. 2001. Arachidonic acid as a bioactive molecule. J. Clin. Invest. 107:1339-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burns, R. A., G. J. Wibert, D. A. Diersen-Schade, and C. M. Kelly. 1999. Evaluation of single-cell sources of docosahexaenoic acid and arachidonic acid: 3-month rat oral safety study with an in utero phase. Food Chem. Toxicol. 37:23-36. [DOI] [PubMed] [Google Scholar]
- 4.Cioli, D., C. Valle, F. Angelucci, and A. E. Miele. 2008. Will new antischistosomal drugs finally emerge? Trends Parasitol. 24:379-382. [DOI] [PubMed] [Google Scholar]
- 5.Contreras, F. X., L. Sánchez-Magraner, A. Alonso, and F. M. Goñi. 2010. Transbilayer (flip-flop) lipid motion and lipid scrambling in membranes. FEBS Lett. 584:1779-1786. [DOI] [PubMed] [Google Scholar]
- 6.Contreras, F. X., V. Villar, A. Alonso, and F. M. Goñi. 2009. Ceramide-induced transbilayer (flip-flop) lipid movement in membranes. Methods Mol. Biol. 462:155-165. [DOI] [PubMed] [Google Scholar]
- 7.Crawford, M. A., I. Golfetto, K. Ghebremeskel, Y. Min, T. Moodley, L. Poston, A. Phylactos, S. Cunnane, and W. Schmidt. 2003. The potential role for arachidonic and docosahexaenoic acids in protection against some central nervous system injuries in preterm infants. Lipids 38:303-315. [DOI] [PubMed] [Google Scholar]
- 8.El Ridi, R., and H. Tallima. 2006. Equilibrium in lung schistosomula sphingomyelin breakdown and biosynthesis allows very small molecules, but not antibody, to access proteins at the host-parasite interface. J. Parasitol. 92:730-737. [DOI] [PubMed] [Google Scholar]
- 9.Gnanasekar, M., A. M. Salunkhe, A. K. Mallia, Y. X. He, and K. Ramaswamy. 2009. Praziquantel affects the regulatory myosin light chain of Schistosoma mansoni. Antimicrob. Agents Chemother. 53:1054-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goñi, F. M., and A. Alonso. 2002. Sphingomyelinases: enzymology and membrane activity. FEBS Lett. 531:38-46. [DOI] [PubMed] [Google Scholar]
- 11.Gryseels, B., K. Polman, J. Clerinx, and L. Kestens. 2006. Human schistosomiasis. Lancet 368:1106-1118. [DOI] [PubMed] [Google Scholar]
- 12.Hannun, Y. A., and C. Luberto. 2000. Ceramide in the eukaryotic stress response. Trends Cell Biol. 10:73-80. [DOI] [PubMed] [Google Scholar]
- 13.Harbige, L. S. 2003. Fatty acids, the immune response, and autoimmunity: a question of n-6 essentiality and the balance between n-6 and n-3. Lipids 38:323-341. [DOI] [PubMed] [Google Scholar]
- 14.Hempenius, R. A., J. M. H. Van Delft, M. Prinsen, and B. A. R. Lina. 1997. Preliminary safety assessment of an arachidonic acid-enriched oil derived from Mortierella alpina: summary of toxicological data. Food Chem. Toxicol. 35:573-581. [DOI] [PubMed] [Google Scholar]
- 15.Huwiler, A., T. Kolter, J. Pfeilschifter, and K. Sandhoff. 2000. Physiology and pathophysiology of sphingolipid metabolism and signaling. Biochim. Biophys. Acta 1485:63-99. [DOI] [PubMed] [Google Scholar]
- 16.Keiser, J., J. Chollet, S. H. Xiao, J. Y. Mei, P. Y. Jiao, J. Utzinger, and M. Tanner. 2009. Mefloquine—an aminoalcohol with promising antischistosomal properties in mice. PLoS Negl. Trop. Dis. 3:e350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keiser, J., M. Vargas, and M. J. Doenhoff. 2010. Activity of artemether and mefloquine against juvenile and adult Schistosoma mansoni in athymic and immunocompetent NMRI mice. Am. J. Trop. Med. Hyg. 82:112-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koskelo, E. K., K. Boswell, L. Carl, S. Lanoue, C. Kelly, and D. Kyle. 1997. High levels of dietary arachidonic acid triglyceride exhibit no subchronic toxicity in rats. Lipids 132:397-405. [DOI] [PubMed] [Google Scholar]
