Abstract
Natural products with macrocyclic structural features often display intriguing biological properties. Molecular design incorporating macrocycles may lead to molecules with unique protein-ligand interactions. We generated novel human immunodeficiency virus type 1 (HIV-1) protease inhibitors (PIs) containing a macrocycle and bis-tetrahydrofuranylurethane. Four such compounds exerted potent activity against HIV-1LAI and had 50% effective concentrations (EC50s) of as low as 0.002 μM with minimal cytotoxicity. GRL-216 and GRL-286 blocked the replication of HIV-1NL4-3 variants selected by up to 5 μM saquinavir, ritonavir, nelfinavir, lopinavir, or atazanavir; they had EC50s of 0.020 to 0.046 μM and potent activities against six multi-PI-resistant clinical HIV-1 (HIVmPIr) variants with EC50s of 0.027 to 0.089 μM. GRL-216 and -286 also blocked HIV-1 protease dimerization as efficiently as darunavir. When HIV-1NL4-3 was selected by GRL-216, it replicated progressively more poorly and failed to replicate in the presence of >0.26 μM GRL-216, suggesting that the emergence of GRL-216-resistant HIV-1 variants is substantially delayed. At passage 50 with GRL-216 (the HIV isolate selected with GRL-216 at up to 0.16 μM [HIV216-0.16 μM]), HIV-1NL4-3 containing the L10I, L24I, M46L, V82I, and I84V mutations remained relatively sensitive to PIs, including darunavir, with the EC50s being 3- to 8-fold-greater than the EC50 of each drug for HIV-1NL4-3. Interestingly, HIV216-0.16 μM had 10-fold increased sensitivity to tipranavir. Analysis of the protein-ligand X-ray structures of GRL-216 revealed that the macrocycle occupied a greater volume of the binding cavity of protease and formed greater van der Waals interactions with V82 and I84 than darunavir. The present data warrant the further development of GRL-216 as a potential antiviral agent for treating individuals harboring wild-type and/or HIVmPIr.
Currently available combination chemotherapy for human immunodeficiency virus type 1 (HIV-1) infection and AIDS typically uses two reverse transcriptase (RT) inhibitors (RTIs) and boosted protease inhibitors (PIs) or an integrase inhibitor, also known as highly active antiretroviral therapy (HAART) (6, 29, 30, 33), and has been shown to suppress the replication of HIV-1 and significantly extend the life expectancy of HIV-1-infected individuals. Indeed, several recent analyses have revealed that the mortality rates for HIV-infected persons have become much closer to the general mortality rates since the introduction of HAART and that first-line HAART with boosted PI-based regimens resulted in less resistance within and across drug classes (2, 20, 21, 35).
However, the ability to provide effective long-term antiretroviral therapy for HIV-1 infection has become a complex issue since those who initially achieved favorable viral suppression to undetectable levels have experienced treatment failure (10, 20, 27). In addition, it is evident that even with these anti-HIV-1 drugs, only partial immunologic reconstitution is attained in patients with advanced HIV-1 infection, and it is likely that HIV-1 will eventually acquire resistance to virtually any antiviral agent. Thus, it appears that the development of potent and drug-resistance-deferring antiviral agents will continue to be required for successful long-term control of HIV-1 infection and AIDS (3, 11).
A number of natural-product-derived small molecules are known to interact with human proteins, and a variety of such molecules have been identified to fulfill significant biochemical functions, permitting the development of a wide range of pharmaceuticals. Macrocycles are representatives of natural-product-derived small molecules. Of note, any molecule containing a ring of seven or more atoms is defined to be a macrocycle (8).
In the present study, we designed and synthesized PIs containing functionalities that interact with the amino acid backbones of the catalytic site of HIV-1 protease along with a flexible macrocyclic group involving P1′-P2′ ligands for effective repacking of the altered PI-binding cavity of protease that emerges upon side chain mutations in PI-resistant HIV-1 variants. We identified a group of novel potent macrocyclic PIs (16). GRL-216, which possesses a dual anti-HIV-1 mechanism, inhibits the catalytic activity and dimerization of HIV-1 protease and exerts potent activity against wild-type HIV-1 as well as a wide spectrum of PI-resistant HIV-1 variants in vitro.
MATERIALS AND METHODS
Cells and viruses.
Human CD4+ MT-2 and MT-4 cell lines were grown in RPMI 1640-based culture medium supplemented with 10% fetal calf serum (PAA Laboratories GmbH, Linz, Austria) plus 50 U of penicillin and 100 μg of kanamycin per ml. The following HIV-1 strains were used for the drug susceptibility assay: HIV-1LAI, HIV-1NL4-3, HIV-2EHO, and HIV-2ROD (5, 32, 36). In addition, we used clinical HIV-1 strains from drug-naive patients with AIDS (HIV-1ERS104pre) (32) and six HIV-1 clinical isolates that were originally isolated from patients with AIDS who had received anti-HIV-1 therapy heavily (for 32 to 83 months) and that were genotypically and phenotypically characterized as multiple-PI-resistant HIV-1 variants (36, 37). To determine whether each clinical HIV-1 isolate used in the present study was a syncytium-inducing (SI) virus or a non-syncytium-inducing (NSI) virus strain, MT-2 cells (105) were exposed to an aliquot of viral stock supernatant containing 100 50% tissue culture infectious doses (TCID50s) of the virus and cultured in six-well culture plates. The cultures were maintained for 4 weeks and were examined under an inverted microscope to determine the syncytium-inducing or non-syncytium-inducing nature of the virus, as described previously (36, 37).
Antiviral agents.
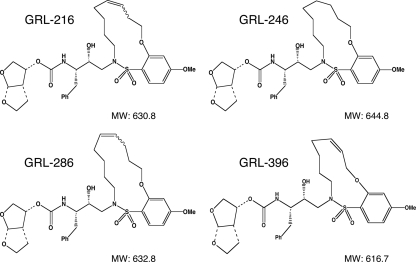
In this work, 40 novel nonpeptidic PIs which contained 3(R),3a(S),6a(R)-bis-tetrahydrofuranylurethane (bis-THF) (14, 15, 24) and a macrocyclic ring (16) were designed and synthesized. Among them, four PIs, GRL-216, GRL-246, GRL-286, and GRL-396 (Fig. 1), with molecular weights of 630.8, 644.8, 632.8, and 616.7, respectively, were chosen for detailed analysis, primarily on the basis of their potent antiviral activity. The method of the synthesis of these four PIs has been described elsewhere by Ghosh and coworkers (16). Saquinavir (SQV) and ritonavir (RTV) were kindly provided by Roche Products, Ltd. (Welwyn Garden City, United Kingdom) and Abbott Laboratories (Abbott Park, IL), respectively. Amprenavir (APV) was a kind gift from GlaxoSmithKline (Research Triangle Park, NC). Nelfinavir (NFV) and lopinavir (LPV) were kindly provided by Japan Energy, Inc., Tokyo, Japan. Indinavir (IDV) was kindly provided by Merck Research Laboratories (Rahway, NJ). Atazanavir (AZV) was a kind gift from Bristol-Myers Squibb (New York, NY). Darunavir (DRV) was synthesized as described previously (17). Tipranavir (TPV) was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, National Institutes of Health.
FIG. 1.
Structures of macrocyclic PIs GRL-216, GRL-246, GRL-286, and GRL-396.
Drug susceptibility assay.
The susceptibilities of HIV-1LAI and primary HIV-1 isolates to various drugs were determined as described previously (24), with minor modifications. Briefly, MT-2 cells (2 × 104/ml) were exposed to 100 TCID50s of HIV-1LAI in the presence or absence of various concentrations of drugs in 96-well microculture plates, and the plates were incubated at 37°C for 7 days. After 100 μl of the medium was removed from each well, 3-(4,5-dimetylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution (10 μl, 7.5 mg/ml in phosphate-buffered saline) was added to each well in the plate, followed by incubation at 37°C for 3 h. After incubation to dissolve the formazan crystals, 100 μl of acidified isopropanol containing 4% (vol/vol) Triton X-100 was added to each well, and the optical density was measured using a kinetic microplate reader (Vmax; Molecular Devices, Sunnyvale, CA). All assays were performed in duplicate or triplicate. To determine the susceptibilities of primary HIV-1 isolates to the drugs, phytohemagglutinin-activated peripheral blood mononuclear cells (PHA-PBMCs; 106/ml) were exposed to 50 TCID50s of each isolate. The target cells were exposed to HIV-1ERS104pre or drug-resistant HIV-1 in the presence or absence of various concentrations of drugs and were incubated for 7 days. Upon the conclusion of the culture, the amounts of p24 Gag protein in the supernatants were determined using a fully automated chemiluminescent enzyme immunoassay system (Lumipulse F; Fujirebio Inc., Tokyo, Japan) (28).
