Abstract
Resistance to the amino alcohol quinine has been associated with polymorphisms in pfnhe, a sodium hydrogen exchanger. We investigated the role of this gene in quinine resistance in vitro in isolates from Kenya. We analyzed pfnhe whole-gene polymorphisms, using capillary sequencing, and pfcrt at codon 76 (pfcrt-76) and pfmdr1 at codon 86 (pfmdr1-86), using PCR-enzyme restriction methodology, in 29 isolates from Kilifi, Kenya, for association with the in vitro activities of quinine and 2 amino alcohols, mefloquine and halofantrine. In vitro activity was assessed as the drug concentration that inhibits 50% of parasite growth (IC50). The median IC50s of quinine, halofantrine, and mefloquine were 92, 22, and 18 nM, respectively. The presence of 2 DNNND repeats in microsatellite ms4760 of pfnhe was associated with reduced susceptibility to quinine (60 versus 227 nM for 1 and 2 repeats, respectively; P < 0.05), while 3 repeats were associated with restoration of susceptibility. The decrease in susceptibility conferred by the 2 DNNND repeats was more pronounced in parasites harboring the pfmdr1-86 mutation. No association was found between susceptibility to quinine and the pfcrt-76 mutation or between susceptibility to mefloquine or halofantrine and the pfnhe gene and the pfcrt-76 and pfmdr1-86 mutations. Using previously published data on the in vitro activities of chloroquine, lumefantrine, piperaquine, and dihydroartemisinin, we investigated the association of their activities with pfnhe polymorphism. With the exception of a modulation of the activity of lumefantrine by a mutation at position 1437, pfnhe did not modulate their activities. Two DNNND repeats combined with the pfmdr1-86 mutation could be used as an indicator of reduced susceptibility to quinine.
The amino alcohol quinine (QN) remains one of the important drugs against malaria. It is the drug of choice for the treatment of severe malaria, and in most African countries, including Kenya, where artemisinin-based combinations (lumefantrine-artemether, amodiaquine-artesunate) are now first-line treatments, 7-day quinine monotherapy has become the second-line treatment for uncomplicated malaria (44). However, there is evidence of selection and spread of QN-resistant parasites or those with reduced susceptibility to QN (1-3, 8, 30, 34). This observation led to the investigation of artesunate (an artemisinin derivative) as an alternative to QN for the treatment of severe malaria (14). However, this option could now be compromised by the emergence of artemisinin resistance (9).
Several studies have been dedicated to understanding the mechanisms of quinine resistance. Polymorphisms in pfmdr, a gene associated with chloroquine (CQ) resistance, modulate QN susceptibility (27, 33, 36). A mutation of the CQ resistance gene pfcrt at codon 76 (pfcrt-76) has been associated with reduced susceptibility to QN in vitro, although transfection studies have shown conflicting results (16, 37). A seminal study on the association of polymorphisms in pfnhe, a sodium hydrogen exchanger gene, and the in vitro activity of QN indicated that an increase in the number of DNNND repeats (1-5) in an ms4760 microsatellite was associated with reduced susceptibility to QN (10), and this finding was confirmed recently (13). Variations in the copy number of this repeat in isolates from areas where QN efficacy is known to be reduced in vivo have also been reported (41).
The initial discovery that the microsatellite ms4760 region was associated with modulation of QN activity was based on the sequencing of the pfnhe gene of reference strains (71 in total) from several areas where malaria is endemic, including Kenya (10). However, the number of strains from each site of malaria endemicity was relatively small; for instance, only 3 strains from Kenya were analyzed. Subsequent investigations on the role of this gene in QN resistance focused on analysis of only the ms4760 region (13, 41), and additional genetic variations of Plasmodium falciparum in local populations may have been overlooked.
With this in mind, we sequenced the whole pfnhe gene of 29 isolates from Kilifi, on the Kenyan coast, and analyzed this gene polymorphism in association with QN activity in vitro, along with the activities of the amino alcohols mefloquine (MFQ) and halofantrine (HLF). Isolates we analyzed in the current study were used in a previous study to investigate polymorphisms of pfcrt at pfcrt-76 and pfmdr1 at codon 86 (pfmdr1-86) for associations with the in vitro activities of CQ, the amino alcohol lumefantrine (LM), and the drugs piperaquine (PQ) and dihydroartemisinin (DHA) (19). We included part of these data to establish the impact of pfnhe polymorphism on the in vitro activities of the aforementioned drugs and the roles of the pfcrt-76 and pfmdr1-86 genotypes on QN activity in vitro.
MATERIALS AND METHODS
CQ was purchased from Sigma Chemical Company (Poole, Dorset, United Kingdom). QN, MFQ, and HAL were gifts from Steve Ward, Liverpool School of Tropical Medicine, Liverpool, United Kingdom.
Parasite adaptation.
P. falciparum parasites were collected from malaria patients as parts of several clinical studies that took place between 2005 and 2008 in the Kilifi district of Kenya, and the in vitro adaptation of parasites was carried out as reported elsewhere (35). Provision of informed consent for the original studies was assured by the Kenya Medical Research Institute, Nairobi.
Chemosensitivity testing.