- 19.Luberto, C., D. F. Hassler, P. Signorelli, Y. Okamoto, H. Sawai, E. Boros, D. J. Hazen-Martin, L. M. Obeid, Y. A. Hannun, and G. K. Smith. 2002. Inhibition of tumor necrosis factor-induced cell death in MCF7 by a novel inhibitor of neutral sphingomyelinase. J. Biol. Chem. 277:41128-41139. [DOI] [PubMed] [Google Scholar]
- 20.Marchesini, N., C. Luberto, and Y. A. Hannun. 2003. Biochemical properties of mammalian neutral sphingomyelinase 2 and its role in sphingolipid metabolism. J. Biol. Chem. 278:13775-13783. [DOI] [PubMed] [Google Scholar]
- 21.Merrill, A. H., Jr. 2002. De novo sphingolipid biosynthesis: a necessary, but dangerous, pathway. J. Biol. Chem. 277:25843-25846. [DOI] [PubMed] [Google Scholar]
- 22.Pica-Mattoccia, L., C. Valle, A. Basso, A. R. Troiani, F. Vigorosi, P. Liberti, A. Festucci, and D. Cioli. 2007. Cytochalasin D abolishes the schistosomicidal activity of praziquantel. Exp. Parasitol. 115:344-351. [DOI] [PubMed] [Google Scholar]
- 23.Rao, B. G., and M. W. Spence. 1976. Sphingomyelinase activity at pH 7.4 in human brain and comparison to activity at pH 5.0. J. Lipid Res. 17:506-515. [PubMed] [Google Scholar]
- 24.Sayed, A. A., A. Simeonov, C. J. Thomas, J. Inglese, C. P. Austin, and D. L. Williams. 2008. Identification of oxadiazoles as new drug leads for the control of schistosomiasis. Nat. Med. 14:407-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simopoulos, A. 2001. Evolutionary aspects of diet and essential fatty acids. World Rev. Nutr. Diet 88:18-27. [DOI] [PubMed] [Google Scholar]
- 26.Smithers, S. R., and R. J. Terry. 1965. The infection of laboratory hosts with cercariae of Schistosoma mansoni and the recovery of the adult worms. Parasitology 55:695-700. [DOI] [PubMed] [Google Scholar]
- 27.Steinmann, P., J. Keiser, R. Bos, M. Tanner, and J. Utzinger. 2006. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect. Dis. 6:411-425. [DOI] [PubMed] [Google Scholar]
- 28.Tallima, H., and R. El Ridi. 2007. Praziquantel binds Schistosoma mansoni adult worm actin. Int. J. Antimicrob. Agents 29:570-575. [DOI] [PubMed] [Google Scholar]
- 29.Tallima, H., M. Salah, and R. El Ridi. 2005. In vitro and in vivo effects of unsaturated fatty acids on Schistosoma mansoni and S. haematobium lung-stage larvae. J. Parasitol. 91:1094-1102. [DOI] [PubMed] [Google Scholar]
- 30.Tallima, H., and R. El Ridi. 2008. Schistosoma mansoni glyceraldehyde 3-phosphate dehydrogenase is a lung-stage schistosomula surface membrane antigen. Folia Parasitol. 55:180-186. [DOI] [PubMed] [Google Scholar]
- 31.Utzinger, J., S. Xiao, E. K. N′Goran, R. Bergquist, and M. Tanner. 2001. The potential of artemether for the control of schistosomiasis. Int. J. Parasitol. 31:1549-1562. [DOI] [PubMed] [Google Scholar]
- 32.Wibert, G. J., R. A. Burns, D. A. Diersen-Schade, and C. M. Kelly. 1997. Evaluation of single cell sources of docosahexaenoic acid and arachidonic acid: a 4-week oral safety study in rats. Food Chem. Toxicol. 35:967-974. [DOI] [PubMed] [Google Scholar]
- 33.Wiest, P. M., S. S. Kunz, and K. R. Miller. 1994. Activation of protein kinase C by phorbol esters disrupts the tegument of Schistosoma mansoni. Parasitology 109:461-468. [DOI] [PubMed] [Google Scholar]
- 34.Xiao, S. H., and B. A. Catto. 1989. In vitro and in vivo studies of the effect of artemether on Schistosoma mansoni. Antimicrob. Agents Chemother. 33:1557-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao, S., J. Utzinger, J. Chollet, Y. Endriss, E. K. N′Goran, and M. Tanner. 2000. Effect of artemether against Schistosoma haematobium in experimentally infected hamsters. Int. J. Parasitol. 30:1001-1006. [DOI] [PubMed] [Google Scholar]
- 36.Zhou, L., and A. Nilsson. 2001. Sources of eicosanoid precursor fatty acid pools in tissues. J. Lipid Res. 42:1521-1542. [PubMed] [Google Scholar]