Abstract
RDEA806 is a novel nonnucleoside reverse transcriptase inhibitor (NNRTI) with potent in vitro activity against wild-type and NNRTI-resistant HIV-1. A phase 2a randomized, double-blind, placebo-controlled, dose-escalating study evaluated the short-term antiviral activity, safety, and pharmacokinetics (PKs) of RDEA806 monotherapy in antiretroviral-naïve, HIV-1-infected subjects. The subjects were randomized to four cohorts comprising four dosage regimens and two formulations of RDEA806 or placebo in a 3:1 ratio within each cohort. The investigators were blinded to the results for each cohort. The subjects received RDEA806 or placebo for 7 days. The primary end point was the change in the HIV RNA load from the baseline to day 9 for each of the four RDEA806 dose regimens compared to that achieved with placebo. The RDEA806 PKs and the immune response to RDEA806 were evaluated along with the safety and tolerability of each dose. Of a total of 48 enrolled subjects, 36 subjects (9 in each cohort) were randomized to RDEA806 study drug, and 12 (3 in each cohort) took placebo. A statistically significant decrease in the viral load from the baseline to day 9 was observed for all RDEA806 treatment groups (P < 0.001). On day 9, the mean changes in the HIV RNA load from that at the baseline were −1.95 log10 copies/ml (400 mg twice a day), −1.39 log10 copies/ml (600 mg once a day [q.d.]), −1.62 log10 copies/ml (800 mg q.d.), and −1.70 log10 copies/ml (1,000 mg q.d.). The pharmacokinetics were linear and dose proportional. Treatment with RDEA806 was well tolerated, and there were no discontinuations due to adverse events. In conclusion, all doses of RDEA806 were safe and well tolerated and exhibited robust antiretroviral activity in this short-term monotherapy study with antiretroviral-naïve HIV-infected subjects. RDEA806 is a potent and promising novel NNRTI.
Preferred antiretroviral therapy regimens utilize combinations of at least two classes of antiretroviral agents to achieve the durable suppression of HIV-1 RNA. These combinations typically contain two nucleoside reverse transcriptase inhibitors (NRTIs) plus either a nonnucleoside reverse transcriptase inhibitor (NNRTI), a boosted protease inhibitor (PI), or an integrase inhibitor (2, 11). The approved NNRTIs, efavirenz, nevirapine, delavirdine, and etravirine, have limitations that may restrict their use or that may lead to treatment discontinuation. Specifically, efavirenz, the most widely used NNRTI, is associated with initial neuropsychiatric or central nervous system side effects and rash, leading to discontinuation in 5 to 10% of clinical trial participants (1, 2). Its use is further restricted by its teratogenic potential and transmitted NNRTI resistance (11). Use of nevirapine is similarly limited by both rash and hepatotoxicity (11). Delavirdine, which is not widely available outside the United States, is also associated with rash (11). Etravirine, the most recently approved NNRTI, is also associated with rash and is approved only for twice-daily (b.i.d.) use in treatment-experienced patients (10). The currently approved NNRTIs, including efavirenz and nevirapine, have a low genetic barrier to resistance, with a clinically relevant loss of susceptibility requiring only a single amino acid substitution (4, 8). The most common single NNRTI mutation, K103N, results in cross-resistance between these agents. While the activity of etravirine is retained against the mutant with the K103N mutation, other single mutations or combinations of mutations may attenuate the activity of this NNRTI (10). NNRTI resistance-associated mutations are the most commonly transmitted resistance mutations (5).
Newer NNRTIs with improved tolerability, improved activity against K103N viruses, lower teratogenic potential, and compact once-daily (q.d.) dosing are needed to expand and improve the viable treatment options for HIV-infected individuals.
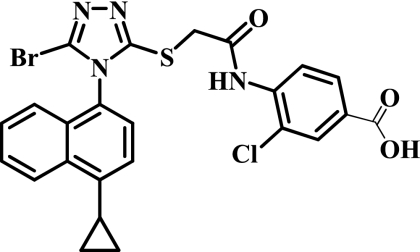
RDEA806 is a novel NNRTI with potent in vitro activity against wild-type HIV-1 and has a 50% effective concentration (EC50) of 1.7 ng/ml and similar activity in the presence of K103N and other common single mutations in the NNRTI binding region (3, 13). The chemical structure of RDEA806 is illustrated in Fig. 1. The agent demonstrates low mutagenic and teratogenic potentials in preclinical studies (15; Ardea Biosciences, Inc., data on file).
FIG. 1.
Chemical structure of RDEA806.
The pharmacokinetics (PKs) of different doses of RDEA806 were characterized in single- and multiple-dose studies with healthy volunteers. RDEA806 was readily absorbed, with the mean times to the maximum concentration of drug in plasma (Tmax) ranging from 1.25 to 3.00 h following the administration of doses of 50, 100, and 300 mg RDEA806 under the fasted condition. Following the administration of 600 mg under the fed condition, the mean Tmax was slightly longer (4.92 h). Systemic exposures to RDEA806 increased linearly following administration of single doses of 50 mg to 300 mg on an empty stomach or following a standard European breakfast. After administration of multiple doses of 300 and 500 mg b.i.d., mean maximum concentrations of drug in plasma (Cmaxs) were 0.667 and 0.746 μg/ml, respectively; mean areas under the plasma concentration-versus-time curves (AUCs) from 0 to 12 h (AUC0-12) were 3.16 and 3.77 μg·h/ml, respectively; and mean trough concentrations of drug in serum (Ctroughs) were 0.131 and 0.181 μg/ml, respectively. Ctrough values were at least five times the EC50 of 1.7 ng/ml.
RDEA806 is highly protein bound, exhibiting >99% binding in human, rat, dog, and monkey plasma. The fraction of an administered dose excreted as unchanged parent compound in urine is low (<0.06% of the dose) and renal clearance is ≤1.0 ml/min, indicating that metabolism plays a significant role in the clearance of RDEA806 and that active secretion is unlikely. Incubation with human liver hepatocytes revealed that RDEA806 is metabolized by glucuronidation and oxidation pathways that yield several inactive metabolites. RDEA806 does not inhibit or induce any single cytochrome P450 isoenzyme. The mean terminal half-lives (t1/2s) after multiple doses of 300 mg and 500 mg were 10.4 to 12.9 h.