We employed the P. falciparum drug-sensitive reference laboratory strain 3D7. Routine cultures were carried out in RPMI 1640 medium (Gibco BRL, United Kingdom) supplemented with 15% (vol/vol) normal human serum, 25 mM bicarbonate, 2 mM glutamine, 25 mM HEPES buffer, and 3.6 nM para-aminobenzoic acid.
The culture was diluted to 0.5% parasitemia and a final hematocrit of 1.5%, and 200 μl of this culture medium was added to each well of a 96-well microtiter plate containing the various drug concentrations and incubated at 37°C in a gas mixture containing 90% N2, 5% CO2, and 5% O2 for 48 h. Thereafter, 25 μl of 0.5 μCi [3H]hypoxanthine was added, and the mixture was incubated for another 18 h. The culture was then harvested, using a Tomtec Harvester (Tomtec, Hamden, CT), transferred to fiberglass paper (Wallac, Turku, Finland), and air dried. This dry fiberglass paper was wrapped in a sealing paper with scintillation fluid (Perkin Elmer, Norwalk, CT), and the amount of ionizing radiation was determined using a Wallac 1450 MicroBeta counter, (Wallac, Turku, Finland). Results were expressed as the drug concentration required for 50% inhibition of [3H]hypoxanthine incorporation into parasite nucleic acid or the concentration that inhibited 50% of parasite growth (IC50), determined by using nonlinear-regression analysis of the dose-response curve (39).
DNA preparation and sequencing.
Parasite DNA was extracted from field isolates adapted in vitro, using a QIAamp DNA blood minikit (Qiagen, United Kingdom). pfnhe (PF13_0019; PlasmoDB [http://www.plasmodb.org/]) was amplified from 30 parasites, using the primers PFNHE1aF (5′-TGTAGCAACACTCAGCTCAG-3′) and PFNHE14bR (5′-ACATATCGGTCCCTATTTTG-3′) to generate the PCR product by using a high-fidelity PCR enzyme (Roche Diagnostics GmbH, Roche Applied Science, Mannheim, Germany) under the following amplification conditions: 10 cycles of denaturation at 94°C for 2 min, denaturation at 94°C for 15 sec, annealing at 55.8°C for 30 sec, extension at 68°C for 4 min and a further 25 cycles of denaturation at 94°C for 15 sec, annealing at 55.8°C for 30 sec, extension at 68°C for 4 min, and a final extension at 72°C for 7 min. PCR products were purified with a QIAquick PCR purification kit (Qiagen, United Kingdom) and sequenced, using the amplification primers and several internal-sequencing primers, a BigDye Terminator version 3.1 cycle sequencing kit (Applied Biosystems, United Kingdom), and an ABI 3130xl capillary sequencer (Applied Biosystems, United Kingdom). Sequences were assembled and edited, using SeqMan, and aligned, using MegAlign (DNAStar Lasergene 7; Madison, WI) and BioEdit version 7.0.9 to identify single-nucleotide polymorphisms (SNPs) and repeat regions in the gene.
Statistical analysis.
All statistical analyses were performed with Stata 11 software (StataCorp LP, College Station, TX). We reported IC50s as median values. The nonparametric Kruskal-Wallis test was used to compare differences in IC50s for different molecular markers (microsatellite variations, codon polymorphisms, and pfcrt/pfmdr-1 genotypes) and antimalarial responses. Statistical significance was defined at the 5% level (P <0.05).
RESULTS AND DISCUSSION
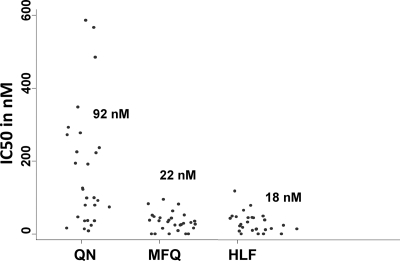
We analyzed the in vitro activities of 29 field isolates against the amino alcohols QN, MFQ, and HLF (Fig. 1). QN, MFQ, and HLF median IC50s were 92 nM with a 95% confidence interval (CI) of 55 to 216 nM, 22 nM with a 95% CI of 18 to 37 nM, and 18 nM with a 95% CI of 10 to 34 nM, respectively. To date, the QN resistance cutoff point has not been defined. Parasites for which the QN IC50 was >800 nM have been proposed to be resistant; however, lower IC50 values (>500 nM) have been suggested as well (26, 40). Based on the lower threshold, all but 2 of our isolates are sensitive in vitro, and based on the high cutoff point, none of the tested isolates is QN resistant; thus, QN is active against isolates from this part of Kenya. Interestingly, studies carried out almost 20 years ago in the coastal region of Kenya (including our study site, Kilifi) showed a similar QN activity range against field isolates, with IC50s of <500 nM (12, 25, 43). Our data are in line with reports from another part of Kenya (Western region) and other African countries (4, 20, 22, 28, 32, 40), although QN-resistant parasites in vitro have also been reported in Africa (6, 15, 18, 26). In vivo, QN is used every 8 h for 7 days, and lack of compliance with this regimen is common (11). Several reports point to a decrease in QN efficacy (1, 3, 17, 24, 30, 31, 34); however, most of these results are based on effectiveness studies. Thus, the observed reduced efficacy could be associated with lack of compliance. In the absence of clear resistance in vivo, it is difficult to define a cutoff point.
