Abstract
The mechanism(s) of daptomycin (DAP) resistance (DAPr) is incompletely defined. Thickened cell walls (CWs) acting as either a mechanical barrier or an affinity trap for DAP have been purported to be a major contributor to the DAPr phenotype. To this end, we studied an isogenic set of methicillin-resistant Staphylococcus aureus (MRSA) isolates (pulsotype USA 300) from the bloodstream of a DAP-treated patient with endocarditis in which serial strains exhibited increasing DAPr. Of interest, the DAPr isolate differed from its parental strain in several parameters, including acquisition of a point mutation within the putative synthase domain of the mprF gene in association with enhanced mprF expression, increased synthesis of lysyl-phosphotidylglycerol, an enhanced positive envelope charge, and reduced DAP surface binding. Transmission electron microscopy (TEM) revealed no significant increases in CW thickness in the two DAPr isolates (MRSA 11/21 and REF2145) compared with that in the DAP-susceptible (DAPs) parental strain, MRSA 11/11. The rates of Triton X-100-induced autolysis were also identical for the strain set. Furthermore, among six additional clinically isolated DAPs/DAPr S. aureus strain pairs, only three DAPr isolates exhibited CWs significantly thicker than those of the respective DAPs parent. These data confirm that CW thickening is neither universal to DAPr S. aureus nor sufficient to yield the DAPr phenotype among S. aureus strains.
Daptomycin (DAP) is a cyclic lipopeptide antibiotic active against a wide range of Gram-positive organisms, including methicillin-resistant Staphylococcus aureus (MRSA) strains, vancomycin (VAN)-intermediate S. aureus (VISA), and vancomycin-resistant S. aureus (VRSA) (23, 24, 28, 32). However, increasing numbers of reports have described development of in vitro DAP resistance (DAPr) in association with DAP clinical treatment failures in a variety of S. aureus infections (2, 9, 12, 26).
The exact mechanism(s) of DAPr remains unknown. However, through previous studies in our laboratory (11, 16, 36, 37), we have found that the pathways which are associated with DAPr appear to be multifactorial and may differ among DAPr S. aureus strains. Recently, using isogenic sets of S. aureus clinical isolates from a DAP-treated patient with recalcitrant endocarditis, we found a variety of cell membrane (CM) and cell envelope alterations associated with the DAPr phenotype (11), including those involving membrane fluidity, membrane phospholipid (PL) composition and asymmetry, surface charge, relative in vitro cross-resistance to certain CM-targeting cationic host defense antimicrobial peptides (CAPs), and DAP binding (11, 36).
In addition to these membrane phenotypic changes, alterations in cell wall (CW) structure and/or function have been proposed to be involved in DAPr. For example, Julian et al. described the reduction of peptidoglycan cross-linking and a reduced degree of muramic acid O-acetylation in DAPr VISA strains (12). Cui et al. (6) also described a positive correlation between DAPr and vancomycin resistance in VISA strains. In both studies, a notable increase in CW thickness was observed, suggesting a potential role for a CW-based physical barrier to DAP reaching its ultimate cell membrane target. We have also observed thickened CWs in one in vitro passage-derived DAPr S. aureus strain (16). However, it is not clear whether such increased CW thickness is a universal phenotype among DAPr S. aureus strains, especially among clinical isolates.
In the present study, we further investigated the putative correlation between CW thickness and DAPr by examining several additional isogenic DAP-susceptible (DAPs) and DAPr sets of S. aureus bloodstream isolates from DAP-treated patients.
(Although the currently accepted term for reduced in vitro susceptibility to daptomycin is “nonsusceptible,” we use the term “daptomycin-resistant” [DAPr] in this paper for a more facile presentation.)
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The primary strain set used in this study was isolated from a patient with recalcitrant endocarditis. The clinical details related to this patient and to the pulsed-field gel electrophoresis (PFGE)-identical, isogenic strain set (strains MRSA 11/11, MRSA 11/17, MRSA 11/21, REF2145) have been described previously (19). This strain set was pulsotype USA 300 (MLST type 8). Strain MRSA 11/11 was the initial bloodstream isolate recovered prior to VAN or DAP therapy and was DAPs. The second strain (MRSA 11/17) was isolated after 5 days of VAN therapy. The last two isolates (MRSA 11/21 and REF2145) were subsequently obtained on days 4 and 7 of DAP therapy, respectively, and found to be DAPr. The DAP MICs for strains MRSA 11/11, MRSA 11/17, MRSA 11/21, and REF2145 in this strain set, determined by standard Etest, were 1, 1, 3, and 4 μg/ml, respectively (19). Of interest, the DAPr strains were found to have a single nucleotide polymorphism within the mprF gene at position 345 (Thr to Ala), which maps to the putative synthase domain of this gene (7, 19). The oxacillin MIC for the initial isolate (isolate 11/11), determined by standard Etest, was 32 μg/ml, while for the last isolate (REF2145), the MIC was 6 μg/ml, demonstrating a “seesaw” effect, as described previously (16). The VAN MICs determined by Etest were 2 μg/ml for all strains in this strain set. The strains in the strain set were also susceptible to the following agents: linezolid, quinupristin-dalfopristin, trimethoprim-sulfamethoxazole, and gentamicin (19).
All strains were grown in either tryptic soy broth (TSB; Difco Laboratories, Detroit, MI) or Mueller-Hinton (MH) broth (Difco Laboratories). Liquid cultures were grown in Erlenmeyer flasks at 37°C with shaking (250 rpm) in a volume that was no greater than 10% of the flask volume. DAP was purchased from Cubist Pharmaceuticals (Lexington, MA) and reconstituted according to the manufacturer's recommendations. All DAP assays were done in the presence of 50 μg/ml calcium, as recommended by the manufacturer.
