Abstract
The present study aimed to determine the frequency of methicillin-resistant Staphylococcus aureus (MRSA)-positive clinical culture among hospitalized adults in different risk categories of a targeted MRSA active surveillance screening program and to assess the utility of screening in guiding empiric antibiotic therapy. We completed a prospective cohort study in which all adults admitted to non-intensive-care-unit locations who had no history of MRSA colonization or infection received targeted screening for MRSA colonization upon hospital admission. Anterior nares swab specimens were obtained from all high-risk patients, defined as those who self-reported admission to a health care facility within the previous 12 months or who had an active skin infection on admission. Data were analyzed for the subcohort of patients in whom an infection was suspected, determined by (i) receipt of antibiotics within 48 h of admission and/or (ii) the result of culture of a sample for clinical analysis (clinical culture) obtained within 48 h of admission. Overall, 29,978 patients were screened and 12,080 patients had suspected infections. A total of 46.4% were deemed to be at high risk on the basis of the definition presented above, and 11.1% of these were MRSA screening positive (colonized). Among the screening-positive patients, 23.8% had a sample positive for MRSA by clinical culture. Only 2.4% of patients deemed to be at high risk but found to be screening negative had a sample positive for MRSA by clinical culture, and 1.6% of patients deemed to be at low risk had a sample positive for MRSA by clinical culture. The risk of MRSA infection was far higher in those who were deemed to be at high risk and who were surveillance culture positive. Targeted MRSA active surveillance may be beneficial in guiding empiric anti-MRSA therapy.
Tremendous disagreement exists about the utility of active surveillance for the detection of methicillin-resistant Staphylococcus aureus (MRSA). The primary rationale for the use of active surveillance culturing is to identify MRSA-colonized patients, followed by institution of contact-isolation precautions and/or decolonization regimens, in order to decrease patient-to-patient transmission. There continues to exist great controversy over its benefit (1). The Society for Healthcare Epidemiology of America (SHEA) strongly advocates for its use (14). Veterans Affairs (VA) hospitals and some states, including Illinois, have mandated that active surveillance be used for all admitted patients deemed to be at high risk (25). However, other organizations, such as the Healthcare Infection Control Practices Advisory Committee (HICPAC), do not recommend routine active surveillance for the detection of MRSA (23). Recent studies have had conflicting results (6, 19). Some studies suggest that targeted surveillance, where swab specimens for active surveillance are not obtained from all patients, may be more efficient (4).
In addition to lowering the rate of patient-to-patient transmission of MRSA, early identification of MRSA-colonized patients via active surveillance, especially with the newly emerged rapid diagnostic methods, such as PCR, for identifying MRSA, could be used to guide more appropriate empiric antibiotic coverage. Rapid testing for MRSA could be used to help guide appropriate empiric antibiotic therapy because S. aureus colonization is known to be a strong risk factor for S. aureus infection (24, 20). Data from previous studies have suggested that patients with S. aureus and MRSA infections who do not receive appropriate empiric therapy or who receive delayed appropriate therapy have worse patient outcomes (9, 11, 12). To our knowledge, no study has assessed the clinical utility and feasibility of targeted MRSA active surveillance to guide empiric anti-MRSA therapy. The aim of the present study was to assess the potential clinical utility and feasibility of a program of targeted MRSA active surveillance in guiding empiric antibiotic therapy by determining the frequency of MRSA-positive clinical cultures of samples among hospitalized patients in different risk categories. Additionally, we calculated the number of individuals who needed to be treated for each risk group so that clinicians can begin to weigh the benefits of treating individual patients with anti-MRSA therapy against the risk of overall population exposure to empiric antibiotics.
MATERIALS AND METHODS
Study design and patient population.
The present study was approved by the institutional review board of the University of Maryland, Baltimore, MD. This study utilized a prospective cohort of adult patients who were admitted to non-intensive-care units (non-ICUs) at the University of Maryland Medical Center (UMMC) from 1 February 2007 to 30 June 2008 and who had no history of MRSA colonization or infection. The hospital is a 648-bed, tertiary-care, academic referral center that serves most of metropolitan Baltimore.