FIG. 1.
In vitro activities of the amino alcohols quinine (QN), mefloquine (MFQ), and halofantrine (HLF). Values on the y axis represent drug concentrations that inhibit 50% of parasite growth (IC50s), in nanomoles. Median IC50s for each drug are also represented.
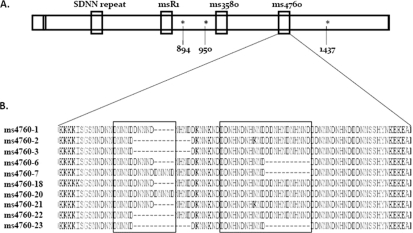
To establish the role of pfnhe in QN susceptibility, we sequenced the whole pfnhe gene from 30 parasites, including the gene of the reference strain 3D7 (GenBank accession numbers HM210746 to HM210771). Information on the gene's structure and polymorphism is summarized in Table 1 and Fig. 2. We identified SNPs (nonsynonymous) at codons 834, (AAT to AAA), 950 (GGG to GTG), and 1437 (TAT to TTT) (Table 1). The first 2 SNPs, at codons 834 and 950, were reported previously (10).
TABLE 1.
IC50s of quinine and pfnhe polymorphisms in P. falciparum
| Isolate | IC50 (nM) | Codon polymorphisms |
Microsatellite polymorphisms |
|||||
|---|---|---|---|---|---|---|---|---|
| 894 | 950 | 1437 | No. of SDNN repeats | msR1 | ms3580 | ms4760 | ||
| 3D7 | 27.1 | AAT | GTG | TAT | 4 | Absent | NIH | ms4760-2 |
| AK127 | 569.5 | AAT | GGG | TAT | 4 | TCDNNMPNNNMSNNN | NIH | ms4760-1 |
| AK182 | 549.2 | AAT | GGG | TTT | 2 | TCDNNMPNNNMSNNN | NIH | ms4760-1 |
| AK227 | 495.3 | AAT | GGG | TAT | 4 | TCDNNMPNNNMSNNN | NIH | ms4760-1 |
| CDA-1553 | 333.3 | AAT | GGG | TTT | 4 | TCDNNMPNNNMSNNN | NIH | ms4760-1 |
| AK150 D-0 | 296.6 | AAT | GGG | TAT | 3 | Absent | NIH | ms4760-1 |
| AK249 | 270.4 | AAT | GTG | TAT | 3 | Absent | NIH | ms4760-1 |
| AK121 | 270.2 | AAT | GGG | TAT | 4 | TCDNNMPNNNMSNNN | NIH | ms4760-21 |
| AK158 | 234.0 | AAT | GGG | TAT | 2 | TCDNNMPNNNMSNNN | NIH | ms4760-1 |
| AK167 | 227.0 | AAT | GGG | TAT | 4 | TCDNNMPNNNMSNNN | NIH | ms4760-1 |
| AK222 | 210.5 | AAT | GGG | TAT | 2 | TCDNNMPNNNMSNNN | NIH | ms4760-1 |
| AK152 D-56 | 207.7 | AAA | GTG | TAT | 2 | Absent | NIH | ms4760-6 |
| AK062 | 181.5 | AAT | GTG | TAT | 4 | TCDNNMPNNNMSNNN | NIH | ms4760-1 |
| AK144 | 145.8 | AAT | GTG | TTT | 4 | Absent | NIH | ms4760-23 |
| AK018 | 141.1 | AAT | GGG | TTT | 2 | Absent | NIH | ms4760-20 |
| AK150 D-42 | 91.6 | AAT | GGG | TAT | 2 | Absent | NIH | ms4760-1 |
| 9067 | 80.1 | AAT | GTG | TAT | 4 | TCDNNMPNNNMSNNN | NIH | ms4760-3 |
| 8885 | 78.2 | AAT | GGG | TAT | 3 | Absent | NIH | ms4760-22 |
| AK183 | 73.9 | AAT | GGG | TTT | 4 | TCDNNMPNNNMSNNN | Absent | ms4760-20 |
| 6816 | 73.3 | AAT | GGG | TAT | 1 | TCDNNMPNNNMSNNN | NIH | ms4760-18 |
| 8966 | 55.8 | AAT | GTG | TAT | 4 | TCDNNMPNNNMSNNN | Absent | ms4760-1 |
| AK033 D-49 | 54.1 | AAT | GGG | TAT | 3 | TCDNNMPNNNMSNNN | NIH | ms4760-7 |
| AK022D-0 | 42.4 | AAT | GGG | TAT | 4 | TCDNNMPNNNMSNNN | NIH | ms4760-22 |
| 9070 | 41.5 | AAT | GGG | TAT | 4 | TCDNNMPNNNMSNNN | NIH | ms4760-22 |
| 8977 | 35.9 | AAT | GGG | TTT | 3 | Absent | NIH | ms4760-7 |
| AK033 D-0 | 32.8 | AAT | GGG | TAT | 4 | Absent | NIH | ms4760-1 |
| 8968 | 30.6 | AAA | GGG | TAT | 4 | Absent | NIH | ms4760-2 |
| 8948 | 27.8 | AAA | GGG | TAT | 4 | Absent | NIH | ms4760-7 |
| AK022 D-28 | 22.0 | AAT | GGG | TAT | 4 | TCDNNMPNNNMSNNN | NIH | ms4760-1 |
| 8973 | 20.0 | AAT | GGG | TAT | 4 | TCDNNMPNNNMSNNN | NIH | ms4760-7 |
FIG. 2.