No clinically significant adverse events (AEs) and no clinically important electrocardiographic (ECG) changes, including no corrected QT (QTc) interval abnormalities, have been observed with RDEA806 in studies with healthy volunteers and in a definitive ECG study (Ardea Biosciences, Inc., data on file). Adverse events have generally been mild to moderate in intensity and do not show a characteristic or dose-dependent pattern (15). A metabolite of RDEA806, identified as RDEA594, increases the urinary excretion of uric acid, leading to exposure-dependent declines in plasma uric acid levels (16). The metabolites of RDEA806 do not show antiretroviral activity at clinically relevant plasma exposure levels. A variety of cytochrome P450 enzymes are responsible for the metabolism of RDEA806, with no significant two-way interactions between RDEA806 and either ritonavir or tenofovir disoproxil fumarate (DF) being observed in volunteers (7).
The phase 2a randomized, double-blind, placebo-controlled, dose-escalating study described here evaluated the short-term antiviral activity, safety, and pharmacokinetics of RDEA806 monotherapy in antiretroviral naïve, HIV-1 infected subjects.
MATERIALS AND METHODS
Study design.
RDEA806-201 was a multicenter, randomized, double-blind, placebo-controlled dose-escalating phase 2a study that used consecutive cohorts of HIV-1 positive, antiretroviral naïve individuals to evaluate the pharmacokinetics, antiviral activity, safety, and pharmacokinetic-pharmacodynamic (PD) relationships of RDEA806 over 7 days of dosing. Eligible subjects were enrolled into four cohorts, and the subjects within each cohort were randomized to receive RDEA806 or placebo in a 3:1 ratio. Blinding was performed within each cohort. The doses for cohorts 1 and 2 were expected to yield exposures significantly greater than the EC90 against wild-type virus. At the completion of the study with cohorts 1 (400 mg b.i.d.) and 2 (600 mg q.d.), a blinded analysis of tolerability (adverse events), PKs, and HIV RNA data was performed. On the basis of the analysis of the results for cohorts 1 and 2, the dose chosen for cohort 3 was an enteric-coated formulation of 800 mg dosed q.d. with food. As the dosing with 800 mg q.d. in cohort 3 was well tolerated and there were no serious or severe AEs (SAEs) possibly related to the study medication, the fourth cohort (cohort 4) was randomized to receive placebo or RDEA806 as 1,000-mg enteric-coated tablets administered once daily. Two different formulations of RDEA806 were used: a 100-mg-strength modified-release formulation in the first two cohorts and a 200-mg-strength enteric-coated formulation in the subsequent two cohorts. The 800-mg dose was formulated as an enteric-coated tablet and was administered with food. The 1,000-mg dose was formulated as an enteric-coated tablet and was administered without food, which was based on data from an earlier study with healthy volunteers that suggested that dosing under a fasted condition reduced intersubject variability without affecting exposures.
(i) Cohort 1.
Nine subjects received RDEA806 400 mg orally b.i.d. under a fasted condition as four modified-release capsules, and three subjects received placebo orally b.i.d. for 7 consecutive days, followed by a single dose on day 8 for PK determinations.
(ii) Cohort 2.
Nine subjects received RDEA806 600 mg orally q.d. under a fasted condition as six modified-release capsules, and three subjects received placebo orally q.d. for 7 consecutive days, followed by a single dose on day 8 for PK determinations.
(iii) Cohort 3.
Nine subjects received RDEA806 800 mg orally q.d. with meals as four enteric-coated tablets, and three subjects received placebo orally q.d. for 7 consecutive days, followed by a single dose on day 8 for PK determinations.
(iv) Cohort 4.
Nine subjects received RDEA806 1,000 mg orally q.d. under a fasted condition as five enteric-coated tablets, and three subjects received placebo orally q.d. for 7 consecutive days, followed by a single dose on day 8 for PK determinations.
Study population.
The study was conducted at three sites, located in United Kingdom, Germany, and Austria. Eligible subjects were men ages 18 to 65 years with documented established HIV-1 infection and screening HIV-1 RNA loads of >5,000 copies/ml, determined with a Roche Amplicor HIV-1 Monitor (version 1.5) system. All subjects were antiretroviral naïve or had received antiretroviral treatment for less than 14 days and not for at least 8 weeks prior to screening. The subjects were required to have no primary reverse transcriptase (RT) or PI mutations associated with resistance (as defined by the International AIDS Society (ISA)—USA Drug Resistance Mutation Group, 2007 [4]), determined by genotypic resistance testing at screening or within the previous 6 months. Subjects were excluded if they had CD4+ counts of <350 cells/mm3 (Germany and Austria), <50 cells/mm3 (United Kingdom, cohort 1), or <200 cells/mm3 (United Kingdom, cohorts 2 to 4) or a life expectancy of <6 months; if they had received an investigational drug within 30 days prior to the trial drug administration or any vaccine within 30 days of the screening visit; or if they had acute hepatitis A virus or acute or chronic hepatitis B or C virus infection or an active AIDS-defining illness. Individuals with any significant concurrent diseases, renal or hepatic impairment, or cardiac dysfunction were excluded.
Study procedures.
The study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice (ICH GCP) and applicable regulatory requirements. All study procedures were approved by local ethics committees, and all participating subjects provided written informed consent.
At screening and prior to dosing, blood and urine collected from the subjects were analyzed for the HIV-1 RNA load and viral resistance and subjected to immunology, biochemistry, and hematology analyses and urinalysis. The screening assessments also included testing for hepatitis A virus IgM antibody, hepatitis B virus surface antigen, and hepatitis C virus antibody; a physical examination; vital sign determinations; and an ECG.
On day 1 (baseline visit), after collection of urine and blood samples, the study treatment was initiated. The patients in cohorts 1, 2, and 4 fasted from 2 h before to 1 h after administration of the study medication, while the patients in cohort 3 received breakfast 30 min before taking the study medication. All morning dosings were witnessed by a member of the investigating team. For cohort 1, the evening doses of the study medication were recorded in a subject diary whenever they were not taken at the site. The plasma viral load was assessed once daily during the treatment period by the use of samples collected just before each morning dose administration and during all of the follow-up visits on days 8, 9, and 10 and at 2 weeks postdosing (day 22). Plasma samples were collected on the last day of dosing (day 8) to determine the full steady-state pharmacokinetic profile of RDEA806. Each subject's CD4+ and CD8+ cell counts were assessed on days 1, 4, and 9 and at 2 weeks postdosing. Genotypic and phenotypic determinations were repeated on day 1 and day 9 and at 2 weeks after the last dose.
Clinical safety was assessed throughout the study. Safety assessments during the treatment period included physical examination (days 1 and 9 and 2 weeks postdosing), vital sign determinations, ECG, and laboratory assessments (hematology, biochemistry, and urinalysis) on days 1, 4, and 9 and during follow-up 2 weeks after the last dose.
