Abstract
Human cytomegalovirus (HCMV) is a widespread pathogen that can cause severe disease in immunologically immature and immunocompromised individuals. Cyclopropavir (CPV) is a guanine nucleoside analog active against human and murine cytomegaloviruses in cell culture and efficacious in mice by oral administration. Previous studies established that the mechanism of action of CPV involves inhibition of viral DNA synthesis. Based upon this action and the structural similarity of CPV to ganciclovir (GCV), we hypothesized that CPV must be phosphorylated to a triphosphate to inhibit HCMV DNA synthesis and that pUL97 is the enzyme responsible for the initial phosphorylation of CPV to a monophosphate (CPV-MP). We found that purified pUL97 phosphorylated CPV 45-fold more extensively than GCV, a known pUL97 substrate and the current standard of treatment for HCMV infections. Kinetic studies with CPV as the substrate for pUL97 demonstrated a Km of 1,750 ± 210 μM. Introduction of 1.0 or 10 nM maribavir, a known pUL97 inhibitor, and subsequent Lineweaver-Burk analysis demonstrated competitive inhibition of CPV phosphorylation, with a Ki of 3.0 ± 0.3 nM. Incubation of CPV with pUL97 combined with GMP kinase [known to preferentially phosphorylate the (+)-enantiomer of CPV-MP] established that pUL97 stereoselectively phosphorylates CPV to its (+)-monophosphate. These results elucidate the mechanism of CPV phosphorylation and help explain its selective antiviral action.
Human cytomegalovirus (HCMV), a betaherpesvirus, is a widespread pathogen infecting between 40 and 80% of the population. Although immunocompetent individuals rarely manifest any symptoms, HCMV can result in severe disease, such as interstitial pneumonia, mental retardation, and hearing loss in immunocompromised and immunologically immature individuals (26, 50). Currently, therapeutic agents such as ganciclovir (GCV), foscarnet (PFA), cidofovir, and fomivirsen are used for the treatment or prophylaxis of HCMV disease (1, 7, 20, 22, 39, 50). However, long-term therapy is generally required due to recurrence of infection upon cessation of therapy, leading to the development of drug resistance and severe adverse effects (4, 13, 16, 23, 33, 41). With the increased use of immunosuppression for cancer chemotherapy and organ transplantation, there is an increasing need for more effective and less toxic drugs to treat HCMV.
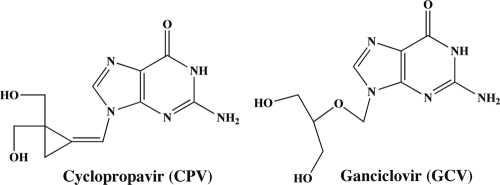
We have demonstrated previously that cyclopropavir (CPV) (Fig. 1), a bis-hydroxymethyl methylenecyclopropane guanosine nucleoside analog, is approximately 10-fold more active in vitro (50% effective concentration [EC50] of 0.46 μM) than GCV (EC50 of 4.1 μM) (51, 52). In addition, CPV is active against several HCMV mutants that are resistant to GCV or PFA (29). Further experimentation in vivo with CPV demonstrated 2- to 5-log10 reductions in titers of murine cytomegalovirus (MCMV), resulting in reduced mortality in severe combined immunodeficient (SCID) mice, and reduced viral replication in human fetal tissue implanted in SCID mice infected with HCMV (28).
FIG. 1.
Structures of cyclopropavir (CPV) and ganciclovir (GCV).
Previous studies have established that the mechanism of action of CPV involves inhibition of viral DNA synthesis (29). Furthermore, the activity of CPV was reduced approximately 20-fold against an HCMV UL97 deletion mutant (29), thereby indicating the importance of this gene product in the action of CPV. Taken together, these results suggest that the mechanism of action of CPV resembles that of GCV, in which the drug is first phosphorylated by viral pUL97, a protein kinase that can phosphorylate nucleoside analogs (32, 36, 46, 47). Upon further phosphorylation by endogenous cellular kinases, the triphosphate of GCV inhibits HCMV DNA polymerase, resulting in inhibition of viral replication (5, 9, 19, 34, 37, 38, 45). Therefore, we investigated the possibility that viral pUL97 also phosphorylates CPV to its monophosphate (CPV-MP). In addition, our previous results demonstrating the enantioselective phosphorylation of (+)-CPV-MP (31) allowed us to determine whether CPV phosphorylation by pUL97 is stereoselective.
MATERIALS AND METHODS
Nucleoside analogs.
Cyclopropavir, or (Z)-9-{[2,2-bis-(hydroxymethyl)cyclopropylidene]-methyl}guanine, the (+)- and (−)-enantiomers of cyclopropavir monophosphate (CPV-MP), and racemic mixtures of CPV di- and triphosphates (CPV-DP and CPV-TP, respectively) (used as high-performance liquid chromatography [HPLC] standards) were provided by J. Zemlicka (31, 52). Compound 1263W94 (maribavir [MBV]) was provided through the courtesy of Karen K. Biron (Burroughs Welcome, Research Triangle Park, NC). Ganciclovir was obtained from Syntex (Roche, Basel, Switzerland). [8-3H]ganciclovir was purchased from Moravek Biochemicals, Inc. (Brea, CA). The radiochemical purity was 99.3%, and the specific activity was 3.4 Ci/mmol.