To determine the drug susceptibilities of certain laboratory HIV-1 strains, MT-4 cells were used as target cells. In brief, MT-4 cells (105/ml) were exposed to 100 TCID50s of drug-resistant HIV-1 strains in the presence or absence of various concentrations of drugs, and on day 7 of culture, the supernatant was harvested and the amounts of p24 Gag protein were determined. The drug concentrations that suppressed the production of p24 Gag protein by 50% (50% effective concentrations [EC50s]) were determined by comparison of the levels produced by drug-treated cell cultures with the levels produced in drug-free control cell cultures. All assays were performed in duplicate or triplicate.
Generation of PI-resistant HIV-1 in vitro.
In the experiments for selecting drug-resistant variants, MT-4 cells were exploited as target cells, since HIV-1 in general replicates at greater levels in MT-4 cells than in MT-2 cells. MT-4 cells (105/ml) were exposed to HIV-1NL4-3 (500 TCID50s) and cultured in the presence of various PIs, each at an initial concentration of its EC50. Viral replication was monitored by determining the amount of p24 Gag produced by MT-4 cells. The culture supernatants were harvested on day 7 and used to infect fresh MT-4 cells for the next round of culture in the presence of increasing concentrations of each drug. When the virus began to propagate in the presence of the drug, the drug concentration was generally increased 2- to 3-fold. Proviral DNA samples obtained from the lysates of infected cells were subjected to nucleotide sequencing of the HIV genome.
Determination of nucleotide sequences.
Molecular cloning and determination of the nucleotide sequences of HIV-1 strains passaged in the presence of anti-HIV-1 agents were performed as described previously (36, 37). In brief, high-molecular-weight DNA was extracted from HIV-1-infected MT-4 cells by using the InstaGene matrix (Bio-Rad Laboratories, Hercules, CA) and was subjected to molecular cloning, followed by sequence determination. The first-round PCR mixture consisted of 1 μl of proviral DNA solution, 10 μl of Premix Taq (Ex Taq version; Takara Bio, Inc., Otsu, Japan), and 10 pmol of each of the first PCR primers in a total volume of 20 μl. The PCR conditions used were an initial 5 cycles of 30 s at 95°C, 2 min at 55°C, and 2 min at 72°C, followed by 15 cycles of 30 s at 95°C, 20 s at 55°C, and 2 min at 72°C. The first-round PCR products were used directly in the second round of PCR. The second-round PCR products were purified with spin columns (MicroSpin S-400 HR columns; Amersham Biosciences Corp., Piscataway, NJ), cloned directly, and subjected to sequencing with a model 3130 automated DNA sequencer (Applied Biosystems, Foster City, CA).
Generation of FRET-based HIV-1 expression system.
The intermolecular fluorescence resonance energy transfer (FRET)-based HIV-1 expression assay employing cyan and yellow fluorescent protein-tagged protease monomers (CFP and YFP, respectively) was performed as described previously (23). In brief, CFP- and YFP-tagged HIV-1 protease constructs were generated using BD Creator DNA cloning kits (BD Biosciences, San Jose, CA). For the generation of full-length molecular infectious clones containing CFP- or YFP-tagged protease, the PCR-mediated recombination method was used (9). A linker consisting of five alanines was inserted between the protease and the fluorescent proteins. The phenylalanine-proline site that HIV-1 protease cleaves was also introduced between the fluorescent protein and RT sites. Thus, the DNA fragments obtained were subsequently joined by using the PCR-mediated recombination reaction performed under the standard conditions for ExTaq polymerase (Takara Bio, Inc.). The amplified PCR products were cloned into the pCR-XL-TOPO vector, according to the manufacturer's instructions (Gateway cloning system; Invitrogen, Carlsbad, CA). PCR products were generated with the pCR-XL-TOPO vector and used as templates, followed by digestion by both ApaI and SmaI; and the ApaI-SmaI fragment was introduced into pHIV-1NLSma (12), generating pHIV-PRWTCFP and pHIV-PRWTYFP (where WT indicates wild type), respectively.
FRET procedure.
COS7 cells plated on an EZ-view cover, glass bottom culture plate (Iwaki, Tokyo) were transfected with pHIV-PRWTCFP and pHIV-PRWTYFP using Lipofectamine 2000 (Invitrogen), according to the manufacturer's instructions, in the presence of various concentrations of each compound, cultured for 72 h, and analyzed under a Fluoview FV500 confocal laser scanning microscope (Olympus Optical Corp., Tokyo, Japan) at room temperature, as described previously (23). When the effect of each compound was analyzed by FRET, the test compounds were added to the culture medium simultaneously with the transfected plasmids.
The results of FRET were determined by measurement of the quenching of the CFP (donor) fluorescence and the increase in the YFP (acceptor) fluorescence (sensitized emission), since part of the energy of CFP is transferred to YFP instead of being emitted. The changes in the CFP and YFP fluorescence intensities in the images of the selected regions were examined and quantified using an FV500 Image software system (Olympus Optical Corp.). The ratios of the intensities of the CFP fluorescence after photobleaching to the CFP fluorescence before photobleaching (CFPA/B ratios) were determined. CFPA/B ratios less than 1 indicated that the association of the two subunits did not occur, and it was interpreted that protease dimerization was inhibited (23).
Structural analysis of interaction of GRL-216 and darunavir.
Counterions were deleted from the crystal structures of HIV-1 protease complexed with GRL-216 (Protein Data Bank [PDB] accession number 3I6O) (16) and darunavir (PDB accession number 2IEN) (13). Bond orders were properly assigned to the inhibitor molecules. Hydrogens were added to all the heavy atoms, and their positions were optimized in an OPLS2005 force field with constraints on the heavy atom positions. A cutoff distance of 3.0 Å between a polar hydrogen and an oxygen or nitrogen atom was used to determine the presence of hydrogen bonds. The structures were analyzed using the Maestro (version 9.0) program (Schrödinger, LLC, New York, NY, 2009).
RESULTS
Generation of mcPIs and their activities against HIV-1LAI.
We designed and synthesized ∼40 PIs containing a variety of macrocyclic components (16). Among those, we identified GRL-216, GRL-246, GRL-286, and GRL-396 (Fig. 1) to be PIs with potent activities against HIV-1 in vitro. As shown in Table 1, GRL-216 and -286 were highly potent against a laboratory wild-type HIV-1 strain, HIV-1LAI, with EC50s of 0.002 ± 0.001 and 0.004 ± 0.001 μM, respectively, determined with the MTT assay using MT-2 cells. Of note, GRL-216 and -286 were more potent against HIV-1LAI than three representative FDA-approved PIs (APV, LPV, and TPV), although the potencies of these two compounds were comparable to the potency of DRV, whose EC50 was 0.003 ± 0.0004 μM. The cytotoxicity of GRL-216 and -286 was seen only at high concentrations, with the 50% cytotoxic concentrations (CC50s) being 48.8 and 33.1 μM, respectively, resulting in reasonably good selectivity indices of 24,400 and 8,280 for these two compounds, respectively. GRL-246 and -396 were also potent and had EC50s of 0.022 ± 0.005 and 0.014 ± 0.001 μM, respectively, and selectivity indices of 1,510 and 3,200, respectively. Thus, in a further study, we primarily examined the pharmacological and virological profiles of the two most potent compounds with greater selectivity indices, GRL-216 and -286.
TABLE 1.
Activities of mcPIs against HIV-1LAI, HIV-2EHO, and HIV-2ROD and cytotoxicitiesa
| Drug | EC50 (μM) |
CC50 (μM) | Selectivity index | ||
|---|---|---|---|---|---|
| HIV-1LAI | HIV-2EHO | HIV-2ROD | |||
| APV | 0.024 ± 0.008 | 0.12 ± 0.03 | 0.42 ± 0.10 | >100 | >4,170 |
| LPV | 0.039 ± 0.006 | 0.035 ± 0.025 | 0.028 ± 0.004 | >100 | >2,560 |
| TPV | 0.17 ± 0.005 | 0.30 ± 0.15 | 0.33 ± 0.07 | 54.1 ± 2.1 | 320 |
| DRV | 0.003 ± 0.0004 | 0.008 ± 0.007 | 0.010 ± 0.004 | >100 | >33,300 |
| GRL-216 | 0.002 ± 0.001 | 0.010 ± 0.007 | 0.017 ± 0.001 | 48.8 ± 1.3 | 24,400 |
| GRL-246 | 0.022 ± 0.005 | ND | ND | 33.3 ± 0.8 | 1,510 |
| GRL-286 | 0.004 ± 0.001 | 0.018 ± 0.013 | 0.025 ± 0.002 | 33.1 ± 2.5 | 8,280 |
| GRL-396 | 0.014 ± 0.001 | ND | ND | 44.9 ± 4.1 | 3,200 |
MT-2 cells (2 × 103) were exposed to 100 TCID50s of HIV-1LAI, HIV-2EHO, or HIV-2ROD and cultured in the presence of various concentrations of each PI. The EC50s were determined by using the MTT assay. All assays were conducted in duplicate, and the data shown represent mean values (±1 standard deviation) derived from the results of three independent experiments. Each selectivity index denotes the ratio of the CC50 to the EC50 against HIV-1LAI. ND, not done.