The primary strain set described above was analyzed for a number of parameters, including cell membrane and cell envelope phenotypes, transcription of two genes involved in maintenance of the cell surface positive charge (mprF and dltA), and CW thickness, determined by transmission electron microscopy (TEM) (see details below). We also studied six additional DAPs/DAPr clinical strain pairs for comparison of their CW thicknesses by TEM (Table 1). Two of these strain pairs have been described previously and were used to provide an overall comparison with the primary strain set in terms of the cell membrane and cell envelope parameters described above, in addition to CW thickness (11, 36, 37). Four other strain sets (courtesy of Aileen Rubio) were selected from the Cubist Pharmaceutical's Registry of DAPs/DAPr strain pairs by an individual not involved in our data analyses and were used exclusively for CW thickness comparisons. The selection of the strains was based on the following prioritization: (i) recent clinical strains (bloodstream isolates) and (ii) strains found to be isogenic by PFGE. Their DAP and VAN MICs were determined by standard Etest and are listed in Table 1. We also included a previously described DAPs/DAPr strain set selected by in vitro passage in DAP as a comparator (8, 16).
TABLE 1.
Cell wall thickness measurements of four recently isolated DAPs and DAPr clinical strain sets studied in addition to the primary strain set
| Strain paira | MIC (μg/ml) |
CW thickness (nm) | Reference or source | |
|---|---|---|---|---|
| DAP | VAN | |||
| CB1482 | 0.5 | 2 | 38.62 ± 4.28 | 8 |
| CB184 | 4 | 2 | 40.02 ± 4.66 | 8 |
| CB5053 | 0.5 | 1 | 33.44 ± 3.42 | This study |
| CB5054 | 2 | 2 | 35.22 ± 3.76 | This study |
| CB5035 | 0.38 | 2 | 38.03 ± 4.15 | This study |
| CB5036 | 2 | 2 | 32.66 ± 3.27 | This study |
| BMC1001 | 0.5 | 2 | 32.28 ± 3.91 | This study |
| BMC1002 | 2 | 4 | 52.40 ± 7.04b | This study |
All clinical strain pairs were isolated from the bloodstream.
P < 0.01 versus the parental strain.
Population analyses.
Population analyses of MRSA 11/11 and REF2145 upon exposure to a range of DAP and VAN concentrations were performed as described before (17). Briefly, after the strains were grown overnight in TSB (∼5 × 109 CFU/ml), serial 10-fold dilutions of the cultures were plated onto MH agar containing DAP or VAN at various concentrations. For DAP and VAN, the range of concentrations tested was 0.0625 to 32 μg/ml, to encompass sublethal to lethal drug levels. The lower limit of detection in these assays was set at 2 log10 CFU/ml. To provide a quantification of this assay, the area under the concentration-time curve (AUC) of the population analysis curves for each strain was determined using the trapezoidal rule (Microcal Origin software, version 5.0; Microcal Software Inc., Northampton, MA) (13).
CAP susceptibility testing.
Recent studies showed a temporal correlation between in vivo development of DAPr and relative in vitro cross-resistance to several mammalian host defense CAPs, including the α-defensin human neutrophil peptide 1 (hNP-1) from polymorphonuclear leukocytes and thrombin-induced platelet microbicidal proteins (tPMPs) from mammalian platelets (11, 36). hNP-1 was purchased from Peptide International (Louisville, KY), and tPMP-1 preparations were obtained as described previously (38, 40). In addition, RP-1 (a synthetic CAP modeled in part upon the microbicidal domain of tPMP-1 and with a mode of action identical to that of the native peptide) (33, 39) was substituted for tPMP-1 in several of the assays requiring higher concentrations of peptide. In vitro bactericidal assays were performed with the tPMP-1 preparation (bioactivity, 1 μg/ml), hNP-1 (20 μg/ml), and RP-1 (3 μg/ml), as described before, using a 2-h CAP exposure time in a microdilution method (11, 34). Data were expressed as the percentage of surviving CFU (±standard deviation [SD]) of CAP-exposed cells compared to the numbers of unexposed control cells over the 2-h exposure period. At least two independent experiments were performed with each peptide.
Peptide binding assays.
To determine the relationship between the peptide association with S. aureus cells and DAPr in the primary strain set, RP-1 (40 μg/ml), DAP (6 μg/ml), or VAN (8 μg/ml) was added to 108 CFU of each S. aureus strain; and the mixture was incubated for 10 min and centrifuged to pellet the cells. The supernatants were then analyzed for residual unbound peptides by a radial diffusion assay, using Bacillus subtilis ATCC 6633 as the indicator strain, as described before (11, 38); the amount of unbound peptide was calculated by comparing the resulting zone sizes with the respective peptide concentration-zone size standard curves. At least two independent assays were performed on separate days.
Comparison of relative net cell surface charge.
Our prior investigations of DAPr S. aureus strains indicated a frequent alteration of the net cell surface charge compared to that of the respective DAPs parental strains (11, 16, 36). For these assays, we compared the relative net cell surface charge of S. aureus strains by quantifying the association of the highly cationic molecule cytochrome c (pI 10; Sigma) to the staphylococcal surface (15, 21). The amount of cytochrome c remaining in the postcentrifugation supernatant after a 10-min binding interaction with S. aureus cells was quantified spectrophotometrically at an optical density at 530 nm (OD530). The more unbound cytochrome c that was detected in the supernatant, the more that a positive charge existed on the bacterial cell surface. The data shown are the means (±SDs) of the amount of unbound cytochrome c from three independent experiments.