Diagram of pfnhe-1 gene (A) and ms4760 microsatellite sequence profiles (B). (A) Asterisks indicate polymorphic codons (894, 950, and 1437). (B) ms4760 profiles. Profiles 1 to 18 were previously described (10, 13), and we identified 4 new profiles, 20 to 23.
We identified 10 ms4760 sequences, as shown in Fig. 2. The majority of the isolates had a single repeat of microsatellites msR1 (TCDNNMPNNNMSNNN) and ms3580 (NIH), while the rest had no repeats (Table 1). Twenty percent (6 out of 29), 76% (22 out 29), and 3% (1 out 29) of isolates had 1, 2, and 3 DDNHNDNHNND repeats (in ms4760), respectively. DNNND (in ms4760) was distributed as follows: of 29 isolates, 17 and 6 isolates had 2 and 1 repeats, respectively, and the remaining 6 isolates had 3 repeats. The pfnhe genetic profile of reference strain 3D7 is identical to one described by Ferdig et al. (10), and overall, the distribution of microsatellites in our field isolates is in line with that of previous reports (10, 13). Interestingly, however, we found repeat variations in the microsatellite SDNN, with the number of repeats changing from 1 to 4 and the majority of isolates (more than 52%) harboring 4 repeats (Table 1).
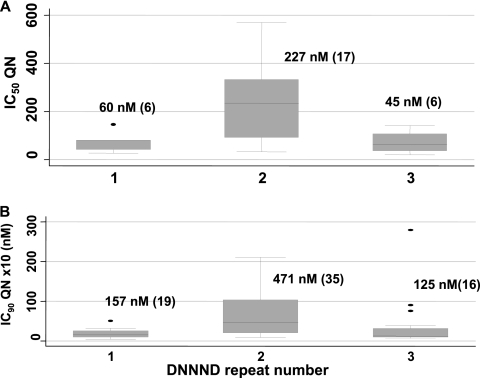
No association was found between the IC50 of QN and SNPs, the repeat number variation in msR1, ms3580, and DDNHNDHHNND (in ms4067), and the new repeat, SDNN. Parasites with 1 DNNND or DDNHNDHHNND repeat (in ms4067) were more susceptible to QN than those with >1 repeat (60 versus 182 nM and 58 versus 141 nM for DNNND and DDNHNDHHNND, respectively), but the difference was not significant. Previous work clearly indicates that the increase in DNNND repeats is associated with reduced susceptibility to QN (10, 13). However, detailed observation of our data shows that the increase in the QN IC50 is observed only for parasites with 2 DNNND repeats. Indeed, the QN IC50 rose from 60 to 227 nM for parasites harboring 1 and 2 repeats, respectively (P < 0.05), and the latter value dropped to 45 nM for parasites with 3 repeats (P < 0.01) (Fig. 3 A). To investigate this further, we reused data from the study of Ferdig et al. (10). As shown in Fig. 3B, the increase from 1 to 2 repeats was associated with decreasing susceptibility to QN (IC90s from 157 to 471 nM; P < 0.01), and the change from 2 to 3 repeats rendered parasites more susceptible (IC90s from 471 to 125 nM; P < 0.01). Thus, these data indicate that the presence of 3 repeats of DNNND restores susceptibility to QN. However, more studies are needed to confirm this observation, since a recent study shows no decrease in QN activity in parasites with 3 DNNND repeats (13).
FIG. 3.
Association between the number of DNNND repeats in pfnhe and the quinine concentration that inhibits 50% (A) or 90% (B) of parasite growth. Isolate numbers are in brackets. (A) Our current data from Kilifi. (B) Data from Ferdig et al. (10) (from Table 1). In both graphs, differences between 1 and 2 and between 2 and 3 repeats were significant (P < 0.0 5), while those between 1 and 3 repeats were not (P > 0.05). In the work of Fergid et al. (10), 1 isolate had 4 copies (IC90 = 582 nM) and was not included.
Early reports demonstrated in vitro cross-resistance between QN and its related drugs, such as CQ, LM and MFQ, and HLF (5, 7, 38, 42), an indication that some of the mechanisms of resistance to these drugs may be common. We therefore investigated the in vitro activities of the quinoline CQ, PQ, the amino alcohol LM, MFQ, and the sesquiterpene DHA in association with pfnhe polymorphism. Detailed information on the in vitro activities of CQ, LM, PQ, and DHA in relation to polymorphisms in pfcrt-76 and pfmdr1-86 was reported elsewhere (19). We reused these data in the current study.