Activities of GRL-216 and GRL-286 against HIV-2 strains in vitro.
The activities of GRL-216 and GRL-286 against two HIV-2 strains, HIV-2EHO and HIV-2ROD, were determined using the MTT assay and MT-2 cells as the target cells, as described previously (1). While the activity of APV against HIV-2EHO and HIV-2ROD was slightly less potent than that of APV against HIV-1LAI (EC50s for HIV-2EHO, HIV-2ROD, and HIV-1LAI, 0.12 μM [3.53-fold less potent], 0.42 μM [12.35-fold less potent], and 0.034 μM, respectively (Table 1), three other PIs (LPV, TPV, and DRV) were comparably potent against both HIV-2 strains and HIV-1LAI. GRL-216 was relatively less potent against HIV-2EHO and HIV-2ROD than against HIV-1LAI (EC50s for HIV-2EHO, HIV-2ROD, and HIV-1LAI, 0.010 μM [5-fold less potent], 0.017 μM [8.5-fold less potent], and 0.002 μM, respectively) (Table 1). Similarly, GRL-286 exerted relatively less activity against the two HIV-2 strains (Table 1) than against HIV-1LAI.
GRL-216 and GRL-286 are potent against PI-selected laboratory HIV-1 variants.
We also examined whether GRL-216 and GRL-286 were active against a variety of HIV-1 variants that had been selected in vitro with each of five FDA-approved PIs (SQV, NFV, APV, LPV and AZV) (Table 2). These HIV-1 variants were obtained by propagating a wild-type laboratory HIV-1 strain, HIV-1NL4-3, in the presence of increasing concentrations of each PI in MT-4 cells over 37 to 60 passages in vitro, and the variants thus obtained were confirmed to have acquired various PI resistance-associated amino acid substitutions in the protease-encoding region of the viral genome (see footnote a of Table 2). Each of the variants (HIV-1SQV-5 μM, HIV-1NFV-5 μM, HIV-1APV-5 μM, HIV-1LPV-5 μM, and HIV-1AZV-5 μM [where the subscripts indicate the drug-drug concentration with which the variant was selected]) (22) was highly resistant to the corresponding PI with which the variant was selected, with the EC50s being >1 μM; and the fold differences in the EC50s compared to the EC50 of each drug against HIV-1NL4-3 ranged from >22 to >250. The activities of GRL-216 against all these variants except HIV-1APV-5 μM were well maintained, with the fold differences being 4 to 8. GRL-216 was virtually inert against HIV-1APV-5 μM, with the EC50 being >1 μM. GRL-286 had potent activity against HIV-1SQV-5 μM, HIV-1NFV-5 μM, and HIV-1AZV-5 μM; but the compound was less potent against HIV-1LPV-5 μM and was virtually inert against HIV-1APV-5 μM.(24). The observation that both GRL-216 and GRL-286 were essentially inert against HIV-1APV-5 μM was presumably due to the structural resemblance among GRL-216A, GRL-286A, and APV, all of which contain a sulfonamide isostere (Fig. 1).
TABLE 2.
Activities of GRL-216 and GRL-286 against laboratory PI-resistant HIV-1 variantsa
| Virus | EC50 (μM) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| SQV | NFV | APV | LPV | AZV | TPV | DRV | GRL-216 | GRL-286 | |
| HIV-1NL4- 3 | 0.005 ± 0.002 | 0.045 ± 0.032 | 0.034 ± 0.001 | 0.046 ± 0.025 | 0.004 ± 0.0004 | 0.32 ± 0.07 | 0.003 ± 0.0006 | 0.005 ± 0.001 | 0.009 ± 0.0006 |
| HIV-1SQV-5 μM | >1 (>200) | 0.51 ± 0.081 (11) | 0.18 ± 0.10 (5) | 0.12 ± 0.02 (2) | 0.21 ± 0.065 (53) | 0.022 ± 0.009 (0.1) | 0.003 ± 0.0002 (1) | 0.020 ± 0.009 (4) | 0.046 ± 0.021 (5) |
| HIV-1NFV-5 μM | 0.003 ± 0.002 (1) | >1 (>22) | 0.028 ± 0.005 (1) | 0.037 ± 0.004 (1) | 0.027 ± 0.005(7) | 0.039 ± 0.041 (0.1) | 0.006 ± 0.0002 (2) | 0.040 ± 0.017 (8) | 0.041 ± 0.006 (5) |
| HIV-1APV-5 μM | 0.026 ± 0.010 (5) | 0.30 ± 0.03 (7) | >1 (>29) | >1 (>22) | 0.006 ± 0.002 (2) | 0.22 ± 0.089 (0.7) | 0.29 ± 0.0018 (97) | >1 (>200) | >1 (>111) |
| HIV-1LPV-5 μM | 0.028 ± 0.007 (6) | 0.32 ± 0.12 (7) | 0.26 ± 0.07 (8) | >1 (>22) | 0.036 ± 0.001 (9) | 0.31 ± 0.030 (1) | 0.025 ± 0.0066 (8) | 0.035 ± 0.005 (7) | 0.35 ± 0.019 (39) |
| HIV-1AZV-5 μM | 0.031 ± 0.005 (6) | >1 (>22) | 0.28 ± 0.06 (8) | >1 (>22) | >1 (>250) | 0.41 ± 0.047 (1) | 0.009 ± 0.0025 (3) | 0.023 ± 0.005 (5) | 0.030 ± 0.006 (3) |
The amino acid substitutions identified in the protease-encoding regions of HIV-1SQV-5 μM, HIV-1NFV-5 μM, HIV-1APV-5 μM, HIV-1LPV-5 μM, and HIV-1AZV-5 μM compared to the consensus type B sequence cited from the Los Alamos database include L10I/G48V/I54V/A71V/I84V/L90 M, L10F/D30N/K45I/A71V/T74S, L10F/M46I/I50V/A71V/I84V/L90 M, L10F/M46I/I54V/V82A, and L23I/E34Q/K43I/M46I/I50L/G51A/L63P/A71V/V82A/T91A, respectively. MT-4 cells (104) were exposed to 100 TCID50s of each HIV-1 isolate, and inhibition of p24 Gag protein production by each drug was used as the end point. Numbers in parentheses represent the n-fold changes in the EC50s for each isolate compared to the EC50s for wild-type isolate HIV-1NL4-3. All assays were conducted in duplicate or triplicate, and the data shown represent mean values (±1 standard deviation) derived from the results of three independent experiments.
GRL-216 and GRL-286 exert potent activities against highly MDR clinical HIV-1 strains.
In our previous work, we isolated highly multiple-drug (PI)-resistant (MDR) clinical HIV-1 strains (HIV-1MDR), including HIV-1MDR/B, HIV-1MDR/C, HIV-1MDR/G, HIV-1MDR/TM, HIV-1MDR/MM, and HIV- 1MDR/JSL, from patients with AIDS who had failed the then-existing anti-HIV regimens after receiving 9 to 11 anti-HIV-1 drugs over 32 to 83 months (36, 37). These clinical strains contained 9 to 14 amino acid substitutions in the protease-encoding region which have reportedly been associated with HIV-1 resistance to various PIs (see footnote a of Table 3). The EC50s of IDV and LPV for these multidrug-resistant clinical HIV-1 isolates were mostly >1 μM, and the activities of the other three PIs (SQV, APV, and AZV) were also found to be significantly compromised, determined using PHA-PBMCs as the target cells and p24 production inhibition as the end point (Table 3). Both TPV and DRV well maintained their activities, and the fold differences between their EC50s against HIV-1ERS104pre (wild-type isolate) and their EC50s against multidrug-resistant clinical isolates ranged from 1 to 9, while it was noteworthy that the highest EC50 of DRV was much lower (0.027 μΜ) than that of TPV (0.38 μM). GRL-216 and -286 exerted potent activities against HIV-1ERS104pre, with the EC50s being as low as 0.005 and 0.007 μM, respectively (Table 3). The potencies of GRL-216 and -286 against five PI-resistant variants (HIV-1MDR/B, HIV-1MDR/C, HIV-1MDR/G, HIV-1MDR/TM, and HIV-1MDR/MM) were well maintained, with the fold differences in the EC50s compared to their EC50s against HIV-1ERS104pre ranging from 4 to 13. However, both GRL-216 and -286 were less potent against HIV-1MDR/JSL, with the differences in the EC50s being 15- and 30-fold, respectively. It was noted that HIV-1MDR/JSL was the most resistant to all other PIs examined except TPV and DRV (Table 3).