On 1 February 2007, UMMC began performing targeted screening for MRSA. On the basis of a previously published rule for prediction of the existence of MRSA, patients were asked on admission two questions as part of the nursing intake triage: (i) have you been admitted to any health care facility in the last 12 months? and (ii) do you have a skin infection (e.g., boil, abscess, spider bite, or cellulitis) at this time? (3). Those who answered “yes” to either question were deemed to be at high risk and were targeted to undergo surveillance nasal swabbing on admission. The nursing intake triage form was administered upon patient admission. Answering either question “yes” automatically generated an order for a swab of the anterior nares for MRSA surveillance to be taken. This was done to increase the compliance and speed of obtaining the surveillance swab results for MRSA.
We chose to analyze several subcohorts of this cohort to assess the frequency of MRSA-positive clinical cultures of samples among hospitalized patients in different risk categories of a targeted MRSA active surveillance program and to assess the potential clinical utility of targeted MRSA active surveillance in guiding empiric antibiotic therapy. The primary subcohort analyzed was (i) patients who had received any oral or parenteral antibiotic within the first 48 h of admission or from whom a sample for clinical culture was obtained within the first 48 h of admission. Other subcohorts analyzed were (ii) patients who had received an antibiotic within the first 48 h of admission and from whom a sample for clinical culture was obtained within the first 48 h of admission, (iii) patients who had received an antibiotic within the first 48 h of admission, and (iv) patients from whom a sample for clinical culture was obtained within the first 48 h of admission. All four analyses yielded similar results. These subcohorts were chosen because they represented the patients in whom clinicians likely suspected an infection and, thus, patients who received empiric antibiotic therapy. For the cohorts and subcohorts, the samples used for clinical culture were those obtained within the first 48 h of admission for nonsurveillance purposes. The antibiotics received within the first 48 h of admission were defined as and determined from the antibiotic orders for the patients in the cohort within 48 h of admission.
Data collection and variables.
All data were abstracted from the UMMC central data repository, which contains the patients' demographic data, microbiological data, and pharmacy data. The validity of these data was assessed by randomly sampling 2% of the patients' electronic data records and comparing them to the original paper medical records. The positive and negative predictive values of this assessment exceeded 99% each, which was similar to the values seen in previous studies with the same data source (5, 7).
The primary outcome variable was the presence of a positive clinical culture result for MRSA for a sample obtained during the same admission, i.e., at any time between hospital admission and hospital discharge. Samples obtained from patients during the same admission in which they underwent screening for MRSA were assessed by clinical culture. Preexisting comorbid conditions were assessed by use of the Charlson comorbidity index (2). We then determined what proportion of the clinical culture-positive samples represented actual infection, as defined using National Healthcare Safety Network (NHSN) definitions. To accomplish this, a senior infection control practitioner (L.J.C.) reviewed each medical record and classified each sample tested by clinical culture as being infected with MRSA or not (8, 15).
Microbiological methods.
Surveillance specimens of the anterior nares were obtained upon admission using one swab for both the right and the left nares. Nasal swabs were processed for MRSA detection using a GeneOhm MRSA assay (Becton Dickinson, Franklin Lakes, NJ), according to the manufacturer's instructions.
Statistical analyses.
All statistical analyses were performed using SAS statistical software, version 9.1 (SAS Institute Inc., Cary, NC). Means and frequency distributions were used to describe the characteristics of the study population. We calculated negative predictive values to assess the ability of the targeted active surveillance program to identify patients from whom samples for clinical culture for MRSA detection were not obtained during the same hospital admission.
RESULTS
During the 17-month study period, 29,978 patients were admitted to non-ICU wards and were asked the targeted MRSA screening questions. The demographics of these patients are as follows. The mean age of the patients was 45 years, and 52% of the patients were male. The mean length of stay in the hospital was 4.9 days, and the median was 2.9 days. The mean comorbidity score, measured from the Charlson comorbidity index, was 1.65, and the median was 1.00. A total of 2,681 (9%) patients were already known to be MRSA positive on the basis of the results of clinical or surveillance cultures from previous admissions and were not tested for MRSA independently of being asked questions on the nursing intake triage admission form.