GMP kinase.
Porcine GMP kinase (GMPK) at a concentration of 3.1 U/ml was purchased from Sigma Aldrich (St. Louis, MO). (A unit is defined as the amount of enzyme necessary to convert 1.0 μmol of GMP to GDP in 1.0 min at pH 7.5 and 30°C.)
Preparation and purification of GST-UL97 (pUL97).
Glutathione S-transferase (GST)-UL97 was prepared and purified as previously described (25), with some modifications. Briefly, baculoviruses were generated using a Bac-to-Bac system (Invitrogen, Inc., Carlsbad, CA) with customized pFASTBAC transfer vectors incorporating an N-terminal glutathione S-transferase tag, as previously described (24). The resulting baculovirus was used to express the GST-UL97 fusion protein in Sf9 insect cells. Sf9 cells expressing GST-UL97 were lysed in 50 mM Tris, pH 8.0, 1 M NaCl, 10 mM EDTA, 0.5% NP-40, 10 mM EDTA containing one Complete (Roche) EDTA-free protease inhibitor tablet per 50 ml. After clarification by centrifugation, the lysate was loaded on glutathione Sepharose 4 Fast Flow resin (GE Health Sciences), washed extensively in lysis buffer lacking NP-40, and eluted in buffer containing 15 mM reduced glutathione in 50 mM Tris-HCl, pH 8.0, 50 mM NaCl, 2 mM dithiothreitol (DTT). This eluate was then loaded on a Q-Sepharose anion-exchange column (GE Health Sciences) and eluted using a 50 mM to 1 M linear NaCl gradient in the same buffer system (50 mM Tris-HCl, pH 8.0, 2 mM DTT) with an automated fraction collector on a BioLogic LP system (Bio-Rad). Fractions containing purified GST-UL97 were pooled and dialyzed into storage buffer (100 mM NaCl, 20 mM Na-HEPES, pH 7.4, 2 mM DTT, 0.5 mM EDTA). Protein concentrations were determined by amino acid analysis at the Molecular Biology Core Facility, Dana-Farber Cancer Institute. A K355Q mutant version of this enzyme, purified in parallel with the same preparation of wild-type (WT) GST-UL97, has been demonstrated to be devoid of detectable contaminating kinase activities (24, 27).
Kinase assays.
Kinase buffer (0.05 M Tris, pH 7.6, 0.05 M KCl, 5.0 mM MgCl2), ATP (2.0 to 5.0 mM), bovine serum albumin (0.1 mg/ml) (all final concentrations), substrate (GCV, CPV, or CPV-MP; 0.1 to 1.0 mM), and inhibitor (maribavir; 1.0 and 10 nM) were incubated at 37°C for 20 min prior to introduction of enzyme. In experiments in which GCV was the substrate, 2.0 μCi of [3H]GCV per 100 μl of reaction mixture was added. At time zero, pUL97 (5.0 ng/μl) and/or GMP kinase (0.2 U/ml) was added to the solution. At designated times, an aliquot was removed and placed on ice, and proteins were precipitated with 0.04 volumes of 10 N perchloric acid. The samples were centrifuged, and the supernatants were neutralized with KOH and stored at −20°C until analyzed by HPLC.
Reverse-phase high-performance liquid chromatography.
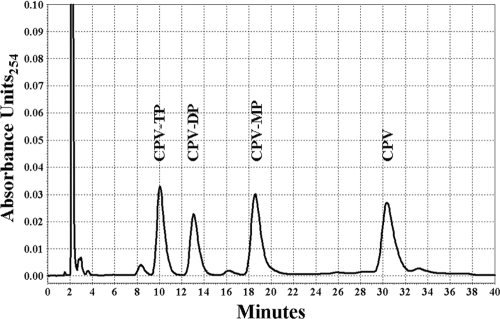
CPV and its phosphorylated derivatives (CPV-MP, CPV-DP, and CPV-TP) were separated and quantified by reverse-phase HPLC (Beckman Coulter [Fullerton, CA] System Gold programmable solvent module 125 and System Gold programmable detector module 166 controlled by 32 Karat software [version 7.0]). Before injection, each sample was centrifuged at 14,000 rpm for 5 min to remove any remaining particulate matter. Samples were loaded onto a 10-μm Alphabond C18 300- by 3.9-mm reverse-phase column (Alltech, Deerfield, IL) at a flow rate of 2.0 ml/min. CPV and its phosphorylated derivatives were eluted using 300 mM ammonium phosphate (pH 3.0) and 100% methanol (linear gradient of 5% methanol over 30 min). Each compound was quantified by comparing the peak area with that of a known amount of the appropriate standard at a wavelength of 254 nm. The retention times for CPV, CPV-MP, CPV-DP, and CPV-TP were 30.3, 18.6, 13.0, and 10.0 min, respectively (Fig. 2).
FIG. 2.
Separation of CPV, CPV-MP, CPV-DP, and CPV-TP by reverse-phase HPLC.
Strong-anion-exchange HPLC.