TABLE 3.
Activities of GRL-216 and GRL-286 against multi-drug-resistant clinical isolates in PHA-PBMsa
| Virus (syncytium formation) | EC50 (μM) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| SQV | IDV | APV | LPV | AZV | TPV | DRV | GRL-216 | GRL-286 | |
| HIV-1ERS104 pre (wild type; SI) | 0.008 ± 0.005 | 0.043 ± 0.004 | 0.030 ± 0.005 | 0.034 ± 0.002 | 0.002 ± 0.001 | 0.12 ± 0.03 | 0.003 ± 0.0002 | 0.005 ± 0.003 | 0.007 ± 0.002 |
| HIV-1MDR/B (SI) | 0.27 ± 0.073 (34) | >1 (>23) | >1 (>33) | >1 (>29) | 0.20 ± 0.10 (100) | 0.18 ± 0.009 (2) | 0.019 ± 0.012 (6) | 0.037 ± 0.016 (7) | 0.089 ± 0.016 (13) |
| HIV-1MDR/C (SI) | 0.032 ± 0.002 (11) | >1 (>23) | 0.37 ± 0.011 (12) | >1 (>29) | 0.065 ± 0.008 (33) | 0.38 ± 0.079 (3) | 0.008 ± 0.006 (3) | 0.044 ± 0.002 (9) | 0.029 ± 0.001 (4) |
| HIV-1MDR/G (SI) | 0.030 ± 0.002 (4) | 0.34 ± 0.14 (5) | 0.43 ± 0.004 (14) | 0.26 ± 0.04 (8) | 0.033 ± 0.024 (17) | 0.24 ± 0.08 (2) | 0.023 ± 0.006 (5) | 0.057 ± 0.012 (11) | 0.028 ± 0.004 (4) |
| HIV-1MDR/TM (SI) | 0.26 ± 0.04 (33) | >1 (>23) | 0.32 ± 0.007 (11) | >1 (>29) | 0.065 ± 0.008 (33) | 0.38 ± 0.05 (3) | 0.004 ± 0.001 (1) | 0.027 ± 0.001 (6) | 0.072 ± 0.014 (10) |
| HIV-1MDR/MM (NSI) | 0.19 ± 0.05 (24) | >1 (>23) | 0.21 ± 0.222 (7) | >1 (>29) | 0.18 ± 0.021 (89) | 0.35 ± 0.06 (3) | 0.011 ± 0.002 (4) | 0.033 ± 0.010 (7) | 0.055 ± 0.025 (8) |
| HIV-1MDR/JSL (NSI) | 0.30 ± 0.02 (37) | >1 (>23) | 0.62 ± 0.02 (21) | >1 (>29) | 0.43 ± 00.036 (215) | 0.23 ± 0.049 (2) | 0.027 ± 0.011 (9) | 0.073 ± 0.07 (15) | 0.21 ± 0.032 (30) |
The amino acid substitutions identified in the protease-encoding region compared to the consensus type B sequence cited from the Los Alamos database include L63P in HIV-1ERS104pre; L10I, K14R, L33I, M36I, M46I, F53I, K55R, I62V, L63P, A71V, G73S, V82A, L90M, and I93L in HIV-1MDR/B; L10I, I15V, K20R, L24I, M36I, M46L, I54V, I62V, L63P, K70Q, V82A, and L89M in HIV-1MDR/C; L10I, V11I, T12E, I15V, L19I, R41K, M46L, L63P, A71T, V82A, and L90 M in HIV-1MDR/G; L10I, K14R, R41K, M46L, I54V, L63P, A71V, V82A, L90M, and I93L in HIV-1MDR/TM; L10I, K43T, M46L, I54V, L63P, A71V, V82A, L90M, and Q92K in HIV-1MDR/MM; and L10I, L24I, I33F, E35D, M36I, N37S, M46L, I54V, R57K, I62V, L63P, A71V, G73S, and V82A in HIV-1MDR/JSL. HIV-1ERS104pre served as a source of wild-type HIV-1. EC50s were determined by using PHA-PBMCs as target cells, and inhibition of p24 Gag protein production by each drug was used as the end point. Numbers in parentheses represent the n-fold changes in the EC50s for each isolate compared to the EC50s for wild-type isolate HIV-1ERS104pre. All assays were conducted in duplicate or triplicate, and the data shown represent the mean values (±1 standard deviation) derived from results of three independent experiments.
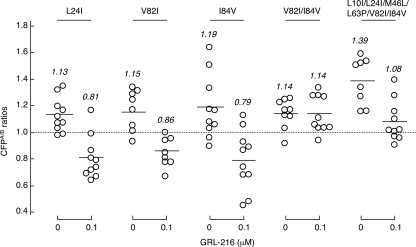
GRL-216 and GRL-286 block the dimerization of HIV-1 protease.
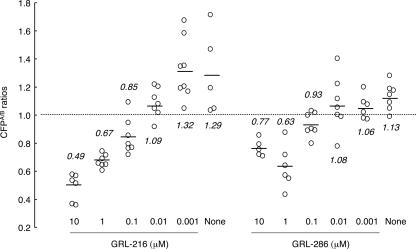
Since the structurally related bis-THF-containing PI DRV effectively blocked the dimerization of HIV-1 protease, determined with the FRET-based HIV-1 expression system as described previously (23), we asked whether GRL-216 and GRL-286 had such protease dimerization-inhibitory activity. In the FRET-based HIV-1 expression system, COS7 cells were transfected with pHIV-PRWTCFP and pHIV-PRWTYFP and exposed to various concentrations of either of the drugs, and the CFPA/B ratios were determined at the end of 72 h of culture. In the absence of drug, virtually all the CFPA/B ratios were above 1.0, with the average figures being 1.29 and 1.13 for GRL-216 and GRL-286, respectively (Fig. 2), indicating that protease dimerization occurred in the system. However, when the transfected COS7 cells were exposed to GRL-216 at a concentration greater than 0.1 μM, all the average CFPA/B ratios were less than 1.0, indicating that GRL-216 effectively blocked HIV-1 protease dimerization (Fig. 2). GRL-286 also effectively blocked dimerization at the same concentration range.
FIG. 2.
GRL-216 and GRL-286 potently block the dimerization of HIV-1 protease. To examine whether GRL-216 and GRL-286 inhibited HIV-1 protease dimerization, the FRET-based HIV-1 expression system was employed (23). COS7 cells were transfected with pHIV-PRWTCFP and pHIV-PRWTYFP and exposed to various concentrations of either of the drugs, and the CFPA/B ratios (y axis) were determined at the end of a 72-h culture. All average CFPA/B ratios were less than 1.0 in the presence of >0.1 μM GRL-216 and GRL-286, indicating that both compounds effectively blocked HIV-1 protease dimerization.
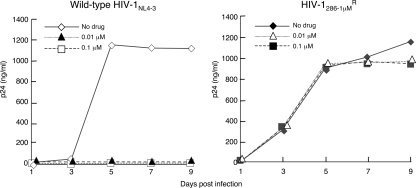
In vitro selection of HIV-1 variants resistant to four mcPIs.
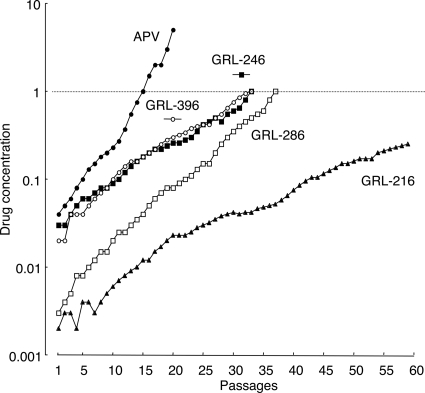
We attempted to select out HIV-1 variants resistant to GRL-216, GRL-246, GRL-286, and GRL-396 by propagating HIV-1NL4-3 in MT-4 cells in the presence of increasing concentrations of each PI, as described previously (1). We also selected HIV-1 variants in the presence of increasing concentrations of APV. As shown in Fig. 3, HIV-1 variants that replicated in the presence of 1 μM APV, GRL-396, GRL-246, and GRL-286 emerged by passages 20, 33, 33, and 37, respectively; however, the virus exposed to GRL-216 continued to be fairly susceptible to GRL-216 even after 50 passages. Beyond approximately 50 passages with GRL-216 exposure, the virus replicated extremely poorly and virtually failed to replicate after exposure to GRL-216 at >0.26 μM, demonstrating that the emergence of a GRL-216-resistant HIV-1 variant is substantially delayed compared to the time to the emergence of resistance to APV and the other mcPIs examined here.