At the end of the trial (i.e., 48 h after the last dose and after completion of all trial-related assessments on day 10), the subjects could start antiretroviral therapy at the discretion of the investigator and according to local treatment guidelines and procedures.
End points.
The primary efficacy end point of the study was the change in the plasma viral load (log10 number of copies/ml HIV-1 RNA) from the baseline to day 9 for each of the four RDEA806 dose regimens compared to the pooled loads for the placebo group. Secondary end points included the proportion of subjects who achieved an undetectable viral load and description of the HIV RNA decay rate. The immunologic response was evaluated by determination of the changes in the CD4+ and CD8+ T-cell counts from the baseline.
Additional secondary end points included changes in the HIV-1 genotype and phenotype, as well as the pharmacokinetics and the pharmacokinetic-pharmacodynamic relationship of RDEA806. Safety assessments included the frequency and severity of AEs, laboratory abnormalities, and discontinuations due to AEs, as recorded at each visit.
Statistical analyses.
The sample size was not based on formal power calculations because the study was designed to provide an initial assessment of the antiviral activity, safety, tolerability, and pharmacokinetics of RDEA806. The sample sizes of 12 subjects for the placebo group (the total number of subjects receiving placebo in the four dose groups) and 9 subjects for each of four RDEA806 dosing groups were sufficient to detect a difference in the viral load of approximately 0.8 log unit, assuming 80% power, a 5% overall two-sided type I error level (with a Bonferroni correction for the comparisons with placebo), and a common standard deviation (SD) of 0.5. Calculations were done using a t test with PASS2002 software to compare the results for the groups. Demographic data and baseline disease characteristics were descriptively presented and tabulated for each group as well as overall. The primary efficacy population included all randomized patients who received one or more doses of the study medications. For efficacy analyses, values below the detection limit were imputed by the value of the detection limit (i.e., 50 HIV RNA copies/ml) for descriptive statistics and the calculation of the changes from the baseline. Follow-up visits were included in the descriptive statistics but not in any of the between-group comparisons.
The primary end point was the change in the log10 plasma HIV RNA load at the end of the treatment period, i.e., at the assessment on day 9, from that at the baseline. In the case of missing data at day 9, the data from the last available postbaseline nonmissing assessment were used. Each dose group was compared to the pooled placebo group.
Additional planned analyses, including within-subject (within-group) changes from the baseline, were also assessed by a paired t test. The change in the log10 viral load was compared between the treatment groups using an analysis of covariance (ANCOVA) model with factors for treatment and the baseline log10 viral load. Because of the small sample size, it was not feasible to add the country or the center as a factor in the ANCOVA model.
Secondary analyses included analyses of the nadir of the log10 viral load, defined as the lowest log10 viral load value that was observed after the baseline (the load at the baseline was not included) during the treatment period (i.e., day 1 through the morning of day 9); the proportions of subjects who, during the treatment period, reached a virologic response, defined as a decline of the viral load of 0.5 log10 unit versus that at the baseline on day 1, a decline of the viral load of 1.0 log10 unit versus that at the baseline on day 1, and a viral load below 400 HIV RNA copies/ml; and the rate of decay of the plasma viral load from day 1 to day 9, as determined by a linear mixed model with random intercept and random time slope and fixed effects for treatment and the baseline log10 viral load. Descriptive statistics were used to analyze the baseline HIV-1 resistance and changes from the baseline.
Changes in CD4+ and CD8+ cell counts and percentages from those at the baseline were analyzed descriptively only. The treatment effect was investigated by means of ANCOVA with the baseline value and the treatment group as covariates. For binary data, Fisher's exact test was used. Each dose group was compared to the pooled placebo group. Within-group changes from the baseline were assessed using a paired t test.
Pharmacokinetic analyses were performed by Ardea Biosciences, Inc. The PK parameters were derived by noncompartmental analysis using the WinNonlin (version 4.0) program (Pharsight Corporation, Palo Alto, CA). Cmax, Tmax, and the AUC over one dosing interval (12 h for cohort 1 [AUC12]and 24 h [AUC24] for the other cohorts) were determined from individual plasma concentration-time profiles for RDEA806 on day 1 and day 8. All concentrations and parameters were summarized descriptively for each dose level. The PK-PD relationship between the efficacy of RDEA806, expressed as the viral load reduction over dosing days, and the steady-state RDEA806 Ctrough values was evaluated for the four dosage regimens. The viral load reduction over time (log10 copy number reduction·day) was calculated as the area under the viral load reduction-day that the curve was below the baseline curve, and reflects the log change in the area above the viral load log reduction over time.
The analysis of safety included all participants who received one or more doses of study medication. During the study, the subjects were monitored for AEs, and the data were analyzed with respect to the overall incidence and severity of the AEs and their potential relationship to the study medication. AEs observed after administration of the study medication were considered treatment-emergent adverse events (TEAEs) and included any AE with onset before initiation of the study medication that increased in severity after treatment initiation. TEAEs were summarized by SOC (System Organ Class) and preferred term (MedDRA [version 10.1] coding) and by the treatment received. A summary of the SAEs and AEs leading to early discontinuation from the trial were presented in listings. ECG parameters were summarized descriptively. Following ICH E14 Guidance for Industry (12), the actual values of the uncorrected QT and the corrected QT (both Bazett's and Fredericia's formulae) were categorized into ≤450 ms, 451 to 480 ms, 481 to 500 ms, and >500 ms. The changes in uncorrected QT and the corrected QT from the reference time point were categorized according to ICH E14 into (12) ≤30 ms, 31 to 60 ms, and >60 ms.
RESULTS
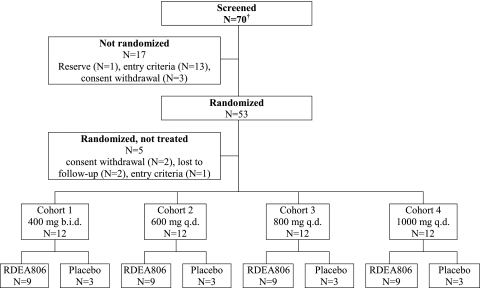
Seventy male subjects were screened across three study sites. Fifty-three subjects were randomized, and 48 subjects received treatment. All treated subjects completed the study after having received the planned treatment (Fig. 2). There were no relevant differences between the randomization groups with respect to any demographic or disease characteristics (Table 1). The patients who were enrolled were predominantly white males across all treatment groups. The lower range of the CD4 T-cell counts differs between the cohorts because the United Kingdom study site initially used the enrollment criterion of CD4 T-cell counts of ≥50 cells/mm3 (for cohort 1) but amended the protocol to CD4 T-cell counts of ≥200 cells/mm3 (for cohorts 2 to 4). Demographic and baseline characteristics are summarized in Table 1. The HIV-1 genotypes at the baseline revealed no IAS primary NNRTI resistance mutations (5). Phenotypic evaluation by the Antivirogram (Virco, Belgium) method indicated that all subjects had virus susceptible to efavirenz, nevirapine, and etravirine.