GCV and GCV-MP were separated by strong-anion-exchange HPLC (Beckman Coulter [Fullerton, CA] System Gold programmable solvent module 125 and System Gold programmable detector module 166 controlled by 32 Karat software [version 7.0]). Before injection, each sample was centrifuged at 14,000 rpm for 5 min to remove any remaining particulate matter. Samples were loaded onto a 5-μm Hypersil 250- by 4.6-mm strong-anion-exchange column (Thermo Scientific, Waltham, MA) at a flow rate of 1.0 ml/min. GCV and GCV-MP were eluted using 1.0 mM ammonium phosphate (pH 3.0) and 500 mM ammonium phosphate (pH 3.0) (linear gradient of 50% 500 mM ammonium phosphate over 100 min). Thirty-second fractions were collected, analyzed, and quantified by liquid scintillation spectrometry using a Tri-Carb liquid scintillation analyzer (Canberra, Meriden, CT).
RESULTS
Phosphorylation of CPV and GCV by pUL97.
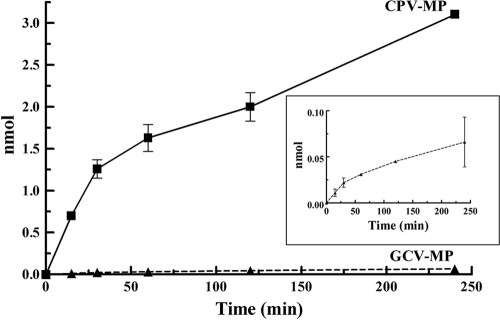
It is known that the mode of action of CPV against HCMV involves inhibition of viral DNA synthesis (29). In conjunction with other results demonstrating that an HCMV UL97 deletion mutant is resistant to CPV (29), we hypothesized that pUL97 can phosphorylate CPV to a monophosphate. To test this, CPV was incubated with pUL97 and compared to GCV, a related guanosine nucleoside analog and a known substrate for pUL97 (32, 46). The results demonstrated an accumulation of CPV monophosphate (CPV-MP) in a time-dependent manner, but surprisingly, no GCV-MP was detected (UV absorption at 254 nm, UV detection). Because any formation of GCV monophosphate (GCV-MP) was below the limit of UV detection (0.1 nmol), radiolabeled GCV was employed to increase sensitivity. Figure 3 compares the phosphorylation of CPV using UV detection with the phosphorylation of [3H]GCV using liquid scintillation spectrometry. The difference in extent of phosphorylation is apparent in that the accumulation of CPV-MP (3.1 nmol) was approximately 45-fold greater at 240 min than that of GCV-MP (0.07 nmol).
FIG. 3.
Phosphorylation of CPV and GCV by pUL97. The time-dependent formation of CPV-MP was determined by UV absorption of the HPLC effluent; that of GCV-MP was measured by liquid scintillation spectrometry. The boxed inset illustrates the time-dependent increase of GCV-MP only. The values represent the means ± standard deviations from at least two experiments.
Kinetic values for CPV as a substrate for pUL97.
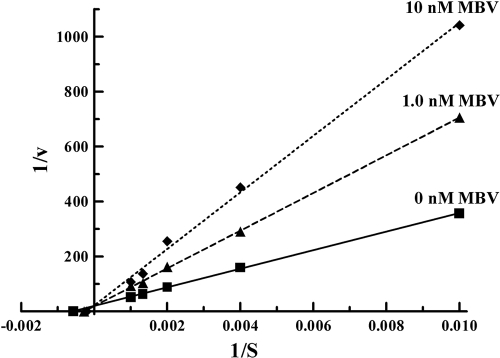
With the demonstration that CPV is a substrate for HCMV pUL97, a kinetic analysis of substrate and enzyme was performed. Preliminary experiments established that all of the conditions for Michaelis-Menten kinetic analysis, including steady state, saturation of substrate, and multiple turnovers, were met. Lineweaver-Burk analysis gave a Km of 1,750 ± 210 μM, a maximum rate of metabolism (Vmax) of 0.050 ± 0.003 nmol/min, a kcat of 0.0012 s−1, and a specificity constant (kcat/Km) of 0.69 s−1 M−1 for CPV phosphorylation (Fig. 4). However, we were unable to do a kinetic value comparison of CPV and GCV because at least one condition for Michaelis-Menten analysis with GCV as a substrate for pUL97 (multiple turnovers) was not observed.
FIG. 4.
Lineweaver-Burk plot for inhibition of CPV phosphorylation by maribavir. Formation of CPV-MP from selected concentrations (100 to 1,000 μM) of CPV was measured in the presence of 0, 1.0, or 10 nM maribavir at 30 min. The Km for formation of CPV-MP from CPV by pUL97 was 1,750 ± 210 μM, with a Vmax of 0.050 ± 0.003 nmol/min, a kcat of 0.0012 s−1, and a specificity constant of 0.69 s−1 M−1. The Ki for maribavir was 3.0 ± 0.3 nM. The values represent the means from at least two experiments. Differences in the y intercepts of the linear regression lines (r2 values of >0.99) are statistically insignificant (over a range of 0 to >1,000, intercepts are 19.4 ± 1.5, 19.3 ± 2.9, and 21.3 ± 12, respectively, for 0, 1.0, and 10 nM maribavir).