Membrane phospholipid profiles.
Previous studies from our laboratory have shown a correlation between altered membrane PL profiles and DAPr. These observations particularly focused upon the proportion of the positively charged PL species lysyl-phosphotidylglycerol (LPG) which was synthesized and/or translocated to the outer cystoplasmic membrane leaflet, i.e., phenotypes encoded by mprF (7, 11, 37). Membrane PLs were extracted from each of the S. aureus isolates in the primary strain set by standard methods (1, 18). The three major PLs (phosphotidylglycerol [PG], LPG, and cardiolipins [CLs]) were separated by two-dimensional thin-layer chromatography (TLC), collected from the plates, and then quantified spectrophotometrically, as described before (11, 18). The proportion of synthesized LPG which was translocated to the outer cell membrane leaflet was quantified spectrophotometrically, as detailed before, using the LPG outer membrane-specific probe fluorescamine (16, 18). The detailed methodologies for detecting and quantifying total synthesized LPG and outer membrane-specific LPG have been published previously (18).
Transcriptional analyses of mprF and dltA.
The transcription of the two genes most frequently linked to DAPr, mprF and dlt, was assessed (7, 11, 36, 37). These genes encode proteins involved in lysinylation of membrane phospholipids and alanylation of CW teichoic acids, respectively, each of which can result in a change in the net cell surface charge in S. aureus (5, 20, 27, 31, 37). For RNA isolation, fresh overnight cultures of S. aureus strains were used to inoculate TSB to an OD600 of 0.1. Cells were harvested during both exponential and stationary growth phases. Total RNA was isolated from the cell pellets by using an RNeasy kit (Qiagen, Valencia, CA) and a Fastprep FP120 instrument (Bio 101, Vista, CA), according to the manufacturers' recommended protocols.
Reverse transcriptase (RT)-PCR was performed as described previously (35). The mprF cDNA products were detected using primer pair mprF-F and mprF-R (37). The dlt cDNA products were detected using primer pair dlt-F and dlt-R (36).
TEM.
To determine the CW thicknesses of the strains, cells were prepared for TEM analyses as described previously (3). For each strain, 100 cell wall thickness measurements were taken from a minimum of 50 cells at ×190,000 magnification (model 100CX; Jeol, Tokyo, Japan), as described previously (16). In addition to the seven strain sets outlined above, we employed MRSA strain MU50, a well-characterized prototypical VISA strain (10) known to have thickened CWs. All CW measurements were performed by one of us (C.C.N.) blinded to the identities of the organisms. Differences for which P values were <0.05 were considered statistically significant.
Statistics.
Data were analyzed by the Kruskal-Wallis analysis of variance (ANOVA) test, and P values of <0.05 were considered significant.
RESULTS
Population analyses.
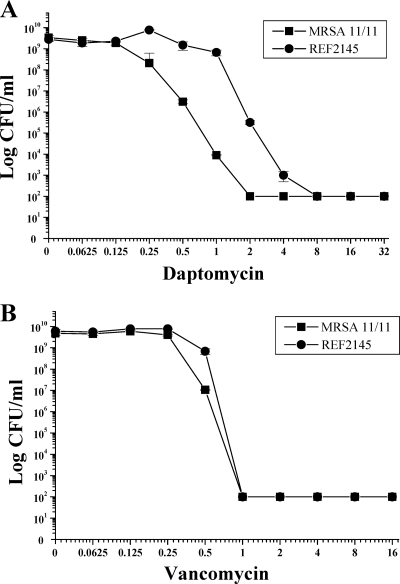
In population analyses upon exposure to a range of DAP concentrations, the population curve for DAPr strain REF2145 was shifted substantially to the right, with heterogeneous subpopulations surviving exposures of between 0.125 and 4 μg/ml DAP (Fig. 1 A). The AUC values for the population analyses with DAP were 8.41 ± 0.64 and 30.64 ± 1.82 for strains MRSA 11/11 and REF2145, respectively. Population analyses upon exposure of MRSA 11/11 and REF2145 to a range of VAN concentrations revealed survival curves and AUCs very similar to those for DAP (Fig. 1B; AUCs, 5.96 ± 0.03 and 6.65 ± 0.14, respectively).
FIG. 1.
Population analyses of MRSA 11/11 and REF2145 strains upon exposure to a range of DAP (A) and VAN (B) concentrations. These data represent the means (±SDs) for two separate assays.
CAP susceptibility.
As shown in Table 2, the parental strain, MRSA 11/11, was highly susceptible to tPMP-1 and RP-1, while strain REF2145 was ∼6-fold and ∼3-fold more resistant to exposures to these peptides, respectively. In contrast, these strains were equally susceptible to the bactericidal activity of hNP-1 (20 μg/ml).
TABLE 2.
In vitro CAP susceptibilities and peptide binding of the primary strain set
| Strain | % survival (mean ± SD) after 2h exposure to: |
Amt of drug bound (μg/ml) |
||||
|---|---|---|---|---|---|---|
| tPMP-1a (1 μg/ml) | hNP-1b (20 μg/ml) | RP-1a (3 μg/ml) | RP-1a (40 μg) | DAPa (6 μg) | VANb (8 μg) | |
| MRSA 11/11 | 4 ± 2.0 | 11 ± 10 | 6.67 ± 0.57 | 10.6 ± 3.3 | 2.03 ± 0.53 | 1.81 ± 0.13 |
| REF2145 | 27 ± 10.0 | 14 ± 11 | 19.33 ± 2.88 | 2.82 ± 2.5 | 0.79 ± 0.45 | 1.78 ± 0.04 |
P < 0.01.