FIG. 2.
Subject disposition.
TABLE 1.
Demographics
| Parameter | Value for each groupa |
|||||
|---|---|---|---|---|---|---|
| Placebo pooled (n = 12) | RDEA806 400 mg b.i.d. (n = 9) | RDEA806 600 mg q.d. (n = 9) | RDEA806 800 mg q.d. (n = 9) | RDEA806 1,000 mg q.d. (n = 9) | Total (n = 48) | |
| Median (range) age (yr) | 37.5 (25-51) | 34.0 (29-44) | 38.0 (34-50) | 30.0 (24-41) | 32.0 (24-50) | 34.5 (24-51) |
| No. (%) of subjects with the following ethnicity: | ||||||
| Caucasian/white | 10 (83.3) | 7 (77.8) | 9 (100) | 7 (77.8) | 8 (88.9) | 41 (85.4) |
| Black | 1 (8.3) | 2 (22.2) | 0 | 1 (11.1) | 1 (11.1) | 5 (10.4) |
| Oriental/Asian | 1 (8.3) | 0 | 0 | 0 | 0 | 1 (2.1) |
| Other | 0 | 0 | 0 | 1 (11.1)b | 0 | 1 (2.1)b |
| Median (range) wt (kg) | 77.5 (60-99) | 83.0 (62-90) | 82.0 (60-95) | 71.4 (64-90) | 73.0 (49-90) | 75.5 (49-99) |
| Median (range) BMIc (kg/m2) | 24.64 (18.4-34.3) | 24.19 (19.3-29.4) | 22.31 (21.2-28.6) | 22.41 (18.2-26.6) | 23.04 (18.0-26.3) | 23.43 (18.0-34.3) |
| Mean (range) HIV-1 RNA (log10 copies/ml) | 4.513 (3.76-5.37) | 4.503 (3.69-5.05) | 4.671 (3.78-5.94) | 4.604 (4.20-5.39) | 4.600 (3.88-5.67) | 4.574 (3.69-5.94) |
| Median (range) CD4+ cell count (cells/mm3) | 360.5 (187-527) | 323.5 (82-553) | 365.3 (219-502) | 292.0 (202-489) | 404.5 (289-614) | 333.3 (82-614) |
| No. (%) of subjects with CDC classification: | ||||||
| A | 11 (92) | 8 (89) | 7 (78) | 7 (78) | 9 (100) | 42 (88) |
| B | 1 (8) | 1 (11) | 1 (11) | 2 (22) | 0 | 5 (10) |
| C | 0 | 0 | 1 (11) | 0 | 0 | 1 (2) |
n, number of subjects per treatment group.
The subject was of mixed origin.
BMI, body mass index.
Virologic response.
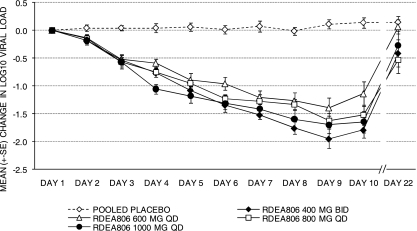
The changes in the log10 HIV RNA loads are summarized in Table 2. A statistically significant decrease in viral load from the baseline to day 9 was observed for all RDEA806 treatment groups (P < 0.001) but not in the pooled placebo group. On day 9, the median changes in the log10 viral loads from the baseline were −1.77 (range, −3.14 to −1.39), −1.33 (range, −2.12 to −0.63), −1.82 (range, −2.03 to −1.04), and −1.76 (range, −2.47 to −0.95) log10 copies/ml for the groups receiving RDEA806 doses of 400 mg b.i.d., 600 mg q.d., 800 mg q.d., and 1,000 mg q.d., respectively, whereas the change was +0.16 log10 copy/ml for the pooled placebo group. The mean changes (±SDs) in the log10 viral load on day 9 from the baseline were −1.95 (±0.53), −1.39 (±0.55), −1.62 (±0.39), and −1.70 (±0.47) log10 copies/ml for the groups receiving RDEA806 doses of 400 mg b.i.d., 600 mg q.d., 800 mg q.d., and 100 mg q.d., respectively, whereas the change was +0.11 log10 copy/ml for the pooled placebo group. From days 3 to 9, the differences in the mean change in the log10 viral load between the RDEA806 treatment groups versus the pooled placebo group were statistically significant (P < 0.0001). The estimates of the treatment differences in the log10 numbers of RNA copies/ml on day 9 were statistically significant (P < 0.0001) for the groups receiving RDEA806 doses of 400 mg b.i.d. (−2.06; standard error [SE], 0.195), 600 mg q.d. (−1.52; SE, 0.196), 800 mg q.d. (−1.74; SE, 0.196), and 1,000 mg q.d. (−1.82; SE, 0.196) compared to the loads for the placebo group. The mean (±SD) decreases in the plasma viral load from the baseline to that at the nadir of the plasma viral load were 2.0 (±0.51), 1.5 (±0.53), 1.7 (±0.36), and 1.8 (±0.48) log10 copies/ml HIV RNA for the groups receiving RDEA806 at doses of 400 mg b.i.d., 600 mg q.d., 800 mg q.d., and 1,000 mg q.d., respectively, whereas the decrease was 0.1 log10 copy/ml for the pooled placebo group. The viral load decay rate for the treatment period from day 1 to day 9 was determined by a linear mixed model with random intercept and random time slope and fixed effects for treatment and baseline log10 HIV RNA levels. The viral load decay rate ranged from −0.25 to −0.18 log10 copy/ml/day for all RDEA806 treatment groups, whereas it was 0.01 log10 copy/ml/day for the pooled placebo group (Fig. 3).
TABLE 2.