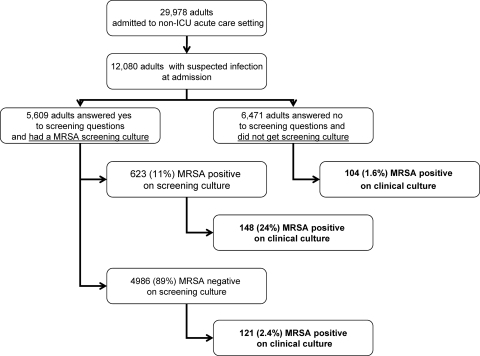
Figure 1, a patient flow diagram, displays the cohort and subcohorts of the patients and the results presented below. A subcohort of 12,080 patients received empiric antibiotics within the first 48 h and/or provided a sample for clinical culture in the first 48 h. Clinicians likely suspected that the patients in this group had an infection. The demographics of these patients are as follows. The mean age of the patients was 45 years, and 52% of the patients were male. The mean length of stay in the hospital was 6.0 days, and the median was 3.6 days. The mean comorbidity score, measured by the Charlson comorbidity index, was 1.75, and the median was 1.00. In the first 48 h, the following proportions of patients received the indicated antibiotics: 2% vancomycin, 16% cephalosporins of any kind, 14% quinolones, 13% cephalosporins cefazolin and cephalexin, and less than 1% carbapenems. In this subcohort, 5,609 (46.4%) answered “yes” to one or both of the questions and thus underwent swabbing for active surveillance screening for MRSA. Of these individuals, 623 (11.1%) were PCR positive for MRSA. Of the 623 MRSA-positive patients, samples obtained from 148 (23.8%) during the same admission were also positive for MRSA by clinical culture. A total of 121 (2.4%) of 4,986 patients who answered “yes” to the screening question but who were negative for MRSA by active surveillance PCR on admission had a subsequent positive culture result for MRSA (negative predictive value, 98%). One hundred four (1.6%) of 6,471 patients who answered “no” to either screening question and who thus did not undergo the active surveillance test had a positive result for MRSA by clinical culture of a sample (negative predictive value, 98%). Of the 2,681 patients who were previously known to be MRSA positive but who were excluded from the screening program, 255 (9.5%) had a positive result for MRSA by clinical culture of a sample obtained on the admission where they were excluded from the screening. The Charlson comorbidity index, either used as a continuous variable (P = 0.18) or a categorical variable, was not statistically significantly associated with a positive result for MRSA by clinical culture of a sample. Table 1 demonstrates the sensitivity, specificity, and positive and negative predictive values for each group, along with the number of individuals in each group who needed to be treated. The number of individuals in each group who needed to be treated is used to indicate the number of patients who need to be treated with anti-MRSA coverage in order to treat one patient who has a positive result for MRSA by clinical culture of a sample. Thus, in this study, 4 patients in the high-risk, surveillance-positive group would need to receive anti-MRSA therapy, while 63 patients in the low-risk group who were screening question negative would need to be treated.
FIG. 1.
Patient flow diagram displaying the cohort and subcohorts of patients and the results below.
TABLE 1.