FIG. 3.
In vitro selection of HIV-1 variants resistant to GRL-216, GRL-246, GRL-286, and GRL-396. HIV-1NL4-3 was propagated in MT-4 cells in the presence of increasing concentrations of APV (•), GRL-216 (▴), GRL-246 (▪), GRL-286 (□), or GRL-396 (○). Each passage of HIV-1 was conducted in a cell-free fashion. APV was employed as a reference compound.
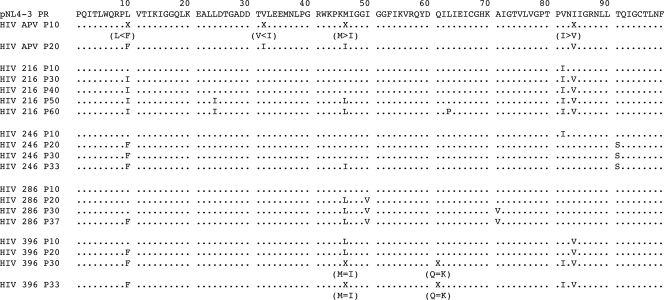
Determination of the nucleotide sequence of the protease-encoding region disclosed that the variants resistant to APV (5 μM, passage 20) had acquired previously reported mutations, such as L10I, V32I, M46I, and I84V (Fig. 4). By passage 5 with GRL-216 exposure, the wild-type protease-encoding sequence in the virus was seen. However, by passage 10 and beyond, the virus was seen to contain the V82I substitution. As the passage proceeded, more amino acid substitutions were acquired. By passage 30, the virus had acquired L10I and I84V substitutions. By passage 50 (P50) with 0.16 μM GRL-216 (HIV216-P50), the virus had further acquired L24I and M46I substitutions. By passage 60, the virus had gained L63P substitutions as well.
FIG. 4.
Amino acid sequences of protease-encoding regions of HIV-1NL4-3 variants selected in the presence of GRL-216, GRL-246, GRL-286, or GRL-396. The amino acid sequence of protease, deduced from the nucleotide sequence of the protease-encoding region of each proviral DNA isolated at each time indicated, is shown. The amino acid sequence of wild-type HIV-1NL4-3 protease is illustrated at the top as a reference.
HIV-1 exposed to GRL-246 (1 μM) had acquired L10F, M46I, and T91S in the protease-encoding region by passage 33. By passage 37, HIV-1 exposed to GRL-286 (1 μM) had acquired L10F, M46L, I50V, and A71V in the protease-encoding region. By passage 33, the HIV-1 isolate selected for resistance to GRL-396 (1 μM) had acquired L10F, M46I, Q61K, V82I, and I84V in the protease-encoding region. We also examined whether the virus acquired mutations in the Gag region after several passages with GRL-216 selection. It was found that by passage 10, the virus had acquired the E107D substitution. By passage 25 and beyond, V35I, R275K, and the p1/p6 cleavage site substitution L449F had emerged. By passage 50, V390D emerged and persisted (data not shown).
We also determined the amino acid sequences of the gag regions of each of the selected HIV variants by direct base sequencing. Two amino acid substitutions were seen in common: the V35I substitution was identified in GRL-216-, GRL-246-, and GRL-286-selected variants (by 25, 10, and 20 passages, respectively); and the L449F substitution, reported by Doyon et al. (7), was identified in GRL-216- and GRL-286-selected variants (by 25 and 20 passages, respectively). Other than these two amino acid substitutions, only sporadic substitutions were identified in the gag region of the four variants (data not shown).
Susceptibilities of mcPI-selected HIV-1 variants to various PIs.
We finally examined the susceptibilities of the four PI-selected HIV-1 variants to six FDA-approved PIs along with the four mcPIs using MT-4 cells (24) as target cells (Table 4), as described previously. HIV216-P50 was substantially resistant to GRL-216, with a 24-fold-greater EC50 (0.094 μM) than the EC50 of GRL-216 against HIV-1NL4-3. HIV216-P50 was more resistant to the other three mcPIs examined, with the fold differences in EC50s against HIV selected with GRL-246 at 1 μM (HIV246-1 μM), HIV286-1 μM, and HIV396-1 μM being 75, >250, and 18, respectively. However, HIV216-P50 was still susceptible to DRV, with the difference in the EC50 for HIV216-P50 relative to that for HIV-1NL4-3 being 3-fold. Interestingly, all four PI-selected HIV-1 variants, including HIV216-P50 were, paradoxically, more susceptible to TPV by factors of 3.3 to 10 relative to the susceptibility of HIV-1NL4-3 to TPV (Table 4), suggesting that the combination of GRL-216 (and the other three PIs as well) and TPV could exert complementarily augmented activity against mcPI-resistant HIV-1 variants. It is not clear at this time how the increased sensitivity to TPV was brought about in those variants. The replication fitness of the GRL-286-resistant variant was examined, and the variant was found to be almost as replication competent as wild-type isolate HIV-1NL4-3 (Fig. 5), suggesting that the possibly compromised replication fitness of the GRL-286-resistant variant was well compensated for by other amino acid substitutions.
TABLE 4.
Activities of various PIs against GRL-216-, GRL-246-, GRL-286-, and GRL-396-selected HIV-1 isolatesa
| Virus | EC50 (μM) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| APV | NFV | LPV | AZV | TPV | DRV | GRL-216 | GRL-246 | GRL-286 | GRL-396 | |
| HIV-1NL4-3 | 0.032 ± 0.001 | 0.040 ± 0.007 | 0.035 ± 0.004 | 0.0032 ± 0.0001 | 0.29 ± 0.05 | 0.003 ± 0.001 | 0.004 ± 0.001 | 0.033 ± 0.003 | 0.006 ± 0.005 | 0.017 ± 0.005 |
| HIV-1216-P50 | 0.26 ± 0.03 (8) | 0.13 ± 0.04 (4) | 0.19 ± 0.06 (5) | 0.014 ± 0.003 (4) | 0.029 ± 0.0004 (0.1) | 0.009 ± 0.006 (3) | 0.094 ± 0.075 (24) | 0.26 ± 0.05 (8) | 0.19 ± 0.06 (32) | 0.24 ± 0.06 (14) |
| HIV-1246-1 μM | 0.34 ± 0.015 (11) | 0.29 ± 0.006 (7) | 0.33 ± 0.04 (8) | 0.032 ± 0.003 (10) | 0.095 ± 0.089 (0.3) | 0.029 ± 0.001 (10) | 0.30 ± 0.01 (75) | 0.42 ± 0.09 (12) | 0.33 ± 0.03 (55) | 0.37 ± 0.013 (22) |
| HIV-1286-1 μM | >1 (>31) | 0.37 ± 0.003 (9) | >1 (>23) | 0.021 ± 0.012 (7) | 0.037 ± 0.001 (0.1) | 0.20 ± 0.05 (67) | >1 (>250) | >1 (>30) | >1 (>167) | >1 (>59) |
| HIV-1396-1 μM | 0.35 ± 0.0002 (11) | 0.22 ± 0.02 (6) | 0.28 ± 0.05 (6) | 0.024 ± 0.002 (8) | 0.042 ± 0.002 (0.1) | 0.016 ± 0.001 (5) | 0.073 ± 0.011 (18) | 0.37 ± 0.002 (11) | 0.25 ± 0.05 (42) | 0.52 ± 0.07 (31) |
The amino acid substitutions identified in the protease-encoding region of HIV-1216-P50, HIV-1246-1 μM, HIV-1286-1 μM, and HIV-1396-1 μM compared to the consensus type B sequence cited from the Los Alamos database include L10I/L24I/M46I/V82I/I84V; L10F/M46I/T91S; L10F/M46L/I50V/A71V; and L10F/M46M, I/Q61Q, or K/V82I/I84V, respectively. MT-4 cells (104) were exposed to 100 TCID50s of each HIV-1 isolate, and inhibition of p24 Gag protein production by each drug was used as the end point. Numbers in parentheses represent the n-fold changes in EC50s for each isolate compared to the EC50s for wild-type isolate HIV-1NL4-3. All assays were conducted in duplicate or triplicate, and the data shown represent the mean values (±1 standard deviation) derived from the results of three independent experiments.