Changes in HIV-1 RNA loads and CD4 and CD8 T-cell counts from baseline to day 9
| Parameter | Result for RDEA806 dose ofa: |
Result for placebo group | |||
|---|---|---|---|---|---|
| 400 mg b.i.d. | 600 mg q.d. | 800 mg q.d. | 1,000 mg q.d. | ||
| No. of subjects per treatment group | 9 | 9 | 9 | 9 | 12 |
| Viral load change (log10 HIV RNA copies/ml) from that at baseline | |||||
| Mean ± SD | −1.948 ± 0.525* | −1.394 ± 0.548* | −1.619* ± 0.385 | −1.696* ± 0.474 | 0.113± 0.271 |
| Median (range) | −1.765* (−3.14 to −1.39) | −1.334* (−2.12 to −0.63) | −1.818* (−2.03 to −1.04) | −1.756* (−2.47 to −0.95) | 0.155 (−0.45 to 0.57) |
| Estimate of difference in HIV-1 RNA load (log10 copies/ml) with treatment vs placebob | −2.0608 | −1.5241 | −1.7426 | −1.8189 | NAc |
| Viral load decay rate (log10 copies/ml/day) | −0.25 | −0.18 | −0.20 | −0.22 | 0.01 |
| No. of subjects with <400 HIV RNA copies/ml | 4 | 3 | 5 | 3 | 0 |
| No. of subjects with viral load decrease of at least 1.0 log10 vs that at baseline | 9 | 7 | 9 | 9 | 0 |
| Mean (range) change in CD4 T-cell count (cells/mm3) from that at baselined | |||||
| CD4 cells | 19.22 (−59.0 to 159.5) | −14.44 (−127.0 to 169.5) | 100.74** (−36.3 to 261.3) | 90.48** (−16.5 to 246.0) | 43.07 (−139.7 to 620.0) |
| CD8 cells | −39.17 (−470.5 to 627.0) | 64.54 (−317.0 to 337.5) | 79.41 (−519.0 to 368.5) | 71.04 (−275.0 to 400.5) | 146.00 (−305.5 to 1,080.0) |
*, P < 0.001 for the difference versus the results on day 1 (t test); **, P < 0.05 for the difference versus the results on day 1 (two-sided paired t test).
The P value for the difference versus the results for the placebo group was <0.0001 for the four treatment groups, determined using an ANCOVA model that factors treatment and the baseline log10 viral load and with a Bonferroni correction applied.
NA, not applicable.
Data for percentages of CD4 and CD8 T cells are not shown.
FIG. 3.
Mean change in viral load.
In the placebo group, there were no responders for any of the virologic response categories analyzed except one subject with a decline of at least 0.5 log10 copy/ml HIV RNA. All RDEA806-treated subjects achieved a reduction in viral load of at least 0.5 log10 copy/ml RNA, and all except two subjects in the groups receiving RDEA806 at 600 mg q.d. achieved at least 1.0-log10-unit reduction in viral load during treatment. Fifteen RDEA806-treated subjects (41.7%) achieved viral loads of <400 HIV RNA copies/ml during treatment, but no subjects in the pooled placebo group did so. Three subjects (i.e., two subjects in the group receiving RDEA806 at 400 mg b.i.d. and one subject in the group receiving RDEA806 at 1,000 mg q.d. group) achieved viral loads of <50 HIV copies/ml during treatment.
Immunologic response.
Changes in mean CD4 T-cell counts were highly variable. The mean changes in total CD4 T-cell counts from the baseline through day 9 were 19 (range, −59 to 159), −14 (range, −127 to 169), 100 (range, −36 to 261), and 90 (range, −16 to 246) cells/mm3 for the groups receiving RDEA806 at 400 mg b.i.d., 600 mg q.d., 800 mg q.d., and 1,000 mg q.d., respectively, whereas the mean change was 43 (range, −139 to 620) cells/μl for the pooled placebo group (Table 2). On day 9, statistically significant mean increases in CD4 T-cell counts compared with those at the baseline were observed for the groups receiving RDEA806 at 800 mg q.d. and 1,000 mg q.d. (P < 0.05). Changes in CD8 T-cell counts from the baseline to day 9 were also variable, and the mean changes ranged from −39 to +79 cells/mm3 across the four RDEA806 treatment groups.
Resistance determinations.
Susceptibility to antiretroviral drugs was determined for each subject at screening, at the baseline, on day 9, and at 2 weeks postdosing using the genotype (Virco type HIV-1) and phenotype (Antivirogram) methods. Virco type HIV-1 and Antivirogram data were available for all except one subject, who was in the group receiving RDEA806 at 1,000 mg q.d. and for whom data for day 9 and 2 weeks postdosing were not available. One additional subject, who was in the group receiving RDEA806 at 400 mg b.i.d., had no Antivirogram phenotype data at 2 weeks postdosing.
HIV-1 genotypic analysis over the time course of treatment with RDEA806 revealed that newly acquired mutations were rare. No evidence of genotypic resistance to any NNRTI was observed on day 9, and there were no previously identified NNRTI resistance mutations or consensus mutations selected in vitro by RDEA806.
Two subjects who had no baseline resistance to NNRTIs had virus with phenotypic resistance to two of the three tested NNRTIs on day 9. One subject (RDEA806 at 800 mg q.d.) had virus showing minor resistance to efavirenz and etravirine, and one subject (RDEA806 at 1,000 mg q.d.) had virus with minor resistance to efavirenz and nevirapine. However, at 2 weeks postdosing, the HIV-1 isolates from these subjects were again susceptible to the NNRTIs to which resistance was demonstrated on day 9. For the subject in the group receiving RDEA806 at 800 mg q.d., there were no RT amino acid sequence changes from the baseline to 2 weeks postdosing, and the pretreatment phenotypic analysis showed elevations in EC50s, albeit they were below the cutoff values for resistance, resulting in a small difference in EC50s for efavirenz and etravirine between the baseline and day 9. The amino acid sequence of virus from the subject in the group receiving RDEA806 at 1,000 mg q.d. differed at four RT residues on day 9 compared to the sequences at the baseline and 2 weeks postdosing. None of the observed changes was a primary IAS-defined NNRTI resistance mutation or a consensus mutation selected by RDEA806 in vitro. For this subject, the pretreatment phenotypic analysis also showed some elevation in EC50s of efavirenz and nevirapine, resulting in a <2.6-fold change in the EC50s for efavirenz and etravirine between the baseline and day 9. In vitro phenotypic susceptibility to RDEA806 at day 9 for isolates from both of these subjects was similar to that at the baseline.
In addition, one subject (RDEA806 600 mg q.d.) had virus with low-level resistance to efavirenz and high-level resistance to nevirapine at 2 weeks postdosing. The genotype of virus from this subject at 2 weeks postdosing revealed the emergence of a single amino acid mutation, Y181C/Y, which could be responsible for this phenotype. This residue was wild type at screening, on day 1 and day 9, and at the end of treatment. This subject also had a mixed population of T69N/T, a known NRTI treatment-selected mutation, at day 1 and 2 weeks postdosing. Neither Y181C nor T69N was a consensus mutation selected by RDEA806 in a recent resistance selection study using wild-type HIV-1. No specific consensus mutations selected in vitro by RDEA806 were detected in this subject. A small change in the RDEA806 EC50 (3-fold) compared to that at the baseline was observed at 2 weeks postdosing.