Previous studies established that maribavir (MBV), a benzimidazole l-riboside and a highly specific pUL97 inhibitor (8, 39), inhibits autophosphorylation of pUL97 as well as pUL97-mediated phosphorylation of exogenous substrates, including pUL44, histones, lamin A/C, and the retinoblastoma tumor suppressor protein (3, 6, 24, 27, 30, 49). Previous studies have shown that the combination of MBV and GCV results in an additive antiviral effect (21, 44), whereas others have reported antagonism (14), thereby suggesting inhibition of nucleoside analog phosphorylation. Therefore, to determine if MBV inhibits the phosphorylation of CPV by pUL97, 1.0 and 10 nM MBV were incubated with concentrations of CPV ranging from 100 to 1,000 μM. A Lineweaver-Burk double-reciprocal plot of the resulting data demonstrated competitive inhibition of CPV phosphorylation, with a Ki of 3.0 ± 0.3 nM for MBV (Fig. 4). This value is consistent with previous results which showed that MBV inhibits protein phosphorylation by pUL97 with a 50% inhibitory concentration of 3.0 nM (6).
Enantioselectivity of CPV phosphorylation by pUL97.
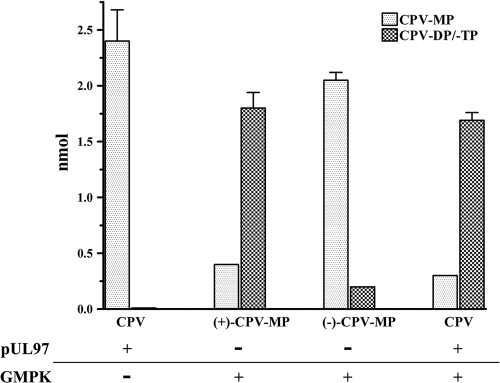
It is well known that stereoisomers of the same drug can have significantly different pharmacological properties (48). Therefore, we investigated which of the hydroxyl groups attached to the cyclopropane moiety of CPV is phosphorylated by pUL97. Because CPV-MP was the only product of CPV phosphorylation by pUL97, one hydroxyl group or the other (but not both) must be phosphorylated. If both were phosphorylated equally on different CPV molecules, the result would be a racemic mixture. If not, either the (+)- or the (−)-enantiomer would form. We have demonstrated previously that (+)-CPV-MP is the preferred substrate of GMP kinase (GMPK) over (−)-CPV-MP (31). Therefore, to determine whether phosphorylation of CPV by pUL97 results in the creation of a racemic mixture or one of the enantiomers, CPV was incubated with pUL97 and GMPK. Results were compared to those obtained by incubating equivalent quantities of (+)- and (−)-CPV-MP with GMPK alone (Fig. 5). Incubation of (+)-CPV-MP with GMPK resulted in the formation of 1.8 nmol of cyclopropavir di- and triphosphates (CPV-DP/-TP), whereas incubation of (−)-CPV-MP with GMPK resulted in only 0.2 nmol of CPV-DP/-TP. As expected, incubation of CPV with pUL97 resulted in a complete lack of CPV-DP/-TP formation. In contrast, incubation of CPV with both pUL97 and GMPK resulted in 1.7 nmol of CPV-DP/-TP. Since the phosphorylation profile of CPV incubated with both pUL97 and GMPK closely resembled that of (+)-CPV-MP incubated with GMPK alone, we conclude that pUL97 stereoselectively phosphorylates CPV to its (+)-enantiomer. The preponderance of CPV-DP/-TPs over CPV-MP in the reaction with both enzymes suggests that the initial phosphorylation of CPV by pUL97 is the rate-limiting step in the formation of CPV-TP, the hypothesized form of CPV responsible for inhibition of viral DNA synthesis.
FIG. 5.
Phosphorylation of CPV by pUL97 and GMPK. CPV, (+)-CPV-MP, and (−)-CPV-MP were used as substrates for pUL97 and/or GMPK, as indicated by the plus and minus symbols at the bottom. In the reaction with both enzymes and CPV as the substrate, CPV-MP was enzymatically produced by pUL97; the resulting amount of CPV-DP/-TP formed by GMPK in the same reaction is compared to those formed by the (+)- and (−)-enantiomers of CPV-MP incubated with GMPK alone. Samples were taken at 240 min, and the values represent the means ± standard deviations from at least two experiments.
DISCUSSION
CPV is a known inhibitor of viral DNA synthesis and replication (29, 52). HCMV isolates with UL97 mutations are resistant to CPV (11, 29), thereby implicating pUL97 in the antiviral action of the drug. The lack of resistance of such isolates to a CPV-MP homolog that circumvents the need for pUL97, cyclopropavir phosphonate (35), provides further support for this conclusion. Our current results establish that purified pUL97 phosphorylates CPV and, in these biochemical experiments, is the rate-limiting step for the formation of CPV triphosphate. CPV is phosphorylated to a much greater extent, approximately 45-fold more, than GCV, a known substrate of pUL97 and the current standard of treatment for HCMV (7, 32, 46). We conclude that this substrate selectivity may explain the greater activity of CPV against HCMV (52). Based on our current results, we hypothesize that pUL97 phosphorylation of CPV to CPV-MP is the rate-limiting step in the formation of CPV-TP (the active compound) in HCMV-infected cells. If this is the case, we further hypothesize that levels of CPV triphosphate in virus-infected cells are greater than those of GCV triphosphate, which very likely contributes to the increased potency of CPV compared to that of GCV. However, other factors may also contribute to the increased potency of CPV, including, but not limited to, the half-life of triphosphate in HCMV-infected cells, the incorporation of drug into viral DNA, and the concentration of drug necessary to inhibit the viral DNA polymerase. All of these factors can affect viral replication and warrant further investigation in order to fully understand the mechanism of action of CPV.