FIG. 5.
Replication kinetics of GRL-286-resistant HIV-1. MT-4 cells (2.4 × 105) were exposed to an HIV-1286-1 μM or a wild-type HIV-1NL4-3 preparation containing 30 ng p24 in six-well culture plates for 3 h, and the MT-4 cells were divided into three fractions, each of which was cultured with or without GRL-286 (104 MT-4 cells/ml and drug concentrations of 0, 0.01, and 0.1 μM). The amounts of p24 were measured every 2 days for up to 9 days.
Substitutions V82I and I84V prevent GRL-216 from blocking protease dimerization and play a role in HIV-1 resistance to GRL-216.
Our observation that the GRL-216- and GRL-396-selected HIV-1 variants had acquired various amino acid substitutions prompted us to examine whether such amino acid substitutions affected the protease dimerization inhibition of GRL-216. We generated in the setting of the FRET-based HIV-1 expression assay (23) a pair of recombinant clones (one with CFP and the other with YFP) containing one of the following mutations: L24I, V82I, I84V, V82I/I84V, or L10I/L24I/M46L/L63P/V82I/I84V. With L24I, V82I, or I84V alone, the protease dimerization-blocking activity of 0.1 μM GRL-216 was not affected; however, with either set of the V82I/I84V or L10I/L24I/M46L/L63P/V82I/I84V substitutions, GRL-216 failed to block the dimerization (Fig. 6). Thus, the V82I and I84V substitutions prevented GRL-216 from blocking protease dimerization and played a role in the emergence of HIV-1 resistance to GRL-216.
FIG. 6.
GRL-216 fails to block protease dimerization with V82I and I84V substitutions. To examine whether an amino acid substitution(s) emerged upon selection of HIV-1NL4-3 with GRL-216, recombinant clones containing the L24I, V82I, I84V, V82I/I84V, or L10I/L24I/M46L/L63P/V82I/I84V mutation(s) were generated in the setting of the FRET-based HIV-1 expression assay (23). With L24I, V82I, or I84V alone, the protease dimerization-blocking activity of 0.1 μM GRL-216 was not affected; however, with either set of the V82I/I84V or L10I/L24I/M46L/L63P/V82I/I84V substitutions, GRL-216 failed to block the dimerization.
Structural analysis of interactions with GRL-216 with HIV-1 protease.
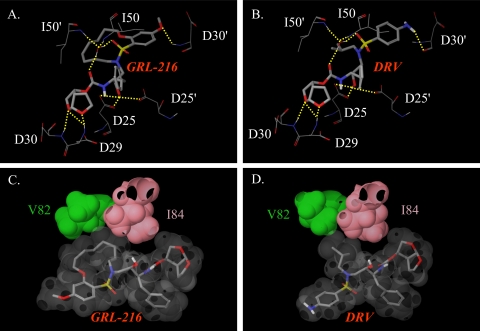
The crystal structures of GRL-216 (PDB accession number 3I6O) and DRV (PDB accession number 2IEN) (34) were analyzed to gain insight into the similarities and differences in their interactions with HIV-1 protease (Fig. 7). Hydrogens were added to the coordinates obtained from PDB and minimized with constraints on the heavy atoms in an OPLS2005 force field using the Maestro (version 9.0) program (Schrödinger, LLC).
FIG. 7.
Interactions of GRL-216 with HIV-1 protease compared to those of DRV. (A and B) Selected hydrogen bond interactions of GRL-216 and DRV. Both inhibitors have hydrogen bond interactions with Asp29, Asp30, Asp25, and Asp25′ and water-mediated hydrogen bond interactions with Ile50 and Ile50′ in the flap. Both inhibitors form hydrogen bond interactions with different backbone atoms of Asp30′; GRL-216 forms a hydrogen bond with the NH, while DRV forms hydrogen bonds with the oxygen of the carbonyl. (C and D) The van der Waals surfaces of GRL-216, DRV, and protease residues V82 and I84 are shown. The macrocyclic ring of GRL-216 appears to have greater van der Waals contact with V82 and I84 than the corresponding isopropyl of darunavir. All figures were generated using the Maestro (version 9.0) program (Schrödinger, LLC).
The bis-THF component of GRL-216 forms a hydrogen-bonding interaction with D29 and D30 of the protease. Hydrogen bond interactions with D25 and D25′ and water molecule-mediated hydrogen bond interactions with I50 and I50′ were also observed (Fig. 7A). These hydrogen bond interactions were also observed for DRV (Fig. 7-B). The P2′ ligands of DRV and GRL-216 were different, and both of them formed hydrogen bond interactions with different backbone atoms of D30′. The oxygen atom in the methoxybenzene of GRL-216 formed a hydrogen bond with the backbone NH of D30′, while the aniline nitrogen of darunavir forms a hydrogen bond with the oxygen of the backbone carbonyl. While the polar interactions of these molecules were similar, there appeared to be subtle differences in the nonpolar interaction due to the presence of the macrocyclic ring in GRL-216.
The macrocyclic ring occupied more volume at the S1′-S2′ binding cavity of the protease and formed more van der Waals interactions with V82 and I84 than the corresponding isopropyl group of the structurally related bis-THF-containing compound DRV (Fig. 7C and D), strongly suggesting that the binding of GRL-216 to V82 and V84 along with the polar interactions described earlier significantly contribute to its activity against HIV-1 protease. This finding should explain well why the V82I and I84V substitutions emerged during the in vitro selection process (Fig. 4). However, the observation that the V82I and I84V substitutions prevented GRL-216 from blocking protease dimerization cannot be explained by structural analysis of the interactions of GRL-216 with the mature and dimerized protease discussed above. Elucidation of the mechanism by which the V82I and I84V substitutions results in the loss of the dimerization blockade of GRL-216 requires detailed structural data on the interactions of GRL-216 and the monomer protease subunit.
DISCUSSION
Macrocycles represent a group of functional moieties inherent to a number of bioactive natural products.
We have designed and synthesized a series of PIs containing functionalities (such as bis-THF) that interact with the amino acid backbones of the catalytic site of the HIV-1 protease along with flexible macrocyclic groups involving P1′-P2′ ligands for effective repacking of the altered PI-binding cavity of protease that emerges upon side chain mutations in PI-resistant HIV-1 variants (16). Here, we report on a novel potent PI, GRL-216, which exerted potent activity against wild-type HIV-1 as well as a wide spectrum of PI-resistant HIV-1 variants in vitro.
We first chose four PIs (GRL-216, GRL-246, GRL-286, and GRL-396) and subsequently focused on GRL-216 and -286, which contain 8 and 9 carbon atoms in the macrocyclic moiety, respectively. Both of these compounds turned out to be reasonably active against HIV-2 (Table 1). Against laboratory-generated PI-resistant HIV-1 variants, both GRL-216 and -286 were somewhat less potent than DRV against a few HIV-1 variants (Table 2); however, GRL-216 had potency comparable to that of DRV against the multidrug-resistant clinical HIV-1 strains examined (Table 3). When we attempted to select HIV-1 variants resistant to each of the four PIs, HIV-1 acquired moderate levels of resistance to GRL-246, -286, and -396. It was noted that the acquisition of resistance to these three PIs was significantly delayed compared to that to APV (Fig. 3). The acquisition of resistance to GRL-216 was the most delayed, and even at passage 60, HIV-1 was only poorly replicating in the presence of 0.26 μM GRL-216 (Fig. 3). As expected, all four PIs were much less active against the HIV-1 variants selected with GRL-246, -286, and -396 (designated HIV-1246-1 μM, HIV-1286-1 μM, and HIV-1396-1 μM, respectively), although the four PIs remained relatively active against the HIV-1 isolates selected with GRL-216 (HIV-1216-P50) (Table 4). It was of note that all FDA-approved PIs employed in this study (APV, NFV, LPV, AZV, and DRV) remained substantially active against HIV-1216-P50, HIV-1246-1 μM, and HIV-1396-1 μM, although APV, LPV, and DRV were much less active against HIV-1286-1 μM. These different resistance profiles between the conventional PIs and the PIs examined are likely to stem at least in part from the presence of the macrocyclic moiety. The four PIs drove the evolution of the resistance mutations commonly observed with FDA-approved PIs (e.g., V82I and I84V). However, since the GRL compounds do interact with the catalytic site amino acids, such as V82 and I84 (Fig. 7), it is expected that mutations in such amino acid residues would occur. Nevertheless, the antiviral activities of the GRL compounds appear to differ from the antiviral activity of DRV (see below).