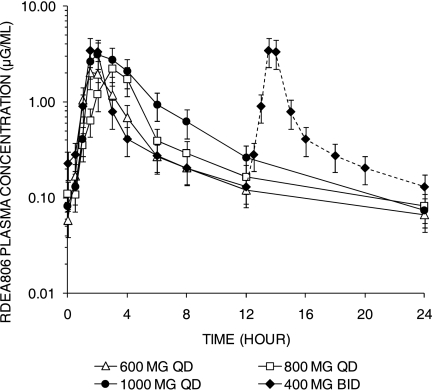
Pharmacokinetics.
The pharmacokinetic parameters for the four dosage forms are summarized in Table 3, and the steady-state profiles are illustrated in Fig. 4. The exposures (AUCs) were greatest for the 1,000-mg-q.d. and 400-mg-b.i.d. dosage regimens, but significant interpatient variability in the AUCs was observed with all dose groups, regardless of whether the study drug was taken with or without meals. The RDEA806 Cτ (maximum concentration after the first dose interval) was the greatest with the 400-mg-b.i.d. dose compared to those for the three other doses. However, the median Cτ values for all doses were greater than the 0.020-μg/ml threshold, consistent with a viral load reduction of at least 1 log10 HIV-1 RNA copy/ml, and were greater than the EC50 of 1.7 ng/ml (0.017 μg/ml). On the basis of trough concentration profiles, steady state was achieved on study day 3. On day 8, the t1/2 values of RDEA806 ranged from 7.09 h (600 mg q.d.) to 11.3 h (400 mg b.i.d., which was administered only once on day 8).
TABLE 3.
Steady-state pharmacokinetics of four RDEA806 dosage regimens on day 8a
| Cohort, dosage regimen | Tmax (h) | Cmax (μg/ml) | Css,avg (μg/ml)b | Cτ (μg/ml) |
|---|---|---|---|---|
| Cohort 1, 400 mg b.i.d. | 1.50 (1.50-6.00) | 2.99 (1.37, 6.53) | 0.505 (0.286, 0.892) | 0.113 (0.0731, 0.176) |
| Cohort 2, 600 mg q.d. | 2.00 (1.00-3.00) | 2.42 (1.35, 4.32) | 0.276 (0.181, 0.422) | 0.0404 (0.0180-0.0909) |
| Cohort 3, 800 mg q.d. | 3.00 (3.00-23.60) | 1.91 (0.83, 4.38) | 0.310 (0.165, 0.581) | 0.0654 (0.0382, 0.112) |
| Cohort 4, 1,000 mg q.d. | 3.00 (1.50-8.00) | 3.70 (1.50, 9.12) | 0.515 (0.267, 0.992) | 0.0615 (0.0372, 0.102) |
Summarized data are geometric means (95% confidence intervals) for all parameters except Tmaxs, which are medians (ranges). Each cohort had nine subjects.
Css,avg, AUC over a dose interval at steady state divided by the duration of the dosing interval (i.e., AUCτ/24 h for q.d. dosing or AUCτ/12 h for b.i.d. dosing)
FIG. 4.
Steady-state plasma concentrations of 4 doses of RDEA806 at day 8. Plasma concentrations of RDEA806 at steady state (day 8) following q.d. dosing of RDEA806 at 600 mg, 800 mg, and 1,000 mg and b.i.d. dosing at 400 mg are shown. For illustration purposes, the plasma profile of the 400-mg-b.i.d. dose level at 12 to 24 h (dashed line) was duplicated from the profile at 0 to 12 h, as only a single dose was given on the last day (day 8).
Pharmacodynamics.
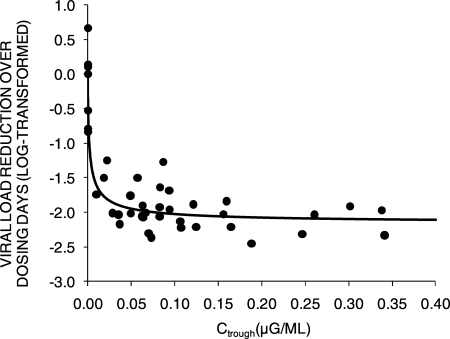
The pharmacokinetic-pharmacodynamic relationship of the four RDEA806 dosage regimens with the HIV RNA response was explored. There was a relationship between the levels of RDEA806 exposure and the reduction in HIV RNA loads. The greatest exposures were attained with doses of 400 mg b.i.d. (median AUC24, 12.36 μg·h/ml) and 1,000 mg q.d. (median AUC24, 17.4 μg·h/ml) and were associated with the greatest changes in the HIV RNA loads from those at the baseline (−1.948 and −1.696 log10 copies/ml/day, respectively) (Fig. 4 and 5). Ctrough exposures of RDEA806 of ≥0.020 μg/ml were consistently associated with declines in HIV RNA loads of ≥1 log10 copies (Fig. 4). The relationship between the steady-state RDEA806 Ctrough levels and viral load reduction demonstrated that Ctrough levels of ∼0.02 μg/ml (20 ng/ml) were associated with a >1-log-unit reduction in the viral load (Fig. 5). The greater median Ctrough exposures achieved with 1,000-mg-q.d. dosing produced similar median changes in HIV RNA loads compared to those observed with 800-mg-q.d. dosing. The AUC and Ctrough values were proportional and were associated with changes in viral loads for each dose. However, because of the small numbers of patients in each cohort, it is not possible to determine the difference between the AUC and Ctrough values needed to predict changes in viral loads for each dose.
FIG. 5.
Correlation of RDEA806 Ctrough and HIV-1 RNA response. The pharmacokinetic-pharmacodynamic relationship between RDEA806 and efficacy, expressed by the viral load reduction over dosing days versus the steady-state Ctrough of RDEA806, is shown. Viral load reduction over time (log10 copy number reduction·day) was calculated as the area of the viral load reduction-day that the curve was below the baseline curve. A Ctrough of ∼0.02 μg/ml (20 ng/ml) is needed to achieve a >1-log-unit reduction in viral load.
Safety.
Treatment with RDEA806 was generally well tolerated. No severe or potentially life-threatening events were observed, and no subjects discontinued the study due to adverse events. All treatment-emergent adverse events were mild or moderate in intensity. A total of 98 adverse events observed in 28 subjects were mild in intensity, and 16 TEAEs observed in 10 subjects were moderate in intensity. No moderate adverse events were observed in more than one subject in any treatment group. The most frequently reported treatment-emergent adverse events were diarrhea, upper abdominal discomfort, fatigue, headache, and erythema. Grade 1 and 2 diarrhea and upper abdominal discomfort were more frequently reported in subjects receiving RDEA806 than in subjects receiving placebo. Diarrhea was reported by 11 subjects (31%) and upper abdominal pain occurred in 8 subjects (22%) following treatment with RDEA806. Treatment-emergent adverse events of moderate intensity or greater were reported in eight patients administered RDEA806, but there were no clinically relevant differences between treatment groups (Table 4).