Sensitivity, specificity, negative predictive value, positive predictive value, and number of different screening groups needed to treat to predict MRSA clinical culture result among patients for whom clinical culture of a sample was performed or who received antibiotics in the first 48 h of admission
| Group | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) | No. needed to treat |
|---|---|---|---|---|---|
| Screening question answers “yes” and MRSA surveillance test positive | 39.7 | 95.9 | 23.8 | 98.0 | 4 |
| Screening question answers “yes” and MRSA surveillance test negative | 32.4 | 58.4 | 2.4 | 96.5 | 42 |
| Screening question answers “no” and thus not tested for MRSA | 27.9 | 45.6 | 1.6 | 95.2 | 63 |
Information about the samples from the 12,080 patients positive for MRSA by clinical culture is as follows. During the same admission (between the times of hospital admission and hospital discharge), 373 patients provided 537 samples that were clinical culture positive for MRSA; of these, 50 (9.3%) were blood samples, 7 (1.3%) were obtained during bronchoscopy, and 1 was a cerebrospinal fluid specimen. There were 314 (58.5%) wound specimens and 57 (10.6%) sputum specimens that were cultured. Using the NHSN definitions outlined in Materials and Methods, we found that 305 (82%) of the 373 patients had clinical infections: 159 had skin and soft tissue infections, 53 had surgical site infections, 33 had bloodstream infections, and 24 had pneumonia or lower respiratory tract infections. The first samples from the 373 patients positive by clinical culture were obtained a median of 12 h after admission, and 75% of the first samples positive by clinical culture were obtained by 43 h after admission.
We performed an additional analysis of the subcohort of 3,097 patients who received empiric antibiotic treatment within the first 48 h and from whom a sample for clinical culture was obtained in the first 48 h (Table 2). Clinicians suspected the individuals in this group of having an infection, and the antibiotics used were chosen empirically. Of the individuals in this subcohort, 1,751 (45%) answered “yes” to one or both of the screening questions and thus underwent swabbing for active surveillance for MRSA. Of these 1,751 patients, 202 (12%) were positive for MRSA by active surveillance. Of the 202 positive patients, 60 (30%) were also positive for MRSA by clinical culture of a sample on the same admission. Fifty-three (3.4%) of the patients who answered “yes” to the screening question but who had a negative active surveillance PCR result had a positive result for MRSA by clinical culture of a sample (negative predictive value, 96%). Thirty-five patients (2.6%) who answered “no” to both screening questions and thus for whom the active surveillance test was not performed had a positive result for MRSA by clinical culture of a sample (negative predictive value, 97%). To test the generalizability of our findings outside the empiric therapy cohorts, we completed a sensitivity analysis using the entire cohort and a subcohort of individuals who received antibiotics but for whom samples for culture were not obtained during the first 2 days of admission. In these analyses, we found very similar results for both the whole cohort of 29,978 patients and the subcohort of 8,022 patients that included only patients who received antibiotics in the first 48 h (data not shown).
TABLE 2.
Sensitivity, specificity, negative predictive value, positive predictive value, and number of different screening groups needed to treat among patients for whom clinical culture of a sample was performed and who received antibiotics in the first 48 h of admission
| Group | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) | No. needed to treat |
|---|---|---|---|---|---|
| Screening question answers “yes” and MRSA surveillance test positive | 40.5 | 95.2 | 29.7 | 97.0 | 3 |
| Screening question answers “yes” and MRSA surveillance test negative | 35.8 | 49.3 | 3.4 | 93.9 | 29 |
| Screening question answers “no” and thus not tested for MRSA | 23.7 | 55.5 | 2.6 | 93.6 | 38 |
DISCUSSION
In the study described here, we demonstrate that a targeted program of active surveillance for MRSA in the non-ICU setting may be useful in guiding empiric antibiotic therapy. We found that 24% of screening swab-positive, MRSA-colonized patients had a positive result for MRSA by clinical culture of a sample during the same admission, whereas only 2.4% of patients who answered “yes” to the screening questions but who were screening swab negative for MRSA had a positive result for MRSA by clinical culture of a sample during the same admission. Only 1.6% of the patients who answered “no” to both screening questions had a positive result for MRSA by clinical culture of a sample. If only the patients known to be colonized with MRSA received empiric anti-MRSA antibiotics, four patients would be treated for every one patient who had a positive result for MRSA by clinical culture of a sample. If the whole cohort received anti-MRSA antibiotics, 32 patients would be treated for every 1 patient who had a positive result for MRSA by clinical culture of a sample. The negative predictive value for both the screening test-negative group and the group that answered “no” to the screening questions were 98%. However, the sensitivity of a positive screening test result for identifying patients with a positive result for MRSA by clinical culture of a sample was 39.7%.