The median IC50s of CQ, PQ, LM, and DHA for the 29 field isolates we used were 56 nM with a 95% CI of 26 to 88 nM, 56 nM with a 95% CI of 43 to 69 nM, 58 nM with a 95% CI of 49 to 881 nM, and 1 nM with a 95% CI of 0.7 to 1 nM, respectively. Neither pfnhe SNPs nor microsatellite repeats modulated susceptibility to these 4 drugs, except that LM activity decreased from 52 to 104 nM (P < 0.005) in the presence of an SNP at position 1437 (TAT to TTT). However, more studies are needed to confirm whether pfnhe, in addition to pfcrt-76 and pfmdr1-86, contributes to a decrease in the median LM susceptibility value (19).
We also investigated the role of the pfcrt-76 and pfmdr1-86 mutations in QN resistance (pfcrt and pfmdr1 are the 2 genes associated with CQ resistance). The pfcrt-76 mutation did not affect QN activity (IC50s were 164 [n = 16] and 142 nM [n = 9] for the mutant and wild type, respectively; P > 0.05). However, a trend toward a decrease in QN activity in parasites carrying the pfmdr1-86 mutation was observed, although the difference was not significant (74 [n = 16] versus 208 nM [n = 9] for the wild type and mutant, respectively; P > 0.05). Transfection studies showed that polymorphisms in pfmdr1, mainly those at codons N1042D and D1246Y, modulate QN activity (16, 33). We limited our studies to codon 86 of pfmdr1; thus, this mutation may have been selected with those at N1042D and D1246Y, though these mutations are predominantly found in South America (8). Interestingly, the presence of wild-type pfmdr1-86 has been associated with reduced susceptibility to QN but in association with an increase in the number of pfmdr1 copies (8). However, the isolates we analyzed in the present study have 1 pfmdr1 copy (a finding from our previous work [19]); thus, pfmdr1 point mutations also modulate QN activity, as previously reported (16, 21, 33).
Our data indicate that pfcrt-76 does not affect quinine activity, which is in line with a recent report (23), although modulation of QN activity by pfcrt has been supported by transfection studies (16, 37).
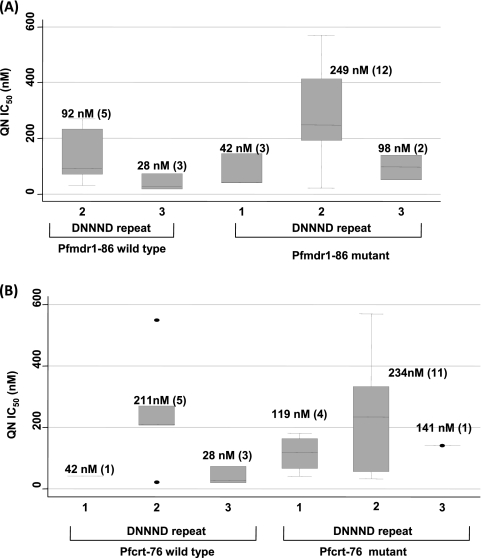
We also sought to establish the contributions of pfmdr1-86 and pfcrt-76 in the context of DNNND polymorphisms in QN resistance. As shown in Fig. 4 A, parasites with 2 DNNND repeats on a background of wild-type pfmdr1-86 have QN IC50s of 92 nM, while this value rises by almost 3-fold in the pfmdr1-86 mutant parasites (QN IC50 of 249 nM). In contrast, no difference was found with regard to pfcrt-76 (211 versus 234 nM) (Fig. 4B). These data indicate that pfmdr1 is an important contributing factor in QN resistance. Thus, monitoring QN resistance should involve both the pfnhe and pfmdr1 genes.
FIG. 4.
Relationship between drug concentrations that inhibit 50% of parasite growth (IC50s) and the genotype combination of DNNND repeat number and pfmdr1-86 (A) or pfcrt-76 (B). On a backdrop of 2 repeats of DNNND, the pfcrt-76 genotype did not affect QN activity, while a 3-fold increase in the IC50 was observed for the pfmdr1-86 mutants.
Our isolates were sensitive to MFQ and HLF, with IC50s of <25. These drugs are not commonly used in Africa because of their relatively high cost and the reported potential side effects, mainly cardiotoxicity and neuropsychiatric complications for HLF and MFQ, respectively. pfcrt-76, pfmdr1-86, and pfnhe did not modulate their in vitro activities. In Southeast Asia, where MFQ has been used extensively, resistance has been associated with an increase in pfmdr1 copy number (29). All our isolates have 1 copy of the pfmdr1 gene (19), explaining the susceptibility of our parasites to MFQ.
In summary, the presence of 2 repeats of DNNND in ms4760 of the pfnhe and pfmdr1-86 mutants is associated with a decrease in in vitro QN susceptibility, and an increase to 3 repeats of DNNND may restore QN activity, although this observation needs to be confirmed. Thus, pfnhe and pfmdr1 could be used as markers to monitor QN resistance.
Acknowledgments
We thank the director of the Kenya Medical Research Institute for permission to publish the manuscript. We thank Kevin Marsh for helpful comments.
This study was supported by the European Developing Countries Clinical Trials Partnership (EDCTP) and Wellcome Trust grant WT077092 (to Kevin Marsh for core activity support of the KEMRI/Wellcome unit in Kenya).
The authors declare no conflict of interest.
Footnotes
Published ahead of print on 1 June 2010.