MBV is a known inhibitor of pUL97 phosphorylation of viral regulatory proteins (40), but direct inhibition of nucleoside analog phosphorylation had not previously been established. Therefore, our results are the first demonstration that MBV does inhibit pUL97 phosphorylation of a nucleoside analog, CPV. However, the competitive mode of inhibition was unexpected. Competitive inhibition implies that binding of an inhibitor prevents binding of a substrate and vice versa. This is usually, but not always, the result of the substrate and the inhibitor competing for a position within the enzyme substrate-binding domain. Our observation of competitive inhibition was unexpected because MBV can be modeled into the ATP-binding site of pUL97, based on the known structure of similar protein kinases (17). Moreover, mutations that confer resistance to MBV are located in or near the ATP-binding site, not the substrate-binding domain (15-17). Together, these observations imply that inhibition would not be competitive. It is possible that binding of MBV results in an allosteric shift in the enzyme conformation, resulting in the enzyme becoming incapable of supporting CPV binding. However, we find this unlikely, since allosteric changes resulting in competitive inhibition are the exception rather than the rule and are usually associated with noncompetitive inhibition.
A more likely scenario involves the position in which CPV interacts with pUL97. Due to its methylenecyclopropane ring, the chemical properties of CPV are significantly different from those of GCV, which has an aliphatic moiety. Conformational factors may also be involved; both analogs have similar structures, but CPV with five rotatable bonds is significantly more rigid than GCV, which has seven rotatable bonds. Therefore, we hypothesize that CPV interacts with pUL97 in a manner different from that of GCV. Consistent with this hypothesis, single mutations in UL97 that result in GCV resistance do not result in CPV resistance, but a double mutation does (11, 29), suggesting that CPV binds in a location different from that of GCV. Since MBV is thought to interact with both the ATP binding site of pUL97 and regions outside this site (16, 17), if CPV were to interact with pUL97 outside the ATP binding site but in regions that overlap with MBV binding, the result would be competitive inhibition with respect to CPV.
The stereoselectivity of CPV phosphorylation was also somewhat surprising. It is well known that stereochemistry may have a significant effect on the pharmacodynamic, pharmacokinetic, and pharmacological properties of drugs. Although one enantiomer may have a therapeutic effect and the other none, it is also possible that the inactive enantiomer may produce a toxic or even fatal effect (2). The initial phosphorylation of either of the hydroxyl groups attached to the cyclopropane ring of CPV results in the formation of an enantiomer. Based upon our previous results showing that the (+)-enantiomer of CPV-MP is the preferred substrate for GMP kinase (GMPK) over the (−)-enantiomer (31), our results strongly indicate that pUL97 stereoselectively phosphorylated CPV to the (+)-enantiomer. Since (+)-CPV-MP is the preferred substrate of GMPK, (+)-CPV triphosphate would result and be the compound hypothesized to elicit an antiviral response. We also hypothesize that formation of the (+)-enantiomer of CPV triphosphate results in viral DNA synthesis inhibition, whereas formation of the (−)-enantiomer does not. Consistent with this hypothesis, the (S)-(+)-enantiomer of synguanol (a guanosine methylenecyclopropane homolog with only one hydroxymethyl group on the cyclopropane ring) is active against HCMV, whereas the (R)-(−)-enantiomer is not (12, 42, 43, 51).
Although some inactive enantiomers may have toxic side effects, we have no evidence that this is the case with CPV. We suggest that because (R)-(−)-synguanol did not produce cytotoxicity (43), neither would the (−)-enantiomer of CPV triphosphate. However, it may be that the lack of cytotoxicity by (R)-(−)-synguanol is due to a lack of phosphorylation to its triphosphate (31). Therefore, if triphosphates of the (−)-enantiomers were not formed enzymatically within cells, no toxicity would result.
The use of CPV for treatment or prophylaxis of HCMV disease is still premature, but promising. The increased antiviral activity compared to that of GCV (52), the ability to achieve therapeutic concentrations in vivo without prodrug modification (10, 18), and our current results demonstrating that CPV is a better substrate for pUL97 are a few examples of why CPV may be superior to GCV. Further examination into the mechanism of action and preclinical development of this compound appear warranted.
Acknowledgments
We thank Yasuo Yamakoshi and James Simmer for assistance with the use of James Simmer's HPLC system.
This work was supported by grants from the NIH (CA32779 and AI26077) and Microbiotix, Inc., plus funds from the University of Michigan.