We believe that the results of this study are important. Many hospitals in the United States are performing cultures for active surveillance for MRSA for patients both inside and outside intensive care units. Our results are important in helping to guide hospitals with making the decisions about whether they are going to perform active surveillance in all intensive care units or the whole hospital or whether they are going to target active surveillance to certain patient populations. We also believe that by providing data such as the number of patients who need to be treated and the sensitivity of the different screening categories, clinicians will be better able to make decisions about the role of anti-MRSA therapy. To be clear, we are not suggesting that low-risk patients not receive anti-MRSA therapy; rather, we are suggesting that clinicians should use their individual judgment in those cases.
Our results suggest that patients who are MRSA colonized and who are suspected of having a clinical infection should receive empiric antibiotic coverage that includes therapy directed at MRSA. Other conclusions from our results are more dependent on clinicians' attitudes toward certain trade-offs that they face when choosing empiric therapy. Issues to be considered relative to these trade-offs include (i) clinician attitudes toward what an acceptable positive predictive value is (i.e., how many MRSA infections are they willing to miss by not providing empiric anti-MRSA antibiotic coverage?); (ii) clinicians' attitudes toward the amount of individual and societal collateral damage consisting of the development of antimicrobial resistance exists from using broad spectrum anti-MRSA antibiotics; and (iii) the frequency of adverse events, such as Clostridium difficile infections, from using broad-spectrum antibiotics (13, 16-18, 27). If clinicians want their decisions to have a high sensitivity and provide anti-MRSA antibiotics to most, if not all, patients who have a clinical MRSA infection to avoid missing patients who would benefit from anti-MRSA antibiotics, they will provide anti-MRSA antibiotics to many patients who are unlikely to benefit. This type of clinician behavior may contribute to the public health problem of the emergence of antibiotic resistance and increased incidences of side effects in individual patients from the unnecessary use of antibiotics. However, if, for example, clinicians provide anti-MRSA antibiotics only to patients who are MRSA positive by the use of culture of specimens for active surveillance, they must realize that the sensitivity of the test is not optimal and, thus, that there will be patients not receiving anti-MRSA antibiotics who will go on to develop MRSA infections.
Harbarth et al. studied active surveillance for MRSA and a decolonization regimen with more than 10,000 surgical patients in a 20,000-patient randomized trial (6). Their primary outcome was MRSA infection. They concluded that a universal, rapid strategy that uses screening for MRSA on admission and a decolonization regimen did not reduce the incidence of nosocomial MRSA infections in a surgical department where MRSA was endemic. Although it was not a primary outcome of the study, they observed that 5% of the patients newly identified to be positive for MRSA on admission screening and 0.5% of patients negative on admission developed a MRSA infection during their surgical hospitalization. This suggests that a targeted active surveillance screening program could be used to optimize empiric antimicrobial therapy. A study by Wertheim et al. evaluated S. aureus carriage rates and the subsequent S. aureus infection rates (26). They observed that the incidence of nosocomial S. aureus bacteremia was three times more frequent in S. aureus carriers than noncarriers. A systematic review suggested that patients colonized with MRSA are four times more likely to develop a clinical infection than patients colonized with methicillin-susceptible S. aureus (21). As part of a whole-hospital universal (nontargeted) active surveillance program that involved the collection of samples for culture upon hospital admission and then the collection of samples for culture upon transfer to hospital units or chronic care facilities, Robicsek et al. found that patients colonized with MRSA were 12.9 times more likely to have a positive result for MRSA by clinical culture of a sample (20). The findings of these studies support our findings that MRSA carriers are more likely to have a clinical infection due to MRSA.
A limitation of our study is that the targeted surveillance program was not studied by use of a randomized controlled trial, and thus, we were not able to assess the impact of a targeted surveillance program on patient outcomes and the actual choice of empiric antibiotic therapy. Thus, the impact of the targeted surveillance program on clinical outcomes remains unclear. The study was performed at a single institution with a high prevalence of MRSA. This may affect the generalizability of the results of this study to other patient populations, especially populations with different prevalences of MRSA colonization on hospital admission. However, future economic evaluations could utilize these results to estimate the cost-effectiveness of targeted surveillance strategies in settings with higher or lower prevalences.