REFERENCES
- 1.Achan, J., J. K. Tibenderana, D. Kyabayinze, F. Wabwire Mangen, M. R. Kamya, G. Dorsey, U. D'Alessandro, P. J. Rosenthal, and A. O. Talisuna. 2009. Effectiveness of quinine versus artemether-lumefantrine for treating uncomplicated falciparum malaria in Ugandan children: randomised trial. BMJ 339:b2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aché, A., M. Escorihuela, E. Vivas, E. Paez, L. Miranda, A. Matos, W. Perez, O. Diaz, and E. Izarra. 2002. In vivo drug resistance of falciparum malaria in mining areas of Venezuela. Trop. Med. Int. Health 7:737-743. [DOI] [PubMed] [Google Scholar]
- 3.Adam, I., D. M. Ali, W. Noureldien, and M. I. Elbashir. 2005. Quinine for the treatment of chloroquine-resistant Plasmodium falciparum malaria in pregnant and non-pregnant Sudanese women. Ann. Trop. Med. Parasitol. 99:427-429. [DOI] [PubMed] [Google Scholar]
- 4.Agnamey, P., P. Brasseur, P. E. de Pecoulas, M. Vaillant, and P. Olliaro. 2006. Plasmodium falciparum in vitro susceptibility to antimalarial drugs in Casamance (southwestern Senegal) during the first 5 years of routine use of artesunate-amodiaquine. Antimicrob. Agents Chemother. 50:1531-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basco, L. K., and J. Le Bras. 1992. In vitro activity of halofantrine and its relationship to other standard antimalarial drugs against African isolates and clones of Plasmodium falciparum. Am. J. Trop. Med. Hyg. 47:521-527. [DOI] [PubMed] [Google Scholar]
- 6.Brasseur, P., J. Kouamouo, O. Brandicourt, R. Moyou-Somo, and P. Druilhe. 1988. Patterns of in vitro resistance to chloroquine, quinine, and mefloquine of Plasmodium falciparum in Cameroon, 1985-1986. Am. J. Trop. Med. Hyg. 39:166-172. [DOI] [PubMed] [Google Scholar]
- 7.Brasseur, P., J. Kouamouo, R. S. Moyou, and P. Druilhe. 1992. Mefloquine resistant malaria in Cameroon and correlation with resistance to quinine. Mem. Inst. Oswaldo Cruz 87(Suppl. 3):271-273. [DOI] [PubMed] [Google Scholar]
- 8.Chaijaroenkul, W., R. Wisedpanichkij, and K. Na-Bangchang. 2010. Monitoring of in vitro susceptibilities and molecular markers of resistance of Plasmodium falciparum isolates from Thai-Myanmar border to chloroquine, quinine, mefloquine and artesunate. Acta Trop. 113:190-194. [DOI] [PubMed] [Google Scholar]
- 9.Dondorp, A. M., F. Nosten, P. Yi, D. Das, A. P. Phyo, J. Tarning, K. M. Lwin, F. Ariey, W. Hanpithakpong, S. J. Lee, P. Ringwald, K. Silamut, M. Imwong, K. Chotivanich, P. Lim, T. Herdman, S. S. An, S. Yeung, P. Singhasivanon, N. P. Day, N. Lindegardh, D. Socheat, and N. J. White. 2009. Artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 361:455-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferdig, M. T., R. A. Cooper, J. Mu, B. Deng, D. A. Joy, X. Z. Su, and T. E. Wellems. 2004. Dissecting the loci of low-level quinine resistance in malaria parasites. Mol. Microbiol. 52:985-997. [DOI] [PubMed] [Google Scholar]
- 11.Fungladda, W., E. R. Honrado, K. Thimasarn, D. Kitayaporn, J. Karbwang, P. Kamolratanakul, and R. Masngammueng. 1998. Compliance with artesunate and quinine + tetracycline treatment of uncomplicated falciparum malaria in Thailand. Bull. World Health Organ. 76(Suppl. 1):59-66. [PMC free article] [PubMed] [Google Scholar]
- 12.Haruki, K., P. A. Winstanley, W. M. Watkins, and K. Marsh. 1998. Quinine sensitivity of isolates of Plasmodium falciparum from the coast of Kenya. Trans. R. Soc. Trop. Med. Hyg. 92:195-196. [DOI] [PubMed] [Google Scholar]
- 13.Henry, M., S. Briolant, A. Zettor, S. Pelleau, M. Baragatti, E. Baret, J. Mosnier, R. Amalvict, T. Fusai, C. Rogier, and B. Pradines. 2009. Plasmodium falciparum Na+/H+ exchanger 1 transporter is involved in reduced susceptibility to quinine. Antimicrob. Agents Chemother. 53:1926-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones, K. L., S. Donegan, and D. G. Lalloo. 17 October 2007, posting date. Artesunate versus quinine for treating severe malaria. Cochrane Database Syst. Rev. CD005967. doi: 10.1002/14651858.CD005967.pub2. [DOI] [PubMed]
- 15.Kilimali, V. A. 1990. The in vitro response of Plasmodium falciparum to amodiaquine, quinine and quinidine in Tanga region, Tanzania. East Afr. Med. J. 67:336-340. [PubMed] [Google Scholar]