Interestingly, all four mcPI-selected variants were significantly more susceptible to TPV by 3.3- to 10-fold. Of note, none of the mcPI-selected variants carried any of the three major amino acid substitutions, L33I/V/F, I54V, and V82T/L (31), which confer on HIV-1 resistance to TPV. Although the exact mechanism for the enhanced activity of TPV against PI-resistant HIV-1 variants remains to be determined, it is suggested that combination chemotherapy with PI and TPV may bring about favorable clinical efficacy, considering that the combination of 3′-thia-2′,3′-dideoxycytidine (3TC) and azidothymidine, which shows increased potency against M184V-containing HIV-1 variants (4, 18, 19, 26), still plays a crucial role in effective HAART regimens.
Dimerization of the HIV-1 protease subunits is essential for the proteolytic activity of HIV-1, which plays a critical role in HIV-1 replication (23). Hence, the inhibition of protease dimerization represents a unique target for potential intervention against HIV-1. We previously developed a FRET-based HIV-1 expression system employing cyan and yellow fluorescent protein-tagged protease monomers. Using this system, in 2007, we reported on a group of nonpeptidyl small-molecule inhibitors of protease, including DRV (23). We therefore asked in the present study whether GRL-216 and GRL-286, both of which contain the bis-THF moiety, like DRV does, could block the dimerization of the HIV-1 protease in the system. All average CFPA/B ratios proved to be less than 1.0 in the presence of certain concentrations of GRL-216 and GRL-286, indicating that both compounds effectively blocked HIV-1 protease dimerization. It is thought that this protease dimerization activity, along with protease catalysis-inhibitory activity, should render GRL-216 and GRL-286 potent against diverse HIV-1 variants and cause a substantial delay in the time to the emergence of resistance to the compounds (Fig. 3).
The crystal structure of GRL-216 revealed that, as in the case of DRV (16, 25), the bis-THF moiety of GRL-216 forms strong hydrogen-bonding interactions with the backbones of D29 and D30 of protease and the compound has good interactions with D25 and I50, which should give GRL-216 significantly potent activity against diverse wild-type and multidrug-resistant HIV-1 strains. However, the macrocyclic ring was found to occupy more of the binding cavity of protease and form more van der Waals interactions with V82 and I84 than the corresponding isopropyl group of DRV (Fig. 7C and D). This observation should explain why HIV-1 selected with GRL-216 acquired V82I and I84V substitutions early in the selection, although GRL-216 remained fairly potent against HIV-1 variants containing these two substitutions (Table 4) (34).
Of note, if GRL-216 and GRL-286 are directly compared with DRV on the basis of the EC50s alone, the compounds do not appear to possess significant advantages over DRV. However, HIV selected with the PIs examined in the present study had increased susceptibility to TPV, suggesting that combination of such mcPIs and TPV may achieve synergism. Furthermore, macrocycles represent a group of functional moieties derived from natural products, and PIs might have advantages over other PIs, including DRV.
In conclusion, the data obtained in the present study showed that GRL-216, which has dual anti-HIV-1 activity, inhibition of protease dimerization and the catalytic activity of HIV-1 protease, exerts potent activity against a wide spectrum of multidrug-resistant HIV-1 variants and that its macrocyclic moiety contributes to its increased anti-HIV-1 potency compared to that of its structurally related, bis-THF-containing PI DRV. The present data suggest that macrocyclic moieties may play a role in improving the anti-HIV-1 profile of PIs and in the future may contribute to the design of anti-HIV-1 compounds.
Acknowledgments
This work was supported in part by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health; a grant from the National Institutes of Health (grant GM 53386 to A.K.G.); a grant from the Kumamoto University Global Center of Excellence Program, Global Education and Research Center Aiming at the Control of AIDS, supported by the Ministry of Education, Culture, Sports, Science, and Technology (Monbu-Kagakusho); a Grant-in-Aid for Scientific Research (Priority Areas) from Monbu-Kagakusho of Japan; and a Grant for Promotion of AIDS Research from the Ministry of Health, Labor and Welfare (Kosei-Rodosho) of Japan.
Footnotes
Published ahead of print on 3 May 2010.
REFERENCES
- 1.Amano, M., Y. Koh, D. Das, J. Li, S. Leschenko, Y. F. Wang, P. I. Boross, I. T. Weber, A. K. Ghosh, and H. Mitsuya. 2007. A novel bis-tetrahydrofuranylurethane-containing nonpeptidic protease inhibitor (PI), GRL-98065, is potent against multiple-PI-resistant human immunodeficiency virus in vitro. Antimicrob. Agents Chemother. 51:2143-2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhaskaran, K., O. Hamouda, M. Sannes, F. Boufassa, A. M. Johnson, P. C. Lambert, and K. Porter. 2008. Changes in the risk of death after HIV seroconversion compared with mortality in the general population. JAMA 300:51-59. [DOI] [PubMed] [Google Scholar]
- 3.Carr, A. 2003. Toxicity of antiretroviral therapy and implications for drug development. Nat. Rev. Drug Discov. 2:624-634. [DOI] [PubMed] [Google Scholar]
- 4.Chamberlain, P. P., J. Ren, C. E. Nichols, L. Douglas, J. Lennerstrand, B. A. Larder, D. I. Stuart, and D. K. Stammers. 2002. Crystal structures of zidovudine- or lamivudine-resistant human immunodeficiency virus type 1 reverse transcriptases containing mutations at codons 41, 184, and 215. J. Virol. 76:10015-10019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clavel, F., D. Guetard, F. Brun-Vezinet, S. Chamaret, M. A. Rey, M. O. Santos-Ferreira, A. G. Laurent, C. Dauguet, C. Katlama, C. Rouzioux, et al. 1986. Isolation of a new human retrovirus from West African patients with AIDS. Science 233:343-346. [DOI] [PubMed] [Google Scholar]
- 6.De Clercq, E. 2010. Highlights in the discovery of antiviral drugs: a personal retrospective. J. Med. Chem. 53:1438-1450. [DOI] [PubMed] [Google Scholar]
- 7.Doyon, L., G. Croteau, D. Thibeault, F. Poulin, L. Pilote, and D. Lamarre. 1996. Second locus involved in human immunodeficiency virus type 1 resistance to protease inhibitors. J. Virol. 70:3763-3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Driggers, E. M., S. P. Hale, J. Lee, and N. K. Terrett. 2008. The exploration of macrocycles for drug discovery—an underexploited structural class. Nat. Rev. Drug Discov. 7:608-624. [DOI] [PubMed] [Google Scholar]
- 9.Fang, G., B. Weiser, A. Visosky, T. Moran, and H. Burger. 1999. PCR-mediated recombination: a general method applied to construct chimeric infectious molecular clones of plasma-derived HIV-1 RNA. Nat. Med. 5:239-242. [DOI] [PubMed] [Google Scholar]
- 10.Ferrer, E., D. Podzamczer, M. Arnedo, E. Fumero, P. McKenna, A. Rinehart, J. L. Perez, M. J. Barbera, T. Pumarola, J. M. Gatell, and F. Gudiol. 2003. Genotype and phenotype at baseline and at failure in human immunodeficiency virus-infected antiretroviral-naive patients in a randomized trial comparing zidovudine and lamivudine plus nelfinavir or nevirapine. J. Infect. Dis. 187:687-690. [DOI] [PubMed] [Google Scholar]
- 11.Fumero, E., and D. Podzamczer. 2003. New patterns of HIV-1 resistance during HAART. Clin. Microbiol. Infect. 9:1077-1084. [DOI] [PubMed] [Google Scholar]
- 12.Gatanaga, H., Y. Suzuki, H. Tsang, K. Yoshimura, M. F. Kavlick, K. Nagashima, R. J. Gorelick, S. Mardy, C. Tang, M. F. Summers, and H. Mitsuya. 2002. Amino acid substitutions in Gag protein at non-cleavage sites are indispensable for the development of a high multitude of HIV-1 resistance against protease inhibitors. J. Biol. Chem. 277:5952-5961. [DOI] [PubMed] [Google Scholar]