Because this was not a randomized controlled trial, the frequency of antibiotic administration, the frequency of ordering of clinical cultures of samples, and the choice of antibiotics was not controlled but was based on the clinicians' medical judgment. However, we believe that the physicians' knowledge of the MRSA colonization or infection status of their patients likely led them to choose more often antibiotics that covered MRSA; thus, this may have led to a bias that underestimated the frequency of MRSA-positive clinical culture results among the group colonized with MRSA (22). This would lead to an underestimate of the potential utility of a targeted MRSA active surveillance program. A potential concern relative to the use of answers of “no” to the screening questions or a negative PCR result for MRSA to guide empiric therapy is the potential adverse events for patients who do not receive empiric anti-MRSA antibiotic therapy. This concern should be the highest in geographic areas and cities with high MRSA prevalence rates. However, the geographic area where this study was done has one of the highest prevalence rates of MRSA, which would mediate this concern (10). A limitation of the study is that only nasal swab specimens were cultured to identify patients colonized with MRSA. The literature reports that in from 5 to 15% of MRSA-colonized patients, MRSA will be detected in only extranasal sites. As well, the PCR method used in our study is reported to have rates of false-positive results of anywhere from 2 to 10%.
An important variable that could affect the potential benefit of a targeted active surveillance program in guiding empiric antibiotic therapy is the turn-around time of the screening method. In our study, the first positive clinical cultures were obtained at a median of 12 h after admission and 75% of the first positive clinical cultures were obtained by 43 h after admission. Thus, our study suggests that the turnaround time of the active surveillance method must be extremely rapid in order to have a significant potential impact on the choice of empiric antibiotic treatment.
Our study demonstrates that a large percentage of patients who are deemed to be at high risk for MRSA colonization according to their responses to questions on a questionnaire and who are targeted by active screening and found to be MRSA colonized have a positive result for MRSA by clinical culture of a specimen on the same admission. Very few high-risk patients with a negative MRSA screening test and even fewer patients in the low-risk group have a clinical culture positive for MRSA. We conclude that a targeted program of active surveillance for MRSA may be beneficial in guiding empiric therapy for suspected MRSA infections.
Acknowledgments
This research was supported by CDC grant R01 CI000369. This funding source had no involvement in the design, analysis, or interpretation of the results of the or in our decision to submit the manuscript for publication.
None of us has a conflict of interest to report.
We thank Colleen Reilly and Jingkun Zhu for database maintenance and abstraction.
Footnotes
Published ahead of print on 17 May 2010.
REFERENCES
- 1.Calfee, D. P., C. D. Salgado, D. Classen, K. M. Arias, K. Podgorny, D. J. Anderson, H. Burstin, S. E. Coffin, E. R. Dubberke, V. Fraser, D. N. Gerding, F. A. Griffin, P. Gross, K. S. Kaye, M. Klompas, E. Lo, J. Marschall, L. A. Mermel, L. Nicolle, D. A. Pegues, T. M. Perl, S. Saint, R. A. Weinstein, R. Wise, and D. S. Yokoe. 2008. Strategies to prevent transmission of methicillin-resistant Staphylococcus aureus in acute care hospitals. Infect. Control Hosp. Epidemiol. 29(Suppl. 1):S62-S80. [DOI] [PubMed] [Google Scholar]
- 2.Deyo, R. A., D. C. Cherkin, and M. A. Ciol. 1992. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J. Clin. Epidemiol. 45:613-619. [DOI] [PubMed] [Google Scholar]