Footnotes
Published ahead of print on 14 June 2010.
REFERENCES
- 1.Alrabiah, F. A., and S. L. Sacks. 1996. New antiherpesvirus agents: their targets and therapeutic potential. Drugs 52:17-32. [DOI] [PubMed] [Google Scholar]
- 2.Ariens, E. J. 1984. Stereochemistry, a basis for sophisticated nonsense in pharmacokinetics and clinical pharmacology. Eur. J. Clin. Pharmacol. 26:663-668. [DOI] [PubMed] [Google Scholar]
- 3.Baek, M. C., P. M. Krosky, and D. M. Coen. 2002. Relationship between autophosphorylation and phosphorylation of exogenous substrates by the human cytomegalovirus UL97 protein kinase. J. Virol. 76:11943-11952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baldanti, F., N. Lurain, and G. Gerna. 2004. Clinical and biologic aspects of human cytomegalovirus resistance to antiviral drugs. Human Immunol. 65:403-409. [DOI] [PubMed] [Google Scholar]
- 5.Biron, K. K., S. C. Stanat, J. B. Sorrell, J. A. Fyfe, P. M. Keller, C. U. Lambe, and D. J. Nelson. 1985. Metabolic activation of the nucleoside analog 9-{[2-hydroxy-1-(hydroxymethyl)-ethoxy]methyl}guanine in human diploid fibroblasts infected with human cytomegalovirus. Proc. Natl. Acad. Sci. U. S. A. 82:2473-2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biron, K. K., R. J. Harvey, S. C. Chamberlain, S. S. Good, A. A. Smith III, M. G. Davis, C. L. Talarico, W. H. Miller, R. Ferris, R. E. Domsife, S. C. Stanat, J. C. Drach, L. B. Townsend, and G. W. Koszalka. 2002. Potent and selective inhibition of human cytomegalovirus replication by 1263W94, a benzimidazole l-riboside with a unique mode of action. Antimicrob. Agents Chemother. 46:2365-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biron, K. K. 2006. Antiviral drugs for cytomegalovirus diseases. Antiviral Res. 71:154-163. [DOI] [PubMed] [Google Scholar]
- 8.Biron, K. K. 2006. Maribavir: a promising new antiherpes therapeutic agent, p. 309-336. In E. Bogner and A. Holzenburg (ed.), New concepts of antiviral therapy. Springer, Dordrecht, Netherlands.
- 9.Boehme, R. E. 1984. Phosphorylation of the antiviral precursor 9-(1,3-dihydroxy-2-propoxymethyl)guanine monophosphate by guanylate kinase isozymes. J. Biol. Chem. 259:12346-12349. [PubMed] [Google Scholar]
- 10.Bowlin, T. L., J. L. Brooks, and J. Zemlicka. 2009. Preclinical pharmacokinetic, toxicokinetic and toxicology results for cyclopropavir, a promising new agent for the treatment of beta- and gamma-herpesviruses. Antiviral Res. 82:A46-A47. [Google Scholar]
- 11.Breitenbach, J. M., K. Z. Borysko, J. Zemlicka, and J. C. Drach. 2006. Resistance of human cytomegalovirus with single and double mutations in UL97 to first and second generation methylenecyclopropane purines. Antiviral Res. 70:A19. [Google Scholar]
- 12.Chen, X., E. R. Kern, J. C. Drach, E. Gullen, Y. C. Cheng, and J. Zemlicka. 2003. Structure-activity relationship of (S,Z)-2-aminopurine methylenecyclopropane analogues of nucleosides. Variation of purine-6 substituents and activity against herpesviruses and hepatitis B virus. J. Med. Chem. 46:1531-1537. [DOI] [PubMed] [Google Scholar]
- 13.Chou, S., R. H. Waldemer, A. E. Senters, K. S. Michels, G. W. Kemble, R. C. Miner, and W. L. Drew. 2002. Cytomegalovirus UL97 phosphotransferase mutations that affect susceptibility to ganciclovir. J. Infect. Dis. 185:162-169. [DOI] [PubMed] [Google Scholar]
- 14.Chou, S., and G. I. Marousek. 2006. Maribavir antagonizes the antiviral action of ganciclovir on human cytomegalovirus. Antimicrob. Agents Chemother. 50:3470-3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chou, S., L. C. Van Wechel, and G. I. Marousek. 2007. Cytomegalovirus UL97 kinase mutations that confer maribavir resistance. J. Infect Dis. 196:91-94. [DOI] [PubMed] [Google Scholar]
- 16.Chou, S. 2008. Cytomegalovirus UL97 mutations in the era of ganciclovir and maribavir. Rev. Med. Virol. 18:233-246. [DOI] [PubMed] [Google Scholar]
- 17.Chou, S., and G. I. Marousek. 2008. Accelerated evolution of maribavir resistance in a cytomegalovirus exonuclease domain II mutant. J. Virol. 82:246-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Curran, M., and S. Noble. 2001. Valganciclovir. Drugs 61:1145-1150. [DOI] [PubMed] [Google Scholar]