- 16.Lakshmanan, V., P. G. Bray, D. Verdier-Pinard, D. J. Johnson, P. Horrocks, R. A. Muhle, G. E. Alakpa, R. H. Hughes, S. A. Ward, D. J. Krogstad, A. B. Sidhu, and D. A. Fidock. 2005. A critical role for PfCRT K76T in Plasmodium falciparum verapamil-reversible chloroquine resistance. EMBO J. 24:2294-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Molinier, S., P. Imbert, D. Verrot, M. Morillon, D. Parzy, and J. E. Touze. 1994. Plasmodium falciparum malaria: type R1 quinine resistance in East Africa. Presse Med. 23:1494. (In French.) [PubMed] [Google Scholar]
- 18.Mutanda, L. N. 1999. Assessment of drug resistance to the malaria parasite in residents of Kampala, Uganda. East Afr. Med. J. 76:421-424. [PubMed] [Google Scholar]
- 19.Mwai, L., S. M. Kiara, A. Abdirahman, L. Pole, A. Rippert, A. Diriye, P. Bull, K. Marsh, S. Borrmann, and A. Nzila. 2009. In vitro activities of piperaquine, lumefantrine, and dihydroartemisinin in Kenyan Plasmodium falciparum isolates and polymorphisms in pfcrt and pfmdr1. Antimicrob. Agents Chemother. 53:5069-5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ndong, J. M., C. Atteke, A. Aubouy, M. Bakary, J. Lebibi, and P. Deloron. 2003. In vitro activity of chloroquine, quinine, mefloquine and halofantrine against Gabonese isolates of Plasmodium falciparum. Trop. Med. Int. Health 8:25-29. [DOI] [PubMed] [Google Scholar]
- 21.Nsobya, S. L., M. Kiggundu, S. Nanyunja, M. Joloba, B. Greenhouse, and P. J. Rosenthal. 2010. In vitro sensitivities of Plasmodium falciparum to different antimalarial drugs in Uganda. Antimicrob. Agents Chemother. 54:1200-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Odhiambo, R. A., and A. Odulaja. 2005. Parasite lactate dehydrogenase assay for the determination of antimalarial drug susceptibility of Kenyan field isolates. East Afr. Med. J. 82:118-122. [DOI] [PubMed] [Google Scholar]
- 23.Parola, P., B. Pradines, F. Simon, M. P. Carlotti, P. Minodier, M. P. Ranjeva, S. Badiaga, L. Bertaux, J. Delmont, M. Morillon, R. Silai, P. Brouqui, and D. Parzy. 2007. Antimalarial drug susceptibility and point mutations associated with drug resistance in 248 Plasmodium falciparum isolates imported from Comoros to Marseille, France in 2004 2006. Am. J. Trop. Med. Hyg. 77:431-437. [PubMed] [Google Scholar]
- 24.Parola, P., S. Ranque, S. Badiaga, M. Niang, O. Blin, J. J. Charbit, J. Delmont, and P. Brouqui. 2001. Controlled trial of 3-day quinine-clindamycin treatment versus 7-day quinine treatment for adult travelers with uncomplicated falciparum malaria imported from the tropics. Antimicrob. Agents Chemother. 45:932-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pasvol, G., C. R. Newton, P. A. Winstanley, W. M. Watkins, N. M. Peshu, J. B. Were, K. Marsh, and D. A. Warrell. 1991. Quinine treatment of severe falciparum malaria in African children: a randomized comparison of three regimens. Am. J. Trop. Med. Hyg. 45:702-713. [DOI] [PubMed] [Google Scholar]
- 26.Pettinelli, F., M. E. Pettinelli, P. Eldin de Pecoulas, J. Millet, D. Michel, P. Brasseur, and P. Druilhe. 2004. Short report: high prevalence of multidrug-resistant Plasmodium falciparum malaria in the French territory of Mayotte. Am. J. Trop. Med. Hyg. 70:635-637. [PubMed] [Google Scholar]
- 27.Pickard, A. L., C. Wongsrichanalai, A. Purfield, D. Kamwendo, K. Emery, C. Zalewski, F. Kawamoto, R. S. Miller, and S. R. Meshnick. 2003. Resistance to antimalarials in Southeast Asia and genetic polymorphisms in pfmdr1. Antimicrob. Agents Chemother. 47:2418-2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pradines, B., P. Hovette, T. Fusai, H. L. Atanda, E. Baret, P. Cheval, J. Mosnier, A. Callec, J. Cren, R. Amalvict, J. P. Gardair, and C. Rogier. 2006. Prevalence of in vitro resistance to eleven standard or new antimalarial drugs among Plasmodium falciparum isolates from Pointe-Noire, Republic of the Congo. J. Clin. Microbiol. 44:2404-2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Price, R. N., A. C. Uhlemann, A. Brockman, R. McGready, E. Ashley, L. Phaipun, R. Patel, K. Laing, S. Looareesuwan, N. J. White, F. Nosten, and S. Krishna. 