- 3.Furuno, J. P., A. D. Harris, M. O. Wright, J. C. McGregor, R. A. Venezia, J. Zhu, and E. N. Perencevich. 2004. Prediction rules to identify patients with methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci upon hospital admission. Am. J. Infect. Control 32:436-440. [DOI] [PubMed] [Google Scholar]
- 4.Furuno, J. P., J. C. McGregor, A. D. Harris, J. A. Johnson, J. K. Johnson, P. Langenberg, R. A. Venezia, J. Finkelstein, D. L. Smith, S. M. Strauss, and E. N. Perencevich. 2006. Identifying groups at high risk for carriage of antibiotic-resistant bacteria. Arch. Intern. Med. 166:580-585. [DOI] [PubMed] [Google Scholar]
- 5.Furuno, J. P., E. N. Perencevich, J. A. Johnson, M. O. Wright, J. C. McGregor, J. G. Morris, Jr., S. M. Strauss, M. C. Roghman, L. L. Nemoy, H. C. Standiford, J. N. Hebden, and A. D. Harris. 2005. Methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci co-colonization. Emerg. Infect. Dis. 11:1539-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harbarth, S., C. Fankhauser, J. Schrenzel, J. Christenson, P. Gervaz, C. Bandiera-Clerc, G. Renzi, N. Vernaz, H. Sax, and D. Pittet. 2008. Universal screening for methicillin-resistant Staphylococcus aureus at hospital admission and nosocomial infection in surgical patients. JAMA 299:1149-1157. [DOI] [PubMed] [Google Scholar]
- 7.Harris, A. D., J. C. McGregor, J. A. Johnson, S. M. Strauss, A. C. Moore, H. C. Standiford, J. N. Hebden, and J. G. Morris, Jr. 2007. Risk factors for colonization with extended-spectrum beta-lactamase-producing bacteria and intensive care unit admission. Emerg. Infect. Dis. 13:1144-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horan, T. C., M. Andrus, and M. A. Dudeck. 2008. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am. J. Infect. Control 36:309-332. [DOI] [PubMed] [Google Scholar]
- 9.Ibrahim, E. H., G. Sherman, S. Ward, V. J. Fraser, and M. H. Kollef. 2000. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest 118:146-155. [DOI] [PubMed] [Google Scholar]
- 10.Klevens, R. M., M. A. Morrison, J. Nadle, S. Petit, K. Gershman, S. Ray, L. H. Harrison, R. Lynfield, G. Dumyati, J. M. Townes, A. S. Craig, E. R. Zell, G. E. Fosheim, L. K. McDougal, R. B. Carey, S. K. Fridkin, and Active Bacterial Core surveillance (ABCs) MRSA Investigators. 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298:1763-1771. [DOI] [PubMed] [Google Scholar]
- 11.Lodise, T. P., and P. S. McKinnon. 2005. Clinical and economic impact of methicillin resistance in patients with Staphylococcus aureus bacteremia. Diagn. Microbiol. Infect. Dis. 52:113-122. [DOI] [PubMed] [Google Scholar]
- 12.Lodise, T. P., P. S. McKinnon, L. Swiderski, and M. J. Rybak. 2003. Outcomes analysis of delayed antibiotic treatment for hospital-acquired Staphylococcus aureus bacteremia. Clin. Infect. Dis. 36:1418-1423. [DOI] [PubMed] [Google Scholar]
- 13.McGregor, J. C., A. D. Harris, J. P. Furuno, D. D. Bradham, and E. N. Perencevich. 2007. Relative influence of antibiotic therapy attributes on physician choice in treating acute uncomplicated pyelonephritis. Med. Decis. Making 27:387-394. [DOI] [PubMed] [Google Scholar]
- 14.Muto, C. A., J. A. Jernigan, B. E. Ostrowsky, H. M. Richet, W. R. Jarvis, J. M. Boyce, and B. M. Farr. 2003. SHEA guideline for preventing nosocomial transmission of multidrug-resistant strains of Staphylococcus aureus and enterococcus. Infect. Control Hosp. Epidemiol. 24:362-386. [DOI] [PubMed] [Google Scholar]
- 15.National Healthcare Safety Network, Centers for Disease Control and Prevention. 2008. The National Healthcare Safety Network (NHSN) manual: patient safety component protocol. National Healthcare Safety Network, Centers for Disease Control and Prevention, Atlanta, GA.