TABLE 4.
Number of patients with treatment-emergent adverse events of moderate intensity (grade 2) or greatera
| TEAE | No. of patients |
|||||
|---|---|---|---|---|---|---|
| RDEA806 group |
Placebo group (n = 12) | |||||
| 400 mg b.i.d. (n = 9) | 600 mg q.d. (n = 9) | 800 mg q.d. (n = 9) | 1,000 mg q.d. (n = 9) | All active (n = 36) | ||
| Back pain | 1 | 1 | ||||
| Diarrhea | 1 | 1 | ||||
| Headache | 1 | 1 | 2 | |||
| Insomnia | 1 | 1 | 1 | |||
| Pruritis | 1 | 1 | ||||
| Myalgia | 1 | 1 | ||||
| Hypersensitivity (aggravated allergy) | 1 | 1 | ||||
| Toothache | 1 | |||||
| Eye swelling | 1 | |||||
| Body pain | 1 | |||||
| Increased amylase/lipase levels | 1 | |||||
Adverse events were counted once per patient.
There were no clinically relevant changes in blood chemistry, hematology, or urinalysis findings; and there were no significant differences in treatment-emergent laboratory abnormalities in any of the treatment groups. One placebo recipient experienced a grade 2 lipase and amylase elevation, and one subject in the group receiving RDEA806 at 600 mg q.d. experienced grade 4 lipase and grade 3 amylase elevations. Declines in serum uric acid levels were observed in all RDEA806 dosing groups. The median decreases in the uric acid levels from those at the baseline were 88.0 μmol/liter in the group receiving RDEA806 at 400 mg b.i.d., 81.0 μmol/liter in the group receiving RDEA806 at 600 mg q.d., 58.0 μmol/liter in the group receiving RDEA806 at 800 mg q.d., and 88.0 μmol/liter in the group receiving RDEA806 at 1,000 mg q.d., whereas the decrease was 22.0 μmol/liter in the placebo group. All treatment-emergent laboratory changes were grade 1 or grade 2 and were seen in a maximum of two subjects per treatment group.
Assessment of ECG results revealed that no QT or QTc values of >480 ms were observed at any visit during the trial. No abnormal changes in the QT corrected for heart rate using the formulae of Bazett and Fridericia of more than 60 ms were observed during treatment.
DISCUSSION
In the present study, RDEA806 demonstrated short-term efficacy across a range of both twice- and once-daily dosage schedules. No short-term safety issues were identified, consistent with the findings of previous healthy volunteer studies (15). Specifically, no QT or other ECG abnormalities have been reported, in contrast to the dose-dependent effects reported for the investigational NNRTI rilpivirine (TMC278) and efavirenz (10).
All doses of RDEA806 exhibited potent antiretroviral activity. The mean changes in log10 HIV RNA viral loads, the findings for the nadir of the log10 viral load, and the viral load decay rates displayed statistically significant differences for all doses compared to the results for the placebo group. It is difficult to differentiate the effects of food or formulation on the pharmacokinetics of RDEA806, as the different doses were coadministered with meals in one cohort and were administered under fasting conditions in three cohorts. The RDEA806 doses of 400 mg b.i.d. and 600 mg q.d. were modified-release formulations, while the 800-mg-q.d. and 1,000-mg-q.d. doses were extended-release formulations. The median Tmaxs for the 800-mg and 1,000-mg extended-release formulations were slightly greater than those for the modified-release 400-mg and 600-mg formulations. The slower absorption rate with the enteric-coated tablet may have been affected by both the formulation and food administration (cohort 3), as most of the subjects receiving enteric-coated tablets were administered drug under fed conditions, while those receiving modified-release tablet were administered drug under fasted conditions. However, significant interpatient variability in Tmax was observed with all four formulations.
Consistent with the slow emergence of resistance to RDEA806 in vitro (13), the short-term monotherapy in this study did not lead to the emergence of NNRTI-resistant virus or consistent changes in reverse transcriptase sequences. In vitro, RDEA806 retains activity against common NNRTI-resistant viruses, such as those with the K103N mutation (13). Clinical studies are needed to determine the activity of RDEA806 in individuals with transmitted or acquired NNRTI resistance.
A PK-PD relationship was observed with respect to HIV RNA response. The plasma pharmacokinetics were broadly linear. The greatest exposures were observed with the 400-mg-b.i.d. and 1,000-mg-q.d. formulations, which correlated with greater viral decay and with a virologic response after 9 days. Plasma trough exposures of RDEA806 above 0.020 μg/ml were consistently associated with a ≥1-log10-unit reduction in the HIV RNA load (Fig. 4 and 5). This exposure was maintained at 12 h in all subjects receiving the 400-mg-b.i.d. modified-release formulation and at 24 h in subjects receiving the 800-mg-q.d. and 1,000-mg-q.d. enteric-coated formulation. This supports the further development of RDEA806 as an agent for q.d. administration. While higher doses have been tested in healthy volunteers, without a maximum tolerated dose being identified, the data suggest that the peak of the dose-response curve was reached with the increased RDEA806 exposure achieved with the 1,000-mg-q.d. dose, but that did not result in short-term HIV RNA load reductions greater than those achieved with the 800-mg-q.d. dose.
RDEA806 is metabolized by a range of cytochrome P450 isoforms and is predominantly a substrate only. Therapeutic exposures are not associated with cytochrome P450 inhibition or induction (14). Interaction studies with ritonavir and tenofovir DF did not reveal any significant drug-drug interaction with RDEA806 or with ritonavir or tenofovir (7), suggesting that dose adjustment may not be required when these drugs are used in combination in clinical practice.
A metabolite of RDEA806, identified as RDEA594, does not retain antiretroviral activity at plasma exposure levels but is associated with increased renal excretion of uric acid through inhibition of the URAT-1 transporter (17). This leads to exposure-dependent declines in serum uric acid levels in RDEA806 recipients. Hyperuricemia has been observed in HIV-infected patients with progressive HIV infection and underlying renal disease (6). This may not be of clinical significance to individuals without hyperuricemia or without preexisting renal disease but warrants further evaluation in HIV-infected individuals.