The results were not significantly different.
Cell surface charge and binding assays.
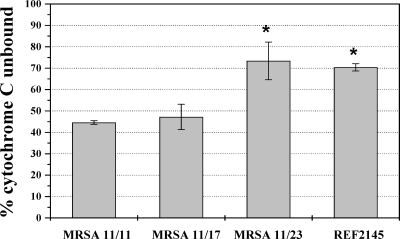
As shown in Fig. 2, the levels of binding of the positively charged molecule cytochrome c to DAPr MRSA strains 11/21 and REF2145 were significantly less than those to parental strain MRSA 11/11 or the MRSA 11/17 strain (P < 0.01). Paralleling the cytochrome c binding data, the levels of binding of both DAP and RP-1 were reduced in DAPr strain REF2145 (Table 2; P < 0.01). In agreement with our previous findings (11, 36, 37), these data suggest that the increased repulsion of DAP and CAPs may be due to an increased net positive surface charge in REF2145. Consistent with the VAN MIC and population analysis data, there was no difference in the levels of VAN binding to the DAPs strains versus those to the DAPr strains.
FIG. 2.
Binding of positively charged cytochrome c to whole S. aureus cells. The graph shows the percentage of cytochrome c unbound after 10 min of incubation with S. aureus at room temperature. Data represent the means and standard deviations from three independent experiments. *, P < 0.01 versus MRSA 11/11.
Membrane PL profiles.
Since the relative proportions of the three major, differentially charged membrane PLs of S. aureus can impact the net surface charge, analysis of the PLs was carried out and the results are shown in Table 3. The proportions of the negatively charged PL CL (net charge, −2) were similar among the strains. In contrast to CL, the proportions of PG (net charge, −1) were ∼10 and 20% lower in DAPr strains 11/21 and REF2145, respectively. Of note, the total amounts of positively charged LPG (net charge, +1) were ∼2-fold higher in the DAPr strains than in the DAPs strains, indicating enhanced synthase function. Importantly, the amount of LPG that was translocated to the outer membrane leaflet was ∼6 to 9% of total LPG in all four strains, suggesting similar levels of MprF translocase (flippase) activity among the strains. The net increases in the total amounts of LPG synthesized and ultimately translocated to the outer membrane are consistent with the relative increases in the overall positive surface charge in the DAPr strains.
TABLE 3.
Phospholipid composition and outer membrane translocation of LPG for the primary strain set
| Strain | % of total phospholipid content (mean ± SD) |
||||
|---|---|---|---|---|---|
| Inner LPG | Outer LPG | Total LPG | PG | CL | |
| MRSA 11/11 | 17.17 ± 1.17 | 1.73 ± 0.24 | 18.91 ± 1.41 | 75.24 ± 1.31 | 5.86 ± 2.71 |
| MRSA 11/17 | 18.16 ± 0.38 | 1.46 ± 0.43 | 19.63 ± 0.81 | 72.57 ± 3.89 | 7.81 ± 3.07 |
| MRSA 11/21 | 32.15 ± 9.08a | 2.72 ± 0.54 | 34.16 ± 8.95a | 62.45 ± 7.93 | 5.68 ± 0.61 |
| REF2145 | 34.67 ± 4.51a | 2.29 ± 0.49 | 36.66 ± 4.95a | 55.25 ± 1.23 | 7.80 ± 6.23 |
P < 0.01 versus MRSA 11/11 or MRSA 11/17.
Transcriptional analyses of mprF and dlt genes.
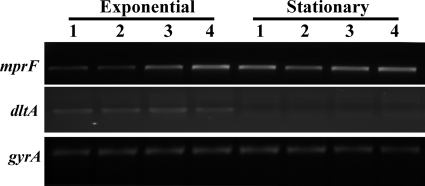
As shown in Fig. 3, RT-PCR analysis revealed that the dlt transcripts were detected to an equivalent extent, with expression occurring only during exponential growth among all four isolates in the primary strain set. In contrast, the level of transcription of the mprF gene was notably increased in the two DAPr strains compared with that in the two DAPs strains during exponential growth. This finding correlated well with the phenotype of increased LPG synthesis observed in these DAPr (see above) strains compared with the phenotype observed in the respective DAPs isolates.
FIG. 3.
RT-PCR analyses of mprF and dlt expression in MRSA 11/11, MRSA 11/17, MRSA 11/23, and REF2145 (lanes 1 to 4, respectively). RNA samples were isolated from exponential- and stationary-phase cultures of the strains and were subjected to RT-PCR to detect transcription of mprF, dltA, and gyrA. The corresponding gels are labeled mprF, dltA, and gyrA, respectively.
TEM analyses.