- 13.Ghosh, A. K., and J. H. Kim. 2004. Stereoselective chloroacetate aldol reactions: syntheses of acetate aldol equivalents and darzens glycidic esters. Org. Lett. 6:2725-2728. [DOI] [PubMed] [Google Scholar]
- 14.Ghosh, A. K., J. F. Kincaid, W. Cho, D. E. Walters, K. Krishnan, K. A. Hussain, Y. Koo, H. Cho, C. Rudall, L. Holland, and J. Buthod. 1998. Potent HIV protease inhibitors incorporating high-affinity P2-ligands and (R)-(hydroxyethylamino)sulfonamide isostere. Bioorg. Med. Chem. Lett. 8:687-690. [DOI] [PubMed] [Google Scholar]
- 15.Ghosh, A. K., K. Krishnan, D. E. Walters, W. Cho, H. Cho, Y. Koo, J. Trevino, L. Holland, and J. Buthod. 1998. Structure based design: novel spirocyclic ethers as nonpeptidal P2-ligands for HIV protease inhibitors. Bioorg. Med. Chem. Lett. 8:979-982. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh, A. K., S. Kulkarni, D. D. Anderson, L. Hong, A. Baldridge, Y. F. Wang, A. A. Chumanevich, A. Y. Kovalevsky, Y. Tojo, M. Amano, Y. Koh, J. Tang, I. T. Weber, and H. Mitsuya. 2009. Design, synthesis, protein-ligand X-ray structure, and biological evaluation of a series of novel macrocyclic human immunodeficiency virus-1 protease inhibitors to combat drug resistance. J. Med. Chem. 52:7689-7705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghosh, A. K., S. Leshchenko, and M. Noetzel. 2004. Stereoselective photochemical 1,3-dioxolane addition to 5-alkoxymethyl-2(5H)-furanone: synthesis of bis-tetrahydrofuranyl ligand for HIV protease inhibitor UIC-94017 (TMC-114). J. Org Chem. 69:7822-7829. [DOI] [PubMed] [Google Scholar]
- 18.Girouard, M., K. Diallo, B. Marchand, S. McCormick, and M. Gotte. 2003. Mutations E44D and V118I in the reverse transcriptase of HIV-1 play distinct mechanistic roles in dual resistance to AZT and 3TC. J. Biol. Chem. 278:34403-34410. [DOI] [PubMed] [Google Scholar]
- 19.Gotte, M., D. Arion, M. A. Parniak, and M. A. Wainberg. 2000. The M184V mutation in the reverse transcriptase of human immunodeficiency virus type 1 impairs rescue of chain-terminated DNA synthesis. J. Virol. 74:3579-3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta, R., A. Hill, A. W. Sawyer, and D. Pillay. 2008. Emergence of drug resistance in HIV type 1-infected patients after receipt of first-line highly active antiretroviral therapy: a systematic review of clinical trials. Clin. Infect. Dis. 47:712-722. [DOI] [PubMed] [Google Scholar]
- 21.Hog, R. 2008. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet 372:293-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koh, Y., D. Das, S. Leschenko, H. Nakata, H. Ogata-Aoki, M. Amano, M. Nakayama, A. K. Ghosh, and H. Mitsuya. 2009. GRL-02031, a novel nonpeptidic protease inhibitor (PI) containing a stereochemically defined fused cyclopentanyltetrahydrofuran potent against multi-PI-resistant human immunodeficiency virus type 1 in vitro. Antimicrob. Agents Chemother. 53:997-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koh, Y., S. Matsumi, D. Das, M. Amano, D. A. Davis, J. Li, S. Leschenko, A. Baldridge, T. Shioda, R. Yarchoan, A. K. Ghosh, and H. Mitsuya. 2007. Potent inhibition of HIV-1 replication by novel non-peptidyl small molecule inhibitors of protease dimerization. J. Biol. Chem. 282:28709-28720. [DOI] [PubMed] [Google Scholar]
- 24.Koh, Y., H. Nakata, K. Maeda, H. Ogata, G. Bilcer, T. Devasamudram, J. F. Kincaid, P. Boross, Y. F. Wang, Y. Tie, P. Volarath, L. Gaddis, R. W. Harrison, I. T. Weber, A. K. Ghosh, and H. Mitsuya. 2003. Novel bis-tetrahydrofuranylurethane-containing nonpeptidic protease inhibitor (PI) UIC-94017 (TMC114) with potent activity against multi-PI-resistant human immunodeficiency virus in vitro. Antimicrob. Agents Chemother. 47:3123-3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kovalevsky, A. Y., A. K. Ghosh, and I. T. Weber. 2008. Solution kinetics measurements suggest HIV-1 protease has two binding sites for darunavir and amprenavir. J. Med. Chem. 51:6599-6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larder, B. A., S. D. Kemp, and P. R. Harrigan. 1995. Potential mechanism for sustained antiretroviral efficacy of AZT-3TC combination therapy. Science 269:696-699. [DOI] [PubMed] [Google Scholar]
- 27.Little, S. J., S. Holte, J. P. Routy, E. S. Daar, M. Markowitz, A. C. Collier, R. A. Koup, J. W. Mellors, E. Connick, B. Conway, M. Kilby, L. Wang, J. M. Whitcomb, N. S. Hellmann, and D. D. Richman. 2002. Antiretroviral-drug resistance among patients recently infected with HIV. N. Engl. J. Med. 347:385-394. [DOI] [PubMed] [Google Scholar]
- 28.Maeda, K., K. Yoshimura, S. Shibayama, H. Habashita, H. Tada, K. Sagawa, T. Miyakawa, M. Aoki, D. Fukushima, and H. Mitsuya. 2001. Novel low molecular weight spirodiketopiperazine derivatives potently inhibit R5 HIV-1 infection through their antagonistic effects on CCR5. J. Biol. Chem. 276:35194-35200. [DOI] [PubMed] [Google Scholar]
- 29.Mitsuya, H., and J. Erickson. 1999. Discovery and development of antiretroviral therapeutics for HIV infection, p. 751-780. In T. C. Merigan, J. G. Bartlet, and D. Bolognesi (ed.), Textbook of AIDS medicine. The Williams & Wilkins Co., Baltimore, MD.
- 30.Murphy, E. L., A. C. Collier, L. A. Kalish, S. F. Assmann, M. F. Para, T. P. Flanigan, P. N. Kumar, L. Mintz, F. R. Wallach, and G. J. Nemo. 2001. Highly active antiretroviral therapy decreases mortality and morbidity in patients with advanced HIV disease. Ann. Intern. Med. 135:17-26. [DOI] [PubMed] [Google Scholar]
- 31.Naeger, L. K., and K. A. Struble. 2007. Food and Drug Administration analysis of tipranavir clinical resistance in HIV-1-infected treatment-experienced patients. AIDS 21:179-185. [DOI] [PubMed] [Google Scholar]
- 32.Shirasaka, T., R. Yarchoan, M. C. O'Brien, R. N. Husson, B. D. Anderson, E. Kojima, T. Shimada, S. Broder, and H. Mitsuya. 1993. Changes in drug sensitivity of human immunodeficiency virus type 1 during therapy with azidothymidine, dideoxycytidine, and dideoxyinosine: an in vitro comparative study. Proc. Natl. Acad. Sci. U. S. A. 90:562-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siliciano, J. D., and R. F. Siliciano. 2004. A long-term latent reservoir for HIV-1: discovery and clinical implications. J. Antimicrob. Chemother. 54:6-9. [DOI] [PubMed] [Google Scholar]
- 34.Tie, Y., P. I. Boross, Y. F. Wang, L. Gaddis, A. K. Hussain, S. Leshchenko, A. K. Ghosh, J. M. Louis, R. W. Harrison, and I. T. Weber. 2004. High resolution crystal structures of HIV-1 protease with a potent non-peptide inhibitor (UIC-94017) active against multi-drug-resistant clinical strains. J. Mol. Biol. 338:341-352. [DOI] [PubMed] [Google Scholar]
- 35.Walensky, R. P., A. D. Paltiel, E. Losina, L. M. Mercincavage, B. R. Schackman, P. E. Sax, M. C. Weinstein, and K. A. Freedberg. 2006. The survival benefits of AIDS treatment in the United States. J. Infect. Dis. 194:11-19. [DOI] [PubMed] [Google Scholar]
- 36.Yoshimura, K., R. Kato, M. F. Kavlick, A. Nguyen, V. Maroun, K. Maeda, K. A. Hussain, A. K. Ghosh, S. V. Gulnik, J. W. Erickson, and H. Mitsuya. 2002. A potent human immunodeficiency virus type 1 protease inhibitor, UIC-94003 (TMC-126), and selection of a novel (A28S) mutation in the protease active site. J. Virol. 76:1349-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoshimura, K., R. Kato, K. Yusa, M. F. Kavlick, V. Maroun, A. Nguyen, T. Mimoto, T. Ueno, M. Shintani, J. Falloon, H. Masur, H. Hayashi, J. Erickson, and H. Mitsuya. 1999. JE-2147: a dipeptide protease inhibitor (PI) that potently inhibits multi-PI-resistant HIV-1. Proc. Natl. Acad. Sci. U. S. A. 96:8675-8680. [DOI] [PMC free article] [PubMed] [Google Scholar]