Limitations with regard to safety, tolerability, teratogenicity, a genetic barrier to resistance, and activity against commonly transmitted mutants exist with approved NNRTIs. These short-term data combined with preclinical data for RDEA806 suggest that this novel agent has the potential to address these issues in HIV-infected patients.
In conclusion, a short-term monotherapy course of four different dosage regimens of the novel NNRTI RDEA806 in antiretroviral-naïve HIV-infected subjects resulted in a rapid decline in viral loads, evidenced by decreases in HIV-1 RNA loads of 1.39 to 1.95 log10 copies/ml. RDEA806 was well tolerated, did not result in significant adverse events or clinically relevant ECG changes, and did not lead to any notable decreases in susceptibility to currently approved NNRTIs. RDEA806 represents a promising highly potent NNRTI candidate for continued development.
Acknowledgments
We acknowledge Mark I. Becker for his efforts with helping to prepare the manuscript.
G.M. has received consultancy fees from Ardea Biosciences, Inc. M.B., A.S., and A.R. have no conflicts of interest. Z.S., K.M., B.S., V.H., A.R., M.N., T.N., V.O., L.-T.Y., and B.Q. are employees of Ardea Biosciences, Inc.
The Clinicaltrials.gov identifier for this study is NCT00617526.
Footnotes
Published ahead of print on 24 May 2010.
REFERENCES
- 1.Domingo, P., I. Suárez-Lozano, F. Torres, R. Teira, J. Lopez-Aldeguer, F. Vidal, A. Muñoz, P. Viciana, F. Lozano, A. Vergara, B. Roca, M. L. García Alcalde, J. Cosín, A. Terrón, M. J. Galindo, P. Geijo, E. Ribera, J. Gonzalez, T. Sanchez, J. R. Lacalle, and M. Garrido. 2008. First-line antiretroviral therapy with efavirenz or lopinavir/ritonavir plus two nucleoside analogues: the SUSKA study, a non-randomized comparison from the VACH cohort. J. Antimicrob. Chemother. 61:1348-1358. [DOI] [PubMed] [Google Scholar]
- 2.European AIDS Clinical Society. Guidelines for the clinical management and treatment of HIV infected adults in Europe. http://www.europeanaidsclinicalsociety.org. Accessed 10 September 2009.
- 3.Hamatake, R., Z. Zhang, W. Xu, D. Bellows, A. Raney, J.-L. Girardet, and B. Quart. 2007. RDEA806 a potent NNRTI with a high genetic barrier to resistance, poster H-1041, abstr. 1662. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 4.Johnson, V., F. Brun-Vézinet, B. Clotet, H. Günthard, D. Kuritzkes, D. Pillay, J. Schapiro, and D. Richman. 2007. Update of the drug resistance mutations in HIV-1: 2007. International AIDS Society—USA. Top. HIV Med. 15:119-125. [PubMed] [Google Scholar]
- 5.Little, S. J., S. Holte, J. P. Routy, E. S. Daar, M. Markowitz, and A. C. Collier. 2002. Antiretroviral drug resistance among patients recently infected with HIV. N. Engl. J. Med. 347:385-394. [DOI] [PubMed] [Google Scholar]
- 6.Maesaka, J. K., A. J. Cusano, H. L. Thies, F. P. Siegal, and A. W. Dreisbach. 1990. Hypouricemia in acquired immunodeficiency syndrome. Am. J. Kidney Dis. 15:252-257. [DOI] [PubMed] [Google Scholar]
- 7.Moyle, G., M. Boffito, Z. Shen, K. Manhard, B. Sheedy, V. Hingorani, M. Nguyen, T. Nguyen, B. Quart, L. Yeh, and V. Ong. 2008. RDEA806, a novel HIV non-nucleoside reverse transcriptase inhibitor, shows positive outcome in treatment of naïve HIV patients, abstr. H-893. Abstr. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA) 46th Annu. Meet. American Society for Microbiology and Infectious Diseases Society of America, Washington, DC.
- 8.Rousseau, M. N., L. Vergne, B. Montes, M. Peeters, J. Reynes, E. Delaporte, and M. Segondy. 2001. Patterns of resistance mutations to antiretroviral drugs in extensively treated HIV-1-infected patients with failure of highly active antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 26:36-43. [DOI] [PubMed] [Google Scholar]
- 9.Reference deleted.
- 10.Tibotec Therapeutics, L. P. Division of Centocor Ortho Biotech Products. 2008. Intelence® (etravirine) U.S. package insert. Tibotec Therapeutics, Raritan, NJ.
- 11.U.S. Department of Health and Human Services, Panel on Antiretroviral Guidelines for Adults and Adolescents. 2008. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents, p. 1-139. http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed 10 September 2009.
- 12.U.S. Food and Drug Administration. 2005. ICH E14 guidance for industry. Clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs. Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research, U.S. Food and Drug Administration, Rockville, MD.
- 13.Xu, W., B. Groschel, R. Straney, Z. Zhang, D. Bellows, R. Hamatake, J.-L. Girardet, B. Quart, and A. Raney. 2008. Resistance to RDEA806 requires multiple mutations which have limited cross-resistance to other NNRTIs, poster H-1222. Abstr. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA) 46th Annu. Meet. American Society for Microbiology and Infectious Diseases Society of America, Washington, DC.
- 14.Yeh, L., R. Hamatake, H. Kim, S. Gunawan, R. Daswani, K. Tieu, C. Dadson, and B. Quart. 2007. RDEA806, a potent non-nucleoside reverse transcriptase inhibitor with less potential for drug-drug interactions, abstr. 3390. Abstr. 47th. Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 15.Yeh, L., S. Ramael, S. Comhaire, V. Hingorani, K. Manhard, M. Nguyes, K. Tieu, and B. Quart. 2007. Safety and pharmacokinetics of ascending single oral doses of RDEA806, a novel HIV non-nucleoside reverse transcriptase inhibitor, in healthy volunteers, poster A-1429, abstr. 1609. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 16.Yeh, L., V. Hingorani, K. Manhard, V. Borges, M. Nguyen, K. Tieu, and B. Quart. 2008. Safety and uric acid lowering effect in humans following multiple doses of RDEA806, a novel prodrug for the potential treatment of hyperuricemia, poster THU0356. Annual EULAR 2008 Eur. Congr. Rheumatol.
- 17.Yeh, L., Z. Shen, B. Kerr, I. Tamai, V. Hingorani, V. Ong, T. Nguyen, M. Nguyen, B. Sheedy, K. Manhard, and B. Quart. 2009. RDEA594: a potent URAT1 inhibitor without affecting other important renal transporters, OAT1 and OAT3, abstr. THU0452. Abstr. Eur. Congr. Rheumatol.