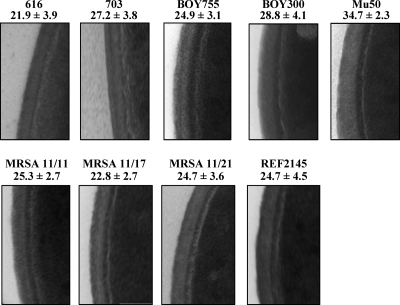
As shown in Fig. 4, DAPr strain 703 (11) had significantly thicker CWs (27.2 ± 3.8 nm) than its DAPs parental strain, strain 616 (21.9 ± 3.9 nm; P < 0.05). Similarly, DAPr strain BOY 300 exhibited thicker CWs than its DAPs parental strain, strain BOY 755 (24.9 ± 3.1 versus 28.8 ± 4.1 nm; P < 0.05). Importantly, unlike the clinical DAPr strain sets, strains MRSA 11/21 and REF2145 did not exhibit increased CW thickness measurements compared to those of DAPs strains (P > 0.10) MRSA11/11 and MRSA 11/17 from the same strain set. In examining four other recent DAPs/DAPr pairs of clinical strain, there were no significant increases (P < 0.05) in CW thickness relative to that of the respective DAPs strain in three of the four DAPr strains compared (Table 1). The CWs of one DAPr strain were significantly thicker than the CWs of the DAPs parental strain. Thus, among the seven pairs of clinical strains studied, less than 50% (three of seven) of the DAPr strains exhibited significantly thicker CWs than the respective DAPs parental strain. The CWs of the one DAPr strain selected by in vitro passage in DAP were significantly thicker than the CWs of the DAPs parental strain (31.3 ± 2.7 nm and 20.4 ± 2.7 nm, respectively (P < 0.05) (16).
FIG. 4.
TEM analyses of the DAPs and DAPr S. aureus strain sets. The thicknesses of the cell walls were measured at ×190,000 magnification.
Of note, there was no obvious relationship between the levels of the DAP MICs in the DAPr strains and either the absolute CW thickness measurements or the relative CW thickness differences when the results for individual DAPr strains and their respective DAPs parental strains were compared.
DISCUSSION
DAP initiates its bactericidal activity by targeting bacterial CMs, causing rapid CM depolarization and potassium leakage, resulting in DNA, RNA, and protein synthesis inhibition and eventual cell death (24, 25, 29, 30). There have been a number of recent reports of studies with both MSSA and MRSA isolates of the in vivo development of DAPr phenotypes in association with clinical failures (9, 12, 14, 19, 22, 26). However, it appears that there are multiple different mechanism(s) of DAPr in S. aureus, including (i) an enhanced surface positive charge, which results in a charge-repulsive milieu causing reduced DAP binding (11, 36), and (ii) altered membrane fluidity, which perhaps impedes DAP-CM interactions (11, 16).
To expand our knowledge base related to the pathways involved in acquisition of DAPr phenotypes during DAP treatment failures, we investigated a recent DAPs/DAPr strain pair for a range of phenotypic CM and cell surface perturbations, as well as by focused genetic profiling. Several interesting observations emerged from this analysis. There were some similarities in phenotypic assay outcomes between the current strain set and our prior strain set, including enhancement of the cell surface positive charge, a reduction in the level of surface binding by cationic antibiotics and innate host defense CAPs, and the relative resistance to selected CAPs concomitantly with DAPr (11, 36). One notable difference between the phenotypic characteristics of the strain set from the current study and those described previously was seen in terms of LPG synthesis and the outer membrane translocation of LPG. In prior studies, mprF point mutations were associated with the synthesis of equivalent proportional amounts of total LPG between DAPs and DAPr isogenic pairs but enhanced outer membrane flipping of LPG (7, 11). In contrast, the current data demonstrate that our DAPr isolates synthesized increased total amounts of LPG compared with the amounts synthesized by the DAPs parental strain. However, on a proportional basis, the percentages of the total synthesized LPG which was then translocated to the outer CM leaflet were similar for the DAPs and DAPr isolates, suggesting no gain in flipping function among the DAPr isolates (MRSA 11/21 and REF2145). This enhanced synthesis of LPG in the DAPr strains, with the overall translocation percentage being maintained at a level equivalent to that in the DAPs parental isolate, would be expected to yield a net increase in the total number of LPG molecules translocated to the outer membrane leaflet. This notion is in line with the increased cell surface positive charge, as well as the increased CAP- and DAPr-repulsive phenotypes documented above for the current DAPr isolates.
One consistent feature of the DAPr phenotype in VISA strains has been their frequent development of increased CW thickness (4, 6, 16). It has not been determined whether such increased CW thickness in DAPr strains simply acts as a mechanical barrier to the penetration of this agent so that it may reach its CM target or as a more specific affinity-trapping mechanism for DAP, as suggested for VAN in VISA strains (6). In addition, at this juncture it is not clear whether the biochemical or the genetic pathway is involved in producing such thick CWs in DAPr strains. To investigate the correlation between CW thickness and DAPr, TEM analyses were performed on three previously published isogenic sets of S. aureus isolates from the bloodstreams of DAP-treated patients in which serial strains exhibited increasing DAPr in the face of clinical treatment failures (11, 36). Similar to the previous studies of in vitro-selected DAPr (4, 6, 16), strains DAPr 703 and BOY 300 demonstrated significantly thicker CWs than their DAPs parental strains, strains 616 and BOY 755, respectively. However, in contrast to the findings for these strain sets, the currently studied strains, DAPr strains MRSA 11/21 and REF2145, showed no difference in CW thickness from that of their parental MRSA 11/11 strain. These findings suggest that the development of DAPr in this strain set does not require significant CW perturbations. Substantiating this interpretation, phenotypic readouts of CW function assessed from the Triton X-100-induced autolysis profiles of DAPs strains MRSA 11/11 and MRSA 11/17 were virtually identical to those of DAPr strains MRSA 11/21 and REF2145 (data not shown). Thus, these data support the notion that CW abnormalities do not underlie the DAPr phenotype in the present strain set. Furthermore, three of four additional recent clinical DAPs/DAPr strain pairs showed no evidence of thickened CWs among the DAPr isolates. Transcriptomic and proteomic comparisons of DAPs/DAPr strain pairs are in progress to clarify whether specific metabolic pathways have a potential role in the thick CW phenotype.