- 19.Elion, G. B., P. A. Furman, J. A. Fyfe, P. De Miranda, L. Beauchamp, and H. J. Schaeffer. 1977. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl)guanine. Proc. Natl. Acad. Sci. U. S. A. 74:5716-5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Emery, V. C., and A. F. Hassan-Walker. 2002. Focus on new drugs in development against human cytomegalovirus. Drugs 62:1853-1858. [DOI] [PubMed] [Google Scholar]
- 21.Evers, D. L., G. Komazin, D. Shin, D. D. Hwang, L. B. Townsend, and J. C. Drach. 2002. Interactions among antiviral drugs acting late in the replication cycle of human cytomegalovirus. Antiviral Res. 56:61-72. [DOI] [PubMed] [Google Scholar]
- 22.Faulds, D., and R. C. Heel. 1990. Ganciclovir: a review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy in cytomegalovirus infections. Drugs 39:597-638. [DOI] [PubMed] [Google Scholar]
- 23.Gilbert, C., and G. Boivin. 2005. Human cytomegalovirus resistance to antiviral drugs. Antimicrob. Agents Chemother. 49:873-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamirally, S., J. P. Kamil, Y. M. Ndassa-Colday, A. J. Lin, W. J. Jahng, M. C. Baek, S. Noton, L. A. Silva, M. Simpson-Holley, D. M. Knipe, D. E. Golan, J. A. Marto, and D. M. Coen. 2009. Viral mimicry of Cdc2/cyclin-dependent kinase 1 mediates disruption of nuclear lamina during human cytomegalovirus nuclear egress. PLoS Pathog. 5:e1000275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He, Z., Y. S. He, Y. Kim, L. Chu, C. Ohmstede, K. K. Biron, and D. M. Coen. 1997. The human cytomegalovirus UL97 protein is a protein kinase that autophosphorylates on serines and threonines. J. Virol. 71:405-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ho, M. 2008. The history of cytomegalovirus and its diseases. Med. Microbiol. Immunol. 197:65-73. [DOI] [PubMed] [Google Scholar]
- 27.Hume, A. J., J. S. Finkel, J. P. Kamil, D. M. Coen, M. R. Culbertson, and R. F. Kalejta. 2008. Phosphorylation of retinoblastoma protein by viral protein with cyclin-dependent kinase function. Science 320:797-799. [DOI] [PubMed] [Google Scholar]
- 28.Kern, E. R., D. J. Bidanset, C. B. Harline, Z. Yan, J. Zemlicka, and D. C. Quenelle. 2004. Oral activity of a methylenecyclopropane analog, cyclopropavir, in animal models for cytomegalovirus infections. Antimicrob. Agents Chemother. 48:4745-4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kern, E. R., N. L. Kushner, C. B. Hartline, S. L. Williams-Aziz, E. A. Harden, S. Zhou, J. Zemlicka, and M. N. Prichard. 2005. In vitro activity and mechanism of action of methylenecyclopropane analogs of nucleosides against herpesvirus replication. Antimicrob. Agents Chemother. 49:1039-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krosky, P. M., M. C. Baek, W. J. Jahng, I. Barrera, R. J. Harvey, K. K. Biron, D. M. Coen, and P. B. Sethna. 2003. The human cytomegalovirus UL44 protein is a substrate for the UL97 protein kinase. J. Virol. 77:7720-7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li, C., B. G. Gentry, J. C. Drach, and J. Zemlicka. 2009. Synthesis and enantioselectivity of cyclopropavir phosphates for cellular GMP kinase. Nucleosides Nucleotides Nucleic Acids 28:795-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Littler, E., A. D. Stuart, and M. S. Chee. 1992. Human cytomegalovirus UL97 open reading frame encodes a protein that phosphorylates the antiviral nucleoside analogue ganciclovir. Nature 358:160-162. [DOI] [PubMed] [Google Scholar]
- 33.Lurain, N. S., A. Weinberg, C. S. Crumpacker, and S. Chou. 2001. Sequencing of cytomegalovirus UL97 gene for genotypic antiviral resistance testing. Antimicrob. Agents Chemother. 45:2775-2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahony, W. B., B. A. Domin, and T. P. Zimmerman. 1991. Ganciclovir permeation of the human erythrocyte membrane. Biochem. Pharmacol. 41:263-271. [DOI] [PubMed] [Google Scholar]
- 35.Mhaske, S. B., B. Ksebati, M. N. Prichard, J. C. Drach, and J. Zemlicka. 2009. Phosphonate analogues of cyclopropavir phosphates and their E-isomers. Synthesis and antiviral activity. Bioorg. Med. Chem. 17:3892-3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michel, D., I. Pavic, A. Zimmermann, E. Haupt, K. Wunderlich, M. Heuschmid, and T. Mertens. 1996. The UL97 gene product of human cytomegalovirus is an early-late protein with a nuclear localization but is not a nucleoside kinase. J. Virol. 70:6340-6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller, W. H., and R. L. Miller. 1980. Phosphorylation of acyclovir (acycloguanosine) monophosphate by GMP kinase. J. Biol. Chem. 255:7204-7207. [PubMed] [Google Scholar]