2004. Mefloquine resistance in Plasmodium falciparum and increased pfmdr1 gene copy number. Lancet 364:438-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pukrittayakamee, S., A. Chantra, S. Vanijanonta, R. Clemens, S. Looareesuwan, and N. J. White. 2000. Therapeutic responses to quinine and clindamycin in multidrug-resistant falciparum malaria. Antimicrob. Agents Chemother. 44:2395-2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pukrittayakamee, S., W. Supanaranond, S. Looareesuwan, S. Vanijanonta, and N. J. White. 1994. Quinine in severe falciparum malaria: evidence of declining efficacy in Thailand. Trans. R. Soc. Trop. Med. Hyg. 88:324-327. [DOI] [PubMed] [Google Scholar]
- 32.Quashie, N. B., N. O. Duah, B. Abuaku, and K. A. Koram. 2007. The in-vitro susceptibilities of Ghanaian Plasmodium falciparum to antimalarial drugs. Ann. Trop. Med. Parasitol. 101:391-398. [DOI] [PubMed] [Google Scholar]
- 33.Reed, M. B., K. J. Saliba, S. R. Caruana, K. Kirk, and A. F. Cowman. 2000. Pgh1 modulates sensitivity and resistance to multiple antimalarials in Plasmodium falciparum. Nature 403:906-909. [DOI] [PubMed] [Google Scholar]
- 34.Roche, J., A. Guerra-Neira, J. Raso, and A. Benito. 2003. Surveillance of in vivo resistance of Plasmodium falciparum to antimalarial drugs from 1992 to 1999 in Malabo (Equatorial Guinea). Am. J. Trop. Med. Hyg. 68:598-601. [DOI] [PubMed] [Google Scholar]
- 35.Sasi, P., A. Abdulrahaman, L. Mwai, S. Muriithi, J. Straimer, E. Schieck, A. Rippert, M. Bashraheil, A. Salim, J. Peshu, K. Awuondo, B. Lowe, M. Pirmohamed, P. Winstanley, S. Ward, A. Nzila, and S. Borrmann. 2009. In vivo and in vitro efficacy of amodiaquine against Plasmodium falciparum in an area of continued use of 4-aminoquinolines in East Africa. J. Infect. Dis. 199:1575-1582. [DOI] [PubMed] [Google Scholar]
- 36.Sidhu, A. B., S. G. Valderramos, and D. A. Fidock. 2005. pfmdr1 mutations contribute to quinine resistance and enhance mefloquine and artemisinin sensitivity in Plasmodium falciparum. Mol. Microbiol. 57:913-926. [DOI] [PubMed] [Google Scholar]
- 37.Sidhu, A. B., D. Verdier-Pinard, and D. A. Fidock. 2002. Chloroquine resistance in Plasmodium falciparum malaria parasites conferred by pfcrt mutations. Science 298:210-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simon, F., J. Le Bras, G. Charmot, P. M. Girard, C. Faucher, F. Pichon, and B. Clair. 1986. Severe chloroquine-resistant falciparum malaria in Gabon with decreased sensitivity to quinine. Trans. R. Soc. Trop. Med. Hyg. 80:996-997. [DOI] [PubMed] [Google Scholar]
- 39.Sixsmith, D. G., W. M. Watkins, J. D. Chulay, and H. C. Spencer. 1984. In vitro antimalarial activity of tetrahydrofolate dehydrogenase inhibitors. Am. J. Trop. Med. Hyg. 33:772-776. [DOI] [PubMed] [Google Scholar]
- 40.Tinto, H., C. Rwagacondo, C. Karema, D. Mupfasoni, W. Vandoren, E. Rusanganwa, A. Erhart, C. Van Overmeir, E. Van Marck, and U. D'Alessandro. 2006. In-vitro susceptibility of Plasmodium falciparum to monodesethylamodiaquine, dihydroartemisinin and quinine in an area of high chloroquine resistance in Rwanda. Trans. R. Soc. Trop. Med. Hyg. 100:509-514. [DOI] [PubMed] [Google Scholar]
- 41.Vinayak, S., M. T. Alam, M. Upadhyay, M. K. Das, V. Dev, N. Singh, A. P. Dash, and Y. D. Sharma. 2007. Extensive genetic diversity in the Plasmodium falciparum Na+/H+ exchanger 1 transporter protein implicated in quinine resistance. Antimicrob. Agents Chemother. 51:4508-4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Warsame, M., W. H. Wernsdorfer, D. Payne, and A. Bjorkman. 1991. Susceptibility of Plasmodium falciparum in vitro to chloroquine, mefloquine, quinine and sulfadoxine/pyrimethamine in Somalia: relationships between the responses to the different drugs. Trans. R. Soc. Trop. Med. Hyg. 85:565-569. [DOI] [PubMed] [Google Scholar]
- 43.Watkins, W. M., R. E. Howells, A. D. Brandling-Bennett, and D. K. Koech. 1987. In vitro susceptibility of Plasmodium falciparum isolates from Jilore, Kenya, to antimalarial drugs. Am. J. Trop. Med. Hyg. 37:445-451. [DOI] [PubMed] [Google Scholar]
- 44.World Health Organization. 2010. Guidelines for the treatment of malaria, 2nd ed. http://whqlibdoc.who.int/publications/2010/9789241547925_eng.pdf. [PubMed]