Lastly, our laboratory has shown that the profiles of the enhanced expression of the mprF and/or dlt gene are intimately related to the maintenance of a relatively high positive surface charge in DAPr (compared with that in DAPs) S. aureus strains (11, 36, 37). Unlike previously characterized DAPr strain BOY 300 (36), RT-PCR analysis revealed comparable levels of dlt expression between DAPs (MRSA 11/11 and MRSA 11/17) and DAPr (MRSA 11/21 and REF2145) strains. In contrast to dlt expression, the two DAPr strains evaluated in the current study exhibited increased levels of mprF transcription compared with those of the respective DAPs strains during exponential growth, correlating proportionally with increased levels of synthesis of LPG in these strains. This mprF overexpression profile of MRSA 11/21 and REF2145 differs from that of previously characterized DAPr strains 701 and 703. In these DAPr strains, mprF overexpression occurred during stationary growth phase, was associated with a point mutation in the putative translocase domain of the mprF gene product (Ser to Leu at position 295), and was phenotypically associated with enhanced LPG flipping to the outer membrane leaflet of the DAPr isolate (11, 37). Interestingly, the point mutation noted within the mprF gene of MRSA 11/21 and REF2145 resides in the putative synthase domain of the MprF protein (Thr to Ala at position 345) (7, 19), consistent with enhanced LPG synthesis relative to that in the DAPs parental strains. Therefore, these data reinforce our previous hypothesis that DAP resistance is associated with mprF mutations that result in a gain of function for either LPG synthesis and/or translocation (11, 37). Whether mprF point mutations can fully account for DAPr is the topic of current studies in our laboratory, utilizing mutated mprF genes from DAPr strains cloned into DAPs background strains.
We have summarized the current and previous studies from our laboratory in Table 4 (11, 16, 36, 37). The information in Table 4 relates CW thickness, dlt and mprF mutations, gains in mprF function in PL synthesis/translocation, as well as surface charge and peptide binding perturbations. These comparative data underscore the concept that DAPr is a final common pathway that may be arrived at by multiple and distinct mechanisms.
TABLE 4.
Correlates of DAPr among recent S. aureus isolates: comparison with current DAPs and DAPr primary strain set
| DAPr strain(s) | Source | Increased positive surface charge | Potential mechanism(s) of increased positive surface | Genetic factor(s) potentially involved in surface charge alterations | CW thickness | Reference |
|---|---|---|---|---|---|---|
| CB2205 (MRSA MW2 USA 400) | In vitro selection by serial passage in DAP | No | NAa | NA | ↑b | 16 |
| 701, 703 (MSSAc) | Clinical isolates - endocarditis | Yes | Increased LPG translocation to outer membrane leaflet | Increased mprF transcription (stationary phase), S295L point mutation | ↑ | 11, 37 |
| BOY300 (MSSA) | Clinical isolate - endocarditis | Yes | Increased teichoic acid alanylation | Increased dlt transcription | ↑ | 36 |
| MRSA 11/21, REF2145 (MRSA USA 300) | Clinical isolates - endocarditis | Yes | Increased total LPG synthesis | Increased mprF transcription (exponential phase), T345A point mutation | Equivalent | This study |
NA, not applicable.
↑, increased.
MSSA, methicillin-susceptible S. aureus.
In summary, although a common accompaniment of DAPr in S. aureus, CW thickening is not a universal finding. This suggests that additional pathways and mechanisms leading to the DAPr phenotype manifested by the current strain set are in play. We speculate that the best explanation for the DAPr phenotype in the latter strain set involves the excess proportional synthesis of LPG with an ultimately increased net outer leaflet localization of this cationic PL, yielding reduced DAP access to or an association with vulnerable targets on the organism's surface.
Acknowledgments
This research was supported by grants from the National Institutes of Health to A.S.B. (grant AI39018) and M.R.Y. (grant AI39001).
Footnotes
Published ahead of print on 24 May 2010.