- 38.Miller, W. H., and R. L. Miller. 1982. Phosphorylation of acyclovir diphosphate by cellular enzymes. Biochem. Pharmacol. 31:3879-3884. [DOI] [PubMed] [Google Scholar]
- 39.Noble, S., and D. Faulds. 1998. Ganciclovir: an update of its use in the prevention of cytomegalovirus infection and disease in transplant recipients. Drugs 56:115-146. [DOI] [PubMed] [Google Scholar]
- 40.Prichard, M. N. 2009. Function of human cytomegalovirus UL97 kinase in viral infection and its inhibition by maribavir. Rev. Med. Virol. 19:215-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Puch, S. S., C. Ochoa, G. Carballal, C. Zala, P. Cahn, R. Brunet, H. Salomon, and C. Videla. 2003. Cytomegalovirus UL97 mutations associated with ganciclovir resistance in immunocompromised patients from Argentina. J. Clin. Virol. 30:271-275. [DOI] [PubMed] [Google Scholar]
- 42.Qiu, Y. L., M. B. Ksebati, R. G. Ptak, B. Y. Fan, J. M. Breitenbach, J. S. Lin, Y. C. Cheng, E. R. Kern, J. C. Drach, and J. Zemlicka. 1998. (Z)- and (E)-2-((hydroxymethyl)-methyladenine and -guanine: new nucleoside analogues with broad-spectrum antiviral activity. J. Med. Chem. 41:10-23. [DOI] [PubMed] [Google Scholar]
- 43.Qiu, Y. L., F. Geiser, T. Kira, E. Gullen, Y. C. Cheng, R. G. Ptak, J. M. Breitenbach, J. C. Drach, C. B. Hartline, E. R. Kern, and J. Zemlicka. 2000. Synthesis and enantioselectivity of the antiviral effects of (R,Z)-, (S,Z)-methylenecyclopropane analogues of purine nucleosides and phosphoralaninate prodrugs: influence of heterocyclic base, type of virus and host cells. Antivir. Chem. Chemother. 11:191-202. [DOI] [PubMed] [Google Scholar]
- 44.Selleseth, D. W., C. L. Talarico, T. Miller, M. W. Lutz, K. K. Biron, and R. J. Harvey. 2003. Interactions of 1263W94 with other antiviral agents in inhibition of human cytomegalovirus replication. Antimicrob. Agents Chemother. 47:1468-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smee, D. F., R. Boehme, M. Chernow, B. P. Binko, and T. R. Matthews. 1985. Intracellular metabolism and enzymatic phosphorylation of 9-(1,3-dihydroxy-2-propoxymethyl)guanine and acyclovir in herpes simplex virus-infected and uninfected cells. Biochem. Pharmacol. 34:1049-1056. [DOI] [PubMed] [Google Scholar]
- 46.Sullivan, V., C. L. Talarico, S. C. Stanat, M. Davis, D. M. Coen, and K. K. Biron. 1992. A protein kinase homologue controls phosphorylation of ganciclovir in human cytomegalovirus-infected cells. Nature 358:162-164. [DOI] [PubMed] [Google Scholar]
- 47.Talarico, C. L., T. C. Burnette, W. H. Miller, S. L. Smith, M. G. Davis, S. C. Stanat, T. I. Ng, Z. He, D. M. Coen, B. Roizman, and K. K. Biron. 1999. Acyclovir is phosphorylated by the human cytomegalovirus UL97 protein. Antimicrob. Agents Chemother. 43:1941-1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tracy, T. S. 1995. Stereochemistry in pharmacotherapy: when mirror images are not identical. Ann. Pharmacother. 29:161-165. [DOI] [PubMed] [Google Scholar]
- 49.Trofe, J., L. Pote, E. Wade, E. Blumberg, and R. D. Bloom. 2008. Maribavir: a novel antiviral agent with activity against cytomegalovirus. Ann. Pharmacother. 42:1447-1457. [DOI] [PubMed] [Google Scholar]
- 50.Whitley, R. J., M. A. Jacobson, D. N. Friedberg, G. N. Holland, D. A. Jabs, D. T. Dieterich, W. D. Hardy, M. A. Polis, T. A. Deutsch, J. Feinberg, S. A. Spector, S. Walmsley, W. L. Drew, W. G. Powderly, P. D. Griffiths, C. A. Benson, and H. A. Kessler. 1998. Guidelines for the treatment of cytomegalovirus diseases in patients with AIDS in the era of potent antiretroviral therapy. Arch. Intern. Med. 158:957-969. [DOI] [PubMed] [Google Scholar]
- 51.Zemlicka, J., and X. Chen. 2004. Methylenecyclopropane analogs of nucleosides as antiviral agents, p. 267-307. In R. F. Schinazi and D. C. Liotta (ed.), Frontiers in nucleosides and nucleic acids. IHL Press, College Park, GA.
- 52.Zhou, S., J. M. Breitenbach, K. Z. Borysko, J. C. Drach, E. R. Kern, E. Gullen, Y. C. Cheng, and J. Zemlicka. 2004. Synthesis and antiviral activity of (Z)- and (E)-2,2-[bis(hydroxymethyl)-cyclopropylidene]methylpurines and -pyrimidines: second-generation methylene-cyclopropane analogues of nucleosides. J. Med. Chem. 47:566-575. [DOI] [PubMed] [Google Scholar]