- 16.Perencevich, E. N., A. D. Harris, K. S. Kaye, D. D. Bradham, D. N. Fisman, L. A. Liedtke, and L. J. Strausbaugh. 2005. Physicians' acceptable treatment failure rates in antibiotic therapy for coagulase-negative staphylococcal catheter-associated bacteremia: implications for reducing treatment duration. Clin. Infect. Dis. 41:1734-1741. [DOI] [PubMed] [Google Scholar]
- 17.Perencevich, E. N., K. S. Kaye, L. J. Strausbaugh, D. N. Fisman, and A. D. Harris. 2004. Acceptable rates of treatment failure in osteomyelitis involving the diabetic foot: a survey of infectious diseases consultants. Clin. Infect. Dis. 38:476-482. [DOI] [PubMed] [Google Scholar]
- 18.Ranji, S. R., M. A. Steinman, K. G. Shojania, and R. Gonzales. 2008. Interventions to reduce unnecessary antibiotic prescribing: a systematic review and quantitative analysis. Med. Care 46:847-862. [DOI] [PubMed] [Google Scholar]
- 19.Robicsek, A., J. L. Beaumont, S. M. Paule, D. M. Hacek, R. B. Thomson, Jr., K. L. Kaul, P. King, and L. R. Peterson. 2008. Universal surveillance for methicillin-resistant Staphylococcus aureus in 3 affiliated hospitals. Ann. Intern. Med. 148:409-418. [DOI] [PubMed] [Google Scholar]
- 20.Robicsek, A., M. Suseno, J. L. Beaumont, R. B. Thomson, Jr., and L. R. Peterson. 2008. Prediction of methicillin-resistant Staphylococcus aureus involvement in disease sites by concomitant nasal sampling. J. Clin. Microbiol. 46:588-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Safdar, N., and E. A. Bradley. 2008. The risk of infection after nasal colonization with Staphylococcus aureus. Am. J. Med. 121:310-315. [DOI] [PubMed] [Google Scholar]
- 22.Schweizer, M. L., J. P. Furuno, A. D. Harris, J. C. McGregor, K. A. Thom, J. K. Johnson, M. D. Shardell, and E. N. Perencevich. 2008. Clinical utility of infection control documentation of prior methicillin-resistant Staphylococcus aureus colonization or infection for optimization of empirical antibiotic therapy. Infect. Control Hosp. Epidemiol. 29:972-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siegel, J. D., E. Rhinehart, M. Jackson, and L. Chiarello. 2007. Management of multidrug-resistant organisms in health care settings, 2006. Am. J. Infect. Control 35:S165-S193. [DOI] [PubMed] [Google Scholar]
- 24.von Eiff, C., K. Becker, K. Machka, H. Stammer, and G. Peters. 2001. Nasal carriage as a source of Staphylococcus aureus bacteremia. Study Group. N. Engl. J. Med. 344:11-16. [DOI] [PubMed] [Google Scholar]
- 25.Weber, S. G., S. S. Huang, S. Oriola, W. C. Huskins, G. A. Noskin, K. Harriman, R. N. Olmsted, M. Bonten, T. Lundstrom, M. W. Climo, M. C. Roghmann, C. L. Murphy, T. B. Karchmer, Society for Healthcare Epidemiology of America, and Association of Professionals in Infection Control and Epidemiology. 2007. Legislative mandates for use of active surveillance cultures to screen for methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci: position statement from the Joint SHEA and APIC Task Force. Infect. Control Hosp. Epidemiol. 28:249-260. [DOI] [PubMed] [Google Scholar]
- 26.Wertheim, H. F., M. C. Vos, A. Ott, A. van Belkum, A. Voss, J. A. Kluytmans, P. H. van Keulen, C. M. Vandenbroucke-Grauls, M. H. Meester, and H. A. Verbrugh. 2004. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet 364:703-705. [DOI] [PubMed] [Google Scholar]
- 27.Wood, F., S. Simpson, and C. C. Butler. 2007. Socially responsible antibiotic choices in primary care: a qualitative study of GPs' decisions to prescribe broad-spectrum and fluoroquinolone antibiotics. Fam. Pract. 24:427-434. [DOI] [PubMed] [Google Scholar]