REFERENCES
- 1.Bharat, L. D., and M. G. Chhitar. 1998. Role of the actin cytoskeleton in regulating the outer phosphatidylethanolamine levels in yeast plasma membrane. Eur. J. Biochem. 254:202-206. [DOI] [PubMed] [Google Scholar]
- 2.Boucher, H. W., and G. Sakoulas. 2007. Antimicrobial resistance: perspectives on daptomycin resistance, with emphasis on resistance in Staphylococcus aureus. Clin. Infect. Dis. 45:601-608. [DOI] [PubMed] [Google Scholar]
- 3.Brunskill, E. W., and K. W. Bayles. 1996. Identification and molecular characterization of a putative regulatory locus that affects autolysis in Staphylococcus aureus. J. Bacteriol. 178:611-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Camargo, I. L, H.-M. Neoh, L. Cui, and K. Hiramatsu. 2008. Serial daptomycin selection generates daptomycin-nonsusceptible Staphylococcus aureus strains with a heterogeneous vancomycin-intermediate phenotype. Antimicrob. Agents Chemother. 52:4289-4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins, L. V., S. A. Kristian, C. Weidenmaier, M. Faigle, K. P. M. van Kessel, J. A. G. van Strijp, F. Gotz, B. Neumeister, and A. Peschel. 2002. Staphylococcus aureus strains lacking d-alanine modifications of teichoic acids are highly susceptible to human neutrophil killing and are virulence attenuated in mice. J. Infect. Dis. 186:214-219. [DOI] [PubMed] [Google Scholar]
- 6.Cui, L., E. Tominaga, H.-M. Neoh, and K. Hiramatsu. 2006. Correlation between reduced daptomycin susceptibility and vancomycin resistance in vancomycin-intermediate Staphylococcus aureus. Antimicrob. Agents Chemother. 50:1079-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ernst, C., P. Staubitz, N. N. Mishra, S. J. Yang, G. Hornig, H. Kalbacher, A. S. Bayer, D. Kraus, and A. Peschel. 2009. The bacterial defensin resistance protein MprF consists of separable domains for lipid lysinylation and antimicrobial peptide repulsion. PLos Pathog. 5:e1000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedman, L., J. D. Alder, and J. A. Silverman. 2006. Genetic changes that correlate with reduced susceptibility to daptomycin in Staphylococcus aureus. Antimicrob. Agents Chemother. 50:2137-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayden, M. K., K. Rezai, R. A. Hayes, K. Lolans, J. P. Quinn, and R. A. Weinstein. 2005. Development of daptomycin resistance in vivo in methicillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 43:5285-5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hiramatsu, K., H. Hanaki, T. Ino, K. Yabuta, T. Oguri, and F. C. Tenover. 1997. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J. Antimicrob. Chemother. 40:135-136. [DOI] [PubMed] [Google Scholar]
- 11.Jones, T., M. R. Yeaman, G. Sakoulas, S. J. Yang, R. A. Proctor, H. G. Sahl, J. Schrenzel, Y. Q. Xiong, and A. S. Bayer. 2008. Failures in clinical treatment of Staphylococcus aureus infection with daptomycin are associated with alterations in surface charge, membrane phospholipid asymmetry, and drug binding. Antimicrob. Agents Chemother. 52:269-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Julian, K., K. Kosowska-Shick, C. Whitener, M. Roos, H. Labischinski, A. Rubio, L. Parent, L. Ednie, L. Koeth, T. Bogdanovich, and P. C. Appelbaum. 2007. Characterization of a daptomycin-nonsusceptible vancomycin-intermediate Staphylococcus aureus strain in a patient with endocarditis. Antimicrob. Agents Chemother. 51:3445-3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lalani, T., J. J. Federspiel, H. W. Boucher, T. H. Rude, I.-G. Bae, M. J. Rybak, G. T. Tonthat, G. R. Corey, M. E. Stryjewski, G. Sakoulas, V. H. Chu, J. Alder, J. N. Steenbergen, S. A. Luperchio, M. Campion, C. W. Woods, and V. G. Fowler. 2008. Associations between the genotypes of Staphylococcus aureus bloodstream isolates and clinical characteristics and outcomes of bacteremic patients. J. Clin. Microbiol. 46:2890-2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mariani, P. G., H. S. Sader, and R. N. Jones. 2006. Development of decreased susceptibility to daptomycin and vancomycin in a Staphylococcus aureus strain during prolonged therapy. J. Antimicrob. Chemother. 58:481-483. [DOI] [PubMed] [Google Scholar]
- 15.Meehl, M., S. Herbert, F. Gotz, and A. Cheung. 2007. Interaction of the GraRS two-component system with the VraFG ABC transporter to support vancomycin-intermediate resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 51:2679-2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mishra, N. N., S. J. Yang, A. Sawa, A. Rubio, C. C. Nast, M. R. Yeaman, and A. S. Bayer. 2009. Analysis of cell membrane characteristics of in vitro-selected daptomycin-resistant strains of methicillin-resistant Staphylococcus aureus (MRSA). Antimicrob. Agents Chemother. 53:2312-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore, M. R., F. Perdreau-Remington, and H. F. Chambers. 2003. Vancomycin treatment failure associated with heterogeneous vancomycin-intermediate Staphylococcus aureus in a patient with endocarditis and in the rabbit model of endocarditis. Antimicrob. Agents Chemother. 47:1262-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mukhopadhyay, K., W. Whitmire, Y. Q. Xiong, J. Molden, T. Jones, A. Peschel, P. Staubitz, J. Adler-Moore, P. J. McNamara, R. A. Proctor, M. R. Yeaman, and A. S. Bayer. 2007. In vitro susceptibility of Staphylococcus aureus to thrombin-induced platelet microbicidal protein-1 (tPMP-1) is influenced by cell membrane phospholipid composition and asymmetry. Microbiology 153:1187-1197. [DOI] [PubMed] [Google Scholar]
- 19.Murthy, M. H., M. E. Olson, R. E. Wickert, P. D. Fey, and Z. Jalali. 2008. Daptomycin non-susceptible methicillin-resistant Staphylococcus aureus USA 300 isolate. J. Med. Microbiol. 57:1036-1038. [DOI] [PubMed] [Google Scholar]
- 20.Oku, Y., K. Kurokawa, N. Ichihashi, and K. Sekimizu. 2004. Characterization of the Staphylococcus aureus mprF gene, involved in lysinylation of phosphatidylglycerol. Microbiology 150:45-51. [DOI] [PubMed] [Google Scholar]
- 21.Peschel, A., M. Otto, R. W. Jack, H. Kalbacher, G. Jung, and F. Gotz. 1999. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J. Biol. Chem. 274:8405-8410. [DOI] [PubMed] [Google Scholar]
- 22.Pillai, S. K., H. S. Gold, G. Sakoulas, C. Wennersten, R. C. Moellering, Jr., and G. M. Eliopoulos. 2007. Daptomycin nonsusceptibility in Staphylococcus aureus with reduced vancomycin susceptibility is independent of alterations in MprF. Antimicrob. Agents Chemother. 51:2223-2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakoulas, G., G. M. Eliopoulos, J. Alder, and C. T. Eliopoulos. 2003. Efficacy of daptomycin in experimental endocarditis due to methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 47:1714-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schriever, C. A., C. Fernandez, K. A. Rodvold, and L. H. Danziger. 2005. Daptomycin: a novel cyclic lipopeptide antimicrobial. Am. J. Health Syst. Pharm. 62:1145-1158. [DOI] [PubMed] [Google Scholar]
- 25.Silverman, J. A., N. G. Perlmutter, and H. M. Shapiro. 2003. Correlation of daptomycin bactericidal activity and membrane depolarization in Staphylococcus aureus. Antimicrob. Agents Chemother. 47:2538-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skiest, D. J. 2006. Treatment failure resulting from resistance of Staphylococcus aureus to daptomycin. J. Clin. Microbiol. 44:655-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Staubitz, P., H. Neumann, T. Schneider, I. Wiedemann, and A. Peschel. 2004. MprF-mediated biosynthesis of lysylphosphatidylglycerol, an important determinant in staphylococcal defensin resistance. FEMS Microbiol. Lett. 231:67. [DOI] [PubMed] [Google Scholar]
- 28.Steenbergen, J. N., J. Alder, G. M. Thorne, and F. P. Tally. 2005. Daptomycin: a lipopeptide antibiotic for the treatment of serious Gram-positive infections. J. Antimicrob. Chemother. 55:283-288. [DOI] [PubMed] [Google Scholar]
- 29.Straus, S. K., and R. E. W. Hancock. 2006. Mode of action of the new antibiotic for Gram-positive pathogens daptomycin: comparison with cationic antimicrobial peptides and lipopeptides. Biochim. Biophys. Acta 1758:1215. [DOI] [PubMed] [Google Scholar]
- 30.Tally, F. P., and M. F. DeBruin. 2000. Development of daptomycin for Gram-positive infections. J. Antimicrob. Chemother. 46:523-526. [DOI] [PubMed] [Google Scholar]
- 31.Weidenmaier, C., A. Peschel, V. A. J. Kempf, N. Lucindo, M. R. Yeaman, and A. S. Bayer. 2005. DltABCD- and MprF-mediated cell envelope modifications of Staphylococcus aureus confer resistance to platelet microbicidal proteins and contribute to virulence in a rabbit endocarditis model. Infect. Immun. 73:8033-8038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wootton, M., A. P. MacGowan, and T. R. Walsh. 2006. Comparative bactericidal activities of daptomycin and vancomycin against glycopeptide-intermediate Staphylococcus aureus (GISA) and heterogeneous GISA isolates. Antimicrob. Agents Chemother. 50:4195-4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiong, Y. Q., A. S. Bayer, L. Elazegui, and M. R. Yeaman. 2006. A synthetic congener modeled on a microbicidal domain of thrombin-induced platelet microbicidal protein-1 recapitulates staphylocidal mechanisms of the native molecule. Antimicrob. Agents Chemother. 50:3786-3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiong, Y. Q., K. Mukhopadhyay, M. R. Yeaman, J. Adler-Moore, and A. S. Bayer. 2005. Functional interrelationships between cell membrane and cell wall in antimicrobial peptide-mediated killing of Staphylococcus aureus. Antimicrob. Agents Chemother. 49:3114-3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang, S.-J., K. C. Rice, R. J. Brown, T. G. Patton, L. E. Liou, Y. H. Park, and K. W. Bayles. 2005. A LysR-type regulator, CidR, is required for induction of the Staphylococcus aureus cidABC operon. J. Bacteriol. 187:5893-5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang, S. J., B. N. Kreiswirth, G. Sakoulas, M. R. Yeaman, Y. Q. Xiong, A. Sawa, and A. S. Bayer. 2009. Enhanced expression of dltABCD is associated with development of daptomycin nonsusceptibility in a clinical endocarditis isolate of Staphylococcus aureus. J. Infect. Dis. 200:1916-1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang, S. J., Y. Q. Xiong, P. M. Dunman, J. Schrenzel, P. Francois, A. Peschel, and A. S. Bayer. 2009. Regulation of mprF in daptomycin-nonsusceptible Staphylococcus aureus. Antimicrob. Agents Chemother. 53:2636-2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yeaman, M. R., A. S. Bayer, S.-P. Koo, W. Foss, and P. M. Sullam. 1998. Platelet microbicidal proteins and neutrophil defensin disrupt the Staphylococcus aureus cytoplasmic membrane by distinct mechanisms of action. J. Clin. Invest. 101:178-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yeaman, M. R., K. D. Gank, A. S. Bayer, and E. P. Brass. 2002. Synthetic peptides that exert antimicrobial activities in whole blood and blood-derived matrices. Antimicrob. Agents Chemother. 46:3883-3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yeaman, M. R., S. M. Puentes, D. C. Norman, and A. S. Bayer. 1992. Partial characterization and staphylocidal activity of thrombin-induced platelet microbicidal protein. Infect. Immun. 60:1202-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]